Abstract
Tobacco is a highly addictive drug and is one of the most widely abused drugs in the world. The first part of this review explores the role of stressors and stress-associated psychiatric disorders in the initiation of smoking, the maintenance of smoking, and relapse after a period of abstinence. The reviewed studies indicate that stressors facilitate the initiation of smoking, decrease the motivation to quit, and increase the risk for relapse. Furthermore, people with depression or an anxiety disorder are more likely to smoke than people without these disorders. The second part of this review describes animal studies that investigated the role of brain stress systems in nicotine addiction. These studies indicate that corticotropin-releasing factor, Neuropeptide Y, the hypocretins, and norepinephrine play a pivotal role in nicotine addiction. In conclusion, the reviewed studies indicate that smoking briefly decreases subjective stress levels but also leads to a further dysregulation of brain stress systems. Drugs that decrease the activity of brain stress systems may diminish nicotine withdrawal and improve smoking cessation rates.
Keywords: Tobacco, nicotine, addiction, dependence, depression, anxiety, CRF, NPY, hypocretin, norepinephrine
1. Introduction
Tobacco is one of the most widely abused drugs in the world. It has been estimated that worldwide there are about 1 billion males who smoke and 250 million females (World Health Organization, 2011). Half of the smokers die as a direct consequence of their tobacco addiction. Worldwide about 5.4 million people die each year from smoking including 400,000 people in the United States and 640,000 in Europe (ASPECT Consortium, 2005; Mokdad et al., 2004; World Health Organization, 2011). It has been estimated that about 600,000 people die each year from exposure to second hand tobacco smoke (World Health Organization, 2009). The World Health Organization has indicated that the tobacco pandemic is moving from Western countries to developing nations and has estimated that about 80 percent of the people who die from smoking now live in low and middle income countries (World Health Organization, 2011). Considering the large number of smokers and the highly addictive properties of tobacco, a better understanding of the environmental, genetic, and neurobiological factors that contribute to the development and maintenance of a tobacco addiction is warranted.
Several lines of evidence suggest that the positive reinforcing effects of cigarettes play a pivotal role in the initiation of smoking (Finkenauer et al., 2009; Wise, 1996). The positive reinforcing effects of smoking include mild euphoria, relaxation, and improved attention and working memory (Ague, 1973; Benowitz, 1988; Wesnes and Warburton, 1983). Discontinuation of smoking leads to negative affective symptoms such as depressed mood, increased anxiety, and impaired memory and attention (Hughes et al., 1991; Hughes and Hatsukami, 1986). The negative affective symptoms associated with smoking cessation may increase the risk for relapse to smoking (Bruijnzeel and Gold, 2005; Koob, 2008). Preclinical studies suggest that nicotine is the main component of tobacco that leads to smoking and prevents people from quitting smoking (Bardo et al., 1999; Crooks and Dwoskin, 1997; Stolerman and Jarvis, 1995). There is, however, evidence that other components in tobacco smoke may also have positive reinforcing effects and / or potentiate the effects of nicotine (Fowler et al., 2003; Talhout et al., 2007). Acetaldehyde is one of the compounds in smoke that may contribute to the development of a tobacco addiction. The pyrolysis of carbohydrates in cigarettes leads to the formation of acetaldehyde and this compound is self-administered by rodents and induces conditioned place preference (Brown et al., 1979; Myers et al., 1982; Smith et al., 1984). Self-administration studies show that acetaldehyde also potentiates the positive reinforcing effects of nicotine in rats (Belluzzi et al., 2005). Furthermore, tobacco smoke contains high concentrations of the β-carbolines norharman and harman which inhibit monoamine oxidase (MAO)-A and MAO-B (Hauptmann and Shih, 2001; Herraiz and Chaparro, 2005; Totsuka et al., 1999). Positron emission tomography imaging studies show that smoking inhibits MAO-A and MAO-B in the human brain (Fowler et al., 1996; Fowler et al., 1998). In humans, MAO-A metabolizes norepinephrine, serotonin and dopamine and MAO-B metabolizes phenylethylamine and dopamine (Shih et al., 1999). Norharman and harman have antidepressant-like effects in rodents (Aricioglu and Altunbas, 2003; Farzin and Mansouri, 2006) and clinical studies indicate that drugs that inhibit MAO-A, but not MAO-B, have antidepressant effects in humans (Blier and de Montigny C., 1994). Tobacco smoke-induced MAO-B inhibition may also explain the fact that smoking decreases the risk for Parkinson’s disease in humans (Chen et al., 2010; Morens et al., 1995). Therefore, in addition to nicotine, many other compounds in tobacco smoke affect brain function.
During the last decades, several treatments have been developed to help people quit smoking. The U.S. Food and Drug Administration approved nicotine replacement therapy, varenicline (brand name Chantix) and bupropion (brand name Zyban) for smoking cessation. Varenicline is a partial agonist of α4β2 nicotinic acetylcholine receptors (nAChRs). This drug may improve smoking cessation rates by inhibiting the positive reinforcing effects of nicotine, attenuating nicotine withdrawal, and decreasing craving for cigarettes (Rollema et al., 2007). The precise pharmacological mechanisms by which bupropion improves smoking cessation rates are not known. However, it may improve smoking cessation rates by blocking nAChRs, inhibiting the reuptake of dopamine, norepinephrine, and serotonin, or inhibiting the firing of noradrenergic neurons (Cryan et al., 2003a). A recent literature review suggests that varenicline may be slightly more effective in preventing relapse to smoking than bupropion or nicotine replacement therapy (Cahill et al., 2010). Although the aforementioned drugs help people quit smoking, relapse rates are still very high (80–85 percent over 1-year period) among people receiving treatment for smoking cessation (Gonzales et al., 2006). Moreover, bupropion increases the risk for seizures and treatment with varenicline may lead to depressed mood, suicidal thoughts, drowsiness, and aggressive behavior in a subgroup of smokers (Davidson, 1989; Johnston et al., 1991; Moore and Furberg, 2009). Varenicline use in humans also leads to an increased risk for cardiovascular events such as stroke and congestive heart failure (Singh et al., 2011). Therefore, despite the fact that significant progress has been made in the development of treatments for tobacco addiction, there remains an urgent need for safer and more effective treatment options.
This review explores the role of brain stress systems in tobacco addiction. The first part of this review examines the role of stressors in the onset of smoking, maintenance of smoking, and relapse to smoking after a period of abstinence. The comorbidity between smoking and stress-associated psychiatric disorders is also discussed. Specifically, it will be investigated if depression, post-traumatic stress disorder (PTSD), and other anxiety disorders increase the risk for smoking and / or if people with these disorders are more likely to experiment with cigarettes and develop a tobacco addiction. The second part of this review provides an overview of studies that investigated the role of brain stress systems in animal models for tobacco addiction. This review focuses mainly on the role of neuropeptides in nicotine addiction. During the last decades, extensive progress has been made in the understanding of the role of neuropeptides in modulating behavioral, endocrine, and autonomic responses. One of the first milestones in this field was the observation by David de Wied that endocrine hormones produced in the pituitary also serve as precursors for peptides that have effects in the central nervous system (i.e., neuropeptide concept) (De Wied D., 1969; De Wied, 1977). Another major milestone was the isolation of corticotropin-releasing factor (CRF) from the ovine hypothalamus (Vale et al., 1981). CRF plays an important role in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis and also affects behavioral responses independent of its affect on the HPA axis (Eaves et al., 1985). Pioneering studies by Nemeroff, Koob and others showed that CRF plays a critical role in depression and drug addiction (Baldwin et al., 1991; Koob, 1996; Nemeroff et al., 1984). More recent studies have provided evidence for a role of neuropeptide Y and the hypocretins in the regulation of mood states and drug addiction (Boutrel et al., 2005; Gilpin et al., 2003). In the second half of this review, the role of CRF, hypocretins, neuropeptide Y (NPY), norepinephrine, and the HPA axis in nicotine addiction will be discussed.
It should be noted that in addition to the aforementioned neuropeptides and neurotransmitters, other cholinergic and non-cholinergic brain systems have also been implicated in the rewarding effects of nicotine, nicotine withdrawal, and the reinstatement of extinguished nicotine-seeking behavior. It is, however, beyond the scope of this review to discuss all the brain systems that may play a role in nicotine addiction. For an overview of the role of acetylcholine, dynorphin, and other neurotransmitters in nicotine addiction, the readers are referred to previous reviews (Balfour, 2009; Bruijnzeel, 2009; Castane et al., 2005; Dani and Balfour, 2011; Maldonado and Berrendero, 2010; Markou, 2007).
2. Tobacco Addiction and Stress Systems; Insights from Human Studies
2.1. Stressors and Smoking
Extensive evidence indicates that brain stress systems play a critical role in the initiation of smoking, the maintenance of smoking, and relapse to smoking after a period of abstinence. Smokers indicate in surveys that stress relief and relaxation are their main reasons for smoking (Ikard et al., 1969). In one study with 16 year old female smokers, about 50 percent of the girls indicated that they started smoking because they experienced a lot of stress in their lives and they believed that smoking helped them to relax (Nichter et al., 1997). In a study conducted by Fidler and West, 51 percent of the smokers indicated that they smoked for enjoyment and 47 percent indicated that they smoked to cope with stress (Fidler and West, 2009). This is in line with another study in which smokers reported that they smoke for stress relief (3.9 on a scale of 1 to 5), boredom relief (3.7), and enjoyment (3.6) (McEwen et al., 2008). Furthermore, smokers often do not want to quit smoking because they experience their life as too stressful (Lader, 2007).
Studies with humans in laboratory settings confirm that exposure to stressors increases craving for cigarettes and smoking. The desire to smoke in regular smokers is greater when conducting a stressful computer task than when working on a non-stressful control task (Perkins and Grobe, 1992). Exposure of test subjects to loud noises has also been shown to increase smoking (Cherek, 1985). In addition, exposure of test subjects to an anxiety-provoking stage fright test leads to an increase in smoking (Rose et al., 1983). Taken together, these studies indicate that smokers report stress relief as one of their main reasons for smoking and exposure to stressors leads to increased craving for cigarettes and smoking. Several studies have also reported increased smoking after large-scale anxiety-provoking events. Increased smoking has been reported in New York City residents after the September 11th, 2001, terrorist attacks; in survivors of the Herald of Free Enterprise disaster off the coast of Belgium in 1987; in people who were exposed to the bushfires in Australia in 2003; in survivors of Hurricane Katrina in New Orleans in 2005; and in people in Florida who were affected by the 2004 hurricanes (Amstadter et al., 2009; Flory et al., 2009; Joseph et al., 1993; Nandi et al., 2005; Parslow and Jorm, 2006; Vlahov et al., 2004a; Vlahov et al., 2004b). In contrast to the aforementioned studies, another study reported that exposure to a traumatic event, a fire in a bar in The Netherlands that killed 14 adolescents and wounded 250, did not lead to an increase in smoking (Reijneveld et al., 2003). Exposure to this extremely stressful event did, however, lead to an increase in alcohol intake and aggressive behavior.
Overall, smokers indicate that stress relief is one of their main reasons for smoking and exposure to stressors often increases the number of cigarettes smoked. However, although smokers indicate that they smoke for stress relief and relaxation, some research suggests that smoking increases subjective stress levels and that smoking does not lead to stress relief but merely reverses the negative mood state caused by nicotine deprivation (Parrott, 1999). Stress levels of smokers vary widely throughout the day. Smokers have decreased subjective stress levels immediately after smoking a cigarette and increased stress levels between cigarettes when nicotine levels are low (Parrott, 1994; Parrott, 1995). Although there is general consensus that the discontinuation of smoking leads to negative mood states (Hughes et al., 1991; Hughes and Hatsukami, 1986), the hypothesis that smoking leads to increased subjective stress levels is not supported by all studies. For example, a study with 1,364 adolescents showed that negative affect leads to increased smoking but not the other way around (Wills et al., 2002).
2.2. Depression and Smoking
2.2.1. Childhood / Adolescent Depression and Smoking Later in Life
Several studies have investigated the effects of childhood or adolescent depression on the likelihood of smoking later in life. Prinstein and La Greca conducted an extensive longitudinal study that investigated the association between childhood depressive symptoms and adolescent cigarette use (Prinstein and La Greca, 2009). They assessed depressive symptoms with the Children’s Depression Inventory and peer aggression with a sociometric peer nomination procedure during grades 4 – 6 (9 – 12 years of age) and smoking during grades 10 – 12 (15– 18 years of age) in 250 children. It was shown that both childhood depression and peer aggression significantly increased the likelihood of smoking during adolescence. The same study also indicated that childhood depression increases the risk for depression during adolescence (Prinstein and La Greca, 2009). There is some evidence that suggests that negative mood states may only contribute to smoking when certain environmental conditions are met. For example, Patton and colleagues reported that increased depression and/or anxiety during adolescence increases the risk for the initiation of smoking in subjects who reported that their peers smoked but not in subjects whose peers did not smoke (Patton et al., 1998). Taken together, the above discussed studies suggest that childhood and adolescent depression in combination with specific environmental factors increases the risk for smoking later in life.
2.2.2. Smoking and Depression Risk and Vice Versa
Numerous large studies have investigated the relationship between depression and smoking in adolescents and adults. Fergusson and colleagues investigated the association between major depression and smoking in young adults (16 – 21 years of age) by using data from 1265 children from New Zealand that were included in the Christenchurch Health and Development Study (Fergusson et al., 2003). This study showed that there is a strong association between depression and smoking in adolescents and young adults (age 16 years odds ratio [OR] = 5.12; age 18 years OR = 2.52; age 21 years OR = 2.52) and this association was still present (age 16–21 years OR = 1.75) when the association was adjusted for confounding factors such as anxiety disorders and parental smoking. This observation is in agreement with another large study that investigated the association between tobacco smoking and depression in upstate New York (Johnson et al., 2000). In this study, 688 adolescents were interviewed from 1985 to 1986 at the age of 16 and from 1991 to 1993 at the age of 22. The results of this study indicated that depressive disorders in 16 year old adolescents increases the risk for heavy smoking during the same developmental stage (OR = 4.07). Taken together, these studies indicate that there is a strong association between depression and smoking in adolescents. The association between depression and smoking remains throughout adulthood. In a study by Mathew and colleagues, 61 percent of depressed patients (mean age 29.6 years) smoked and only 27 percent of the non-depressed controls smoked (Mathew et al., 1981). Another study reported that the smoking rate among psychiatric outpatients (mean age 31.9 years) with major depression was 49 percent and the smoking rate was 30 percent in a population-based control sample (Hughes et al., 1986). Because the two aforementioned studies used relatively small sample sizes, another study was conducted by using data from the St. Louis Epidemiologic Catchment Area Survey. In this survey, 3212 subjects (mean age 42.5 years) reported on their smoking and depression histories. Analyses of the data indicated that a lifetime occurrence of major depression greatly increases the risk (OR = 2.38) for lifetime smoking (Glassman et al., 1990). More recent large population-based studies have confirmed that the smoking rate in depressed adults is about twice as high as in control subjects without a mental illness (Lasser et al., 2000; Lawrence et al., 2009). Epidemiological studies suggest that the association between smoking and depression is bidirectional. Thus, depression may not only increase the risk for smoking but smoking in non-depressed subjects may also increase the risk for developing depression. Goodman and Capitman investigated the effects of adolescent smoking on depression later in life by analyzing the data from 8704 adolescents (mean age 15.3 years) with low depression scores on the Center for Epidemiologic Studies Depression Scale prior to the onset of smoking (Goodman and Capitman, 2000). It was shown that current cigarette smoking was an extremely strong predictor of developing severe depressive symptoms (OR = 3.9) later in life. This finding is in agreement with another study that investigated the effects of smoking on the development of depression in 1731 children and adolescents who were not depressed prior to the onset of smoking (Wu and Anthony, 1999). It was shown that smoking (8–9 years through 13–14 years) was associated with a modestly increased risk for the development of depressed mood. Taken together, these large epidemiological studies demonstrate that smoking increases the risk for developing depression (see also Figure 1).
Figure 1.
