Abstract
Lower levels of serum bicarbonate and a higher anion gap have been associated with insulin resistance and hypertension in the general population. Whether these associations extend to other cardiovascular disease risk factors is unknown. To clarify this, we examined the association of serum bicarbonate and anion gap with cardiorespiratory fitness in 2714 adults aged 20–49 years in the 1999–2004 National Health and Nutrition Examination Survey. The mean serum bicarbonate was 24.6 mEq/L and the mean anion gap was 10.26 mEq/L, with fitness determined by submaximal exercise testing. After multivariable adjustment, gender, length of fasting, soft drink consumption, systolic blood pressure, serum phosphate, and hemoglobin were independently associated with both the serum bicarbonate and the anion gap. Low fitness was most prevalent among those in the lowest quartile of serum bicarbonate or highest quartile of anion gap. After multivariable adjustment, a one standard deviation higher serum bicarbonate or anion gap was associated with an odds ratio for low fitness of 0.80 (95% CI 0.70–0.91) and 1.30 (95% CI 1.15–1.48), respectively. The association of bicarbonate with fitness may be mediated by differences in lean body mass. Thus, lower levels of serum bicarbonate and higher levels of anion gap are associated with lower cardiorespiratory fitness in adults aged 20–49 years in the general population.
INTRODUCTION
Chronic metabolic acidosis as a manifestation of chronic kidney disease (CKD) is believed to adversely affect skeletal muscle protein breakdown.1 Chronic acidosis may have other sequelae, including contributing to insulin resistance2 and the progression of kidney disease.3, 4 These effects may extend to the non-CKD population. A low-level chronic acidosis, partly due to aging and the acidogenic Western diet, has been associated with pathophysiologic consequences in older persons without CKD, including urinary nitrogen wasting5 and reduced muscle strength.6, 7
Cross-sectional studies of healthy persons have demonstrated an association of lower levels of serum bicarbonate and higher levels of anion gap (AG) with greater insulin resistance and higher blood pressure.8–10 Whether these associations extend to other cardiovascular disease (CVD) risk factors is unknown. Low cardiorespiratory fitness, or aerobic capacity, is associated with an increased prevalence of CVD risk factors11 and with mortality and incident CVD.12, 13 Reduced muscle mass or function due to metabolic acidosis could reduce skeletal muscle oxygen uptake and thus cardiorespiratory fitness.
We hypothesized that lower levels of serum bicarbonate and higher levels of AG would be associated with lower cardiorespiratory fitness in the general population. We tested these hypotheses in adult participants aged 20–49 years who completed submaximal exercise testing in the National Health and Nutrition Examination Survey (NHANES) 1999–2004.
RESULTS
Participant Characteristics
The mean serum bicarbonate was 24.6 mEq/L (SE 0.1) and the mean AG was 10.26 mEq/L (SE 0.18). Participants with lower serum bicarbonate were more likely to be women, had higher body-mass index (BMI), greater poverty, and higher estimated net endogenous acid production (NEAP), were less likely to have fasted 2 hours or less prior to phlebotomy, were more likely to report consuming soft drinks and to have hypertension, and had higher levels of AG and C-reactive protein (CRP) and lower serum albumin, calcium, and hemoglobin, and lower % lean body mass (%LBM) (Table 1). Participants with higher levels of AG had higher BMI, greater poverty, were more likely to report soft drink consumption and to have hypertension and diabetes, had higher systolic blood pressure, were more likely to have albuminuria, and had lower serum bicarbonate and higher hemoglobin, total cholesterol, and CRP levels, and lower %LBM (Table 2). No participants had CVD by self-report.
Table 1.
Participant Characteristics by Quartiles of Serum Bicarbonate
| Characteristic | Serum Bicarbonate (mEq/L)
|
P | |||
|---|---|---|---|---|---|
| < 24 | 24–25 | 25.1–26.9 | ≥ 27 | ||
| Number | 833 | 661 | 747 | 473 | |
| Age (years) | 33.8 (0.4) | 33.7 (0.5) | 34.3 (0.4) | 34.2 (0.6) | 0.43 |
| Women (%) | 60.8 (3.2) | 50.0 (2.8) | 40.1 (2.0) | 26.6 (2.6) | <0.001 |
| Race/Ethnicity (%) | 0.14 | ||||
| Non-Hispanic White | 70.8 (2.2) | 73.0 (2.3) | 69.9 (2.5) | 76.4 (2.0) | |
| Mexican American | 11.3 (2.3) | 9.4 (1.2) | 10.5 (1.3) | 5.4 (0.9) | |
| Non-Hispanic Black | 8.2 (1.3) | 10.2 (1.7) | 11.1 (1.3) | 10.5 (1.5) | |
| Body-mass index (kg/m2) | 28.2 (0.3) | 27.0 (0.3) | 26.7 (0.2) | 25.5 (0.2) | <0.001 |
| % Lean body mass | 62.5 (0.3) | 65.0 (0.4) | 66.7 (0.4) | 69.1 (0.5) | <0.001 |
| Poverty (<100% poverty index, %) | 12.8 (1.6) | 12.3 (1.9) | 11.0 (1.4) | 7.2 (1.5) | 0.01 |
| Less than high-school diploma (%) | 12.1 (1.3) | 13.5 (1.5) | 14.6 (1.6) | 11.0 (2.3) | 0.97 |
| Activity Level (MET-min/wk, %) | 0.08 | ||||
| 0 | 9.5 (1.1) | 8.5 (1.1) | 12.0 (1.6) | 6.8 (1.4) | |
| < 500 | 22.9 (2.1) | 19.7 (1.8) | 17.7 (1.7) | 17.6 (2.2) | |
| 500–2000 | 35.1 (1.9) | 37.9 (3.2) | 38.8 (2.1) | 35.6 (2.0) | |
| > 2000 | 32.5 (2.6) | 33.8 (2.6) | 31.5 (2.3) | 40.0 (2.8) | |
| Smoking (%) | 0.24 | ||||
| Never | 55.0 (3.1) | 55.0 (2.4) | 58.4(2.3) | 61.6 (2.9) | |
| Former | 15.4 (1.7) | 17.4 (2.0) | 15.5 (1.4) | 17.2 (1.7) | |
| Current | 29.6 (2.6) | 27.6 (2.0) | 25.9 (2.3) | 21.3 (2.4) | |
| Estimated NEAP (mEq/day) | 60.4 (1.2) | 62.9 (1.0) | 59.8 (1.2) | 55.1 (1.1) | 0.005 |
| Fasting length ≤2 hours (%) | 1.4 (0.4) | 2.5 (0.8) | 2.7 (0.5) | 4.4 (1.9) | 0.04 |
| Soft drink consumption (%) | 0.003 | ||||
| None | 45.6 (3.2) | 56.7 (3.2) | 43.9 (2.6) | 55.9 (3.6) | |
| 1 | 31.9 (2.9) | 23.2 (3.0) | 27.9 (1.9) | 27.7 (2.8) | |
| ≥ 2 | 22.5 (1.9) | 20.1 (2.4) | 28.2 (1.8) | 16.5 (2.7) | |
| Diuretic use (%) | 0.9 (0.5) | 1.0 (0.4) | 2.3 (0.9) | 1.4 (0.6) | 0.23 |
| Cholesterol-lowering medication (%) | 1.4 (0.5) | 2.2 (0.9) | 2.6 (0.8) | 1.7 (0.9) | 0.59 |
| Hypertension (%) | 19.8 (1.9) | 9.6 (1.5) | 13.6 (1.7) | 10.9 (1.9) | 0.002 |
| Diabetes Mellitus (%) | 1.7 (0.6) | 0.9 (0.4) | 1.5 (0.6) | 1.2 (0.6) | 0.64 |
| Systolic Blood Pressure (mm Hg) | 115.5 (0.6) | 115.0 (0.7) | 114.7 (0.7) | 115.3 (0.7) | 0.60 |
| eGFR (mL/min/1.73m2) | 103.3 (1.0) | 102.6 (0.9) | 102.8 (0.8) | 102.8 (1.2) | 0.73 |
| UACR > 30 mg/g (%) | 4.9 (0.8) | 5.5 (1.2) | 3.4 (1.0) | 2.9 (1.2) | 0.13 |
| Serum anion gap (mEq/L) | 11.6 (0.3) | 10.4 (0.2) | 9.7 (0.2) | 8.5 (0.2) | <0.001 |
| Serum albumin (g/dL) | 4.37 (0.01) | 4.43 (0.02) | 4.46 (0.02) | 4.56 (0.03) | <0.001 |
| Serum calcium (mg/dL) | 9.41 (0.02) | 9.52 (0.02) | 9.51 (0.02) | 9.54 (0.04) | 0.001 |
| Serum phosphate (mg/dL) | 3.71 (0.03) | 3.75 (0.03) | 3.67 (0.03) | 3.70 (0.03) | 0.44 |
| Hemoglobin (g/dL) | 14.48 (0.11) | 14.60 (0.10) | 14.66 (0.07) | 14.94 (0.08) | 0.005 |
| Total cholesterol (mg/dL) | 191.3 (1.6) | 191.5 (1.8) | 193.2 (2.3) | 193.6 (2.6) | 0.38 |
| HDL cholesterol (mg/dL) | 50.1 (0.8) | 52.5 (0.8) | 50.7 (0.7) | 52.4 (1.0) | 0.16 |
| C-reactive protein ≥ 1.0 mg/dL (%) | 7.8 (0.9) | 5.8 (0.9) | 4.9 (1.0) | 5.2 (1.2) | 0.04 |
Abbreviations: MET, metabolic equivalent; NEAP, net endogenous acid production; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-creatinine ratio.
