Abstract
Objectives
(1) To test whether single nucleotide polymorphisms (SNPs) of the FRZB gene are associated with hip shape. (2) To determine whether FRZB variant alleles affect the relationship between hip shape and radiographic hip osteoarthritis (RHOA).
Methods
A nested case-control study of Caucasian women aged ≥ 65 years in the Study of Osteoporotic Fractures (SOF) was performed. Cases (n = 451) demonstrated incident RHOA during follow-up (mean 8.0 ± 0.4 years). Controls (n = 601) had no RHOA at baseline or follow-up. Statistical shape modeling (SSM) of digitized hip radiographs was used to assess proximal femur shape, and center-edge angle and acetabular depth were used to assess acetabular shape. The association of the rs288326 and rs7775 FRZB variant alleles with hip shape was analyzed using linear regression. The effect of these alleles on the relationship between hip shape and RHOA was analyzed using logistic regression with and without interaction terms.
Results
The rs288326 and rs7775 alleles were associated with shape of the proximal femur (SSM Mode 2). There was a significant interaction between the rs288326 SNP and proximal femur shape (Mode 2) in predicting RHOA (p for interaction = 0.022). Among subjects with the rs288326 variant allele, there were increasing odds of RHOA with increasing quartiles of proximal femur shape Mode 2 (OR for 4th quartile of Mode 2 = 2.5, 95% confidence interval 1.2–5.3; p for linear trend = 0.02).
Conclusions
The rs288326 and rs7775 FRZB SNPs are associated with the shape of the proximal femur. The presence of the rs288326 SNP alters the relationship between proximal femur shape and incident RHOA. Together, these findings suggest that FRZB may serve an important role in determining hip shape and may modify the relationship between hip shape and OA.
Keywords: FRZB, WNT pathway, osteoarthritis, joint shape, active shape modeling, bone
INTRODUCTION
Osteoarthritis (OA) is a major cause of disability in older adults worldwide (1, 2). The prevalence of OA in the United States is expected to rise from an estimated 15% of the population in 1995 to an estimated 18.2% of the population by the year 2020 (3). In the Framingham study, the prevalence of radiographic knee OA among subjects aged 63 to 93 years was 33.0 cases per 100 (4), and in the NHANES I study, the prevalence of radiographic hip OA among subjects aged 55 to 74 years was 3.2 cases per 100 (3, 5). Currently, no disease-modifying treatment for osteoarthritis is available (6), and a complete understanding of the pathogenesis of this disease is still evolving.
The development of OA appears to be mediated by both genetic and environmental factors. Classic twin studies demonstrate that approximately 60% of the variation in hip osteoarthritis in women is associated with genetic factors (7). Among the biological pathways implicated in the pathogenesis of OA is the WNT/β-catenin signaling pathway (8). This pathway is of key importance during skeletal patterning in embryogenesis, and it also appears to be involved in the homeostasis of bone and cartilage in adult life (9–13). Modifying the WNT pathway has thus become a target of new therapeutic development for OA and other forms of arthritis (14).
The importance of the WNT pathway in bone and cartilage homeostasis is highlighted by the observation that animals deficient in the WNT antagonist FRZB demonstrate increased susceptibility to experimental OA and sclerosis of cortical bone (9). In humans, reports from two groups, including ours, have demonstrated a link between polymorphisms in the FRZB gene (rs288326 and rs7775) and OA of the hip (15, 16). Not all reports have replicated this finding, however, and a recent meta-analysis failed to find a sizeable association between FRZB variant alleles and hip OA (17). Since the source of this discrepancy has not yet been resolved, the biological role of the WNT signaling pathway in bone and joint homeostasis remains under investigation.
Previous work has shown that WNT signaling in chondrocytes and osteoblasts is up-regulated in response to increased mechanical loading (19). Assuming that mechanical forces in bone are distributed in part according to bone shape, and given the well-established link between bone shape and the development of OA (20–23), we performed an exploratory analysis examining possible interactions between FRZB variant alleles and both hip shape and radiographic hip OA.
PATIENTS AND METHODS
Study Subjects, Case Definition, and Control Selection
Cases and controls were randomly sampled from the Study of Osteoporotic Fractures (SOF) database, a longitudinal, multi-center cohort study of 9,704 Caucasian, post-menopausal ambulatory women recruited from population lists in 4 U.S. cities (24). Subjects were enrolled at age ≥ 65 years between September 1986 and October 1988. Subjects with bilateral hip replacements at baseline were excluded. The study was approved by the institutional review boards at each of the institutions involved. All subjects provided written informed consent at enrollment and at each clinical examination.