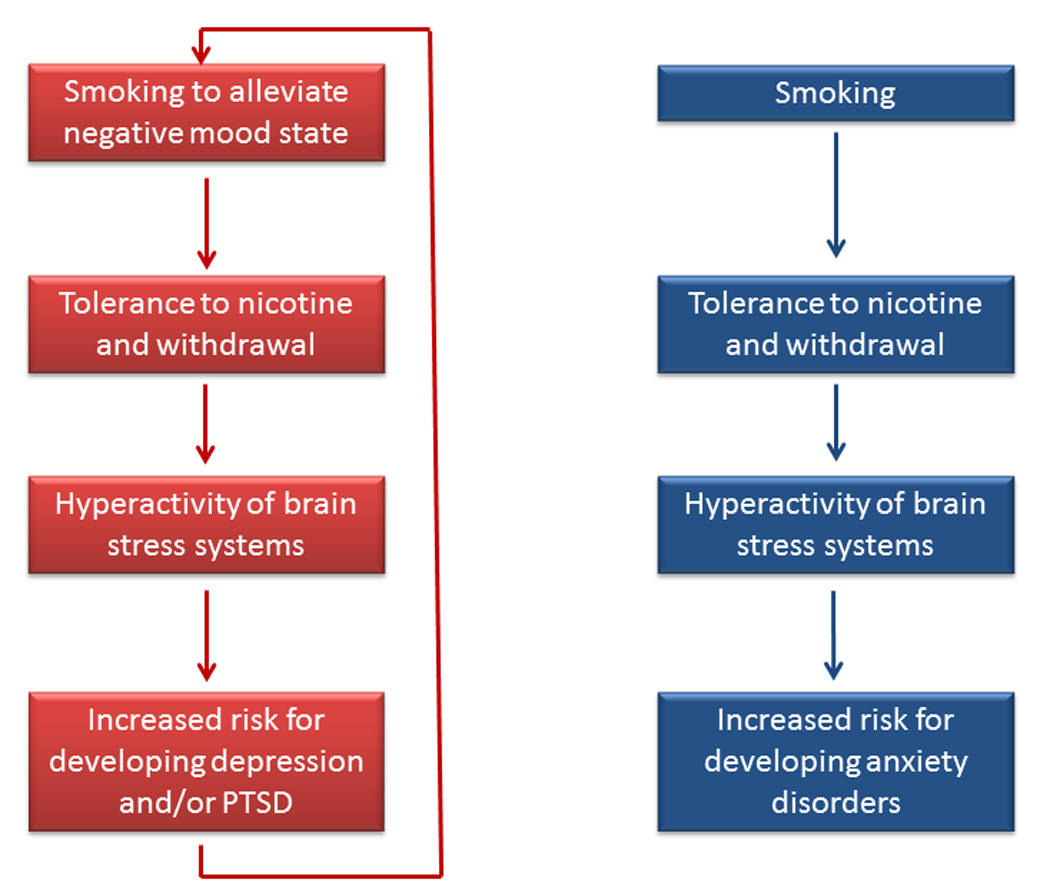
Role of smoking in developing depression, PTSD, and other anxiety disorders. The left side of the figure shows that there is a bidirectional relationship between smoking and depression and PTSD; smoking increases the risk for developing depression and PTSD and vice versa. The right side of the figure indicates that smoking increases the risk for developing an anxiety disorder (PTSD not included). Most anxiety disorders do not increase the risk for smoking.
2.3. Post-Traumatic Stress Disorder and Smoking
There is strong evidence for an association between PTSD and smoking (Feldner et al., 2007). First of all, the smoking rate in people with PTSD is higher than the smoking rate in people without a mental illness. The smoking rate in PTSD patients is 44.6 percent compared to 22.5 percent in people without a mental illness (Lasser et al., 2000). This in line with data from the National Women’s study which indicated that the current smoking rate for women with PTSD was 40.5 percent and 24.8 percent for women without PTSD (Acierno et al., 1996). Beckham and colleagues investigated the prevalence of smoking in Vietnam combat veterans with and without PTSD (Beckham et al., 1997). The smoking rates were the same among veterans with (53 percent) and without PTSD (45 percent). However, the veterans with PTSD reported a higher rate of heavy smoking compared to the veterans without PTSD. Forty eight percent of the veterans with PTSD reported to smoke more than 25 cigarettes per day and only 28 percent of the veterans without PTSD smoked more than 25 cigarettes per day. On a similar note, Cook and colleagues reported that Iraq and Afghanistan combat veterans with high levels of overall PTSD symptoms were more likely (OR = 1.65) to report heavy smoking (≥ 20 cigarettes per day) than PTSD patients with relatively less severe symptoms (Cook et al., 2009).
Some evidence suggests that the development of PTSD, but not exposure to trauma by itself, increases the risk for smoking. This is supported by the analysis of smoking and PTSD data from 6744 subjects who were included in the Vietnam Era Twin Registry (Koenen et al., 2005). This analysis demonstrated that in veterans who were not nicotine dependent prior to a traumatic experience, the development of PTSD was associated with an increased risk for the development of nicotine dependence (OR=1.73). When non-nicotine dependent veterans were exposed to trauma and did not develop PTSD then there was no increased risk for the development of nicotine dependence (OR=0.90). Interestingly, veterans who were nicotine dependent prior to exposure to the traumatic experience were more likely to develop PTSD than veterans who were not nicotine dependent prior to the traumatic experience (OR = 2.24). This indicates that subjects who were addicted to cigarettes at the time of the traumatic experience were twice as likely to develop PTSD as subjects who did not smoke when exposed to the trauma. Recent studies also support the hypothesis that smoking increases the risk for developing PTSD. Van der Velden and colleagues investigated the association between smoking and the development of PTSD in rescue workers who were involved in the Enschede fireworks explosion that killed 23 people and destroyed 500 homes (Van der Velden et al., 2008). The rescue workers were surveyed 2–3 weeks after the fireworks explosion and 18 months after the explosion. Rescue workers who smoked when the first survey was done were more likely to experience PTSD symptoms such as re-experiencing the trauma, avoiding reminders of the trauma, hostility, and depression at the time of the second survey. The association between smoking and mental health problems was also investigated among residents exposed to the Enschede fireworks explosion (Van der Velden et al., 2007). In this study, the first survey was conducted 18 months after the explosion and the second survey 4 years after the explosion. The subjects who smoked during the first survey were at a greater risk to suffer from severe anxiety (OR = 2.32), severe hostility (OR = 1.84), and disaster-related PTSD (OR = 2.64) during the second survey. Taken together, the present studies suggest that PTSD increases the risk for smoking and vice versa.
Animal studies have been conducted to investigate the role of nicotine in the development of PTSD. It has been suggested that certain aspects of PTSD can be investigated with the Pavlovian fear conditioning procedure (Charney et al., 1993). In this procedure, an emotionally neutral conditioned stimulus (CS), such as a tone or light, is paired with an aversive unconditioned stimulus (US) (LeDoux, 2000). After the pairing, the CS induces a fear response that is similar to the one induced by the US. Extensive evidence indicates that the acute and chronic administration of nicotine improves learning and memory (Gould, 2006; Rezvani and Levin, 2001). Several studies have shown that the administration of nicotine before fear conditioning training and testing enhances contextual fear conditioning in mice (Gould and Higgins, 2003; Gould and Wehner, 1999). Nicotine withdrawal has the opposite effect and impairs fear conditioning (Davis et al., 2005). In addition, the administration of nicotine during training and extinction sessions impairs extinction learning in mice (Elias et al., 2010). Based on these studies it has been suggested that nicotine could potentially contribute to the development of PTSD by enhancing the consolidation of aversive memories and delaying the extinction of aversive memories.
2.4. Anxiety Disorders and Smoking
2.4.1. Association between Smoking and Anxiety Disorders
Extensive evidence indicates that there is a strong positive association between smoking and anxiety disorders. Population surveys indicate that smoking rates in people with an anxiety disorder are higher than those in people without a mental illness. Lasser and colleagues analyzed the data from 4411 subjects who were between 15 and 54 year of age and participated in the 1991–1992 National Comorbidity Survey. It was shown that 22.5 percent of the respondents without a mental illness smoked and 54.6 percent of the subjects with generalized anxiety disorder smoked (Lasser et al., 2000). Similar results were obtained by analyzing the data from a sample of young adults, 21–30 years of age, in the Detroit area (Breslau et al., 1994). It was shown that smokers were more likely to have panic disorder (OR males = 3.2, OR females = 2.2), obsessive-compulsive disorder (OR males = 4.3, OR females = 3.7), and phobia (OR males = 2.5, OR females = 2.1) than non smokers. In a more recent study, Lawrence and colleagues studied the association between smoking and psychiatric disorders by analyzing data from the 2007 Australian Survey of Mental Health, the 2001–2003 US National Comorbidity Survey-Replication, and the 2007 US National Health Interview Survey (Lawrence et al., 2009). In Australian adults, 18.8 percent of the respondents without a mental disorder smoked and 45.8 percent of the respondents with generalized anxiety disorder smoked. Smoking rates were also very high in respondents with other anxiety disorders such as: panic disorder (39.6 percent); agoraphobia (37 percent); and obsessive-compulsive disorder (41.1 percent). Similar smoking rates were observed in American adults. In the United States, 21 percent of the adults without a mental illness smoked and 45.2 percent of the subjects with generalized anxiety disorder smoked (Lawrence et al., 2009). The association between anxiety and smoking has also been reported in a large study that was conducted in China and included 4724 adolescents (Weiss et al., 2008). It was shown that a high level of anxiety increased the risk (OR = 1.28) for lifetime smoking.
2.4.2. Smoking and Risk for Anxiety Disorders
Although the studies in the previous section indicate that there is an association between anxiety disorders and smoking, these studies do not indicate whether smoking increases the risk for anxiety disorders or the other way around. Epidemiological studies suggest that smoking increases the likelihood of developing an anxiety disorder. Breslau and Klein compared the risk (hazard ratio, HR) for first panic attack in adults who smoked daily and people who did not smoke (Breslau and Klein, 1999). It was shown that smoking greatly increased the risk for (HR = 3.96) for first panic attack in smokers versus non smokers. In addition, smoking increased the risk for developing a panic disorder (HR = 4.73). It is interesting to note that quitting smoking significantly decreased the risk of first panic attack. A separate analysis indicated that the hazard ratio for first panic attack was 4.71 for people who continued smoking and 0.21 for people who quit smoking. Johnson and colleagues investigated the longitudinal association between smoking and anxiety disorders in adolescents and young adults (Johnson et al., 2000). For this prospective longitudinal investigation, 688 adolescents were interviewed at the age of 16 (1985–1986) and again at the age of 22 (1991–1993). Interestingly, during adolescence there was no association between anxiety disorders and smoking. However, heavy smoking (≥20 cigarettes /day) during adolescence (age 16 years) was associated with an increased risk for agoraphobia (OR = 6.79), generalized anxiety disorder (OR = 5.53) and panic disorder (OR = 15.58) in early adulthood at the age of 22. Thus, these studies suggest that smoking increases the risk for developing an anxiety disorder.
2.4.3. Anxiety Disorders and Risk for Smoking
In contrast to the clear effects of smoking on the development of anxiety disorders, conflicting findings have been reported with regard to the effects of anxiety disorders on the onset of smoking and the development of a tobacco addiction. Sonntag and colleagues analyzed the data from 3,021 subjects that participated in the Early Developmental Stages of Psychopathology Study to investigate the effects of social fear on the likelihood of developing a nicotine dependency (Sonntag et al., 2000). The respondents were 14–24 years of age during the first interview and were interviewed again 4–5 years later. This study showed that social fears and social phobia at baseline increases the risk for becoming nicotine dependent later in life (OR = 3.85). Dierker and colleagues studied the temporal onset of anxiety disorders and smoking by using data from the Yale Longitudinal High Risk Study (Dierker et al., 2001). The 192 participants were between 7 and 17 years old. It was shown that there was a strong positive association between anxiety disorders and nicotine dependence (OR = 4.5) but there was no association between having an anxiety disorder and experimenting with cigarettes and regular use. In addition, it was shown that the onset of an anxiety disorder mostly (73 percent of cases) precedes the onset of smoking or precedes becoming nicotine dependent (60 percent of cases). A separate analysis demonstrated that anxiety disorders do not predict the transition from experimenting with cigarettes to developing a nicotine dependency. The results of this study are in line with another study that reported that anxiety disorders during adolescence do not increase the risk for smoking during early adulthood (Johnson et al., 2000). Taken together, these studies indicate that there is a high comorbidity between smoking and anxiety disorders. This effect may be due to the fact that smoking increases the likelihood of developing an anxiety disorder. Conflicting findings have been reported with regard to the role of anxiety disorders in the development of a nicotine dependency. Most studies suggest that anxiety disorders do not increase the risk for developing a tobacco addiction. However, one study suggests that social fears and phobias during adolescence increase the risk for becoming nicotine dependent in early adulthood (Sonntag et al., 2000).
2.5. Anxiety, Depression, and Relapse to Smoking
Smoking cessation leads to a relatively mild somatic withdrawal syndrome and a severe affective withdrawal syndrome that is characterized by a decrease in positive affect, an increase in negative affect, craving for tobacco, irritability, anxiety, difficulty concentrating, hyperphagia, restlessness, and a disruption of sleep (Caan et al., 1996; Cook et al., 2004; Hughes and Hatsukami, 1986; Jorenby et al., 1996; Zhdanova and Piotrovskaya, 2000). Smoking during the acute withdrawal phase reduces craving for cigarettes and returns cognitive abilities to pre-smoking cessation levels (Bell et al., 1999). A majority of the smokers relapses during the first week of abstinence when the withdrawal symptoms are most severe (Hughes et al., 2004; Jarvis, 2004). It has been estimated that 49–76 percent of the subjects who quit on their own without medication relapses within one week, 72–85 percent within one month, and 80–90 percent within three months (Hughes et al., 2004). Approximately 3–5 percent of the smokers who quit on their own are able to maintain abstinent for 6–12 months. There is a high comorbidity between smoking and psychiatric disorders such as anxiety disorders and depression. Extensive evidence indicates that these disorders have a negative effect on smoking cessation rates. Anda and colleagues used data from the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study to investigate the effects of depression on smoking cessation (Anda et al., 1990). This was a 9 year prospective study that was conducted with 1167 smokers and smoking cessation was defined as not smoking for at least one year. It was shown that 17.7 percent of the nondepressed smokers were able to quit for at least one year and only 9.9 percent of the depressed smokers was able to quit for at least one year during the 9-year study period. This indicates that depressed smokers are 40 percent less likely to be able to quit for at least one year compared to nondepressed smokers. This observation is in line with another prospective study that investigated the effects of nicotine gum on smoking cessation rates in depressed subjects and nondepressed controls (Kinnunen et al., 1996). In this study, about 90 percent of the untreated depressed smokers relapsed within one month and about 65 percent of the untreated nondepressed smokers relapsed during the same time period. Furthermore, Niaura and colleagues demonstrated in three separate experiments that even very low levels of depression prior to the onset of smoking cessation decreases the amount of time that smokers can maintain abstinence (Niaura et al., 2001).
Heightened anxiety levels have also been shown to increase the risk for relapse to smoking. Piper and colleagues investigated the role of anxiety in relapse by using the data from 1,504 female smokers who participated in the Wisconsin Smokers’ Health Study (Piper et al., 2010). It was shown that women who had been diagnosed with an anxiety disorder at one point in their life were less likely to be abstinent 8 weeks (OR = 0.72) and 6 months after quitting smoking (OR = 0.72). Zvolensky and colleagues conducted a relatively small study to investigate the effects of PTSD (n = 47) or any other anxiety disorder (n=33) on smoking cessation. It was shown that the PTSD patients were more likely to have a lapse during the first week after quitting compared to the patients with other anxiety disorders and the control subjects. However, both the PTSD patients and the patients with other anxiety disorders were more likely to relapse than the control subjects and there was no difference in relapse rates between the PTSD patients and the patients with other anxiety disorders (Zvolensky et al., 2008). It has also been reported that increased anxiety sensitivity (i.e., fear of being anxious) increases the risk for a lapse to smoking during the first 2 weeks after quitting but does not affect full blown relapse to smoking during the same time period (Zvolensky et al., 2009). Taken together, the studies described in this section indicate that depression and anxiety disorders increase the risk for relapse to smoking.