Data are expressed as mean (standard error (SE)) or percent (SE).
Table 2.
Participant Characteristics by Quartiles of Serum Anion Gap
| Characteristic | Serum Anion Gap (mEq/L)
|
P | |||
|---|---|---|---|---|---|
| < 8.60 | 8.60–10.25 | 10.26–11.90 | ≥ 11.91 | ||
| Number | 653 | 735 | 691 | 635 | |
| Age (years) | 34.7 (0.5) | 33.6 (0.5) | 33.8 (0.4) | 33.8 (0.4) | 0.26 |
| Women (%) | 42.5 (2.0) | 47.8 (2.5) | 48.3 (3.4) | 47.0 (2.3) | 0.18 |
| Race/Ethnicity (%) | 0.46 | ||||
| Non-Hispanic White | 75.4 (2.4) | 68.8 (2.6) | 73.6 (2.2) | 71.0 (2.8) | |
| Mexican American | 8.9 (1.3) | 10.2 (1.5) | 9.2 (1.4) | 9.8 (2.1) | |
| Non-Hispanic Black | 9.5 (1.3) | 11.5 (1.5) | 9.4 (1.3) | 9.0 (1.9) | |
| Body-mass index (kg/m2) | 26.4 (0.3) | 26.9 (0.2) | 27.1 (0.3) | 27.8 (0.4) | 0.001 |
| % Lean body mass | 66.6 (0.4) | 65.3 (0.4) | 65.2 (0.4) | 64.5 (0.4) | <0.001 |
| Poverty (<100% poverty index, %) | 7.3 (1.2) | 12.6 (1.5) | 11.9 (1.4) | 12.9 (2.2) | 0.05 |
| Less than high-school diploma (%) | 12.0 (2.3) | 13.4 (1.4) | 14.5 (1.4) | 11.5 (1.6) | 0.98 |
| Activity Level (MET-min/wk, %) | 0.58 | ||||
| 0 | 7.2 (1.0) | 10.2 (1.4) | 11.9 (1.6) | 8.2 (1.2) | |
| < 500 | 20.1 (2.5) | 20.8 (2.0) | 19.0 (2.2) | 19.2 (2.6) | |
| 500–2000 | 37.1 (2.5) | 36.8 (2.8) | 37.2 (2.5) | 36.1 (2.7) | |
| > 2000 | 35.6 (2.0) | 32.2 (2.5) | 31.9 (1.8) | 36.6 (3.4) | |
| Smoking (%) | 0.16 | ||||
| Never | 61.9 (2.7) | 54.7 (2.2) | 56.7 (2.7) | 55.2 (4.1) | |
| Former | 17.3 (1.7) | 17.5 (1.7) | 14.2 (1.7) | 16.0 (2.0) | |
| Current | 20.8 (2.4) | 27.8 (1.6) | 29.1 (2.4) | 28.8 (3.2) | |
| Estimated NEAP (mEq/day) | 58.7 (1.3) | 59.0 (1.3) | 60.5 (1.3) | 61.3 (1.4) | 0.16 |
| Fasting length ≤2 hours (%) | 3.4 (1.2) | 3.1 (0.9) | 1.5 (0.5) | 2.3 (0.7) | 0.21 |
| Soft drink consumption (%) | 0.004 | ||||
| None | 55.6 (3.6) | 56.6 (3.3) | 44.8 (3.1) | 41.7 (3.6) | |
| 1 | 26.4 (2.8) | 25.2 (2.8) | 28.7 (2.4) | 31.7 (2.7) | |
| ≥ 2 | 17.9 (2.0) | 18.2 (2.3) | 26.5 (2.2) | 26.6 (2.8) | |
| Diuretic use (%) | 1.0 (0.4) | 1.4 (0.8) | 1.4 (0.6) | 1.7 (0.7) | 0.36 |
| Cholesterol-lowering medication (%) | 2.2 (0.9) | 2.1 (0.7) | 2.0 (0.6) | 1.6 (0.6) | 0.58 |
| Hypertension (%) | 10.0 (1.3) | 13.4 (1.9) | 16.1 (1.8) | 16.8 (2.3) | 0.003 |
| Diabetes Mellitus (%) | 0.2 (0.1) | 1.5 (0.6) | 1.1 (0.2) | 2.6 (0.8) | 0.005 |
| Systolic Blood Pressure (mm Hg) | 113.3 (0.6) | 114.8 (0.7) | 116.0 (0.6) | 116.5 (0.8) | 0.001 |
| eGFR (mL/min/1.73m2) | 103.1 (1.1) | 103.8 (0.9) | 101.9 (0.8) | 102.8 (1.0) | 0.51 |
| UACR > 30 mg/g (%) | 2.9 (0.8) | 3.6 (0.8) | 5.9 (1.2) | 4.9 (1.2) | 0.05 |
| Serum bicarbonate (mEq/L) | 26.1 (0.2) | 24.9 (0.1) | 24.1 (0.2) | 23.3 (0.2) | <0.001 |
| Serum albumin (g/dL) | 4.48 (0.02) | 4.43 (0.02) | 4.43 (0.02) | 4.44 (0.02) | 0.40 |
| Serum calcium (mg/dL) | 9.47 (0.03) | 9.49 (0.02) | 9.49 (0.02) | 9.50 (0.04) | 0.47 |
| Serum phosphate (mg/dL) | 3.65 (0.03) | 3.72 (0.03) | 3.68 (0.03) | 3.79 (0.03) | 0.01 |
| Hemoglobin (g/dL) | 14.55 (0.09) | 14.50 (0.08) | 14.71 (0.09) | 14.81 (0.09) | 0.008 |
| Total cholesterol (mg/dL) | 187.9 (1.5) | 190.4 (2.0) | 193.6 (2.1) | 197.5 (2.6) | 0.003 |
| HDL cholesterol (mg/dL) | 51.6 (0.6) | 52.3 (0.7) | 51.4 (0.9) | 49.6 (0.8) | 0.09 |
| C-reactive protein ≥ 1.0 mg/dL (%) | 3.2 (1.0) | 5.3 (1.2) | 6.9 (0.9) | 9.0 (1.6) | 0.001 |
Abbreviations: MET, metabolic equivalent; NEAP, net endogenous acid production; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-creatinine ratio.