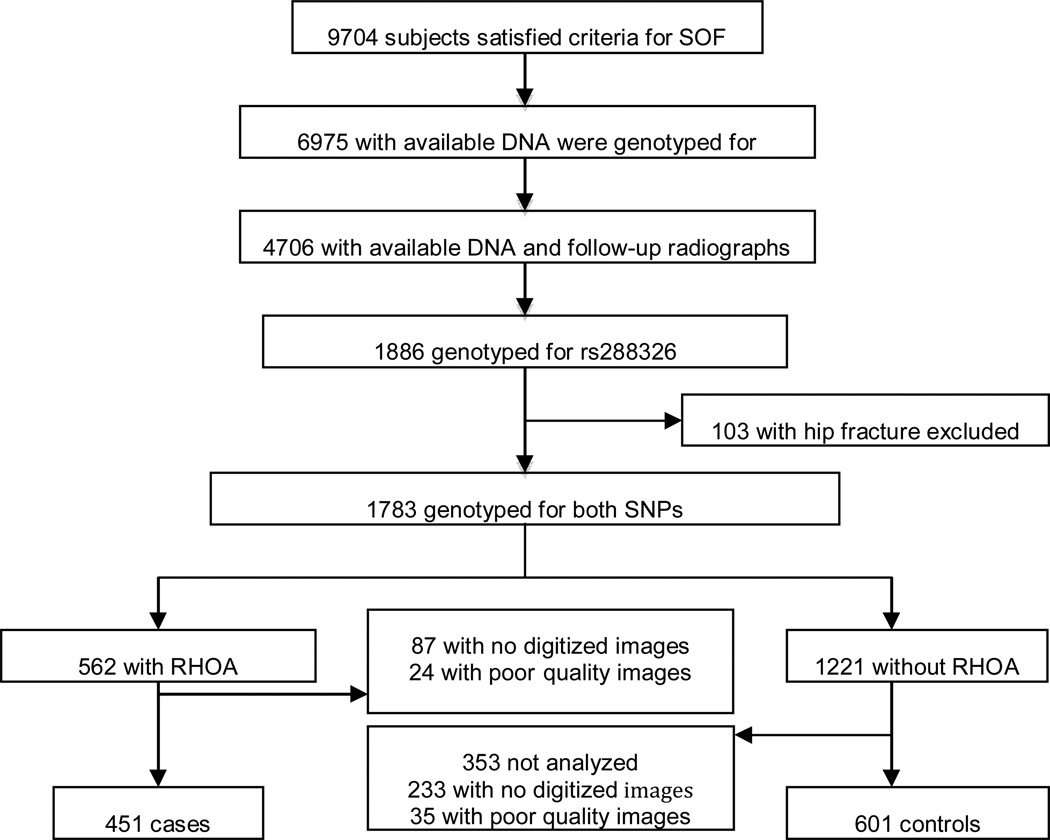
The subjects for this study were selected as described in Figure 1. Subjects with available DNA (n = 6,975) were genotyped for the rs7775 SNP as part of a previous study (15). Of these, 4706 subjects had available baseline and follow-up radiographs (15). From this group of 4706 women with baseline and follow-up X-rays, 1886 incident radiographic hip OA (RHOA) cases and controls were selected to undergo additional genotyping of the rs288326 SNP in a 1:2 ratio (15). 1783 women were eligible for the study after excluding those with incident hip fractures. 954 women had available digitized good quality images and genotype data for both SNPs. For controls, radiographs were read in numeric order based on study identification number with the first 601 controls selected for the current analysis. 562 cases were eligible for inclusion in the study with available digitized images, of which 451 were read for hip shape.
Figure 1. Flow chart of study subject selection.
RHOA = radiographic hip osteoarthritis.
Measurements
Height and weight at baseline visit were recorded by using a wall-mounted Harpenden stadiometer (Holtan, Dyfed, UK) and balance beam scale, respectively (24). All participants completed a questionnaire at baseline that assessed self-reported health status, hours sedentary each day, and current medication use (21). Physical activity was assessed by using a modified Paffenberger survey (25). Bone mineral density (BMD) of the femoral neck and total hip was measured at second follow-up visit by dual energy X-ray absorptiometry (DXA) with calibrated scanners (Hologic QDR 1000, Waltham, MA). All scanners employed the same measurement program, and control phantoms were scanned daily to ensure proper calibration (26).
Radiographic Assessment
Subjects obtained a supine anterioposterior pelvic radiograph at baseline and at the fifth follow-up visit (mean 8.3 years after baseline visit) (27). All radiographs were digitized with a VIDAR digitizer (VIDAR Systems Corp, Herndon, VA) at a resolution of 0.169 mm (150 dpi) and stored as 16-bit DICOM images for further analysis.
Details of the radiographic scoring methods for hip OA have been previously published (28, 29). Subjects were considered to have incident RHOA if they satisfied at least 1 of the following 3 criteria at follow-up visit 5 and not at baseline: severe joint space narrowing (JSN defined by the Osteoarthritis Research Society International [OARSI] atlas (30) feature grade ≥3), definite osteophytes and JSN (both OARSI grades ≥2), or any 3 or more individual radiographic features (summary grade ≥3). Osteophyte-predominant OA was defined as femoral osteophyte grade ≥2 and JSN grade ≤2. Control subjects were free of RHOA at baseline and follow-up radiographs according to the above definition. Radiographs were scored for OA by a single reader (NEL) who was blinded to all subject characteristics.
Statistical Shape Modeling (SSM) and Acetabular Measurements
To measure proximal femur shape, we utilized the technique of statistical shape modeling (SSM), which utilizes principal components analysis to generate “modes of variation” of shape within a given population (31). Since its introduction by Cootes and Taylor (31), SSM has been widely applied in medical imaging (32–37), including modeling of the shape of the proximal femur (21, 38). SSM was performed using the method of Cootes and colleagues (26, see http://www.isbe.man.ac.uk/val/asmtk/ASMInfo.html). Digitized radiographs were evaluated by a reader (JAL), who outlined the shape of the femoral head and neck by placing a series of 60 evenly-spaced points in between two anchor points: the inferior margin of the lesser trochanter and the corresponding point on the lateral aspect of the femoral shaft (17). The algorithms used were identical to those of Gregory et al. (27) except for the number of points used (60 rather than 16) and inclusion of the entire proximal femur to the level of the lesser trochanter rather than only the femoral head and neck (17). Images were deemed unacceptable for analysis if greater and lesser trochanters were not fully visualized; using these criteria, approximately 5% were deemed unacceptable for active shape modeling analysis.