2.6. Hypothalamic-Pituitary-Adrenal Axis and Smoking
2.6.1. Smoking and Hypothalamic-Pituitary-Adrenal Axis Activation
Extensive evidence indicates that smoking and smoking cessation affect the release of ACTH and cortisol. Smoking activates the HPA axis and the magnitude of the effect depends on the amount of nicotine in the cigarette, the number of cigarettes smoked, and the interval between smoking cigarettes (Kirschbaum et al., 1992; Mendelson et al., 2005; Steptoe and Ussher, 2006). Throughout this review we will use the terms nicotine yield and total amount of nicotine. The nicotine yield is indicative of the amount of nicotine that is inhaled by the smoker and this is about 15 percent of the total amount of nicotine in cigarettes. For example, in 2005 the average nicotine yield per cigarette was 1.9 mg and the total amount of nicotine per cigarette was 13.9 mg (Connolly et al., 2007). During the 1990s regular commercial cigarettes had a yield of approximately 1 mg and therefore cigarettes with a similar nicotine yield were often used in studies during this period (Federal Trade Commission, 2000). There is overwhelming evidence that cortisol levels are higher in smokers than in non-smokers (Steptoe and Ussher, 2006; Wilkins et al., 1982). However, conflicting findings have been reported with regard to the effects of smoking a small number of cigarettes with a yield of 1 mg of nicotine per cigarette on cortisol levels. It has been reported that smoking 2 cigarettes with a nicotine yield of 1 mg does not affect cortisol levels (Gilbert et al., 1992) or increases cortisol levels (Kirschbaum et al., 1992). In contrast, all studies consistently show that smoking cigarettes with a somewhat higher nicotine yield, 2 mg or more, leads to an increase in cortisol levels (Gilbert et al., 1992; Mendelson et al., 2005; Mendelson et al., 2008; Winternitz and Quillen, 1977). Although earlier studies only investigated the effects of smoking on cortisol levels, recent studies have demonstrated that smoking also affects ACTH levels. Smoking a single low-nicotine cigarette with a total nicotine content of 1 mg does not increase ACTH levels (Mendelson et al., 2005; Mendelson et al., 2008). In contrast, smoking one Marlboro red cigarette with a total nicotine content of 15.5 mg induces a dramatic increase in ACTH levels (Mendelson et al., 2005; Mendelson et al., 2008). The nicotine yield was not reported in the aforementioned studies but it can be estimated based on the relationship between total nicotine content and nicotine yield. Previous studies have shown that cigarettes with approximately 13.5 – 14.5 mg of nicotine have a yield of about 1.9 mg / cigarette. Therefore, it might be expected that the high nicotine Marlboro red cigarettes in this study had a yield that was approximately 2 mg per cigarette. A recent report by the Massachusetts Department of Public Health classified the Marlboro red as a regular cigarette. Cigarettes in this group have an average yield of 2.16 mg per cigarette (Massachusetts Department of Public Health, 2010). A recent study investigated the effects of smoking three Marlboro red cigarettes, with one hour intervals between each cigarette, on plasma ACTH and cortisol levels (Mendelson et al., 2008). It was shown that the first, second, and third cigarette increased cortisol levels. The first and third cigarette also increased ACTH levels. It was suggested that the second cigarette might not have increased ACTH levels because the cortisol levels were still elevated from smoking the first cigarette and elevated cortisol levels decrease the release of ACTH (Reader et al., 1982). Taken together, these studies demonstrate that smoking cigarettes leads to an increased release of ACTH and cortisol and cigarettes with a high nicotine yield have a greater effect on the HPA axis than cigarettes with a low nicotine yield.
2.6.2. Hypothalamic-Pituitary-Adrenal Axis Activation and Rewarding Effects of Smoking
At this point, relatively little research has been conducted to investigate whether smoking-induced activation of the HPA axis affects the rewarding effects of nicotine. One study has systematically investigated the relationship between smoking-induced activation of the HPA axis and feelings of high and rush as scored with the Visual Analogue Scale (Mendelson et al., 2005). It was shown that the level of “rush” and “high” was highest during the first few minutes after the onset of smoking. These positive feelings rapidly dissipated over a 30-minute time period. ACTH levels started to rise a few minutes after the onset of smoking and peaked 20 minutes after the onset of smoking. The cortisol levels started to rise 10 minutes after the onset of smoking and peaked 60 minutes after the onset of smoking. The time course of these events indicates that the positive subjective feeling of “rush” and “high” precedes the activation of the HPA axis. Therefore, this pattern of results would suggest that the activation of the HPA axis does not play a role in the positive effects that smokers experience immediately after the onset of smoking a cigarette. Smoking a cigarette decreased craving for cigarettes and this effect was maximal 20 minutes after the onset of smoking. This indicates that craving for cigarettes is minimal when ACTH levels peak. Therefore, additional studies are warranted to investigate if ACTH or ACTH fragments could attenuate craving for cigarettes.
2.6.3. Hypothalamic-Pituitary-Adrenal Axis and Smoking Cessation
Clinical studies suggest that tobacco withdrawal-induced distress and craving are most severe in subjects with the lowest HPA-activity during the withdrawal phase. Frederick and colleagues demonstrated that two weeks after quitting smoking cortisol levels are 40 percent lower than during active smoking (Frederick et al., 1998). The subjects who had the greatest drop in cortisol levels (smoking baseline compared to 2 weeks post cessation) experienced the most distress two weeks after quitting smoking. Subjects with the smallest drop in cortisol levels during the first two weeks after quitting were somewhat more likely to be abstinent 4 weeks after quitting, however, this effect did not reach statistical significance (P = 0.09). The decrease in cortisol levels on the first day of abstinence might be a better predictor of relapse than cortisol levels two weeks after quitting smoking. It has been shown that smokers who relapse during the first week have a greater decrease in cortisol levels on the first day of abstinence compared to smokers who are able to maintain abstinence for at least one week (al'Absi et al., 2004). In the same study, the women with the greatest drop in cortisol level on day 1 experienced the most distress during the first day of abstinence and more severe affective withdrawal symptoms as assessed with the Minnesota Nicotine Withdrawal Scale. The correlation between the drop in cortisol levels and affective and somatic withdrawal symptoms was not detected in men. The negative relationship between cortisol levels and withdrawal symptoms is also present in abstinent smokers who are treated with 15 mg nicotine patches (Ussher et al., 2006). Ussher and colleagues showed that quitting smoking leads to a dramatic decrease in cortisol levels in subjects treated with nicotine patches. The saliva cortisol levels during ad libitum smoking were about 7 nmol/l and this level dropped to 2.5 nmol/l on the first day after quitting smoking. The cortisol level gradually increased to 4 nmol/l during the first 6 weeks after quitting smoking. The increase in cortisol levels over the 6 week withdrawal period was not significant and the cortisol level remained significantly lower compared to pre-abstinence baseline levels. The subjects with the lowest absolute cortisol levels on the first day after quitting smoking reported the strongest urges to smoke, withdrawal symptoms, and stress during the first week of abstinence. There was also a nonsignificant trend (P=0.73) towards an increased risk for relapse in the subjects who displayed the greatest decline (smoking baseline minus withdrawal day 1) in cortisol levels. Smokers who relapse early not only have low post-smoking cessation cortisol levels but also display a decreased HPA axis responsivity to stressors during the acute withdrawal phase. In one study, the relationship between stress-induced activation of the HPA axis and relapse over a 4-week period was investigated (al'Absi et al., 2005). Subjects who relapsed within 4 weeks after quitting smoking had a decreased release of ACTH and cortisol in response to public speaking and a stressful arithmetic test on the first day of abstinence. Furthermore, a decreased stress-induced ACTH response, increased withdrawal symptoms, increased anxiety, perceived stress, and anger were predictive of early relapse. The aforementioned studies indicate that a hypoactivity of the HPA axis during withdrawal increases the risk for severe withdrawal symptomatology and early relapse. At this point in time, there is no evidence for a causal relationship between low cortisol levels and withdrawal symptomatology and it is not known how cortisol affects withdrawal and relapse. It can be speculated that cortisol may affect tobacco withdrawal by modulating nAChRs. Smoking leads to a desensitization and upregulation of nAChRs (Dani and Heinemann, 1996). Smoking cessation leads to an activation of the desensitized nAChR and this in combination with the upregulation of the nAChRs may lead to withdrawal and craving. Corticosterone has been shown to desensitize nAChRs in mice and therefore low cortisol levels may exacerbate withdrawal symptomatology by facilitating the reactivation of nAChRs (Pauly et al., 1988; Robinson et al., 1996).
Taken together, the studies in this section indicate that smoking increases the release of ACTH and cortisol. A time-sequence analyses indicated that the positive mood state associated with smoking precedes the activation of the HPA axis. Therefore, the activation of the HPA axis does not play a role in the positive mood state associated with smoking. Finally, smoking cessation leads to a hypoactivity of the HPA axis which may contribute to withdrawal symptomatology and relapse to smoking.
3. Tobacco Addiction and Stress Systems; Insights from Animal Studies
3.1. Corticotropin-Releasing Factor and Nicotine Addiction
3.1.1. Brain Corticotropin-Releasing Factor Systems
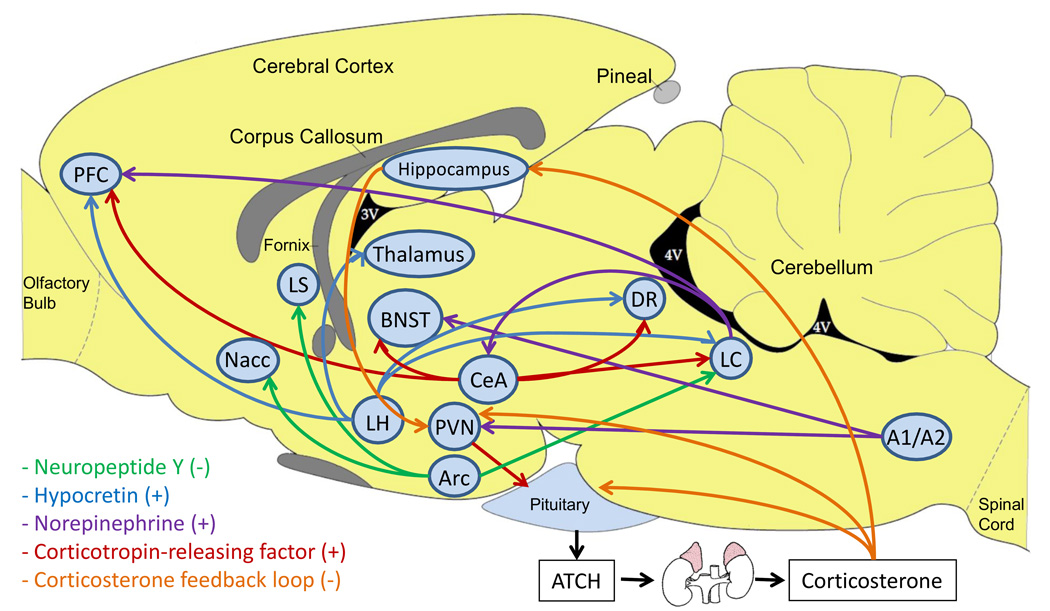
CRF is a 41-amino acid neuropeptide that was first isolated from the ovine hypothalamus (Figure 2)(Vale et al., 1981). CRF-immunoreactive cells have been detected in the paraventricular nucleus of the hypothalamus (PVN) and in other brain areas such as the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), and locus coeruleus (LC) (Swanson et al., 1983). Scattered CRF-immunoreactive cells have also been found throughout the neocortex (Swanson et al., 1983). PVN CRF neurons project to the median eminence and play an important role in the release of ACTH and β-endorphin from the anterior pituitary. ACTH is transported via the blood to the adrenal cortex where it stimulates the synthesis of corticosterone and its release into the circulation. Corticosterone prepares the body for acute stressors by mobilizing energy stores and suppressing physiological processes that are temporarily unessential for survival (McEwen, 2000; Sapolsky, 1992).
Figure 2.
Dysregulation of brain stress systems and tobacco addiction. A dysregulation of brain stress systems may play a role in transitioning from experimenting with cigarettes to habitual smoking, the dysphoria associated with smoking cessation, and relapse to smoking. CRF neurons project from the CeA to the prefrontal cortex, BNST, dorsal raphe nucleus, and LC (Swanson et al., 1983). CRF neurons also project from the PVN to the median eminence. NPY neurons project from the arcuate hypothalamic nucleus to the nucleus accumbens, lateral septum, and the LC (Holmes et al., 2003; Kask et al., 2002). Hypocretin neurons project from the later hypothalamus to the prefrontal cortex, thalamus, dorsal raphe nucleus, and the LC (Lambe et al., 2007). Norepinephrine neurons project from the LC to the prefrontal cortex and the CeA and from the A1/A2 region to the BNST (Aston-Jones and Cohen, 2005; Delfs et al., 2000). A2 noradrenergic neurons also play an important role in stimulating CRF neurons in the PVN and thereby activating the HPA axis (Matta et al., 1993b). Corticosterone inhibits the activity of the HPA axis by stimulating glucocorticoid receptors in the pituitary, PVN, and hippocampus (de Kloet et al., 1998a). Inhibitory GABAergic neurons project from the hippocampus to the PVN (de Kloet et al., 1998b). Abbreviations: Arc, arcuate hypothalamic nucleus; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; DR, dorsal raphe nucleus; LC, locus coeruleus; LS, lateral septum; Nacc, nucleus accumbens; PFC, prefrontal cortex; PVN, paraventricular nucleus of the hypothalamus.
Extrahypothalamic CRF orchestrates behavioral and autonomic responses to stressors (Koob and Heinrichs, 1999; Nijsen et al., 2001). Some of these effects of CRF are independent of its effects on the HPA axis (Eaves et al., 1985; Sutton et al., 1982). Two CRF receptors have been cloned, the CRF1 and CRF2 receptor (Chen et al., 1993; Lovenberg et al., 1995; Perrin et al., 1993). Both the CRF1 and CRF2 receptor are G-protein-coupled receptors and are positively coupled to adenylyl cyclase (Chalmers et al., 1996; Lewis et al., 2001). There are at least 8 splice variants of the CRF1 receptor (α, β, c, d, e, f, g and h) and 4 splice variants of the CRF2 receptor (α, β, γ, soluble 2α, and a soluble form of the first extracellular domain of the mouse CRF2β receptor) (Chen et al., 2005; Kostich et al., 1998; Lovenberg et al., 1995; Pisarchik and Slominski, 2001; Zmijewski and Slominski, 2010). Pharmacological studies suggest that CRF serves as an endogenous ligand for the CRF1 receptor and that urocortin 2 and urocortin 3 serve as endogenous ligands for the CRF2 receptor (Lewis et al., 2001). Urocortin 1 binds with a slightly higher affinity to the CRF1 receptor than to the CRF2 receptor (Lewis et al., 2001). Evidence suggests that stress-induced psychopathology and drug withdrawal-induced behavioral and physiological changes are predominantly mediated by the activation of the CRF1 receptors (Koob, 1999; Steckler and Holsboer, 1999). Conflicting findings have been reported with regard to the role of the CRF2 receptor in stress-induced behavioral changes and drug withdrawal (see (Bruijnzeel and Gold, 2005) for a review on this topic). At this point, it has not been investigated if one of the urocortins affects the rewarding effects of nicotine or nicotine withdrawal.
3.1.2. Nicotine Withdrawal and Depressive and Anxiety-Like Behavior
Preclinical studies indicate that the discontinuation of nicotine administration to rodents has extensive behavioral effects. Cessation of chronic nicotine administration leads to a somatic nicotine withdrawal syndrome in rats and mice (Isola et al., 1999; Malin et al., 1992). The somatic nicotine withdrawal syndrome can last up to 4 days and is characterized by teeth chattering, facial fasciculations, abdominal constrictions, increased eye blinks, and ptosis (Malin et al., 1992). Nicotine withdrawal also leads to negative affective signs. The effects of nicotine withdrawal on the state of the brain reward system have been investigated with the intracranial self-stimulation procedure (ICSS) and the forced swim test. Elevations in brain reward thresholds in the ICSS procedure are indicative of a decreased sensitivity to rewarding electrical stimuli and have been suggested to reflect a depressive-like state (Barr et al., 2002). The discontinuation of chronic nicotine administration and the administration of nAChR antagonists to nicotine dependent rats has been shown to lead to elevations in brain reward thresholds in rats (Epping-Jordan et al., 1998; Watkins et al., 2000). In a recent study, it was demonstrated that the discontinuation of nicotine administration also leads to elevations in brain reward thresholds in mice (Johnson et al., 2008). The elevations in brain reward thresholds associated with nicotine withdrawal in rats and mice can last up to 3–4 days (Epping-Jordan et al., 1998; Johnson et al., 2008). The rat forced swim test is widely used to screen for novel antidepressant drugs and to investigate the effects of stressors on the emotional state of rats (Cryan et al., 2005; Porsolt et al., 1977). In this test the rats are placed in a cylinder with water on two consecutive days (15 minutes first session and 5 minutes second session) and the duration that the rats spend swimming, climbing, and immobile is assessed on the second day. Antidepressants that block the reuptake of noradrenaline decrease immobility and increase climbing and antidepressants that block the reuptake of serotonin decrease immobility and increase swimming (Cryan et al., 2002a). Treatments that induce a negative mood state such as amphetamine withdrawal, footshocks, or social defeat increase immobility in the forced swim test (Cryan et al., 2003b; Rygula et al., 2005; Weiss et al., 1981). In a recent study it was reported that the discontinuation of nicotine administration also leads an increase in immobility in the rat forced swim test (Zaniewska et al., 2010). This observation is in line with previous ICSS studies that suggest that nicotine withdrawal leads to a negative mood state (Bruijnzeel et al., 2007; Epping-Jordan et al., 1998).
Nicotine withdrawal also leads to increased anxiety-like behavior in rodents. Rats that are withdrawing from nicotine display increased anxiety-like behavior in the elevated plus maze test, the social interaction test, the acoustic startle test, and the defensive burying test (George et al., 2007; Helton et al., 1993; Irvine et al., 1999; Irvine et al., 2001). Nicotine withdrawal-induced anxiety-like behavior in rats may only be detected under relatively stressful testing conditions. This is supported by the observation that nicotine withdrawing rats display an increased startle response compared to control rats in a brightly lit test environment but not in a dark test environment (Jonkman et al., 2008). Nicotine withdrawing mice have also been shown to display increased anxiety-like behavior in the elevated plus maze test and the light-dark box test (Costall et al., 1989; Damaj et al., 2003; Jonkman et al., 2005). The effects of nicotine withdrawal on anxietylike behavior in mice are strain dependent. Nicotine withdrawal leads to increased anxiety-like behavior in C57/BL/6J mice but not in 129/SvEv mice or DBA/2J mice (Damaj et al., 2003; Jonkman et al., 2005).