Data are expressed as mean (standard error (SE)) or percent (SE).
After multivariable adjustment, sex, fasting length, soft drink consumption, systolic blood pressure, serum phosphate, and hemoglobin were independently associated with both serum bicarbonate and AG (Table 3). For each, the direction of association differed for serum bicarbonate and AG, respectively. In addition, age, diuretic use, and serum sodium and chloride were significantly associated with bicarbonate level, and total cholesterol and CRP were significantly associated with AG. Multivariable models were also examined entering sugar-sweetened and diet soft drink consumption simultaneously into the model. Of 1,371 participants reporting consumption of ≥1 soft drink, 351 (25.6%) reported only diet soft drink consumption. Sugar-sweetened soft drink consumption (≥1 versus 0) was associated with a 0.36 mEq/L (95% confidence interval (CI) 0.03–0.70) lower serum bicarbonate and 0.68 mEq/L (95% CI 0.18–1.19) higher AG. Diet soft drink consumption (≥1 versus 0) was associated with a 0.19 mEq/L (95% CI −0.07 to 0.46) lower serum bicarbonate and 0.24 mEq/L (95% CI −0.06 to 0.55) higher AG.
Table 3.
Independent Predictors of Serum Bicarbonate and Anion Gap
| Characteristic | Serum Bicarbonate | Anion Gap | ||
|---|---|---|---|---|
|
| ||||
| Change (mEq/L) | P | Change (mEq/L) | P | |
| Age (per 10 years) | 0.14 (0.01 to 0.26) | 0.03 | −0.17 (−0.35 to 0.01) | 0.06 |
| Women | −1.28 (−1.61–−0.95) | <0.001 | 0.76 (0.33–1.19) | 0.001 |
| Race/Ethnicity | ||||
| Mexican American | −0.31 (−0.72–0.10) | 0.13 | 0.11 (−0.43–to 0.64) | 0.69 |
| Non-Hispanic Black | 0.10 (−0.28 to 0.48) | 0.60 | 0.10 (−0.41 to 0.61) | 0.69 |
| Other | −0.27 (−0.72 to 0.17) | 0.22 | 0.39 (−0.13 to 0.91) | 0.14 |
| Body-mass index (per 1 kg/m2) | −0.03 (−0.05 to −0.01) | 0.003 | 0.003 (−0.02 to 0.03) | 0.83 |
| Poverty (<100% poverty index) | −0.14 (−0.48 to 0.20) | 0.41 | 0.20 (−0.22 to 0.63) | 0.34 |
| Less than high-school diploma | −0.23 (−0.55 to 0.10) | 0.17 | 0.21 (−0.17 to 0.60) | 0.27 |
| Activity Level (MET-min/wk) | ||||
| < 500 | −0.13 (−0.48 to 0.21) | 0.44 | −0.07 (−0.53 to 0.39) | 0.76 |
| 500–2000 | −0.17 (−0.43 to 0.09) | 0.20 | 0.11 (−0.23 to 0.46) | 0.52 |
| > 2000 | −0.25 (−0.53 to 0.04) | 0.09 | 0.25 (−0.10 to 0.61) | 0.16 |
| Smoking | ||||
| Former | −0.11 (−0.33 to 0.12) | 0.35 | 0.11 (−0.20 to 0.42) | 0.48 |
| Current | −0.44 (−0.75 to −0.14) | 0.006 | 0.24 (−0.11 to 0.60) | 0.17 |
| Estimated NEAP (per 10 mEq/day) | −0.04 (−0.08 to 0.01) | 0.08 | 0.02 (−0.03 to 0.07) | 0.48 |
| Fasting length | to | to | ||
| 2–6 hours | −0.61 (−1.37 to 0.16) | 0.12 | 0.76 (−0.06 to 1.57) | 0.07 |
| > 6 hours | −0.69 (−1.32 to −0.06) | 0.03 | 0.67 (0.13 to 1.20) | 0.02 |
| Soft drink consumption | ||||
| 1 | −0.26 (−0.57 to 0.05) | 0.10 | 0.48 (0.06 to 0.90) | 0.03 |
| ≥ 2 | −0.30 (−0.58 to −0.02) | 0.04 | 0.60 (0.17 to 1.04) | 0.008 |
| Diuretic use | 0.94 (0.34 to 1.55) | 0.003 | −0.28 (−1.22 to 0.65) | 0.54 |
| Cholesterol-lowering medication | 0.13 (−0.59 to 0.86) | 0.71 | −0.24 (−1.19 to 0.69) | 0.60 |
| Diabetes Mellitus | −0.15 (−0.92 to 0.63) | 0.71 | 0.64 (−0.25 to 1.53) | 0.15 |
| Systolic BP (per 10 mmHg) | −0.16 (−0.25 to −0.07) | 0.001 | 0.22 (0.09 to 0.35) | 0.002 |
| eGFR | ||||
| 75–89 mL/min/1.73m2 | −0.08 (−0.33 to 0.16) | 0.49 | 0.07 (−0.25 to 0.40) | 0.65 |
| < 75 mL/min/1.73m2 | −0.52 (−1.23 to 0.18) | 0.14 | 0.45 (−0.55 to 1.45) | 0.37 |
| UACR > 30 mg/g (%) | −0.04 (−0.42 to 0.35) | 0.85 | 0.10 (−0.33 to 0.53) | 0.65 |
| Serum albumin (g/dL) | −0.33 (−1.01 to 0.35) | 0.33 | −0.50 (−1.31 to 0.32) | 0.22 |
| Serum sodium (mg/dL) | 0.44 (0.36 to 0.52) | <0.001 | …… | |
| Serum chloride (mg/dL) | −0.33 (−0.42 to −0.24) | <0.001 | …… | |
| Serum potassium (mg/dL) | 0.32 (−0.09 to 0.73) | 0.12 | −0.38 (−1.00 to 0.24) | 0.22 |
| Serum calcium (mg/dL) | 0.20 (−0.38 to 0.77) | 0.50 | 0.08 (−0.62 to 0.79) | 0.81 |
| Serum phosphate (mg/dL) | −0.34 (−0.50 to −0.18) | <0.001 | 0.52 (0.26 to 0.77) | <0.001 |
| Hemoglobin (g/dL) | −0.25 (−0.39 to −0.10) | 0.001 | 0.31 (0.11 to 0.52) | 0.003 |
| Total cholesterol (per 10 mg/dL) | −0.004 (−0.03 to 0.02) | 0.71 | 0.04 (0.01 to 0.07) | 0.01 |
| HDL cholesterol (per 10 mg/dL) | 0.08 (−0.02 to 0.17) | 0.10 | −0.04 (−0.14 to 0.07) | 0.49 |
| C-reactive protein | ||||
| 0.14–0.99 mg/dL | −0.06 (−0.25 to 0.14) | 0.57 | 0.29 (0.05 to 0.54) | 0.02 |
| ≥ 1.0 mg/dL | −0.18 (−0.61 to 0.24) | 0.39 | 0.73 (0.11 to 1.35) | 0.02 |
Abbreviations: MET, metabolic equivalent; BP, blood pressure; NEAP, net endogenous acid production; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-creatinine ratio.