One thousand fifty-two baseline hip radiographs (451 cases, 601 controls) were entered into the SSM program to generate the composite average proximal femur shape of this sample, which formed the point of reference for comparison of variations from this average shape (Figure 3A). The SSM program used principal components analysis to calculate 10 independent “modes of variation” in hip shape, each of which independently described a portion of the overall variance in hip shape. Each of the 10 modes was independent of the others, with no significant interactions between modes. Each individual hip was expressed in terms of standard deviations from the mean value of the 10 modes of variation. Together, the 10 modes of variation explained 95% of the total variance in proximal femur shape (21, 39). Modes 1–5 explained approximately 90% of the overall variance, with modes 6–10 accounting for an additional 5% of the overall variance in hip shape.
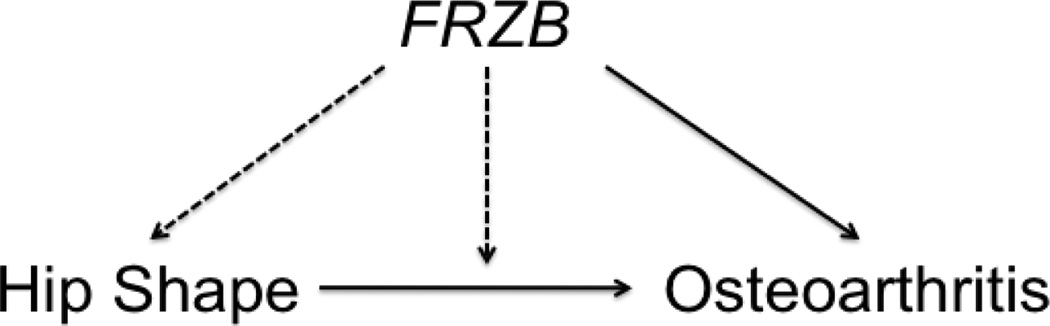
Figure 3. Schematic depicting possible interrelationships between FRZB, hip shape, and osteoarthritis.
Solid arrows represent associations that have previously been reported, while dotted lines represent associations that are under investigation in this study.
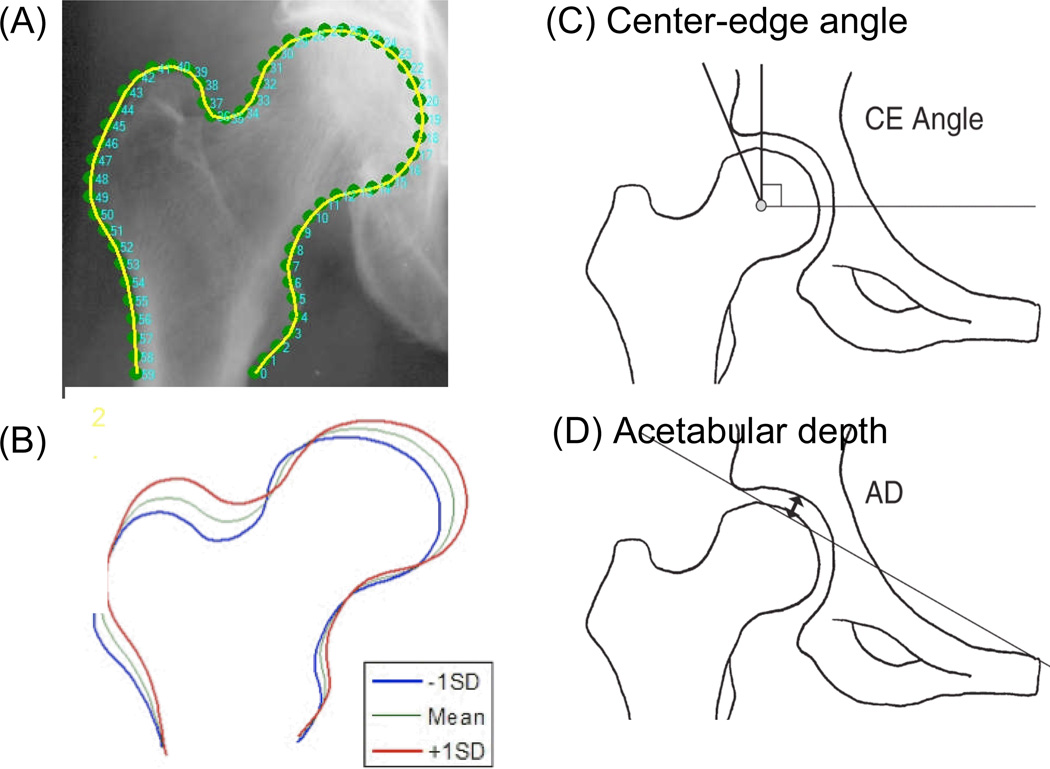
To assess the shape of the acetabulum, two measurements were used: center-edge angle (40) and acetabular depth (41) (Figure 3C and D). Digitized radiographs were evaluated by a reader (JCB) who used digital calipers for all measurements. The center-edge angle was defined as the angle between the line joining the center of the femoral head and the lateral margin of the acetabular roof and a true vertical line, defined as a line perpendicular to the line joining the inferior borders of the bilateral ischial tuberosities, according to published methods (40). The center of the femoral head was located with the aid of an electronic tool producing concentric circles. Acetabular depth was defined as the greatest perpendicular distance from the acetabular roof to a line joining the lateral margin of the acetabular roof to the superior corner of the ipsilateral symphysis pubis, according to published methods (41).
Genotyping
Genotypes for FRZB rs288326 (Arg200Trp) and rs7775 (Arg324Gly) SNPs were obtained by two protocols (Roche Molecular Systems, Alameda, CA). The rs7775 SNP was genotyped for the entire cohort in conjunction with another study of osteoporosis to conserve sample material. The rs288326 SNP was separately determined for subjects with radiographic hip OA and 2.5 times as many randomly-selected controls. Hence, genotypes of 569 subjects with radiographic hip OA and 4,136 controls were obtained for the rs7775 SNP, and genotypes of 570 subjects with radiographic hip OA and 1,317 controls were obtained for the rs288326 SNP.