There is a strong positive association between smoking and anxiety disorders in humans (Lasser et al., 2000; Lawrence et al., 2009). Studies with animal models indicate that acute nicotine administration can have anxiogenic-like effects (Picciotto et al., 2002). However, several studies suggest that animals develop tolerance to the anxiogenic-like effects of nicotine in the elevated plus maze test and the social interaction test after about 7 days of treatment (Irvine et al., 1999; Irvine et al., 2001). Thus, although clinical studies suggest that smoking increases the risk for anxiety disorders, chronic nicotine administration does not lead to a persistent increase in anxiety-like behavior in rats. Additional studies are needed to investigate if nicotine may increase anxiety-like behavior after more prolonged nicotine treatment periods or in different anxiety tests.
3.1.3. Role of Corticotropin-Releasing Factor in Nicotine Withdrawal, Self-Administration, Reinstatement
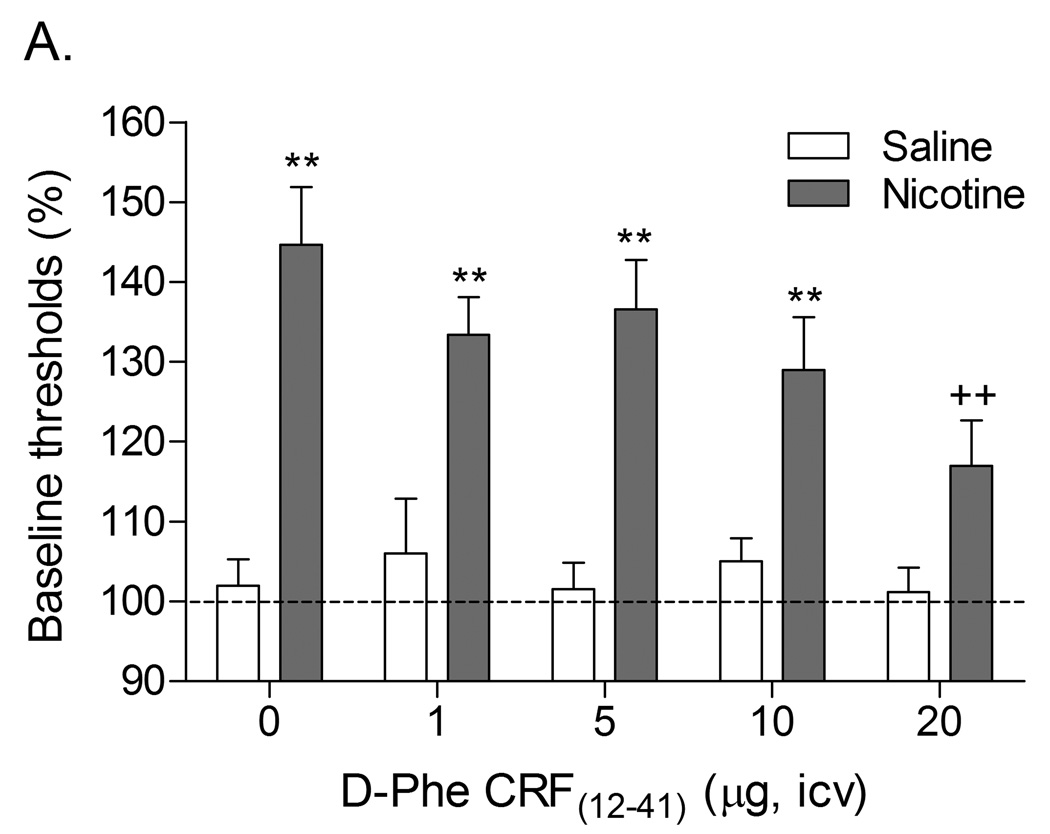
Previous research has demonstrated that the discontinuation of nicotine administration or the administration of nAChR antagonists to nicotine dependent rats leads to elevations in brain reward thresholds in the ICSS procedure (Epping-Jordan et al., 1998; Watkins et al., 2000). In a series of experiments, our laboratory investigated the role of CRF in the elevations in brain reward thresholds associated with spontaneous and precipitated nicotine withdrawal. The first study investigated whether pretreatment with the nonspecific CRF1/CRF2 receptor antagonist D-Phe CRF(12–41) attenuates the elevations in brain reward thresholds associated with nicotine withdrawal (Figure 3)(Bruijnzeel et al., 2007). Pretreatment with the highest dose of D-Phe CRF(12–41), 20 µg icv, prevented the elevations in brain reward thresholds associated with mecamylamine precipitated nicotine withdrawal. The administration of D-Phe CRF(12–41) during spontaneous withdrawal did not reverse the elevations in brain reward thresholds associated with nicotine withdrawal. One major difference between these two studies was that in the precipitated withdrawal experiment the CRF receptor antagonist was administered prior to the onset of withdrawal and in the spontaneous withdrawal experiment the CRF receptor antagonist was administered during the withdrawal phase when the brain reward thresholds were already elevated. This pattern of results would suggest that the administration of CRF receptor antagonists prior to the withdrawal phase attenuates withdrawal but the administration of CRF receptor antagonists during the withdrawal phase does not affect withdrawal.
Figure 3.
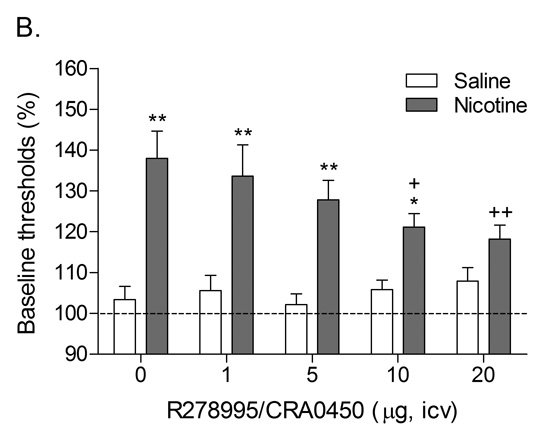
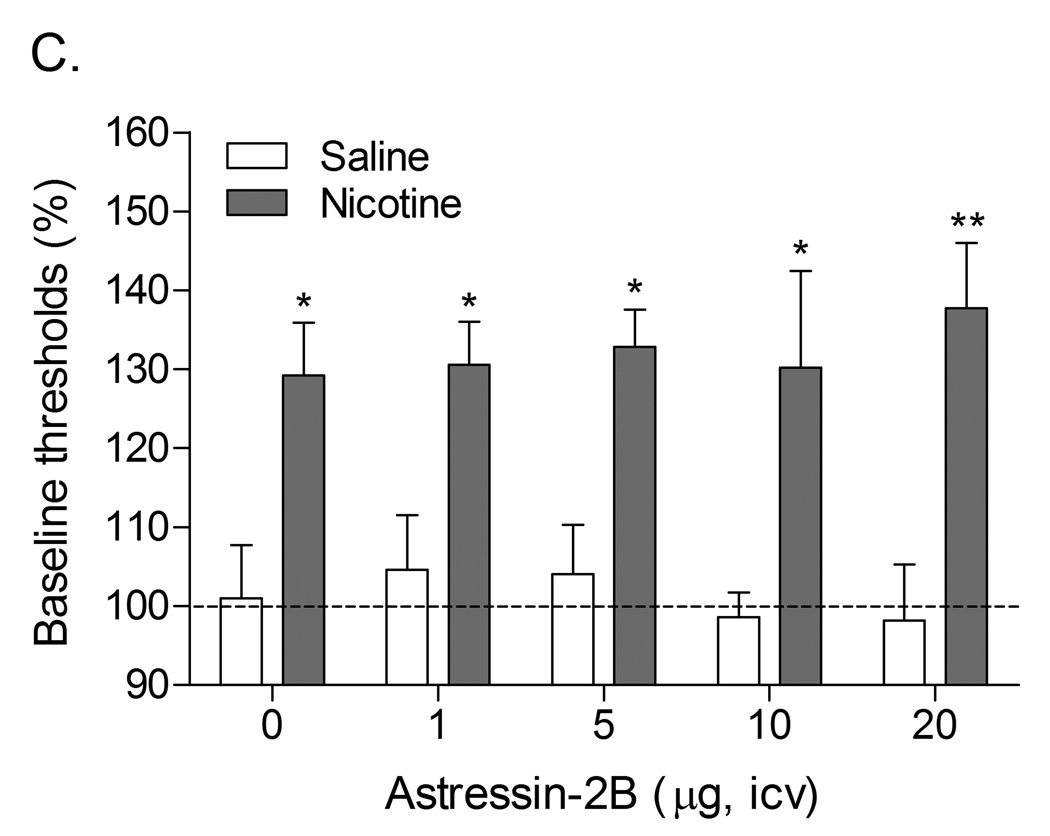
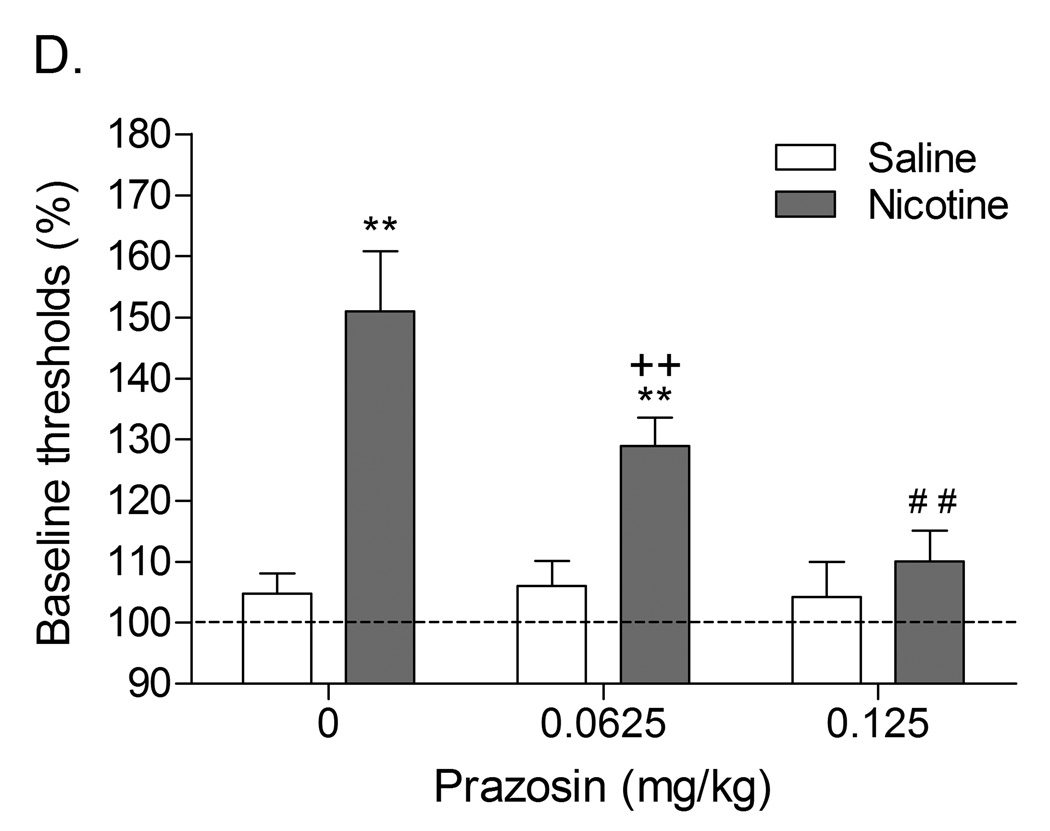
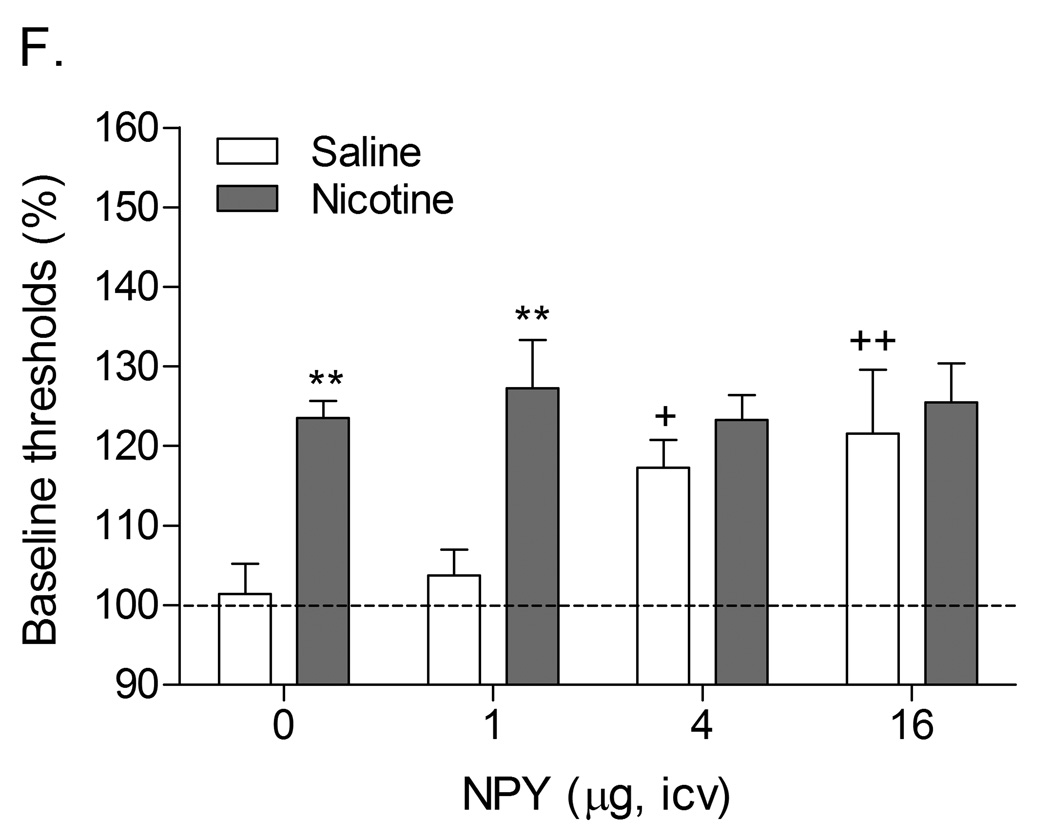
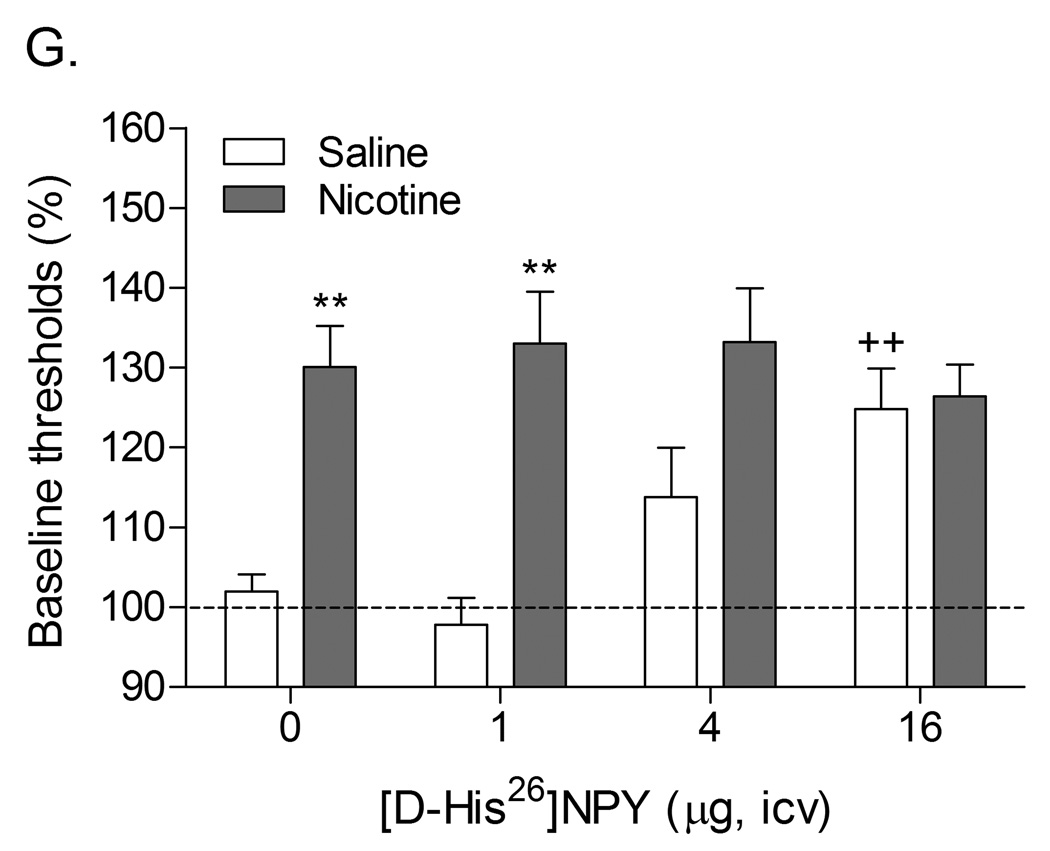
Role of corticotropin-releasing factor, norepinephrine, and neuropeptide Y in precipitated nicotine withdrawal in rats. In all figures, brain reward thresholds were assessed with a discrete trial intracranial self-stimulation procedure and were expressed as a percentage of the pre-test day baselines. (A) Effect of the CRF1/CRF2 receptor antagonist D-Phe CRF(12–41) (icv; saline, n = 8; nicotine, n = 7) on the elevations in brain reward thresholds associated with mecamylamine (3 mg/kg, sc) precipitated nicotine withdrawal. Asterisks (** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Plus signs (++ P<0.01) indicate lower brain reward thresholds compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle (0 µg of D-Phe CRF(12–41)). Reproduced with permission from (Bruijnzeel et al., 2007). (B) Effect of the specific CRF1 receptor antagonist R278995/CRA0450 (icv; saline, n = 12; nicotine, n = 14) on the elevations in brain reward thresholds associated with mecamylamine (3 mg/kg, sc) precipitated nicotine withdrawal. Asterisks (* P<0.05, ** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Plus signs (+ P<0.05, ++ P<0.01) indicate lower brain reward thresholds compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle (0 µg of R278995/CRA0450). Reproduced with permission from (Bruijnzeel et al., 2009). (C) Effect of the specific CRF2 receptor antagonist Astressin-2B (icv; saline, n = 8; nicotine, n = 8) on the elevations in brain reward thresholds associated with mecamylamine (3 mg/kg, sc) precipitated nicotine withdrawal. Asterisks (* P<0.05, ** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Reproduced with permission from (Bruijnzeel et al., 2009). (D) Effect of the α1-adrenoceptor antagonist prazosin (ip; saline, n = 9; nicotine, n = 9) on the elevations in brain reward thresholds associated with mecamylamine (2 mg/kg, sc) precipitated nicotine withdrawal. Asterisks (** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Plus signs (++ P<0.01) indicate lower brain reward thresholds compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle. Pound signs (## P<0.01) indicate lower brain reward thresholds compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle or mecamylamine and 0.0625 mg/kg of prazosin. Reproduced with permission from (Bruijnzeel et al., 2010). (E) Effect of the α2-adrenoceptor antagonist idazoxan (ip; saline, n = 12; nicotine, n = 12) on the elevations in brain reward thresholds associated with DHβE (3 mg/kg, sc) precipitated nicotine withdrawal. The at symbol (@) indicates a statistically significant main effect of precipitated nicotine withdrawal on thresholds (P<0.0001) independent of idazoxan treatment. Reproduced with permission from (Semenova and Markou, 2010). (F) Effect of NPY (icv, saline, n = 8; nicotine, n = 8) on the elevations in brain reward thresholds associated with mecamylamine (2 mg/kg, sc) precipitated nicotine withdrawal. Asterisks (** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Crosses (+ P<0.05, ++ P<0.01) indicate elevations in brain reward thresholds compared to those of rats chronically treated with saline and acutely treated with vehicle (0 µg of NPY). Reproduced with permission from (Rylkova et al., 2008). (G) Effect of the selective Y1 receptor agonist [D-His26]-NPY (icv, saline, n = 9; nicotine, n = 11) on the elevations in brain reward thresholds associated with mecamylamine (2 mg/kg, sc) precipitated nicotine withdrawal. Asterisks (** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Crosses (++ P<0.01) indicate elevations in brain reward thresholds compared to those of rats chronically treated with saline and acutely treated with vehicle (0 µg of [D-His26]-NPY). Reproduced with permission from (Rylkova et al., 2008). In all the figures (A–G), the brain reward thresholds are expressed as means ± SEM.