Multivariable model adjusted for all variables listed in the Table. Reference categories are non-Hispanic white for race/ethnicity; 0 Met-min/wk for activity level; non-smokers for smoking status; fasting length ≤2 hours; no soft drink consumption; eGFR ≥90 mL/min/1.73m2 for eGFR; and CRP <0.14 mg/dL
Association of Serum Bicarbonate and Anion Gap with Cardiorespiratory Fitness
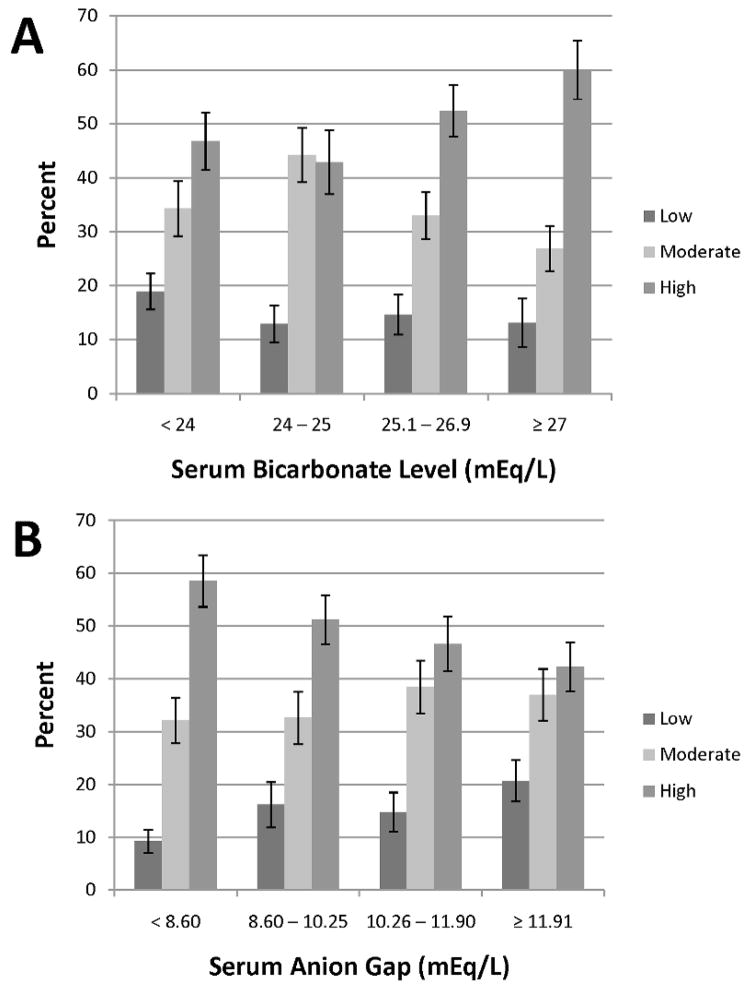
Figure 1 shows the prevalence of cardiorespiratory fitness level by quartiles of serum bicarbonate and AG. Low fitness, based on age- and sex-specific cutpoints, was present among 451 participants and was most prevalent among those in the lowest quartile of serum bicarbonate or highest quartile of AG (18.9% (95% CI 15.6–22.2) and 20.7% (95% CI 16.8–24.6), respectively).
Figure 1.
Percentage of cardiorespiratory fitness level by quartiles of serum bicarbonate (panel A) and serum anion gap (panel B). Error bars signify standard errors.
In unadjusted analysis, a 1 standard deviation (SD) higher serum bicarbonate was associated with a 1.46 mL/kg/min (95% confidence interval (CI) 1.00–1.93) greater estimated maximal oxygen consumption (VO2max), while a 1 SD higher AG was associated with a 1.07 mL/kg/min (95% CI 0.58–1.56) lower estimated VO2max (Table 4, upper panel). Multivariable adjustment somewhat attenuated these associations. Modeling serum bicarbonate and AG as quartiles confirmed significant linear associations of each predictor variable with estimated VO2max (Table 4, upper panel).
Table 4.
Association of Cardiorespiratory Fitness with Serum Bicarbonate and Anion Gap
| Association of Estimated VO2max (mL/kg/min) with Serum Bicarbonate and Anion Gap
| |||
|---|---|---|---|
| Coefficient (95% CI)
|
|||
| Model 1 | Model 2 | Model 3 | |
| Bicarbonate | |||
| Continuous* | 1.46 (1.00 to 1.93) | 0.65 (0.24 to 1.05) | 0.88 (0.44 to 1.31) |
| < 24 mEq/L | [Ref] | [Ref] | [Ref] |
| 24–25 mEq/L | 0.73 (−0.53 to 1.99) | −0.03 (−1.32 to 1.25) | 0.18 (−1.08 to 1.44) |
| 25.1–26.9 mEq/L | 2.72 (1.52 to 3.91) | 1.41 (0.13 to 2.69) | 1.70 (0.41 to 2.98) |
| ≥ 27 mEq/L | 3.76 (2.17 to 5.35) | 1.49 (0.07 to 2.90) | 1.85 (0.38 to 3.32) |
| P for trend | <0.001 | 0.008 | 0.002 |
| Anion Gap | |||
| Continuous* | −1.07 (−1.56 to −0.58) | −1.05 (−1.49 to −0.61) | −0.97 (−1.42 to −0.52) |
| < 8.60 mEq/L | [Ref] | [Ref] | [Ref] |
| 8.60–10.25 mEq/L | −1.54 (−2.81 to −0.27) | −1.19 (−2.35 to −0.03) | −1.04 (−2.08 to 0.01) |
| 10.26–11.90 mEq/L | −2.41 (−3.63 to −1.20) | −2.08 (−3.13 to −1.03) | −1.90 (−2.80 to −0.99) |
| ≥ 11.91 mEq/L | −2.77 (−3.91 to −1.63) | −2.50 (−3.67 to −1.33) | −2.24 (−3.44 to −1.04) |
| P for trend | <0.001 | <0.001 | <0.001 |
| Odds Ratio of Low Cardiorespiratory Fitness by Serum Bicarbonate and Anion Gap
| |||
|---|---|---|---|
| OR (95% CI) | |||
|
| |||
| Model 1 | Model 2 | Model 3 | |
| Bicarbonate | |||
| Continuous* | 0.84 (0.74–0.95) | 0.85 (0.75–0.96) | 0.80 (0.70–0.92) |
| < 24 mEq/L | [Ref] | [Ref] | [Ref] |
| 24–25 mEq/L | 0.64 (0.43–0.94) | 0.64 (0.43–0.95) | 0.62 (0.39–0.99) |
| 25.1–26.9 mEq/L | 0.73 (0.49–1.08) | 0.73 (0.49–1.08) | 0.69 (0.44–1.08) |
| ≥ 27 mEq/L | 0.65 (0.41–1.03) | 0.68 (0.42–1.09) | 0.64 (0.37–1.10) |
| P for trend | 0.07 | 0.10 | 0.11 |
| Anion Gap | |||
| Continuous* | 1.32 (1.16–1.51) | 1.32 (1.15–1.52) | 1.30 (1.15–1.48) |
| < 8.60 mEq/L | [Ref] | [Ref] | [Ref] |
| 8.60–10.25 mEq/L | 1.88 (1.28–2.77) | 1.79 (1.21–2.65) | 1.74 (1.15–2.62) |
| 10.26–11.90 mEq/L | 1.69 (1.19–2.41) | 1.67 (1.16–2.38) | 1.62 (1.16–2.26) |
| ≥ 11.91 mEq/L | 2.54 (1.78–3.62) | 2.48 (1.73–3.55) | 2.33 (1.68–3.22) |
| P for trend | <0.001 | <0.001 | <0.001 |
Abbreviations: OR, odds ratio; CI, confidence interval. Bold values indicate p<0.05.
Model 1: unadjusted
Model 2: adjusted for age, sex, and race/ethnicity
Model 3: Model 2 + adjusted for BMI, poverty, education, activity level, smoking status, estimated dietary potential renal acid load, fasting time, soft drink consumption, diuretic use, use of cholesterol-lowering medications, diagnosis of diabetes mellitus, systolic blood pressure, eGFR, log-transformed urine albumin-creatinine ratio, serum albumin, potassium, calcium, phosphate, hemoglobin, total cholesterol, HDL cholesterol, log-transformed CRP, and (for bicarbonate models) serum sodium and chloride
Per standard deviation higher serum bicarbonate (SD = 2.16 mEq/L) and adjusted anion gap (SD = 2.36 mEq/L), respectively.