The rs288326 SNP was assayed by an allele-specific kinetic polymerase chain reaction (PCR) method previously described (15). The genotypes were analyzed with software developed at Roche Molecular Systems. Each amplification plate used 3 positive controls and 1 negative control. Two hundred seventy-six samples were genotyped in duplicate and with complete concordance. The rs7775 SNP was genotyped in the context of a multiplex PCR amplification followed by allele-specific SNP detection with immobilized oligonucleotide probes in linear arrays, as described previously (42). Sixteen percent of samples were genotyped twice for this SNP, and all results were concordant (15).
Statistical analysis
Statistical analyses were performed using SAS software (version 9.2, SAS Institute, Inc., Cary, NC). Baseline subject characteristics were compared using Student’s t-tests and chi-squared methods. P-values of ≤ 0.05 in two-tailed tests were considered significant. The association of the FRZB rs288326 and rs7775 variant alleles with the 10 modes of variation identified by SSM or acetabular measurements was analyzed using linear regression in the control population. Next, the relationship between the FRZB variant alleles and proximal femur shape as a continuous measure (SSM modes 1–5, which accounted for the majority of the overall variability in proximal femur shape (90.1%)) as predictors of RHOA was analyzed using logistic regression with and without interaction terms. If a significant interaction with a hip shape mode and the FRZB variant was found, we performed stratified analyses by the presence or absence of the FRZB variant allele. The modes were categorized into quartiles using cut-points derived from the control population. We conducted logistic regression in subjects with and without the variant allele to determine the association of quartiles of the hip shape mode and RHOA. The p value for linear trend was assessed in order to determine whether there was a linear trend for increasing odds of RHOA comparing subjects in each ascending quartile of hip shape.
RESULTS
Baseline characteristics of the subjects
Subjects with incident radiographic hip osteoarthritis (RHOA) were slightly older than controls (age 71.5 ± 4.9 vs. 69.9 ± 4.3 years; p < 0.0001; Table 1). Incident RHOA cases had a slightly higher bone mineral density (BMD) at the femoral neck compared to controls (0.68 ± 0.12 vs. 0.66 ± 0.12 g/cm2; p = 0.007; Table 1). There was no statistically significant difference between cases and controls with regard to height, weight, body mass index, BMD of the total hip, estrogen or vitamin D use, self-reported overall health status, self-reported exercise status, or weight change from 25 years of age.
Table 1. Baseline characteristics of subjects and controls†.
| Cases (n = 451) | Controls (n = 601) | |
|---|---|---|
| Age (years) | 71.5 ± 4.9 | 69.9 ± 4.3* |
| Height (cm) | 159.4 ± 5.9 | 159.4 ± 5.7 |
| Weight (kg) | 68.6 ± 12.2 | 68.1 ± 12.35 |
| BMI (kg/m2) | 27.0 ± 4.7 | 26.8 ± 4.7 |
| BMD (g/cm2): | ||
| Total hip | 0.78 ± 0.1 3 | 0.77 ± 0.14 |
| Femoral neck | 0.66 ± 0.12 | 0.68 ± 0.12* |
| Estrogen use (%) | 42.5 | 41.1 |
| Vitamin D supplement use (%) | 54.1 | 55.7 |
| Health status: Good vs. Poor (%) |
99.1 | 99.2 |
| Walks for exercise (%) | 55.2 | 55.7 |
| Weight change from 25 years of age | 12.0 ± 10.7 | 11.5 ± 10.3 |
| rs288326 FRZB allele: | ||
| CC, n (%) | 342 (74.3%) | 462 (76.9%) |
| CT, n (%) | 102 (22.6%) | 127 (21.1%) |
| TT, n (%) | 7 (3.1%) | 12 (2.0%) |
| rs7775 FRZB allele: | ||
| CC, n (%) | 373 (82.6%) | 497 (82.7%) |
| CG, n (%) | 75 (16.7%) | 101 (16.8%) |
| GG, n (%) | 3 (0.7%) | 3 (0.5%) |
Values are mean ± standard deviation unless otherwise indicated.
p < 0.05
Abbreviations: BMD = bone mineral density; BMI = body mass index
Minor allele frequencies in the population
The minor allele frequency of rs288326 was 0.13 in our study population. There were no statistically significant differences between RHOA cases and controls in the frequency of subjects with 0, 1, or 2 copies of the rs288326 minor allele (p = 0.41), with or without adjustment for age. (Table 1)
Due to previous reports demonstrating an additional association between the rs7775 FRZB polymorphism and radiographic hip OA (15, 16), we also examined the frequency of this SNP in the cohort. The minor allele frequency of rs7775 was 0.09 in our study population. There were also no statistically significant differences between RHOA cases and controls in the frequency of subjects with 0, 1, or 2 copies of the rs7775 allele (p = 0.93), and no RHOA cases were homozygous for both the rs288326 and the rs7775 minor alleles (Table 1).
Association between the FRZB rs288326 and rs7775 alleles and hip shape
To assess hip shape, we performed statistical shape modeling (SSM) of the proximal femur and geometric measurements of the acetabulum. Of the 10 SSM modes of proximal femur shape (21), Mode 2 demonstrated a significant association with both the rs288326 and rs7775 FRZB SNPs (Table 2). Neither acetabular depth nor center-edge angle was associated with either of the FRZB variant alleles.
Table 2. Association between proximal femur shape (active shape modeling modes 1–10) and FRZB variant alleles.