A follow-up study was conducted to investigate the effects of the CRF1 receptor antagonist R278995/CRA0450 and the CRF2 receptor antagonist astressin-2B on the elevations in brain reward thresholds associated with precipitated nicotine withdrawal (Bruijnzeel et al., 2009). It was shown that R278995/CRA0450, but not astressin-2B, prevented the elevations in brain reward thresholds associated with precipitated nicotine withdrawal (Table 1). This indicates that the activation of CRF1 receptors, but not CRF2 receptors, plays a pivotal role in the negative mood state associated with nicotine withdrawal. In another series of experiments, it was investigated if the administration of CRF1/CRF2 receptor antagonist D-Phe CRF(12–41) into specific brain sites would prevent the elevations in brain reward thresholds associated with precipitated nicotine withdrawal in rats (Marcinkiewcz et al., 2009). These experiments focused on the role of CRF in the CeA, BNST, and the Nacc shell in nicotine withdrawal. There is strong evidence that CRF transmission in the CeA and BNST plays a role in drug withdrawal. Withdrawal from drugs of abuse such as alcohol, nicotine, cocaine, and cannabis leads to an increased release of CRF in the CeA (George et al., 2007; Merlo Pich et al., 1995; Richter and Weiss, 1999; Rodriguez de Fonseca et al., 1997). Alcohol withdrawal also leads to an increased release of CRF in the BSNT and CRF levels return to baseline levels upon alcohol intake (Olive et al., 2002). Furthermore, the administration of the nonspecific CRF1/CRF2 receptor antagonist α-helical CRF(9–41) into the CeA prevents alcohol withdrawal-induced anxiety-like behavior in the elevated plus maze test (Rassnick et al., 1993). Prior to the onset of our studies there was little evidence for a role of CRF in the Nacc shell in drug withdrawal. However, CRF and CRF receptors have been detected in the Nacc shell (De Souza et al., 1985; Swanson et al., 1983). In addition, the administration of CRF into the Nacc shell induces an increase in locomotor activity, rearing, and grooming in a familiar non-stressful environment (Holahan et al., 1997). A similar behavioral response has been detected in rats that received icv CRF and were subsequently observed under low arousal conditions in their home cage or in another familiar environment (Dunn and Berridge, 1990; Sutton et al., 1982). Therefore, CRF may mediate some of its behavioral effects by stimulating CRF receptors in the Nacc shell. The results of our study demonstrate that the administration of D-Phe CRF(12–41) into the CeA and the Nacc shell, but not the BNST, attenuates the elevations in brain reward thresholds associated with precipitated nicotine withdrawal (Marcinkiewcz et al., 2009). In a recent study it was also shown that the intra-CeA administration of the CRF1 receptor antagonist R278995/CRA0450 prevents the elevations in brain reward thresholds associated with precipitated nicotine withdrawal {Bruijnzeel, 2012 3596 /id}. These findings suggest that the endogenous release of CRF in the CeA and Nacc shell plays a role in the negative mood state associated with nicotine withdrawal.
Table 1.
Role of CRF, hypocretin, NPY, and norepinephrine in nicotine withdrawal.
| Drugs | Depressive-like behavior |
Anxiety-like behavior |
Somatic withdrawal signs |
Increased nicotine intake after period of abstinence |
Stress-induced reinstatement of nicotine seeking |
|---|---|---|---|---|---|
| CRF1 receptor antagonist | ↓ | ↓ | n/a | ↓ | ↓ |
| Hypocretin-1 receptor antagonist | n/a | n/a | ↓ | n/a | – |
| Y1 receptor agonist | – | n/a | ↓ | n/a | n/a |
| α1-adrenoceptor antagonist | ↓ | n/a | – | n/a | n/a |
| α2-adrenoceptor agonist | – | n/a | ↓ | n/a | ↓ |
| β1/β2-adrenoceptor antagonist | – | n/a | ↓ | n/a | n/a |
Arrows (↓) indicate that systemic or intracerebroventricular administration of drugs decreases a specific behavior. Minus signs (–) indicate that the drugs are ineffective. The data in this table are based on previous studies (Bruijnzeel et al., 2009; Bruijnzeel et al., 2010; George et al., 2007; Plaza-Zabala et al., 2010, 2012; Rylkova et al., 2008; Zislis et al., 2007).
Abbreviations: CRF1 receptor, corticotropin releasing factor type 1 receptor; Y1 receptor, Neuropeptide Y type 1 receptor; n/a, data not available.
Some evidence suggests that CRF1 receptors may also play a role in nicotine withdrawal-induced anxiety like behavior. Systemic administration of the CRF1 receptor antagonist MPZP has been shown to diminish nicotine withdrawal-induced anxiety-like behavior in the defensive burying test (George et al., 2007). At this point, it is not known which populations of CRF1 receptors mediate these anxiety-like effects. In addition, it is not known if CRF1 receptor antagonists would also decrease nicotine withdrawal-induced anxiety-like behavior in other behavioral tests.
A large number of studies have investigated the effects of a period of alcohol abstinence on alcohol intake in rats. These studies demonstrated that alcohol intake is increased on the first day after the alcohol deprivation period and then returns to baseline levels (Heyser et al., 1997; Heyser et al., 2003; Sinclair and Senter, 1968). The alcohol intake on the first day of access increases as a function of the deprivation period, which suggests that the reinforcing properties of alcohol increase when the abstinence period increases (Heyser et al., 1997). This is in line with the observation that cue-induced cocaine seeking gradually increases over the time (Grimm et al., 2001). O’Dell and Koob have developed an animal model to investigate the nicotine deprivation effect in rats (O'Dell and Koob, 2007). Rats were allowed to self-administer nicotine for 23 hours per day for 4 consecutive days and then the rats did not have access to nicotine for 3 days. It was shown that the nicotine intake was highest on the first day after the abstinence period and then decreases over the following 3 days of access. A follow-up experiment demonstrated that the CRF1 receptor antagonist MPZP prevented the increased nicotine intake after a period of abstinence (George et al., 2007). In the same study, it was shown that blockade of CRF1 receptors does not affect nicotine self-administration in animals with limited, 1 hour per day, access to nicotine (George et al., 2007). These findings suggest that the endogenous release of CRF and the activation of CRF1 receptors plays an important role in the increased nicotine intake in nicotine dependent animals after a period of abstinence. CRF does not play a role in the intake of small amounts of nicotine in nondependent animals.
Animal models have been developed to investigate relapse to smoking in humans. A detailed discussion about reinstatement models is beyond the scope of this manuscript and therefore the readers are being referred to some excellent reviews about this topic (Epstein et al., 2006; Shaham et al., 2003). In order to investigate the reinstatement of drug seeking behavior, rodents are allowed to self-administer a drug of abuse and then after a specific amount of time (mostly about 14 days) drug self-administration is extinguished by withholding the drug. Extinguished drug seeking can be reinstated by exposure to footshock stress, cues associated with drug taking behavior, or the noncontingent administration of a drug of abuse. Similar to other drugs of abuse, nicotine seeking behavior can be reinstated by exposing rats to footshocks, nicotine, or cues associated with the self-administration of nicotine (Buczek et al., 1999; O'Connor et al., 2010; Paterson et al., 2005). Furthermore, restraint stress has been shown to reinstate nicotine-induced conditioned place preference in rats (Leao et al., 2009). The administration of the nonspecific CRF1/CRF2 receptor antagonist D-Phe CRF(12–41) or the specific CRF1 receptor antagonist R278995/CRA0450 into the lateral ventricles prior to the footshock session attenuates stress-induced reinstatement of extinguished nicotine-seeking behavior (Bruijnzeel et al., 2009; Zislis et al., 2007). In a recent study, it was shown that intra-CeA administration of the CRF1 receptor antagonist R278995/CRA0450 also attenuates stress-induced reinstatement of extinguished nicotine-seeking behavior (Yamada and Bruijnzeel, 2011). The CRF2 receptor antagonist astressin-2B does not prevent footshock-induced reinstatement of nicotine-seeking behavior (Bruijnzeel et al., 2009). This indicates that exposure to a stressor leads to the release of CRF which contributes to nicotine-seeking behavior by activating CRF1 receptors. The CRF1 receptor antagonist CP-154,526 has been shown to attenuate cue and drug (methamphetamine) induced reinstatement of extinguished methamphetamine-seeking behavior (Moffett and Goeders, 2007). At this point, it is not known if blockade of CRF1 receptors would also attenuate cue and drug-induced reinstatement of nicotine-seeking behavior.
3.2. Hypocretins and Nicotine Addiction
3.2.1. Brain Hypocretin Systems
The hypocretins, hypocretin-1 and hypocretin-2, are neuropeptides that are derived from a common precursor protein called prepro-hypocretin (de Lecea et al., 1998). The hypocretins, which are also known as orexins, were discovered around the same time by two independent research groups who each named these peptides differently. De Lecea and colleagues named these peptides hypocretins because the peptides have a similar amino acid sequence as the gut peptide secretin but their expression was restricted to the hypothalamus (de Lecea et al., 1998). Because these peptides stimulate food intake, Sakurai and colleagues called these peptides orexins after the Greek word orexis which means appetite (Sakurai et al., 1998). Hypocretin-1 is a 33 amino acid peptide and hypocretin-2 is a 28 amino acid peptide. There is a high degree of sequence similarity at the c-terminal side of hypocretin-1 and hypocretin-2 whereas the N-terminal sides are quite distinct (Tsujino and Sakurai, 2009). The localization of the hypocretin producing neurons in the brain is extremely restricted. Hypocretin positive neurons have only been detected in the perifornical region of the lateral hypothalamus and the posterior hypothalamic area (Date et al., 1999; Nambu et al., 1999). Although the expression of hypocretin neurons is restricted, hypocretin axons are widely distributed throughout the brain (Date et al., 1999; Nambu et al., 1999). High levels of hypocretin fibers have been detected in brain areas that play a role in the regulation of stress responses such as the CeA, BNST, LC, and PVN. High levels of hypocretin fibers have also been detected in the dorsal and medial raphe nuclei, the hypothalamic arcuate nucleus, area postrema, parabrachial nuclei, and the Barrington’s nucleus (Nambu et al., 1999). The hypocretins mediate their effects via two receptors, the hypocretin 1 receptor and the hypocretin-2 receptor. Hypocretin-1 and hypocretin-2 have a similar affinity for the hypocretin-2 receptor. However, hypocretin-1 has a 2–3 fold higher affinity for the hypocretin-1 receptor than hypocretin-2 (Sakurai et al., 1998).
The hypocretins have been shown to play a pivotal role in the regulation of a variety of behaviors. The hypocretins play a role in the regulation of sleep-wake states and a lack of hypocretin leads to the sleep disorder narcolepsy (Chemelli et al., 1999; Lin et al., 1999; Thannickal et al., 2000). Narcolepsy is a disorder that is characterized by an inability to maintain a wakeful state (Zarcone, 1973). The hypocretin projections from the lateral hypothalamus to cholinergic nuclei of the basal forebrain play an important role in attention as the release of hypocretins in the basal forebrain stimulates to the release of acetylcholine in cortical brain areas (Eggermann et al., 2001). Furthermore, the hypocretins play an important role in feeding behavior and energy homeostasis. Several studies have confirmed the original finding by Sakurai and colleagues that the central administration of hypocretin increases food intake in rodents (Dube et al., 1999; Jain et al., 2000; Sakurai et al., 1998; Yamanaka et al., 1999).
3.2.2. Hypocretins and the Hypothalamic-Pituitary-Adrenal Axis
Since the discovery of the hypocretins, a significant number of studies have provided evidence for a role of these neuropeptides in the regulation of stress responses. Hypocretin receptor mRNA has been detected in the PVN, the anterior pituitary, and the adrenal glands (Date et al., 2000; Johren et al., 2001; Lopez et al., 1999; Marcus et al., 2001). In addition, the central administration of the hypocretins leads to an increased release of ACTH and corticosterone into the peripheral circulation (Jaszberenyi et al., 2000; Kuru et al., 2000; Russell et al., 2001). Experimental evidence indicates that the hypocretins activate the HPA axis by stimulating CRF neurons that project from the PVN to the median eminence. This is supported by the observation that pretreatment with the nonspecific CRF1/CRF2 receptor antagonist alpha-helical CRF(9–41) prevents the hypocretin-1 or hypocretin-2 induced corticosterone release (Jaszberenyi et al., 2000).
Furthermore, hypocretin-1 stimulates the release of CRF from hypothalamic tissue in vitro and the central administration of hypocretin-1 or hypocretin-2 increases the number of c-Fos positive neurons in the parvocellular (i.e., CRF neurons), but not magnocellular, subdivision of the PVN (Date et al., 1999; Russell et al., 2001). The icv administration of the hypocretin-1 receptor antagonist SB-408124 has been shown to attenuate the immobility stress-induced release of ACTH (Samson et al., 2007). This indicates that the activation of hypocretin receptors also plays an important role in the stress-induced activation of the HPA axis. The results of a recent study suggest that hypocretin may also play a role in nicotine-induced activation of neurons in the PVN (Balfour et al., 1975; Plaza-Zabala et al., 2010). Plaza-Zabala and colleagues first demonstrated that subcutaneous nicotine administration increases the expression of c-Fos in the PVN. An increased expression of c-Fos, the protein product of the proto-oncogene c-fos, is indicative of increased neuronal activity (Morgan and Curran, 1995). This effect was attenuated by pretreatment with the hypocretin-1 receptor antagonist SB334867 or prepro-hypocretin gene deletion. Then they demonstrated that nicotine increases the expression of c-Fos in CRF and vasopressin neurons in the PVN and pretreatment with SB334867 attenuates this effect (Plaza-Zabala et al., 2010). Nicotine withdrawal also leads to an increased expression of c-Fos in the PVN and this effect is blocked by pretreatment with the hypocretin-1 receptor antagonist SB334867 (Plaza-Zabala et al., 2012). Taken together, these findings suggest that the activation of hypocretin-1 receptors plays a critical role in nicotine and nicotine-withdrawal induced activation of the PVN.