Serum bicarbonate and AG were associated in opposite directions with low fitness in univariate analysis (Table 4, lower panel). These associations were largely unchanged after multivariable adjustment. Compared with participants in the lowest quartile of serum bicarbonate, there was a uniform decreased likelihood of low fitness among participants with bicarbonate ≥24 mEq/L in the fully adjusted model (OR 0.62 (95% CI 0.39–0.98), 0.69 (95% CI 0.44–1.07), and 0.64 (95% CI 0.37–1.09) for serum bicarbonate 24–25, 25.1–26.9, and ≥ 27 mEq/L, respectively). Higher levels of AG as quartiles were associated with a greater likelihood of low fitness after multivariable adjustment.
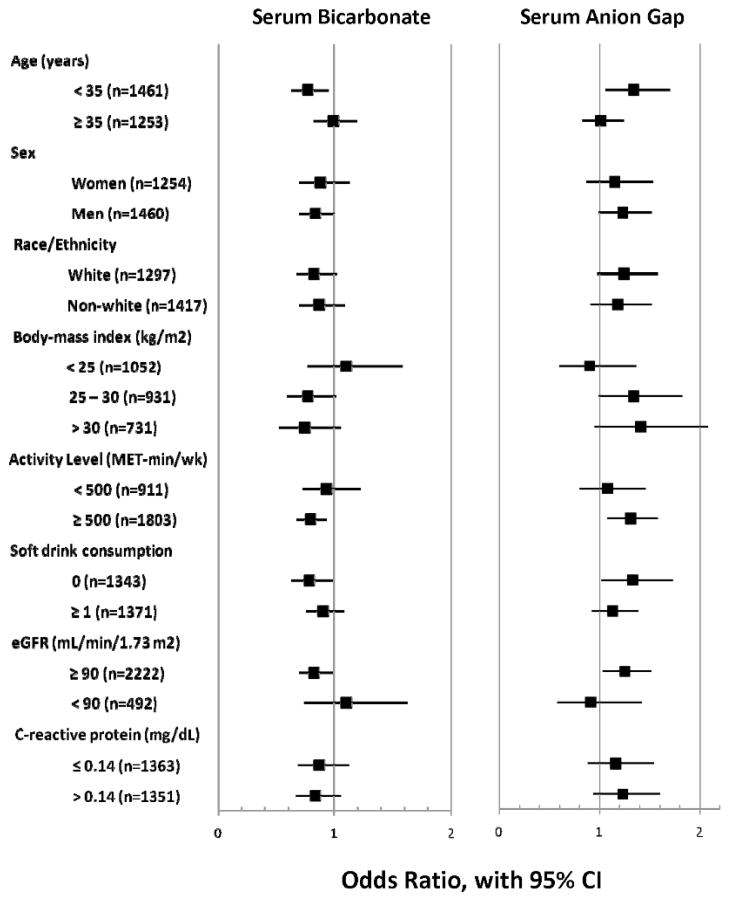
Effect modification of the associations of serum bicarbonate and AG with cardiorespiratory fitness was examined in fully adjusted models for the covariates specified above. For all interactions p>0.2 except for serum bicarbonate with sex (p=0.20), activity level (p=0.003), and CRP (p=0.17). Visual inspection of the forest plot (Figure 2) demonstrates a similar but inverse pattern for the associations of serum bicarbonate and AG within participant subgroups.
Figure 2.
Fully adjusted odds ratios for low cardiorespiratory fitness within participant subgroups. P>0.2 for all interactions except for effect modification of serum bicarbonate with sex (p=0.20), activity level (p=0.003), and C-reactive protein (p=0.17).
Sensitivity Analyses
Adjustment for %LBM rendered associations of serum bicarbonate with fitness non-significant, while associations of AG with fitness were mildly attenuated but remained significant (Table 5). Forty participants had serum bicarbonate <20 mEq/L. There was a trend toward greater fitness with higher serum bicarbonate even among those with low to low-normal bicarbonate levels (Supplementary Table S1, upper panels). Compared with participants with serum bicarbonate >22 mEq/L, those with bicarbonate ≤22 mEq/L had 1.77 (95% CI 0.52–3.01) mL/kg/min lower estimated VO2max and a greater likelihood of low fitness (OR 1.73, 95% CI 1.26–2.37) in multivariable analyses. The association of serum bicarbonate with estimated VO2max was more robust among men than women but the association with low fitness did not differ significantly by sex (Table S2; p for interaction = 0.05 and 0.20, respectively). After calculating AG without adjustment for albumin, we found no substantive difference in our results (Table S1, lower panels). Among participants who reported never being told of a diagnosis of asthma (n=2,505), the multivariable-adjusted OR for low fitness was 0.82 (95% CI 0.72–0.94) per SD higher serum bicarbonate and 1.28 (95% CI 1.12–1.46) per SD higher AG. After defining the cohort without exclusions for missing covariate data or invalid dietary data (n=3,013), the age, sex, and race/ethnicity-adjusted OR for low fitness was 0.84 (95% CI 0.75–0.94) per SD higher serum bicarbonate and 1.30 (95% CI 1.14–1.49) per SD higher AG. Associations with estimated VO2max were also unchanged (data not shown). There was a direct association of higher activity decile with greater estimated VO2max and lower odds of low fitness in unadjusted models (p<0.001 for trend for each). Including activity deciles in multivariable models instead of the previously defined categories did not change the associations of serum bicarbonate and AG with estimated VO2max or low fitness (data not shown).
Table 5.
Association of Cardiorespiratory Fitness with Serum Bicarbonate and Anion Gap after Adjustment for % Lean Body Mass
| Association of Estimated VO2max (mL/kg/min) with Serum Bicarbonate and Anion Gap
| |||
|---|---|---|---|
| Coefficient (95% CI) | Coefficient (95% CI) | ||
| Bicarbonate | Anion Gap | ||
| Continuous* | 0.14 (−0.23 to 0.51) | Continuous* | −0.68 (−1.11 to −0.25) |
| < 24 mEq/L | [Ref] | < 8.60 mEq/L | [Ref] |
| 24–25 mEq/L | −0.58 (−1.93 to 0.76) | 8.60–10.25 mEq/L | −0.83 (−2.00 to 0.34) |
| 25.1–26.9 mEq/L | 0.45 (−0.74 to 1.64) | 10.26–11.90 mEq/L | −1.65 (−2.67 to −0.64) |
| ≥ 27 mEq/L | 0.02 (−1.26 to 1.31) | ≥ 11.91 mEq/L | −1.51 (−2.64 to −0.37) |
| P for trend | 0.57 | P for trend | 0.007 |
| Odds Ratio of Low Cardiorespiratory Fitness by Serum Bicarbonate and Anion Gap
| |||
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| Bicarbonate | Anion Gap | ||
| Continuous* | 0.97 (0.86–1.09) | Continuous* | 1.19 (1.03–1.37) |
| < 24 mEq/L | [Ref] | < 8.60 mEq/L | [Ref] |
| 24–25 mEq/L | 0.71 (0.47–1.10) | 8.60–10.25 mEq/L | 1.69 (1.09–2.64) |
| 25.1–26.9 mEq/L | 0.96 (0.63–1.45) | 10.26–11.90 mEq/L | 1.51 (1.05–2.15) |
| ≥ 27 mEq/L | 0.98 (0.63–1.54) | ≥ 11.91 mEq/L | 1.96 (1.31–2.93) |
| P for trend | 0.98 | P for trend | 0.005 |
Abbreviations: OR, odds ratio; CI, confidence interval. Bold values indicate p<0.05.
Models adjusted for age, sex, race/ethnicity, and % lean body mass.
Per standard deviation higher serum bicarbonate (SD = 2.16 mEq/L) and adjusted anion gap (SD = 2.36 mEq/L), respectively.