Comparisons are between control subjects with 1–2 copies of the indicated allele versus control subjects with no copies (n = 601).
| Hip Shape SSM Mode |
rs288326 allele | rs7775 allele | ||
|---|---|---|---|---|
| Beta Estimate (95% CI) |
p value | Beta Estimate (95% CI) |
p value | |
| 1 | 0.03 (−0.15 to 0.20) |
0.76 | −0.13 (−0.32 to 0.07) |
0.21 |
| 2 | −0.21 (−0.38 to −0.03) |
0.019* | −0.23 (−0.43 to −0.04) |
0.019* |
| 3 | 0.03 (−0.16 to 0.22) |
0.75 | −0.04 (−0.26 to 0.18) |
0.73 |
| 4 | 0.02 (−0.16 to 0.21) |
0.81 | −0.17 (−0.38 to 0.03) |
0.10 |
| 5 | −0.15 (−0.34 to 0.04) |
0.11 | −0.13 (−0.34 to 0.08) |
0.22 |
| 6 | −0.06 (−0.24 to 0.12) |
0.52 | 0.02 (−0.19 to 0.23) |
0.86 |
| 7 | −0.12 (−0.30 to 0.07) |
0.22 | −0.14 (−0.35 to 0.08) |
0.21 |
| 8 | −0.09 (−0.28 to 0.09) |
0.34 | −0.04 (−0.25 to 0.17) |
0.71 |
| 9 | −0.03 (−0.22 to 0.16) |
0.75 | 0.05 (−0.16 to 0.27) |
0.61 |
| 10 | 0.02 (−0.17 to 0.21) |
0.87 | 0.11 (−0.10 to 0.33) |
0.31 |
Models are adjusted for age and femoral neck BMD.
Abbreviations: CI = confidence interval; BMD = bone mineral density
Effect of the FRZB variant alleles on the relationship between hip shape and RHOA
To examine this question, we determined whether there was a statistical interaction between the FRZB variant alleles and hip shape SSM modes in predicting RHOA. In the model without interaction terms, rs288326 was not independently associated with RHOA (p = 0.20), after adjusting for age, femoral neck BMD, and modes 1–5. In the model with interaction terms, there was a significant interaction between Mode 2 and the rs288326 FRZB variant (p value for interaction = 0.022). There was no significant interaction between the rs288326 FRZB variant allele and the other hip shape modes (data not shown). When acetabular depth or center-edge angle were used as predictors of RHOA, no significant interaction with the FRZB rs288326 variant allele was observed (data not shown).
We next repeated this analysis with attention to the rs7775 FRZB SNP. In the model without interaction terms, rs7775 variant allele was not independently associated with RHOA (p = 0.98), after adjusting for age, femoral neck BMD, and modes 1–5. In the model with interaction terms, none of the modes showed a significant interaction with rs7775 variant allele in predicting the outcome of RHOA.
Given the finding that the FRZB rs288326 polymorphism demonstrated an interaction with hip shape SSM Mode 2 and incident radiographic hip OA, we stratified subjects according to whether or not they possessed the rs288326 variant allele. We observed that in subjects with 1 or 2 copies of the FRZB rs288326 variant allele, Mode 2 was associated with incident RHOA (OR 2.46, 95% CI 1.15 to 5.25 for 4th quartile of Mode 2). In addition, higher values of mode 2 were associated with an increased risk of OA in women with 1 or more copies of the variant allele, whereas this association was not observed in subjects lacking the variant allele (Table 3).
Table 3. Relationship between proximal femur shape (SSM Mode 2) and radiographic hip osteoarthritis (RHOA) is modified by the presence or absence of the FRZB rs288326 variant allele.
Odds ratio (OR) is adjusted for age and femoral neck BMD. Quartiles were created based on the control population.
| 1 or 2 copies of rs288326 FRZB variant allele |
No copies of rs288326 FRZB variant allele |
||
|---|---|---|---|
| Quartiles of Mode 2 |
OR (95% CI) |
Quartiles of Mode 2 |
OR (95% CI) |
| 1st Quartile (n = 66; 24 cases, 42 controls) |
1.00 (reference) |
1st Quartile (n = 194; 86 cases, 108 controls) |
1.00 (reference) |
| 2nd Quartile (n = 66; 27 cases, 39 controls) |
1.03 (0.49 to 2.17) |
2nd Quartile (n = 192; 81 cases, 111 controls) |
0.89 (0.59 to 1.36) |
| 3rd Quartile (n = 60; 26 cases, 34 controls) |
1.09 (0.51 to 2.36) |
3rd Quartile (n = 176; 60 cases, 116 controls) |
0.63 (0.40 to 0.97) |
| 4th Quartile* (n = 63; 39 cases, 24 controls) |
2.46 (1.15 to 5.25) |
4th Quartile (n = 235; 108 cases, 127 controls) |
0.90 (0.60 to 1.35) |
|
Test for Linear Trend |
p = 0.02 |
Test for Linear Trend |
p = 0.40 |
P value for interaction using mode two as continuous measure = 0.02.
DISCUSSION
In this longitudinal study of elderly Caucasian women, we found that the FRZB rs288326 and rs7775 variant alleles were associated with the shape of the proximal femur and that the rs288326 variant modified the relationship between hip shape and incident radiographic hip osteoarthritis (RHOA). Considering these observations together, we hypothesize that the WNT pathway may have different functions over a lifespan. In early life, prior to the development of OA, FRZB may play a role in determining the shape of the proximal femur, whereas in later life, FRZB may act via its role in bone homeostasis to influence whether OA develops in a particular architectural setting in response to biomechanical loading forces.
The product of the FRZB gene (sFRP3) is an antagonist of the WNT signaling pathway in both developing embryos and adult organisms. For example, the mouse homolog of the human FRZB gene, Frzb-1, is expressed in the developing limb bud, branchial arteries, and cartilage during development (18, 42) and is important for proper digit formation (43). The chick homolog of FRZB, cFrzb-1, is expressed in developing cartilage, nerves, adrenal glands, gonads, and certain blood cells (44). Mice that have a disruption in the FRZB gene appear to develop normally but have an increased susceptibility to OA in the presence of environmental or experimental triggers such as mechanical stress or inflammation (9). Although FRZB-knockout mice do not appear to have an overt bone phenotype at birth, bone shape in these animals has yet to be studied in detail.