Several other histological studies have provided evidence for the notion that nicotine may affect the brain hypocretin system. The acute administration of nicotine to rats has been shown to increase the number of c-Fos positive hypocretin neurons in the lateral hypothalamus / perifornical area (Pasumarthi et al., 2006). This study suggests that the acute administration of nicotine leads to the activation of hypocretin neurons in the lateral hypothalamus. Chronic administration of nicotine increases the expression of hypocretin and its receptors (Kane et al., 2000). In one study it was shown that chronic (10–14 days) nicotine administration leads to increased prepro-hypocretin mRNA levels, and hypocretin-1 and 2 receptor mRNA levels in whole hypothalamus abstracts. The same treatment regimen also led to increased hypocretin-1 levels in the dorsomedial nucleus of the hypothalamus (DMH) and increased hypocretin-2 levels in the DMH and the PVN (Kane et al., 2000). It is somewhat surprising that chronic nicotine administration increases hypocretin levels in the DMH and PVN because nicotine decreases food intake and the administration of hypocretin-1 in the DMH and the PVN increases food intake (Dube et al., 1999). It has been suggested that this discrepancy is due to the fact that chronic nicotine also leads to a downregulation in high-affinity hypocretin-1 receptors in the hypothalamus which would lead to a decrease in hypocretin signaling (Kane et al., 2001).
3.2.3. Hypocretins, Nicotine, and Behavior
Behavioral studies indicate that the hypocretins play a critical role in mediating some of the effects of nicotine. Numerous studies have demonstrated that the acute administration of relatively high doses of nicotine can have anxiogenic effects in rats and mice (File et al., 1998; Ouagazzal et al., 1999). In a recent study, it was demonstrated that pretreatment with the hypocretin-1 receptor antagonist SB334867 prevents nicotine-induced anxiogenic-like behavior in the elevated plus maze test in mice (Plaza-Zabala et al., 2010). In addition, nicotine-induced anxiogenic-like behavior was attenuated in preprohypocretin knockout mice compared to wild-type C57BL/6J mice (Plaza-Zabala et al., 2010). In the same study, it was also demonstrated that hypocretin-1 induced the reinstatement of extinguished nicotine seeking behavior in rats with a history of nicotine self-administration. This effect was blocked by pretreatment with the hypocretin-1 receptor antagonist SB334867, but not by pretreatment with the CRF1 receptor antagonist antalarmin. Furthermore, SB334867 did not block stress-induced reinstatement of extinguished nicotine seeking behavior. In contrast, CRF1 receptor antagonists have been shown to block stress-induced reinstatement of extinguished nicotine-seeking behavior (Bruijnzeel et al., 2009; Plaza-Zabala et al., 2010). A recent study reported that the hypocretin-1 receptor antagonist SB334867 also attenuates somatic nicotine withdrawal signs (Plaza-Zabala et al., 2012). Taken together, these experiments indicate that the activation of the hypocretin-1 receptor contributes to the anxiogenic effects of nicotine, somatic nicotine withdrawal signs, and may play a role in the reinstatement of extinguished nicotine-seeking behavior.
It might be possible that the hypocretin system also plays a role in mediating the effects of food deprivation on smoking / nicotine self-administration and relapse. Hunger increases smoking in humans and food deprivation increases nicotine self-administration in rats (Cheskin et al., 2005; Franklin et al., 1948; Lang et al., 1977; Singer et al., 1978). The effect of food deprivation on the reinstatement of extinguished nicotine-seeking behavior has not been investigated. However, extensive evidence indicates that food deprivation reinstates heroin and cocaine-seeking behavior (Carroll, 1985; Shalev et al., 2000; Shalev et al., 2003). Furthermore, food deprivation increases hypothalamic preprohypocretin mRNA levels, hypocretin-1 and 2 peptide levels, hypocretin-1 and 2 mRNA and protein levels (Karteris et al., 2005). Therefore, additional studies are warranted to investigate the relationship between hypocretin signaling, food deprivation, and nicotine self-administration and relapse. Based on the aforementioned studies, it would be expected that hypocretin antagonists attenuate a food deprivation-induced increase in nicotine self-administration and food deprivation-induced reinstatement of nicotine-seeking behavior.
Two recent studies have investigated the effects of hypocretin antagonists on the rewarding effects of nicotine (Hollander et al., 2008; LeSage et al., 2010). These studies suggest that the activation of the hypocretin-1 receptor plays a critical role in the positive reinforcing effects of nicotine. It was shown that the selective hypocretin-1 receptor antagonist SB334867 decreases the self-administration of nicotine in rats under a fixed ratio and a progressive ratio schedule of reinforcement at doses that do not affect responding for food pellets (Hollander et al., 2008). The hypocretin-1 receptor antagonist SB334867 also prevented the nicotine-induced potentiation of brain reward function as assessed with the intracranial self-stimulation procedure (Hollander et al., 2008). In another set of experiments, the effects of the same selective hypocretin-1 receptor antagonist SB334867 and the mixed hypocretin-1 and 2 receptor antagonist almorexant on nicotine self-administration was investigated (LeSage et al., 2010). It was shown that SB334867 and almorexant decreased the self-administration of nicotine in rats under a fixed ratio schedule of reinforcement. SB334867 decreased the self-administration of nicotine at a dose that did not affect responding for food pellets. However, the dose of almorexant that decreased responding for nicotine also decreased responding for food pellets although to a lesser degree. It is somewhat surprising to note that the dose-effect curves for the effects of SB334867 on nicotine self-administration in the two aforementioned studies were very different. In the study conducted by Hollander and colleagues, 4 mg/kg of SB334867 significantly decreased the self-administration of nicotine under a fixed ratio schedule of reinforcement while in the study by LeSage and colleagues the lowest dose that decreased the self-administration of nicotine was 30 mg/kg. It was suggested that this discrepancy in the effectiveness of SB334867 to decrease nicotine self-administration was due to the fact that different strains of rats were used (LeSage et al., 2010). Furthermore, the rats in the study by LeSage and colleagues were somewhat food deprived, which led to a higher nicotine intake (15 vs. 9 infusions in 1-hour sessions) and therefore it was suggested that a higher dose of SB334867 might have been needed to decrease the self-administration of nicotine (LeSage et al., 2010). Taken together, the studies in this section indicate that the positive reinforcing effects of nicotine are at least partly mediated by the activation of hypocretin-1 receptors.
3.3. Neuropeptide Y and Nicotine Addiction
3.3.1. Brain Neuropeptide Y Systems
Neuropeptide Y (NPY) is a 36 amino acid peptide that was first isolated from the porcine brain (Tatemoto et al., 1982). Follow-up research indicated that NPY is widely expressed in the human and rodent central and peripheral nervous systems (Adrian et al., 1983; Allen et al., 1983; Lundberg et al., 1983). High concentrations of NPY have been detected in the rodent and human hypothalamus and the suprachiasmatic nucleus. In addition, NPY-positive cells and fibers have been detected in brain areas involved in the regulation of mood such as the amygdala complex and the nucleus accumbens (Chronwall et al., 1985; Walter et al., 1990). Five NPY receptors have been cloned from the rodent and human brain (Y1, Y2, Y4, Y5, Y6; for excellent reviews see (Blomqvist and Herzog, 1997; Michel et al., 1998; Thorsell and Heilig, 2002). All the cloned NPY receptors are G-protein coupled receptors and stimulation of these receptors inhibits the production of cyclic adenosine monophosphate (cAMP). Functional Y6 receptors have been detected in the mouse, but this receptor is absent in the rat and not functional in humans (Burkhoff et al., 1998; Gregor et al., 1996).
3.3.2. Neuropeptide Y and Psychiatric Disorders and Drug Addiction
NPY has been implicated in a range of physiological and behavioral responses that are necessary to maintain homeostasis (Broberger and Hokfelt, 2001; Kalra and Kalra, 2004; Shine et al., 1994; Yannielli and Harrington, 2001). Preclinical and clinical studies suggest that decreased NPY transmission in the brain is involved in the etiology and maintenance of stress related psychiatric disorders (Thiele and Heilig, 2004). There is experimental evidence that suggests that low levels of NPY in the brain may contribute to negative mood states. It has been reported that NPY levels in the cerebrospinal fluid (CSF) are lower in depressed patients than in schizophrenics or healthy controls (Widerlov et al., 1988). A similar pattern of results was observed by Heilig and colleagues (Heilig et al., 2004). They showed that CSF NPY levels are lower in patients with unipolar depression than in control subjects (Heilig et al., 2004). In addition, NPY levels in the frontal cortex and the caudate nucleus are lower in suicide victims than in age matched controls (Widdowson et al., 1992).
The antidepressant-like effects of NPY have been investigated in a variety of depression models, including the rat and mouse forced swim test and the rat olfactory bulbectomy model (Cryan and Mombereau, 2004; Kelly et al., 1997). Similar to selective serotonin reuptake inhibitors and other antidepressant treatments, NPY decreases immobility in the rat and mouse forced swim test (Husum et al., 2000; Redrobe et al., 2002; Stogner and Holmes, 2000). In addition, olfactory bulbectomy in rats results in an increase in ambulation, rearing, grooming, and defecation in a novel open field and these effects can be prevented by chronic treatment with NPY (Song et al., 1996). This pattern of results suggests that NPY has antidepressant-like effects and that NPY receptor agonists may serve as novel pharmacological treatments for depressive disorders. This is further supported by the observation that treatment with antidepressants such as lithium, citalopram, and imipramine increases NPY neurotransmission in a variety of brain areas in the rat (Heilig et al., 1988; Husum et al., 2000; Wahlestedt et al., 1990; Weiner et al., 1992).
As indicated above, heightened NPY levels have been associated with fewer depressive-like symptoms and it has been suggested that NPY counteracts the effects of stressors (Redrobe et al., 2004; Thiele and Heilig, 2004). These findings suggest that NPY may decrease drug withdrawal syndromes. Indeed, it has been reported that NPY decreases irritability, tremor and rigidity associated with spontaneous alcohol withdrawal (Woldbye et al., 2002). In addition, NPY and other NPY receptor agonists such as [Leu31, Pro34]-NPY, NPY 3–36, and peptide YY decrease somatic opioid withdrawal signs in rats (Woldbye et al., 1998). There is also strong evidence for a role of NPY in alcohol self-administration in rodents (Badia-Elder et al., 2001; Thiele et al., 1998). NPY knockout mice have higher alcohol intake than wild type mice and transgenic mice that overexpress NPY have a lower alcohol intake compared to wild type mice (Thiele et al., 1998). NPY also decreases alcohol intake in rats that are bred for a high alcohol preference (alcohol-preferring, P rats) and alcohol dependent Wistar rats but not in rats with a low alcohol preference or nondependent Wistar rats (Badia-Elder et al., 2001) (Thorsell et al., 2005). Recent studies imply an important role for NPY in the CeA in alcohol self-administration. Viral vector-induced overexpression of NPY in the CeA decreases alcohol intake in alcohol dependent animals (Thorsell et al., 2007). Furthermore, the administration of NPY in the CeA decreases alcohol intake in alcohol dependent animals but not in nondependent control animals (Gilpin et al., 2008).
3.3.3. Neuropeptide Y and Nicotine Withdrawal
In order to investigate the role of NPY in nicotine withdrawal, our laboratory investigated the effects of NPY on the elevations in brain reward thresholds associated with precipitated nicotine withdrawal in rats (Rylkova et al., 2008). It has been suggested that NPY’s anxiolytic and antidepressant-like effects are mediated via the Y1 receptor and NPY’s sedative effects via the Y5 receptor (Ki of 0.28 nM for Y1, Ki of 1.5 nM for Y5) (Ishida et al., 2007; Mullins et al., 2001; Redrobe et al., 2002; Sorensen et al., 2004). Therefore, an additional study was conducted to investigate the effect of the selective Y1 receptor agonist [D-His26]-NPY (Ki of 2.0 nM for Y1, Ki of 34.6 nM for Y5) on the elevations in brain reward thresholds associated with nicotine withdrawal (Mullins et al., 2001). It was shown that neither NPY nor [D-His26]-NPY prevented the elevations in brain reward thresholds associated with precipitated nicotine withdrawal in rats. Furthermore, NPY and [D-His26]-NPY elevated the brain reward thresholds of the control rats that were not treated with nicotine. It is interesting to note that a recent study reported that the icv administration of NPY increases the expression of c-fos mRNA in the CeA of rats (Cippitelli et al., 2010). Numerous studies have reported an increased expression of c-Fos protein or c-fos mRNA in the CeA during drug withdrawal and an increased activity of this area has been implicated in negative mood states (Frenois et al., 2002; Panagis et al., 2000). Therefore, it cannot be ruled out the NPY’s effects on the CeA contributed to the NPY and [D-His26]-NPY induced elevations in brain reward thresholds. Previous research from our group demonstrated that the intra-CeA administration of the non-specific CRF1/CRF2 receptor antagonist D-Phe CRF(12–41) or the selective CRF1 receptor antagonist R278995/CRA0450 prevents the elevations in brain reward thresholds associated with nicotine withdrawal {Marcinkiewcz, 2009 2867 /id;Bruijnzeel, 2012 3596 /id}. Therefore, follow-up studies could investigate if the NPY-induced elevations in brain reward thresholds are mediated by increased CRF transmission in the CeA. Although NPY and [D-His26]-NPY did not affect the affective signs of nicotine withdrawal, both neuropeptides attenuated the somatic signs associated with precipitated and spontaneous nicotine withdrawal. This is in line with studies that reported that NPY attenuates the somatic signs associated with morphine and alcohol withdrawal (Woldbye et al., 1998; Woldbye et al., 2002). Thus, these findings suggest that NPY or Y1 receptor agonists diminish the somatic signs of nicotine withdrawal but do not attenuate the dysphoria associated with nicotine withdrawal.
3.4. Norepinephrine and Nicotine Addiction
3.4.1. Brain Norepinephrine Systems
Two distinct noradrenergic cell groups have been located in the rodent and human brainstem (Dahlström and Fuxe, 1964). The noradrenergic neurons in the LC and the sub-coeruleus give rise to the dorsal noradrenergic bundle, which innervates cortical areas, the hippocampus, amygdala, and other forebrain areas. The LC provides the majority of the norepinephrine input to the forebrain areas, and plays an important role in behavioral functions such as attention, arousal, waking, and learning and memory (Aston-Jones, 2005). The noradrenergic cell groups (A1, A2, A5, and A7) located in the lateral tegmentum give rise to the ventral noradrenergic bundle. The ventral noradrenergic bundle provides extensive projections to the hypothalamus, septum, and subcomponents of the extended amygdala such as the CeA and BNST (Moore and Card, 1984). The ventral noradrenergic bundle provides less extensive projections to the forebrain areas than the dorsal noradrenergic bundle.
Norepinephrine mediates its behavioral and physiological effects via the activation of α- and β-adrenoceptors. Based on their anatomical localization, the α-adrenoceptors were divided into two subfamilies: the postsynaptic α1-adrenoceptors and the presynaptic α2-adrenoceptors (Langer, 1980). More recent studies demonstrated that the α2-adrenoceptors are also located postsynaptically (Ruffolo, Jr. and Hieble, 1994). The development of selective α-adrenoceptor ligands allowed a further subdivision of the α1- and α2-adrenoceptors. At this point, three α1-adrenoceptors (α1A, α1B, and α1D) and three α2-adrenoceptors (α2A, α2B, and α2C) have been pharmacologically identified (Bylund et al., 1994; Hieble et al., 1995). Pharmacological studies have led to the discovery of two subtypes of β-adrenoceptors, β1 and β2, and molecular cloning led to the discovery of the β3-adrenoceptor (Bylund et al., 1994; Muzzin et al., 1991). The adrenoceptors are part of the superfamily of seven transmembrane G-protein-coupled receptors. The α1-adrenoceptors are coupled to the Gαq subfamily of G-proteins, the α2-adrenoceptors are coupled to Gαi subfamily, and the β-adrenoceptors are coupled to Gαs subfamily (Duman and Nestler, 2002). The activation of each receptor has specific G-protein dependent intracellular effects. Activation of the α1-adrenoceptor leads to the activation of phospholipases, activation of the α2-adrenoceptor leads to the inhibition of adenylyl cyclase and the inhibition of cAMP formation, and activation of the β-adrenoceptors to the activation of adenylyl cyclase and increased cAMP formation (Duman and Nestler, 2002).