DISCUSSION
Our results demonstrate that lower levels of serum bicarbonate and higher levels of AG are associated with low cardiorespiratory fitness in adults aged 20–49 years in the general US population. To our knowledge, this is the first study to examine these associations on a population-wide level. Furthermore, the cohort examined in our study was composed of relatively young, healthy people. These results are of potential public health importance given the association of low fitness with known CVD risk factors11 and with greater risk of all-cause mortality and CVD events among healthy persons.13
Although oxygen consumption during exercise is determined by a number of factors, changes in skeletal muscle are an important contributor to submaximal endurance performance.14 For example, an increase in VO2max has been seen with strength training, possibly due to adaptations in oxidative capacity and increased mass of the strength-trained muscles.15 The association of lower serum bicarbonate with low fitness may be related to an effect of chronic metabolic acidosis on skeletal muscle. Acidosis impairs signaling by insulin and IGF-1 in muscle and stimulates skeletal muscle proteolysis through several mechanisms, including activation of the ubiquitin-proteasome pathway.16 Metabolic acidosis may also reduce muscle protein synthesis.17–19 Alkali administration has produced improvements in short-term endurance performance and lactate threshold.20, 21 Both reduced muscle mass, due to increased protein breakdown relative to synthesis, and impaired skeletal muscle function could reduce skeletal muscle oxygen uptake, thereby resulting in lower VO2max. Therefore, differences in estimated VO2max may partly reflect skeletal muscle adaptations, and acidosis may be associated with fitness through an effect on muscle. This hypothesis is supported by the disappearance of an association of serum bicarbonate with fitness after adjustment for %LBM, which suggests that lean body mass may be a mediator of the association of acidosis with fitness. Although there was a suggestion that the association of serum bicarbonate with fitness differs among men and women, this finding should be regarded with caution given the differential exclusion of men and women, namely the exclusion of otherwise eligible pregnant women from participation (n=595).
These associations can also partly be explained as the inverse of an effect related to higher levels of AG. The pattern of associations within subgroups for serum bicarbonate appears to be a mirror image of the pattern for AG, suggesting a prominent role for changes in AG in our findings. Previous cross-sectional studies have shown an association of higher levels of serum bicarbonate and lower levels of AG with increased insulin resistance and systolic blood pressure in the general population.8, 10 The etiology for higher AG in such individuals remains unclear. Higher levels of endogenous organic acids are one possible explanation. Animal and human data suggest an association between salt-sensitive hypertension and lower blood pH and bicarbonate,22–24 which might result from greater endogenous acid production. Higher lactate levels have been associated with type 2 diabetes in older adults.25 In a non-diabetic cohort, α-hydroxybutyrate and other organic acids were inversely associated with insulin sensitivity.26 Thus a higher AG may be a marker for factors related to obesity and the metabolic syndrome even in this relatively healthy cohort.
Increased organic anion production may also account for the association of soft drink consumption with higher AG and lower serum bicarbonate. Ingestion of fructose, found in sugar-sweetened beverages, causes an increase in plasma lactate.27 This is in accord with the higher AG seen in association with sugar-sweetened, compared with diet, soft drink consumption in our analysis. However, there was still a trend toward higher AG in association with diet soft drink intake. In addition, the majority of blood samples in our cohort were collected after prolonged fasting. The presence of higher lactate hours after soft drink consumption seems unlikely, especially in individuals believed to be largely free of significant liver disease. Soft drink consumption could be a marker for an unhealthy lifestyle, and such individuals could have higher lactate levels.25 The phosphoric acid in soft drinks may partly explain our findings. Although the participants had largely well-preserved kidney function, a reduction in urinary phosphate excretion may occur with relatively preserved kidney function.28 Retained phosphoric acid could then contribute to the AG. Despite the association of soft drink consumption with serum bicarbonate and AG levels, it does not appear to mediate the associations with low fitness. Our results were independent of the quantity of soft drink consumption, and the associations with low fitness were not stronger among consumers of soft drinks.
Higher dietary acid load has been associated with the development of hypertension29 and can cause low-grade metabolic acidosis.30 However, we did not find an independent association of NEAP with bicarbonate level and our analyses were adjusted for NEAP. Nevertheless, we may have been unable to fully account for dietary factors due to imprecision in the collection of dietary data and in the estimation of NEAP.
VO2max was estimated in our cohort as direct measurements were not feasible in a large epidemiologic study. The estimation of VO2max using heart rate changes may vary from directly measured VO2max by up to 10–20%.31 Nevertheless, it is believed to provide a satisfactory estimate for studies such as ours that are conducted on a group level.31 Estimated VO2max determined in this manner has been associated with mortality risk,13 thereby affording it prognostic significance. Furthermore, inaccuracy in estimation of VO2max leading to misclassification of participants’ fitness levels would be expected to bias our results toward the null hypothesis. Thus we believe our findings are robust despite this potential inaccuracy.
Several important limitations of our analysis should be noted. As levels of arterial pH and pCO2 were unavailable, we cannot exclude lower bicarbonate levels as a marker of alterations in respiratory status. However, participants with chronic obstructive pulmonary disease or respiratory symptoms were not included in our analysis, and excluding those with a diagnosis of asthma did not change our results. Levels of serum bicarbonate and AG were determined from single measurements, and variability in sample handling may affect bicarbonate levels.32–34 We could not account for this possibility, which would be expected to introduce non-differential misclassification and likely bias our results toward the null hypothesis. Analyses of NEAP may have been affected by the limitations of dietary assessment using a single 24-hour recall period. Given the associations of serum bicarbonate and AG with BMI and other markers of the metabolic syndrome, there may be residual confounding related to sedentary lifestyle. Objective measures of physical activity would have added additional information but were unavailable for this cohort. However, physical activity was assessed by detailed questionnaires and did provide meaningful quantitative information, as demonstrated by the expected association with cardiorespiratory fitness, suggesting that our findings were not simply due to imprecise quantification of physical activity. Finally, no causal associations can be inferred due to the cross-sectional nature of our analysis, and we are unable to exclude the possibility of reverse causality. For example, low fitness, if associated with chronic disease, could cause low serum bicarbonate, either from respiratory alkalosis due to occult liver or cardiopulmonary disease or from impaired renal acid excretion. However, given the age and health status of the participants in the treadmill test protocol, a substantial burden of chronic illness seems unlikely. Similarly, predictors of serum bicarbonate and AG might be different among older persons or those with chronic illness than among our participants.
In summary, we have shown that lower levels of serum bicarbonate and higher levels of AG are associated with lower cardiorespiratory fitness in adults aged 20–49 years in the general US population. Further studies are needed to elucidate the determinants of serum bicarbonate and AG in persons without overt kidney disease and to prospectively examine associations of serum bicarbonate and AG with fitness and other CVD risk factors.
METHODS
Study Population
NHANES 1999–2004 was a nationally representative survey of the non-institutionalized civilian population in the United States.35 A stratified, multistage, probability sampling design was used to select participants. Overall, 3 302 adults aged 20–49 years completed the interview and examination components, including the cardiovascular fitness component. We excluded participants with missing serum bicarbonate (n =137); a history of emphysema or chronic bronchitis by self-report (n =101); who were pregnant at the time of examination (n=51); had invalid dietary data (n=74); or with missing covariate data (n =225). No participants had an estimated glomerular filtration rate (eGFR) <15 mL/min/1.73m2, and 4 had eGFR< 60 ml/min/1.73 m2. Thus 2 714 participants were available for analysis. The Committee on Clinical Investigation at the Albert Einstein College of Medicine determined this analysis to be exempt.