We observed an association between the rs288326 FRZB variant allele and hip shape Mode 2 in the control population. In addition, we found a significant interaction between this FRZB variant allele and hip shape Mode 2 in predicting incident RHOA, suggesting that the presence of the rs288326 FRZB variant allele may modify the effect of hip shape on the risk of incident RHOA. The shape of Mode 2 varies depending on the population under study in the statistical shape model. In our population, Mode 2 describes a proximal femur shape in which the width of the femoral head is narrow relative to the width of the femoral neck and shaft. Mode 2 also describes the position of the greater trochanter and femoral head relative to the position of femoral shaft (Figure 3B). Interestingly, the shape of the proximal femur contributes to the distribution of mechanical forces across the hip joint during weight bearing (45). Since WNT signaling is known to be activated in response to biomechanical loading (19), it is possible that the biologic pathway linking WNT and OA involves activation of WNT signaling in response to altered biomechanical loading of the joint.
The shape of the proximal femur is associated with the development of osteoarthritis of the hip in adult life (reviewed in (21,47)). Nonetheless, some individuals fail to develop RHOA despite the presence of this risk factor, which is consistent with the concept that the development of hip OA is a complex, multivariate process involving genetic, environmental, and perhaps epigenetic risk factors.
In this study, we excluded patients with prevalent RHOA at study enrollment, which may have excluded subjects in whom FRZB variant alleles conferred a predisposition to RHOA in childhood or early adulthood. Ideally, the relationship between FRZB variant alleles and hip shape should be verified in young adults, though no such cohort with serial pelvic radiographs is currently available to us.
Despite the well-established role of WNT signaling in skeletal development and bone homeostasis, there has been controversy as to the magnitude of the association between FRZB variant alleles and osteoarthritis. While two studies reported an association between FRZB allelic variant alleles and RHOA of > 3.0 (15, 16), the association was more modest (46–48) or not found (17, 49) in other populations. Loughlin and colleagues (16) studied subjects within families containing multiple members with RHOA and subjects with advanced OA undergoing total hip replacement. Our research group reported a significant association between the rs288326 allele and hip OA defined by the presence of advanced osteophytosis (15). However, in a recent meta-analysis (17) and primary study (47), the magnitude of association between the rs288326 allele and RHOA was far weaker. Both of these latter studies used a relatively broad definition of RHOA including subjects with mild RHOA, which may have diluted the association. The association between the rs288326 allele variant and hip OA is likely to be strengthened by using defined phenotype of OA characterized by femoral osteophyte formation. The recognition of heterogeneity among RHOA studies and the need for stricter definition of RHOA phenotypes was recently the subject of an international OA consortium (50). As data accumulate on the associations between genetic polymorphisms and RHOA, and radiographic OA at other skeletal sites, attention to such phenotyping is likely to be important.
Although this study examines the interrelationships between FRZB variant alleles, hip shape, and RHOA, several important limitations must be considered. First, the findings reported here were studied in a limited population of Caucasian, American, post-menopausal women and thus, the results may not be generalizable to other ethnic, gender, or geographic groups. Secondly, the selection of the controls was not random. The first 601 controls were selected in sequential order based on their numerical ID, resulting in unequal representation among the clinical sites. However, the same protocol was used to obtain the radiographs at all of the study sites to reduce variability. Thirdly, the frequency of subjects homozygous for the FRZB variant alleles was quite low in our population, despite the relative abundance of subjects heterozygous for the alleles. Thus, our study combined subjects with either 1 or 2 copies of the FRZB allele and compared them to subjects with no copies. Whether individuals with homozygosity for this allele show a more severe OA phenotype is currently not known.
In summary, we find that FRZB variant allele rs288326 modified the association of hip shape and incident radiographic hip OA. Additional studies of the WNT signaling pathway and skeletal homeostasis are warranted to confirm or refute these results.
Figure 2. Hip shape measurements.
(A) Outline of the proximal femur used in statistical shape modeling (SSM). A 60-point outline of the proximal femur between two anchor points (the inferior margin of lesser trochanter and the corresponding point on the lateral femoral shaft) was made (green dots), and coordinates of the points were entered into the SSM software to generate the 10 independent modes of hip shape variation by principal components analysis. The outline of the proximal femur generated by SSM is shown in yellow. (B) Illustration of variation in proximal femur shape in Mode 2. Mean shape of Mode 2 (green line) with ± 1 standard deviation (SD) (red and blue lines) is shown. (C and D) Acetabular measurements used in the study.
Acknowledgments
This work was supported by the NIH Academic Rheumatology and Clinical Immunology Training Grant #AR007304 to J.C.B. and by SOF, REF, UCSF training grant, and AR04884, AR052000, and AR043052 to N.E.L.
Footnotes
Conflicts of Interest: The authors state no conflicts of interest.