3.4.2. Norepinephrine and Psychiatric Disorders and Drug Addiction
Central noradrenergic signaling plays an important role in neurobehavioral and physiological responses to stressors. A dysregulation of central noradrenergic systems has been suggested to contribute to the development and maintenance of psychiatric disorders such as anxiety disorders and major depression (Charney, 2003). Evidence for a dysregulation of noradrenergic systems in anxiety disorders is provided by the observation that patients with panic disorder and PTSD display abnormal responses to the administration of the α2-adrenoceptor antagonist yohimbine and the α2-adrenoceptor agonist clonidine. Yohimbine increases norepinephrine levels in the synaptic cleft and clonidine decreases the availability of norepinephrine. Yohimbine induces greater increases in anxiety and plasma levels of the norepinephrine metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG) in patients with panic disorder and PTSD than in healthy controls (Charney et al., 1992; Southwick et al., 1993; Southwick et al., 1997). Patients with panic disorder also display a blunted growth hormone response to the administration of clonidine, which has been suggested to be mediated by presynaptic autoreceptor subsensivity (Charney and Heninger, 1986; Sallee et al., 2000). Several lines of evidence imply a role for noradrenergic systems in depressive disorders. The strongest evidence is provided by studies that investigated the effects of catecholamine depletion on mood states in patients with a history of depression. Treatments that deplete monoamine stores (reserpine and tetrabenazine) or inhibit catecholamine synthesis (α-methylparatyrosine) induce depressive symptoms in patients with a history of depression (Berman et al., 1999; Freis, 1954; Goodwin and Bunney, Jr., 1971; Lingjaerde, 1963). Patients return to baseline levels after the discontinuation of these treatments or after the administration of the catecholamine precursor L-DOPA (Schildkraut, 1965). Depressive disorders have also been associated with alterations in catecholamine receptor functioning. Most research has focused on the role of the α2-adrenoceptor in depression. The chronic administration of the antidepressant and selective norepinephrine reuptake inhibitor desipramine decreases the sensitivity of presynaptic α2-adrenoceptors in depressed patients, which lead to the α2-adrenoceptor supersensitivity hypothesis (Charney et al., 1981; Spyraki and Fibiger, 1980). A more recent study that investigated the effects of selective α2-adrenoceptor agonists on [(35)S]GTPgammaS binding in the frontal cortices of suicide victims with major depression provided additional support for the α2-adrenegic supersensitivity hypothesis. It was shown that the α2-adrenoceptors of the suicide victims were 4.6 times more sensitive to the stimulatory effects of specific α2-adrenergic agonists than those of healthy controls (Gonzalez-Maeso et al., 2002).
Additional evidence for a dysregulation of noradrenergic signaling in mood disorders is provided by studies that indicate that chronic elevation of central norepinephrine levels ameliorates depressive symptomatology. Drugs that preferentially inhibit the reuptake of norepinephrine such as the tricyclic desipramine and the selective norepinephrine reuptake inhibitor reboxetine are as effective in improving depression scores in depressed patients as specific serotonin reuptake inhibitors (Andreoli et al., 2002; Nelson, 1999). Moreover, antidepressants that block both the reuptake of norepinephrine and serotonin have been reported to be more effective in improving depression scores than selective serotonin reuptake inhibitors (Stahl et al., 2002). During the last decades, a great majority of the studies has focused on the role of the LC and its ascending projections in stress-associated psychiatric disorders and drug withdrawal while the role of noradrenergic neurons in the lateral tegmentum has been under-investigated. Research by Lucki and colleagues suggests that the ventral noradrenergic bundle may, however, play a more significant role in mediating the neurobehavioral effects of norepinephrine reuptake inhibitors than the dorsal noradrenergic bundle (Cryan et al., 2002b). They showed that reboxetine, similar to other norepinephrine reuptake inhibitors, decreases immobility and swimming behavior in the forced swim test while simultaneously increasing climbing behavior. Lesioning of the ventral noradrenergic bundle prevents the reboxetine induced decrease in immobility behavior and the increase in climbing behavior. In contrast, lesioning of the dorsal noradrenergic bundle did not affect the behavioral effects of reboxetine in the modified forced swim test. This suggests that noradrenergic cell groups in the lateral tegmentum and possibly their projections to the CeA and BNST may play an important role in mediating the antidepressant effects of norepinephrine reuptake inhibitors.
Several lines of evidence indicate that stressors increase central noradrenergic transmission (Cecchi et al., 2002; Khoshbouei et al., 2002; Morilak et al., 2005; Swanson et al., 2004). Although the great majority of the studies have focused on the role of the LC in stress responses, recent studies also indicate that stressors induce a strong activation of the A1 and A2 noradrenergic cell groups in the medulla (Dayas et al., 2001). The behavioral and neurochemical effects of stressors can be modulated by the administration of drugs that stimulate or block adrenoceptors. Immobilization stress increases extracellular levels of norepinephrine in the CeA and these effects are potentiated by the administration of the α2-adrenoceptor antagonist yohimbine (Khoshbouei et al., 2002). Yohimbine by itself also increases the expression of c-Fos in the CeA and BNST (Singewald et al., 2003). Furthermore, simultaneous blockade of β1 and β2-adrenoceptors in the BNST or the activation of α2-adrenoceptors in this brain site decreases anxiety-like behavior (Cecchi et al., 2002; Schweimer et al., 2005). Discontinuation of chronic opioid administration has been suggested to mediate a similar effect on brain norepinephrine systems as exposure to stressors (Koob et al., 2004). Morphine withdrawal mediates an increased release of norepinephrine in the prefrontal cortex, CeA, and BNST (Devoto et al., 2002; Fuentealba et al., 2000; Watanabe et al., 2003). Drugs that prevent the increase in noradrenergic transmission such as the α2-adrenoceptor agonists clonidine and lofexidine decrease opioid withdrawal symptomatology (Gold et al., 1978; Gold et al., 1981). Recent studies have focused on the neurobiological substrates that may mediate the aversive state associated with the discontinuation of morphine administration. Aston-Jones and colleagues have provided evidence for a role of the ventral, but not of the dorsal, noradrenergic bundle in the negative aversive state associated with morphine withdrawal (Delfs et al., 2000). They also showed that noradrenergic transmission in the CeA and BNST plays a critical role in the negative mood state associate with opioid withdrawal (Aston-Jones and Harris, 2004; Delfs et al., 2000).
3.4.3. Norepinephrine and Nicotine Withdrawal
Clinical and preclinical studies suggest that noradrenergic transmission plays a role in tobacco and nicotine withdrawal. Gourlay and colleagues conducted a meta-analysis on the data of 6 studies that investigated the effects of the α2-adrenoceptor agonist clonidine on smoking cessation (Gourlay et al., 2004). Subjects who were treated with clonidine were more likely to be abstinent 12 weeks after quitting smoking than control subjects who were treated with placebo (OR = 1.89). In addition, it has been reported that clonidine decreases anxiety and irritability in the first week after quitting smoking (Prochazka et al., 1992). Additional evidence for a role of noradrenergic transmission in tobacco withdrawal is provided by studies with the antidepressant and relative selective norepinephrine reuptake inhibitor nortriptyline (serotonin, 570; NE, 3.4; dopamine, 3500, IC50, reuptake inhibition in vivo)(Hyttel, 1994). Nortriptyline has been shown to decrease relapse rates and diminish anxiety, anger, irritability, difficulty concentrating, restlessness, and impatience associated with smoking cessation (Hall et al., 1998; Prochazka et al., 1998; Wagena et al., 2005). It cannot be ruled out that nortriptyline’s effect on the serotonin transporter contributed to its effectiveness as a smoking cessation aid. However, it should be noted that selective serotonin reuptake inhibitors such as fluoxetine, paroxetine, and sertraline do not improve smoking cessation rates (Hughes et al., 2007). Therefore, it is most likely that the effects of nortriptyline are mediated by its actions on the norepinephrine transporter. The antidepressant drug bupropion has also been shown to improve smoking cessation rates and decrease tobacco withdrawal symptoms such as depression, difficulty concentrating, and irritability (Hurt et al., 1997; Jorenby et al., 1999; Shiffman et al., 2000). Although there is evidence that bupropion and its metabolites affect noradrenergic transmission, bupropion also acts upon many other brain systems. Bupropion has been shown to be a weak dopamine and norepinephrine reuptake inhibitor, stimulates norepinephrine release, and blocks nAChRs (Damaj et al., 2004; Dong and Blier, 2001; Ferris et al., 1982; Fryer and Lukas, 1999).
There are only a few animal studies that have investigated the effects of selective adrenoceptor agonists or antagonists on nicotine withdrawal. In our laboratory, the effects of the α1-adrenoceptor antagonist prazosin, the α2-adrenoceptor agonist clonidine, and the nonselective β1/β2-adrenoceptor antagonist propranolol on the elevations in brain reward thresholds associated with nicotine withdrawal was investigated (Bruijnzeel et al., 2010). Pretreatment with low doses of prazosin (0.0625 and 0.125 mg/kg) dose-dependently prevented the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. In a separate experiment, the effect of higher doses of prazosin (0.25–1 mg/kg, ip) was also investigated (Bruijnzeel et al., 2010). These higher doses tended to induce a non-significant increase in the brain reward thresholds of the control rats and were less effective in attenuating the elevations in brain reward thresholds associated with nicotine withdrawal than lower doses. This observation is in line with previous studies that reported that high doses of α1-adrenoceptor antagonists inhibit ICSS responding (Fenton and Liebman, 1982; Liebman et al., 1982; Lin et al., 2007). The above discussed prazosin study suggests that suboptimal, either too low or too high, levels of noradrenergic transmission lead to negative mood states. Pretreatment with the α2-adrenoceptor agonist clonidine or the nonselective β1/β2-adrenoceptor antagonist propranolol did not prevent the elevations in brain reward thresholds associated with nicotine withdrawal (Bruijnzeel et al., 2010). In contrast, clonidine and propranolol, but not prazosin, decreased the total number of somatic signs associated with nicotine withdrawal. Taken together, these studies suggest that antagonism of α1-adrenoceptors attenuates the deficit in brain reward function associated with nicotine withdrawal and that antagonism of β-adrenoceptors or stimulation of α2-adrenoceptors attenuates the somatic symptoms of nicotine withdrawal.
In a recent study, Semenova and Markou investigated the effects of blockade of α2-adrenoceptors on the elevations in ICSS reward thresholds associated with spontaneous and dihydro-beta-erythroidine (DHβE) precipitated nicotine withdrawal (Semenova and Markou, 2010). The α2-adrenoceptor antagonist idazoxan attenuated the elevations in brain reward thresholds associated with spontaneous, but not precipitated, nicotine withdrawal. The discrepancy between the spontaneous and precipitated nicotine withdrawal experiments may have been due to the pretreatment interval. In the spontaneous withdrawal experiment, brain reward thresholds were measured 30 minutes, 24 hours, and 72 hours after the administration of idazoxan. The brain reward thresholds of the nicotine withdrawing rats treated with idazoxan rats were lower than those of the nicotine withdrawing rats treated with saline 24 and 72 hours, but not 30 minutes, after the administration of idazoxan. In the precipitated withdrawal experiment, idazoxan was administered 30 minutes before assessing the brain reward thresholds and idazoxan did not prevent the elevations in brain reward thresholds. This pattern of results suggests that idazoxan has a delayed effect on the elevations in brain reward thresholds associated with nicotine withdrawal. Taken together, these findings would suggest that blockade (Semenova and Markou, 2010), but not stimulation (Bruijnzeel et al., 2010), of α2-adrenoceptors may attenuate the negative mood state associated with smoking cessation.
3.4.4. Norepinephrine and Reinstatement of Nicotine Seeking
At this point, few studies have investigated the role of noradrenergic transmission in the reinstatement of extinguished nicotine-seeking behavior. Our group showed that systemic administration of the α2-adrenoceptor agonist clonidine attenuates footshock-induced reinstatement of nicotine-seeking behavior in rats (Zislis et al., 2007). This observation is in line with a study that showed that clonidine inhibits stress-induced reinstatement of cocaine-seeking behavior in rats (Erb et al., 2000). In a follow-up experiment, we showed that intra-CeA administration of clonidine or another α2-adrenoceptor agonist, dexmedetomidine, attenuates stress-induced reinstatement of nicotine seeking in rats (Yamada and Bruijnzeel, 2011). In contrast, intra-CeA administration of the nonselective β1/β2-adrenoceptor antagonist propranolol or the α1-adrenoceptor antagonist prazosin did not decrease stress-induced reinstatement of nicotine-seeking behavior. A recent study by Forget and colleagues showed that systemic administration of prazosin attenuates nicotine and cue-induced reinstatement of extinguished nicotine seeking behavior (Forget et al., 2010). Additional studies are warranted to investigate if systemic administration of prazosin attenuates stress-induced reinstatement of nicotine-seeking behavior.
3.5. Hypothalamic-Pituitary-Adrenal Axis and Nicotine Addiction
3.5.1. Central Norepinephrine and Hypothalamic-Pituitary-Adrenal Axis
The acute non-contingent administration of nicotine to rats and mice has been shown to stimulate the release of ACTH and corticosterone (Andersson et al., 1983; Balfour et al., 1975; Cam et al., 1979; Lutfy et al., 2006). The self-administration of nicotine has also been shown to increase the release of ACTH and corticosterone in rats (Chen et al., 2008; Donny et al., 2000). Extensive evidence indicates that nicotine activates the HPA axis by stimulating central but not peripheral nAChRs (Matta et al., 1998). This is supported by research conducted with mecamylamine, which readily crosses the blood brain barrier, and hexamethonium, which does not cross the blood brain barrier. The systemic administration of mecamylamine, but not hexamethonium, blocks nicotine-induced ACTH release (Matta et al., 1987). In addition, the intravenous administration of the cholinergic agonist cytisine, which does not cross the blood brain barrier, does not increase ACTH levels (Matta et al., 1987).
Nicotine may activate the HPA axis by stimulating nAChRs in the brain stem. Nicotinic receptors have been detected in noradrenergic brain stem areas (Maley and Seybold, 1993; Pauly et al., 1996) and there are extensive excitatory noradrenergic projections from the brain stem to CRF containing neurons in the parvocellular divisions of the PVN (Pacak et al., 1995). The administration of nicotine into the LC, the A2 (norepinephrine-containing neurons) and C2 (epinephrine-containing neurons) regions of the nucleus of the tractus solitarius, and the A1 region of the ventrolateral medulla stimulates the release of ACTH (Matta et al., 1993b). Nicotine stimulates the release of ACTH in the following rank order: A2 > C2 > LC > A1 > C1. The administration of 2.5 µg of nicotine free-base into the LC induced a similar increase in ACTH levels as the administration of 0.25 µg of nicotine free-base into the A2 region. The administration of nicotine in the C1 region did not induce the release of ACTH and only the administration of very high doses of nicotine (5 and 10 µg, free-base) into the A1 region stimulated the release of ACTH. This suggests that the A2/C2 region of the NTS plays a more important role in the nicotine-induced ACTH release than the LC and A1 and C1 regions of the ventrolateral medulla. This pharmacological study is in line with another experiment that investigated the effects of nicotine on the activation of neurons (c-Fos) in the brain (Matta et al., 1993a). In this study, c-Fos expression in the A2 and C2 regions of the nucleus tractus solitarius and the parvocellular divisions of the PVN was increased after the administration of a low dose of nicotine (0.05 mg/kg, free-base). This low dose of nicotine did not increase the expression of c-Fos in the LC or the A1 and C1 regions of the ventrolateral medulla. A higher dose of nicotine (0.1 mg/kg, free-base) also increased the expression of c-Fos in the LC.
The noncontingent intravenous administration of nicotine to rats induces a dose-dependent increase in NE levels in the PVN and a dose-dependent increase in plasma ACTH levels (Fu et al., 1997). In order to investigate if nicotine induces the release of NE in the PVN via the activation of nAChRs in the PVN or in the brainstem, Fu and colleagues investigated the effects of the administration of mecamylamine in the PVN or in the fourth ventricle on nicotine-induced NE release in the PVN (Fu et al., 1997). The administration of mecamylamine into the fourth ventricle leads to the blockade of nAChRs in the brain stem. The administration of nicotine into the PVN did not affect the iv nicotine-induced NE release in the PVN. In contrast, the administration of mecamylamine into the fourth ventricle completely blocked the iv nicotine-induced release of NE in the PVN. This suggests that nicotine-induced NE release is mediated by the activation of nAChRs in the brain stem and that nAChRs in the PVN do not play a role in nicotine-induced NE release in this brain site. The administration of the α2-adrenoceptor antagonist yohimbine or the α1-adrenoceptor antagonist prazosin, but not the nonspecific β1/β2-adrenoceptor antagonist propranolol, in the third ventricle has been shown to attenuate the nicotine-induced ACTH release (Matta et al., 1990). Based on the aforementioned observation it was concluded that the activation of α1 and α2-adrenoceptors in the PVN plays a role in the nicotine-induced ACTH release (Matta et al., 1990). Taken together, these studies indicate that nicotine stimulates the HPA axis by activating neurons in the A2 region that project to the parvocellular region of the PVN. The release of NE in the parvocellular PVN and the subsequent activation of the CRF neurons leads to the release of CRF into the hypophyseal portal system and the release of ACTH into the peripheral circulation by the anterior pituitary.