Data Collection
Information on household income, education, physical activity, smoking, comorbidities, and medication use in the previous month was obtained by self-report. Race/ethnicity was self-identified. Poverty was defined as <100% of the poverty index based on self-reported household income. Participants were asked about the frequency and duration of walking or bicycling, home or yard work, and moderate or vigorous leisure time physical activity performed within the past 30 days. These responses were used to calculate metabolic equivalents (MET-min/wk) based on intensity values recommended by the National Center for Health Statistics.36 Activity level was classified as 0, <500, 500–2000, or >2000 MET-min/wk. Smoking was classified as never, former, or current smoker. Data on dietary intake were obtained from a 24-hour dietary recall questionnaire. The diet-dependent net acid load was estimated as: NEAP (mEq/d) = [54.5 × protein (g/d)/potassium (mEq/d)] −10.2.37 Soft drink consumption was defined using USDA Food Codes 92400000 through 92411620 and was also categorized as sugar-sweetened or diet. Hypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, physician diagnosis, and/or antihypertensive medication use.38 A participant was considered to have diabetes mellitus if he or she reported a physician diagnosis while not pregnant or the current use of insulin or oral hypoglycemic medications, or had a glycohemoglobin level ≥6.5%.
Serum chemistry values were measured using the Hitachi 917 multichannel analyzer (Roche Diagnostics, Indianapolis, IN) in 1999–2001 and the Beckman Synchron LX20 (Beckman Coulter Inc., Brea, CA) in 2002–2004. Serum bicarbonate was measured in 2 laboratories via the phosphoenolpyruvate carboxylase method from 1999–2001 and with a pH-sensitive electrode in 2002–2004. The coefficient of variation ranged between 2.3–5.6%. Serum bicarbonate levels during these time periods were compared using weighted linear regression. Mean serum bicarbonate was 1.105 ± 0.178 mEq/L higher (p<0.001) among all NHANES participants in 2003–2004 compared with 1999–2002. Therefore, serum bicarbonate levels in 1999–2002 were adjusted by adding 1.105 mEq/L. AG was defined as: AG(initial)=serum sodium(mEq/L) − (serum chloride(mEq/L) + serum bicarbonate(mEq/L)), and was adjusted for serum albumin using the following equation: AG=AG(initial) + 2.5×(4 − serum albumin(g/dL)).39 Serum creatinine was measured by a modified kinetic Jaffé reaction. Values from 1999–2000 were calibrated to the Cleveland Clinic laboratory standard by multiplying by 1.013 and then adding 0.147. Correction of serum creatinine values from 2001–2004 was not necessary. eGFR was calculated using the CKD-EPI equation.40 As ingestion of food may affect serum bicarbonate levels,41 the period of fasting prior to phlebotomy was categorized as ≤2, 2–6, and >6 hours. Body composition was assessed using whole-body dual-energy X-ray absorptiometry (DXA). Due to the pattern of non-response, missing and invalid data were multiply imputed. Details of the DXA protocol, quality control analyses, and the multiple imputation procedure are available (http://www.cdc.gov/nchs/nhanes/dxx/dxa.htm). %LBM was calculated as 100 × total body lean mass (excluding bone mineral content (BMC))/total mass. Repeating the calculation using total body lean mass (including BMC) did not change the results.
Outcome Variables
The cardiovascular fitness component consisted of a submaximal exercise test in individuals aged 12–49 years. Participants were excluded from participation based on physical limitations, cardiovascular conditions and symptoms, asthma symptoms, lung/breathing conditions and symptoms, and medications including beta blockers and calcium channel blockers. The initial goal of the protocol was to elicit a heart rate 75% of the age-predicted maximum and was later modified to allow heart rates of 80% of the age-predicted maximum among adults. Participants were assigned to 1 of 8 treadmill test protocols of varying difficulty based on age, sex, BMI, and self-reported level of physical activity. Maximal oxygen consumption (VO2max, mL/kg/min) was estimated from the heart rate response to reference workloads. Higher VO2max indicates better cardiorespiratory fitness. In addition, as recommended in the NHANES Cardiovascular Fitness Procedure Manual, participants were categorized as having low (<20th percentile), moderate (20th–59th percentile), or high (≥60th percentile) fitness based on age- and sex-specific cutpoints derived from the Aerobics Center Longitudinal Study.42, 43 Details of the protocols and formulas used are available in the Procedure Manual at http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/cv_99-04.pdf. We specifically examined low fitness as an outcome because submaximal exercise testing may not be ideal for determining high fitness as it may understress fit individuals.43
Statistical Analysis
All analyses used NHANES-appropriate sampling weights and accounted for the complex multistage cluster design using the “survey” command in Stata 11.1 (Stata Corporation, College Station, TX, USA). The distributions of participant characteristics were examined by quartiles of serum bicarbonate and AG. Multivariable linear regression models were used to determine independent predictors of serum bicarbonate and AG, separately. Linear and logistic regression models were created to examine separately the associations of serum bicarbonate and AG with estimated VO2max and low cardiorespiratory fitness, respectively. Serum bicarbonate and AG were analyzed as continuous variables and within quartiles to examine non-linear associations with either outcome. A variable was included in the final model based on association with the predictor (bicarbonate or AG) and the outcome (p<0.20) and on a priori determination of confounders of the association of serum bicarbonate or AG with cardiorespiratory fitness. These included known determinants of cardiovascular health and fitness and factors that may affect levels of serum bicarbonate or AG, including relevant laboratory parameters. Variables included in the final models were age, sex, race/ethnicity, BMI, poverty, education, activity level, smoking status, estimated NEAP, fasting length, soft drink consumption, diuretic use, use of cholesterol-lowering medications, diagnosis of diabetes mellitus, systolic blood pressure, eGFR categories, log-transformed urine albumin-to-creatinine ratio, serum albumin, potassium, total calcium (not adjusted for albumin), phosphate, hemoglobin, total cholesterol, HDL cholesterol, log-transformed CRP, and (for bicarbonate models) serum sodium and chloride. Effect modification by age, sex, race/ethnicity, BMI, activity level, soft drink consumption, eGFR, and CRP was tested by including multiplicative interaction terms in the models. A p-value <0.05 was considered statistically significant.
All analyses were repeated using unadjusted serum bicarbonate values from 1999–2002, and also using survey year-specific quartiles of serum bicarbonate and AG. As the results were not materially different, only those using the adjusted serum bicarbonate values from 1999–2002 are presented.
Sensitivity Analyses
Possible mediation by muscle mass of the associations with fitness was assessed by adding %LBM as a covariate to age, sex, and race/ethnicity-adjusted models. We explored the impact of severity of acidosis by sub-categorizing participants in the lowest quartile of serum bicarbonate. We also examined associations of serum bicarbonate with fitness using the clinical cutpoint of serum bicarbonate ≤22 mEq/L. As serum bicarbonate differed significantly by sex, we examined the association of serum bicarbonate with fitness in fully adjusted, sex-stratified models. We calculated AG without adjustment for serum albumin and repeated our analyses to determine if our findings were a result of differences in albumin level and not the AG per se. Although participants were excluded from the cardiovascular fitness component based on respiratory symptoms, some reported a diagnosis of asthma. As differences in serum bicarbonate could be due to respiratory factors, we repeated our analyses after excluding participants who answered yes to the question, “Has a doctor or other health professional ever told you that you have asthma?” To determine if our findings resulted from excluding participants with missing covariate data or invalid dietary data, we redefined the cohort without these exclusions and calculated age, sex, and race/ethnicity-adjusted estimates. Finally, to examine the possibility of residual confounding due to our categorization of activity level, we created deciles of activity based on MET-min/wk and re-examined the fully adjusted models.
Supplementary Material
Acknowledgments
Sources of support: This research was supported by National Institutes of Health (NIH) grants K23DK078774 to Dr. Melamed, R21DK077326, R01DK087783 and RO1DK080123 to Dr. Hostetter, and CTSA grants UL1RR025750, KL2RR025749 and TL1RR025748 from the National Center for Research Resources, a component of the NIH.
Footnotes
Disclosures
Dr. Hostetter has consulted for Bristol Myers Squibb, Eli Lilly, Genzyme, and Wyeth. Neither of the other authors has any financial conflicts to disclose.