References
- 1.Reginster JY. The prevalence and burden of arthritis. Rheumatology (Oxford) 2002;41(Supp 1):3–6. [PubMed] [Google Scholar]
- 2.Reginster JY, Khaltaev NG. Introduction and WHO perspective on the global burden of musculoskeletal conditions. Rheumatology (Oxford) 2002;41(Supp 1):1–2. [PubMed] [Google Scholar]
- 3.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30(8):914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 5.Maurer K. Basic data on arthritis knee, hip, sacroiliac joints in adults ages 25–74 years. Vital Health Stat 11. 1979;(213):1–31. [PubMed] [Google Scholar]
- 6.Hunter DJ. Pharmacologic therapy for osteoarthritis-the era of disease modification. Nat Rev Rheumatol. doi: 10.1038/nrrheum.2010.178. [DOI] [PubMed] [Google Scholar]
- 7.MacGregor AJ, Antoniades L, Matson M, Andrew T, Spector TD. The genetic contribution to radiographic hip osteoarthritis in women: results of a classic twin study. Arthritis Rheum. 2000;43(11):2410–2416. doi: 10.1002/1529-0131(200011)43:11<2410::AID-ANR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 8.Baker-LePain JC, Lane NE. Relationship between joint shape and the development of osteoarthritis. Curr Opin Rheumatol. 2010;22(5):538–543. doi: 10.1097/BOR.0b013e32833d20ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lories RJ, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, et al. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007;56(12):4095–4103. doi: 10.1002/art.23137. [DOI] [PubMed] [Google Scholar]
- 10.Enomoto-Iwamoto M, Kitagaki J, Koyama E, Tamamura Y, Wu C, Kanatani N, et al. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol. 2002;251(1):142–156. doi: 10.1006/dbio.2002.0802. [DOI] [PubMed] [Google Scholar]
- 11.Dong Y, Drissi H, Chen M, Chen D, Zuscik MJ, Schwarz EM, et al. Wnt-mediated regulation of chondrocyte maturation: modulation by TGF-beta. J Cell Biochem. 2005;95(5):1057–1068. doi: 10.1002/jcb.20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M. Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest. 2008;88(3):264–274. doi: 10.1038/labinvest.3700747. [DOI] [PubMed] [Google Scholar]
- 13.Dell'accio F, De Bari C, Eltawil NM, Vanhummelen P, Pitzalis C. Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum. 2008;58(5):1410–1421. doi: 10.1002/art.23444. [DOI] [PubMed] [Google Scholar]
- 14.Beyer C, Schett G. Pharmacotherapy: concepts of pathogenesis and emerging treatments. Novel targets in bone and cartilage. Best Pract Res Clin Rheumatol. 24(4):489–496. doi: 10.1016/j.berh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Lane NE, Lian K, Nevitt MC, Zmuda JM, Lui L, Li J, et al. Frizzled-related protein variants are risk factors for hip osteoarthritis. Arthritis Rheum. 2006;54(4):1246–1254. doi: 10.1002/art.21673. [DOI] [PubMed] [Google Scholar]
- 16.Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci U S A. 2004;101(26):9757–9762. doi: 10.1073/pnas.0403456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evangelou E, Chapman K, Meulenbelt I, Karassa FB, Loughlin J, Carr A, et al. Large-scale analysis of association between GDF5 and FRZB variants and osteoarthritis of the hip, knee, and hand. Arthritis Rheum. 2009;60(6):1710–1721. doi: 10.1002/art.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witte F, Dokas J, Neuendorf F, Mundlos S, Stricker S. Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr Patterns. 2009;9(4):215–223. doi: 10.1016/j.gep.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281(42):31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 20.Doherty M, Courtney P, Doherty S, Jenkins W, Maciewicz RA, Muir K, et al. Nonspherical femoral head shape (pistol grip deformity), neck shaft angle, and risk of hip osteoarthritis: a case-control study. Arthritis Rheum. 2008;58(10):3172–3182. doi: 10.1002/art.23939. [DOI] [PubMed] [Google Scholar]
- 21.Lynch JA, Parimi N, Chaganti RK, Nevitt MC, Lane NE. The association of proximal femoral shape and incident radiographic hip OA in elderly women. Osteoarthritis Cartilage. 2009;17(10):1313–1318. doi: 10.1016/j.joca.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McWilliams DF, Doherty SA, Jenkins WD, Maciewicz RA, Muir KR, Zhang W, et al. Mild acetabular dysplasia and risk of osteoarthritis of the hip: a case-control study. Ann Rheum Dis. 69(10):1774–1778. doi: 10.1136/ard.2009.127076. [DOI] [PubMed] [Google Scholar]
- 23.Wagner S, Hofstetter W, Chiquet M, Mainil-Varlet P, Stauffer E, Ganz R, et al. Early osteoarthritic changes of human femoral head cartilage subsequent to femoro-acetabular impingement. Osteoarthritis Cartilage. 2003;11(7):508–518. doi: 10.1016/s1063-4584(03)00075-x. [DOI] [PubMed] [Google Scholar]
- 24.Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263(5):665–668. [PubMed] [Google Scholar]
- 25.Paffenberger RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314(10):605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 26.Black DM, Steinbuch M, Palermo L, Dargent-Molina P, Lindsay R, Hoseyni MS, et al. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporos Int. 2001;12(7):519–528. doi: 10.1007/s001980170072. [DOI] [PubMed] [Google Scholar]
- 27.Lane NE, Nevitt MC, Hochberg MC, Hung YY, Palermo L. Progression of radiographic hip osteoarthritis over eight years in a community sample of elderly white women. Arthritis Rheum. 2004;50(5):1477–1486. doi: 10.1002/art.20213. [DOI] [PubMed] [Google Scholar]
- 28.Lian K, Zmuda JM, Nevitt MC, Lui L, Hochberg MC, Greene D, et al. Type I collagen alpha1 Sp1 transcription factor binding site polymorphism is associated with reduced risk of hip osteoarthritis defined by severe joint space narrowing in elderly women. Arthritis Rheum. 2005;52(5):1431–1436. doi: 10.1002/art.21011. [DOI] [PubMed] [Google Scholar]