References
- 1.Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005;45:978–993. doi: 10.1053/j.ajkd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Beckles AD. Glucose intolerance following chronic metabolic acidosis in man. Am J Physiol. 1979;236:E328–334. doi: 10.1152/ajpendo.1979.236.4.E328. [DOI] [PubMed] [Google Scholar]
- 3.Shah SN, Abramowitz M, Hostetter TH, et al. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis. 2009;54:270–277. doi: 10.1053/j.ajkd.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Brito-Ashurst I, Varagunam M, Raftery MJ, et al. Bicarbonate Supplementation Slows Progression of CKD and Improves Nutritional Status. J Am Soc Nephrol. 2009;20:2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frassetto L, Morris RC, Jr, Sebastian A. Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. J Clin Endocrinol Metab. 1997;82:254–259. doi: 10.1210/jcem.82.1.3663. [DOI] [PubMed] [Google Scholar]
- 6.Dawson-Hughes B, Castaneda-Sceppa C, Harris SS, et al. Impact of supplementation with bicarbonate on lower-extremity muscle performance in older men and women. Osteoporos Int. 2010;21:1171–1179. doi: 10.1007/s00198-009-1049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramowitz MK, Hostetter TH, Melamed ML. Association of serum bicarbonate levels with gait speed and quadriceps strength in older adults. Am J Kidney Dis. 2011;58:29–38. doi: 10.1053/j.ajkd.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farwell WR, Taylor EN. Serum bicarbonate, anion gap and insulin resistance in the National Health and Nutrition Examination Survey. Diabet Med. 2008;25:798–804. doi: 10.1111/j.1464-5491.2008.02471.x. [DOI] [PubMed] [Google Scholar]
- 9.Forman JP, Rifas-Shiman SL, Taylor EN, et al. Association between the serum anion gap and blood pressure among patients at Harvard Vanguard Medical Associates. J Hum Hypertens. 2008;22:122–125. doi: 10.1038/sj.jhh.1002286. [DOI] [PubMed] [Google Scholar]
- 10.Taylor EN, Forman JP, Farwell WR. Serum anion gap and blood pressure in the national health and nutrition examination survey. Hypertension. 2007;50:320–324. doi: 10.1161/HYPERTENSIONAHA.107.092643. [DOI] [PubMed] [Google Scholar]
- 11.Carnethon MR, Gulati M, Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. JAMA. 2005;294:2981–2988. doi: 10.1001/jama.294.23.2981. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta: 1996. [Google Scholar]
- 13.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 14.Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Frontera WR, Meredith CN, O’Reilly KP, et al. Strength training and determinants of VO2max in older men. J Appl Physiol. 1990;68:329–333. doi: 10.1152/jappl.1990.68.1.329. [DOI] [PubMed] [Google Scholar]
- 16.Mitch WE, Du J. Cellular mechanisms causing loss of muscle mass in kidney disease. Semin Nephrol. 2004;24:484–487. doi: 10.1016/j.semnephrol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 17.England BK, Chastain JL, Mitch WE. Abnormalities in protein synthesis and degradation induced by extracellular pH in BC3H1 myocytes. Am J Physiol. 1991;260:C277–282. doi: 10.1152/ajpcell.1991.260.2.C277. [DOI] [PubMed] [Google Scholar]
- 18.Ballmer PE, McNurlan MA, Hulter HN, et al. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J Clin Invest. 1995;95:39–45. doi: 10.1172/JCI117668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleger GR, Turgay M, Imoberdorf R, et al. Acute metabolic acidosis decreases muscle protein synthesis but not albumin synthesis in humans. Am J Kidney Dis. 2001;38:1199–1207. doi: 10.1053/ajkd.2001.29215. [DOI] [PubMed] [Google Scholar]
- 20.Edge J, Bishop D, Goodman C. Effects of chronic NaHCO3 ingestion during interval training on changes to muscle buffer capacity, metabolism, and short-term endurance performance. J Appl Physiol. 2006;101:918–925. doi: 10.1152/japplphysiol.01534.2005. [DOI] [PubMed] [Google Scholar]
- 21.McNaughton L, Backx K, Palmer G, et al. Effects of chronic bicarbonate ingestion on the performance of high-intensity work. Eur J Appl Physiol Occup Physiol. 1999;80:333–336. doi: 10.1007/s004210050600. [DOI] [PubMed] [Google Scholar]
- 22.Batlle DC, Sharma AM, Alsheikha MW, et al. Renal acid excretion and intracellular pH in salt-sensitive genetic hypertension. J Clin Invest. 1993;91:2178–2184. doi: 10.1172/JCI116444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma AM, Cetto C, Schorr U, et al. Renal acid-base excretion in normotensive salt-sensitive humans. Hypertension. 1993;22:884–890. doi: 10.1161/01.hyp.22.6.884. [DOI] [PubMed] [Google Scholar]
- 24.Sharma AM, Kribben A, Schattenfroh S, et al. Salt sensitivity in humans is associated with abnormal acid-base regulation. Hypertension. 1990;16:407–413. doi: 10.1161/01.hyp.16.4.407. [DOI] [PubMed] [Google Scholar]
- 25.Crawford SO, Hoogeveen RC, Brancati FL, et al. Association of blood lactate with type 2 diabetes: the Atherosclerosis Risk in Communities Carotid MRI Study. Int J Epidemiol. 2010;39:1647–1655. doi: 10.1093/ije/dyq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gall WE, Beebe K, Lawton KA, et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5:e10883. doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 28.Slatopolsky E, Bricker NS. The role of phosphorus restriction in the prevention of secondary hyperparathyroidism in chronic renal disease. Kidney Int. 1973;4:141–145. doi: 10.1038/ki.1973.92. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Curhan GC, Forman JP. Diet-dependent net acid load and risk of incident hypertension in United States women. Hypertension. 2009;54:751–755. doi: 10.1161/HYPERTENSIONAHA.109.135582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurtz I, Maher T, Hulter HN, et al. Effect of diet on plasma acid-base composition in normal humans. Kidney Int. 1983;24:670–680. doi: 10.1038/ki.1983.210. [DOI] [PubMed] [Google Scholar]
- 31.Achten J, Jeukendrup AE. Heart rate monitoring: applications and limitations. Sports Med. 2003;33:517–538. doi: 10.2165/00007256-200333070-00004. [DOI] [PubMed] [Google Scholar]
- 32.Bray SH, Tung RL, Jones ER. The magnitude of metabolic acidosis is dependent on differences in bicarbonate assays. Am J Kidney Dis. 1996;28:700–703. doi: 10.1016/s0272-6386(96)90251-6. [DOI] [PubMed] [Google Scholar]
- 33.Kirschbaum B. Spurious metabolic acidosis in hemodialysis patients. Am J Kidney Dis. 2000;35:1068–1071. doi: 10.1016/s0272-6386(00)70041-2. [DOI] [PubMed] [Google Scholar]
- 34.Laski ME. Penny wise and bicarbonate foolish. Am J Kidney Dis. 2000;35:1224–1225. doi: 10.1016/s0272-6386(00)70063-1. [DOI] [PubMed] [Google Scholar]
- 35.About the National Health and Nutrition Examination Survey. National Center for Health Statistics; [Accessed December 21, 2010.]. Available at: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. [Google Scholar]
- 36.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 37.Frassetto LA, Todd KM, Morris RC, Jr, et al. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68:576–583. doi: 10.1093/ajcn/68.3.576. [DOI] [PubMed] [Google Scholar]
- 38.Muntner P, Woodward M, Mann DM, et al. Comparison of the Framingham Heart Study hypertension model with blood pressure alone in the prediction of risk of hypertension: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2010;55:1339–1345. doi: 10.1161/HYPERTENSIONAHA.109.149609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. 2007;2:162–174. doi: 10.2215/CJN.03020906. [DOI] [PubMed] [Google Scholar]
- 40.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cullen GE, Earle IP. STUDIES OF THE ACID-BASE CONDITION OF BLOOD. Journal of Biological Chemistry. 1929;83:545–559. [Google Scholar]
- 42.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, et al. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 43.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams and Wilkins Company; Philadelphia: 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.