- 29.Lin K, Wang S, Julius MA, Kitajewski J, Moos M, Jr, Luyten FP. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc Natl Acad Sci U S A. 1997;94(21):11196–11200. doi: 10.1073/pnas.94.21.11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckland-Wright C. Protocols for precise radio-anatomical positioning of the tibiofemoral and patellofemoral compartments of the knee. Osteoarthritis Cartilage. 1995;3(Suppl A):71–80. [PubMed] [Google Scholar]
- 31.Cootes TF, Taylor CJ, Cooper DH, Graham J. Active shape models -- their training and application. Comput Vis Image Underst. 1995;61(1):38–59. [Google Scholar]
- 32.Beymer D, Syeda-Mahmood T. Cardiac disease recognition in echocardiograms using spatio-temporal statistical models. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:4784–4788. doi: 10.1109/IEMBS.2008.4650283. [DOI] [PubMed] [Google Scholar]
- 33.Cosio FA. Automatic initialization of an active shape model of the prostate. Med Image Anal. 2008;12(4):469–483. doi: 10.1016/j.media.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Heimann T, Wolf I, Meinzer HP. Active shape models for a fully automated 3D segmentation of the liver--an evaluation on clinical data. Med Image Comput Comput Assist Interv. 2006;9(Pt 2):41–48. doi: 10.1007/11866763_6. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Lim JH, Liu J, Wong TY. Towards automatic grading of nuclear cataract. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:4961–4964. doi: 10.1109/IEMBS.2007.4353454. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Shen D. Hierarchical shape statistical model for segmentation of lung fields in chest radiographs. Med Image Comput Comput Assist Interv. 2008;11(Pt 1):417–424. doi: 10.1007/978-3-540-85988-8_50. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel M, Hahn DA, Daum V, Wasza J, Hornegger J. Segmentation of kidneys using a new active shape model generation technique based on non-rigid image registration. Comput Med Imaging Graph. 2009;33(1):29–39. doi: 10.1016/j.compmedimag.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Gregory JS, Testi D, Stewart A, Undrill PE, Reid DM, Aspden RM. A method for assessment of the shape of the proximal femur and its relationship to osteoporotic hip fracture. Osteoporos Int. 2004;15(1):5–11. doi: 10.1007/s00198-003-1451-y. [DOI] [PubMed] [Google Scholar]
- 39.Baker-Lepain JC, Luker KR, Lynch JA, Parimi N, Nevitt MC, Lane NE. Active shape modeling of the hip in prediction of incident hip fracture. J Bone Miner Res. 2010 doi: 10.1002/jbmr.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiberg G. A measuring method for distinguishing between a normal and a maldevelopmental acetabulum. Acta Chir Scand. 1939;83:28–38. [Google Scholar]
- 41.Murray RO. The aetiology of primary osteoarthritis of the hip. Br J Radiol. 1965;38(455):810–824. doi: 10.1259/0007-1285-38-455-810. [DOI] [PubMed] [Google Scholar]
- 42.Hoang BH, Thomas JT, Abdul-Karim FW, Correia KM, Conlon RA, Luyten FP, et al. Expression pattern of two Frizzled-related genes, Frzb-1 and Sfrp-1, during mouse embryogenesis suggests a role for modulating action of Wnt family members. Dev Dyn. 1998;212(3):364–372. doi: 10.1002/(SICI)1097-0177(199807)212:3<364::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 43.Chimal-Monroy J, Montero JA, Ganan Y, Macias D, Garcia-Porrero JA, Hurle JM. Comparative analysis of the expression and regulation of Wnt5a, Fz4, and Frzb1 during digit formation and in micromass cultures. Dev Dyn. 2002;224(3):314–320. doi: 10.1002/dvdy.10110. [DOI] [PubMed] [Google Scholar]
- 44.Duprez D, Leyns L, Bonnin MA, Lapointe F, Etchevers H, De Robertis EM, et al. Expression of Frzb-1 during chick development. Mech Dev. 1999;89(1–2):179–183. doi: 10.1016/s0925-4773(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 45.Lenaerts G, Bartels W, Gelaude F, Mulier M, Spaepen A, Van der Perre G, et al. Subject-specific hip geometry and hip joint centre location affects calculated contact forces at the hip during gait. J Biomech. 2009;42(9):1246–1251. doi: 10.1016/j.jbiomech.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 46.Lories RJ, Boonen S, Peeters J, de Vlam K, Luyten FP. Evidence for a differential association of the Arg200Trp single-nucleotide polymorphism in FRZB with hip osteoarthritis and osteoporosis. Rheumatology (Oxford) 2006;45(1):113–114. doi: 10.1093/rheumatology/kei148. [DOI] [PubMed] [Google Scholar]
- 47.Min JL, Meulenbelt I, Riyazi N, Kloppenburg M, Houwing-Duistermaat JJ, Seymour AB, et al. Association of the Frizzled-related protein gene with symptomatic osteoarthritis at multiple sites. Arthritis Rheum. 2005;52(4):1077–1080. doi: 10.1002/art.20993. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Lopez J, Pombo-Suarez M, Liz M, Gomez-Reino JJ, Gonzalez A. Further evidence of the role of frizzled-related protein gene polymorphisms in osteoarthritis. Ann Rheum Dis. 2007;66(8):1052–1055. doi: 10.1136/ard.2006.065938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kerkhof JM, Uitterlinden AG, Valdes AM, Hart DJ, Rivadeneira F, Jhamai M, et al. Radiographic osteoarthritis at three joint sites and FRZB, LRP5, and LRP6 polymorphisms in two population-based cohorts. Osteoarthritis Cartilage. 2008;16(10):1141–1149. doi: 10.1016/j.joca.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Kerkhof HJ, Meulenbelt I, Akune T, Arden NK, Aromaa A, Bierma-Zeinstra SM, et al. Recommendations for standardization and phenotype definitions in genetic studies of osteoarthritis: the TREAT-OA consortium. Osteoarthritis Cartilage. doi: 10.1016/j.joca.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]