3.5.2. Corticosterone and Behavioral Effects of Nicotine
Experimental evidence suggests that corticosterone may potentiate some of the behavioral effects of psychostimulants such as cocaine and amphetamine. For example, it has been suggested that corticosterone plays a role in the acquisition of amphetamine self-administration in rats that have a low baseline intake of this drug and corticosterone has also been shown to increase the intake of low doses of cocaine (Goeders, 2002; Piazza et al., 1991). Corticosterone potentiates the acute locomotor effects of cocaine and plays a role in cocaine-induced locomotor sensitization (Piazza et al., 1994). Finally, it has been reported that corticosterone is necessary for the footshock-induced increase in cocaine self-administration (Mantsch and Katz, 2007). It is beyond the scope of this review to fully discuss the role of corticosterone in psychostimulant addiction and therefore the readers are referred to some excellent reviews (Goeders, 2002; Piazza and Le Moal, 1996). Animal studies suggest that the effects of corticosterone on the behavioral and physiological effects of nicotine are opposite to those of other psychostimulants. Corticosterone has been reported to decrease the behavioral and physiological effects of nicotine. Pauly and colleagues investigated the effects of adrenalectomy (ADX), which leads to extremely low corticosterone levels, on the nicotine-induced changes in the acoustic startle response, Y-maze activity, heart rate, and body temperature (Pauly et al., 1988). Adrenalectomy potentiated the nicotine-induced decrease in Y-maze crosses, body temperature, and heart rate. In addition, ADX potentiated the nicotine-induced increase in startle responses. The administration of corticosterone to the ADX animals prevented the hypersensitivity to nicotine. Adrenalectomy has also been shown to potentiate the locomotor response to a low dose of nicotine and potentiate the locomotor depressant effects of high doses of nicotine (Shoaib and Shippenberg, 1996). The effects of the administration of corticosterone are opposite to those of ADX. The administration of corticosterone to intact animals (no-ADX) decreases the sensitivity to nicotine-induced changes in the acoustic startle procedure, Y maze test, heart rate response, and body temperature (Robinson et al., 1996). These findings suggest that high levels of corticosterone cause a hyporesponsivity to nicotine and the absence of corticosterone leads to a hypersensitivity to nicotine.
Chronic exposure to tobacco smoke or nicotine leads to tolerance to the physiological (e.g., decrease in heart rate and body temperature) and behavioral (e.g., decrease in locomotor activity and rearing) effects of nicotine and an upregulation of α4β2 and α7-containing nAChRs in rats and mice (Pauly et al., 1991; Pauly et al., 1992; Small et al., 2010; Stolerman et al., 1973; Yates et al., 1995). The upregulation of nAChRs is considered a hallmark feature of nicotine dependence (Dani and Heinemann, 1996). Because corticosterone, like nicotine, decreases the sensitivity to nicotinic receptor agonists, several studies have investigated if corticosterone has similar effects on brain nAChR levels as nicotine. The levels of α4β2 nAChRs are often assessed by measuring [3H]-nicotine binding and α7 nAChRs levels are often assessed by measuring [125I]-α-bungarotoxin binding (Barrantes et al., 1995; Zoli et al., 1998). Autoradiographic studies have shown that the effects of corticosterone on nAChR levels are very different than the effects of nicotine. It has been reported that corticosterone does not affect [3H]-nicotine binding or it increases [3H]-nicotine binding in only a small percentage of the brain sites investigated (Pauly et al., 1990; Pauly and Collins, 1993). Studies that investigated the effects of corticosterone on [125I]-α-bungarotoxin binding consistently show that corticosterone decreases [125I]-α-bungarotoxin binding (Pauly et al., 1990; Pauly and Collins, 1993; Robinson et al., 1996). Thus, both nicotine and corticosterone induce tolerance to the effects of nicotine. However, nicotine increases α4β2 and α7 nAChR levels and corticosterone only marginally increases α4β2 nAChR levels in a few brain sites and decreases α7 nAChR levels. A better understanding of the nicotine-induced activation, desensitization, and upregulation of nAChRs has been considered to be the key to understanding tolerance to the effects of nicotine and nicotine dependence (De Biasi and Dani, 2010). Additional studies are warranted to investigate the possible mechanisms by which corticosterone mediates tolerance to the behavioral and physiological effects of nicotine.
4. Concluding Remarks
The first part of this review investigated the role of stressors and stress-associated psychiatric disorders in tobacco addiction. The epidemiological and clinical studies that were discussed indicate that brain stress systems play a critical role in tobacco addiction. Smokers indicate that they smoke for stress relief and to relax. Exposure to stressors in the real world or in the laboratory increases the number of cigarettes smoked. There is also a high comorbidity between smoking and stress-associated psychiatric disorders such as depression, PTSD, and other anxiety disorders. The smoking rate in people with these disorders is about twice as high as in the general population. The reviewed studies suggest that there is a bidirectional relationship between depression and smoking. Children and adolescents who are depressed are more likely to start smoking than their nondepressed peers. Smokers who did not have a history of depression when they started smoking are more likely to become depressed than nonsmokers. Most anxiety disorders do not increase the risk for smoking but there is some evidence that social fears increase the risk for smoking. In contrast, smoking increases the risk for developing anxiety disorders such a panic disorder, agoraphobia, and generalized anxiety disorder. People who smoke are also more likely to develop PTSD after exposure to a traumatic event. Smoking and smoking cessation affects the HPA axis. Smoking leads to an increased release of ACTH and cortisol and smoking cessation leads to a dramatic decrease in cortisol levels. The smoking cessation-induced decrease in cortisol levels may play a role in relapse to smoking as people with the largest decrease in cortisol levels are the most likely to relapse. Smoking leads to an upregulation and desensitization of nAChRs and after people quit smoking the nAChRs recover to a responsive state. The excessive number of responsive nAChRs may contribute to craving for cigarettes and relapse. Animal studies show that corticosterone decreases the sensitivity of nAChRs. Therefore, the smoking-cessation induced decrease in cortisol levels may exacerbate withdrawal symptomatology and relapse by facilitating the recovery of nAChRs.
The reviewed studies focused on the role of stressors, depression, and anxiety disorders in the onset of smoking and smoking cessation. It should be noted that in addition to the discussed parameters, age and sex can also affect the onset of smoking and smoking cessation. For example, the reviewed studies indicate that there is a strong association between smoking and anxiety disorders in adults but not in adolescents (Johnson et al., 2000; Lawrence et al., 2009). Furthermore, females are more likely to relapse to smoking than males (Bjornson et al., 1995; Swan et al., 1993). Therefore, future studies that investigate the association between anxiety, depression or other brain disorders and smoking should pay close attention to the age and sex of the test subjects.
The second part of this review described studies that used animal models to investigate the role of brain stress systems in tobacco addiction. The animal studies indicate that brain stress systems play a critical role in all stages of the addiction cycle. Drugs that block CRF1 receptors prevent the increased nicotine intake after a period of abstinence, decrease the negative mood state and anxiety-like behavior associated with nicotine withdrawal, and prevent stress-induced reinstatement of extinguished nicotine-seeking behavior. The reviewed studies also suggest that noradrenergic transmission plays a critical role in nicotine addiction. Blockade of α1-adrenoceptors and stimulation of α2-adrenoceptors diminishes the negative mood state associated with nicotine withdrawal. Stimulation of α2-adrenoceptors also prevents stress-induced relapse to nicotine seeking. Blockade of hypocretin-1 receptors has been shown to decrease the positive reinforcing effects of nicotine and decreases nicotine-withdrawal induced anxiety-like behavior. Finally, NPY or Y1 receptor agonists attenuate the somatic signs associated with nicotine withdrawal.
Taken together, the reviewed studies indicate that smoking induces a feeling of stress relief and relaxation. However, contrary to the subjective experience, smoking activates brain stress systems and over time induces a dysregulation of these systems. This can lead to an increase in perceived stress levels and an increased risk for developing depression, PTSD, and other anxiety-disorders. Animal studies suggest that drugs that counteract the increased activity in brain stress systems may help people to quit smoking and prevent relapse to smoking after a period of abstinence.
Stressors increase the number of cigarettes smoked and increase the risk for relapse
Depression increases the risk for smoking and vice versa
Smoking increases the risk for developing an anxiety disorder
A hyperactivity of brain stress system plays a role in nicotine withdrawal in rats
Drugs that decrease the activity of brain stress systems may help people quit smoking
Acknowledgements
This research was supported by the National Institute on Drug Abuse (DA023575) and the Flight Attendant Medical Research Institute (Grant 52312).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acierno RA, Kilpatrick DG, Resnick HS, Saunders BE, Best CL. Violent assault, posttraumatic stress disorder, and depression. Risk factors for cigarette use among adult women. Behav. Modif. 1996;20:363–384. doi: 10.1177/01454455960204001. [DOI] [PubMed] [Google Scholar]
- Adrian TE, Allen JM, Bloom SR, Ghatei MA, Rossor MN, Roberts GW, Crow TJ, Tatemoto K, Polak JM. Neuropeptide Y distribution in human brain. Nature. 1983;306:584–586. doi: 10.1038/306584a0. [DOI] [PubMed] [Google Scholar]
- Ague C. Nicotine and smoking: effects upon subjective changes in mood. Psychopharmacologia. 1973;30:323–328. doi: 10.1007/BF00429191. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, Polak JM. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J. Association between COMT, PTSD, and increased smoking following hurricane exposure in an epidemiologic sample. Psychiatry. 2009;72:360–369. doi: 10.1521/psyc.2009.72.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking. A national perspective. JAMA. 1990;264:1541–1545. [PubMed] [Google Scholar]
- Andersson K, Siegel R, Fuxe K, Eneroth P. Intravenous injections of nicotine induce very rapid and discrete reductions of hypothalamic catecholamine levels associated with increases of ACTH, vasopressin and prolactin secretion. Acta Physiol Scand. 1983;118:35–40. doi: 10.1111/j.1748-1716.1983.tb07237.x. [DOI] [PubMed] [Google Scholar]
- Andreoli V, Caillard V, Deo RS, Rybakowski JK, Versiani M. Reboxetine, a new noradrenaline selective antidepressant, is at least as effective as fluoxetine in the treatment of depression. J. Clin. Psychopharmacol. 2002;22:393–399. doi: 10.1097/00004714-200208000-00010. [DOI] [PubMed] [Google Scholar]
- Aricioglu F, Altunbas H. Harmane induces anxiolysis and antidepressant-like effects in rats. Ann. N.Y. Acad. Sci. 2003;1009:196–201. doi: 10.1196/annals.1304.024. [DOI] [PubMed] [Google Scholar]
- ASPECT Consortium. Tobacco or Health in the European Union: Past, Present and Future. 2005 [Google Scholar]
- Aston-Jones G. Brain structures and receptors involved in alertness. Sleep Med. 2005;6(Suppl 1):S3–S7. doi: 10.1016/s1389-9457(05)80002-4. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Suppl 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li TK. Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and -nonpreferring (NP) rats. Alcohol Clin.Exp.Res. 2001;25:386–390. [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the "anxiogenic" response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handb. Exp. Pharmacol. 2009:209–233. doi: 10.1007/978-3-540-69248-5_8. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Khullar AK, Longden A. Effects of nicotine on plasma corticosterone and brain amines in stressed and unstressed rats. Pharmacol.Biochem.Behav. 1975;3:179–184. doi: 10.1016/0091-3057(75)90145-8. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A, Phillips AG. A 'crash' course on psychostimulant withdrawal as a model of depression. Trends Pharmacol. Sci. 2002;23:475–482. doi: 10.1016/s0165-6147(02)02086-2. [DOI] [PubMed] [Google Scholar]
- Barrantes GE, Rogers AT, Lindstrom J, Wonnacott S. alpha-Bungarotoxin binding sites in rat hippocampal and cortical cultures: initial characterisation, colocalisation with alpha 7 subunits and up-regulation by chronic nicotine treatment. Brain Res. 1995;672:228–236. doi: 10.1016/0006-8993(94)01386-v. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Kirby AC, Feldman ME, Hertzberg MA, Moore SD, Crawford AL, Davidson JR, Fairbank JA. Prevalence and correlates of heavy smoking in Vietnam veterans with chronic posttraumatic stress disorder. Addict. Behav. 1997;22:637–647. doi: 10.1016/s0306-4603(96)00071-8. [DOI] [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine. Tob. Res. 1999;1:45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N. Engl. J. Med. 1988;319:1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Berman RM, Narasimhan M, Miller HL, Anand A, Cappiello A, Oren DA, Heninger GR, Charney DS. Transient depressive relapse induced by catecholamine depletion: potential phenotypic vulnerability marker? Arch. Gen. Psychiatry. 1999;56:395–403. doi: 10.1001/archpsyc.56.5.395. [DOI] [PubMed] [Google Scholar]
- Bjornson W, Rand C, Connett JE, Lindgren P, Nides M, Pope F, Buist AS, Hoppe-Ryan C, O'Hara P. Gender differences in smoking cessation after 3 years in the Lung Health Study. Am. J. Public Health. 1995;85:223–230. doi: 10.2105/ajph.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Blomqvist AG, Herzog H. Y-receptor subtypes--how many more? Trends Neurosci. 1997;20:294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de LL. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. DSM-III-R nicotine dependence in young adults: prevalence, correlates and associated psychiatric disorders. Addiction. 1994;89:743–754. doi: 10.1111/j.1360-0443.1994.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Breslau N, Klein DF. Smoking and panic attacks: an epidemiologic investigation. Arch. Gen. Psychiatry. 1999;56:1141–1147. doi: 10.1001/archpsyc.56.12.1141. [DOI] [PubMed] [Google Scholar]
- Broberger C, Hokfelt T. Hypothalamic and vagal neuropeptide circuitries regulating food intake. Physiol Behav. 2001;74:669–682. doi: 10.1016/s0031-9384(01)00611-4. [DOI] [PubMed] [Google Scholar]
- Brown ZW, Amit Z, Rockman GE. Intraventricular self-administration of acetaldehyde, but not ethanol, in naive laboratory rats. Psychopharmacology (Berl) 1979;64:271–276. doi: 10.1007/BF00427509. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW. kappa-Opioid receptor signaling and brain reward function. Brain Res. Rev. 2009;62:127–146. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Bishnoi M, van Tuijl IA, Keijzers KF, Yavarovich KR, Pasek TM, Ford J, Alexander JC, Yamada H. Effects of prazosin, clonidine, and propranolol on the elevations in brain reward thresholds and somatic signs associated with nicotine withdrawal in rats. Psychopharmacology (Berl) 2010;212:485–499. doi: 10.1007/s00213-010-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Ford J, Rogers JA, Scheick S, Ji Y, Bishnoi M, Alexander JC. Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats. Pharmacol. Biochem. Behav. 2012;101:62–68. doi: 10.1016/j.pbb.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res. Brain Res. Rev. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-Releasing Factor-1 Receptor Activation Mediates Nicotine Withdrawal-Induced Deficit in Brain Reward Function and Stress-Induced Relapse. Biol. Psychiatry. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Buczek Y, Le AD, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Burkhoff A, Linemeyer DL, Salon JA. Distribution of a novel hypothalamic neuropeptide Y receptor gene and it's absence in rat. Brain Res. Mol. Brain Res. 1998;53:311–316. doi: 10.1016/s0169-328x(97)00302-1. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr, Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- Caan B, Coates A, Schaefer C, Finkler L, Sternfeld B, Corbett K. Women gain weight 1 year after smoking cessation while dietary intake temporarily increases. J. Am. Diet. Assoc. 1996;96:1150–1155. doi: 10.1016/S0002-8223(96)00296-9. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane. Database. Syst. Rev. 2010 doi: 10.1002/14651858.CD006103.pub2. CD006103. [DOI] [PubMed] [Google Scholar]
- Cam GR, Bassett JR, Cairncross KD. The action of nicotine on the pituitary-adrenal cortical axis. Arch. Int. Pharmacodyn. Ther. 1979;237:49–66. [PubMed] [Google Scholar]
- Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug Alcohol Depend. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Castane A, Berrendero F, Maldonado R. The role of the cannabinoid system in nicotine addiction. Pharmacol. Biochem. Behav. 2005;81:381–386. doi: 10.1016/j.pbb.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, De Souza EB. Corticotrophin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol. Sci. 1996;17:166–172. doi: 10.1016/0165-6147(96)81594-x. [DOI] [PubMed] [Google Scholar]
- Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr. Scand. Suppl. 2003:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Arch. Gen. Psychiatry. 1993;50:295–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR. Abnormal regulation of noradrenergic function in panic disorders. Effects of clonidine in healthy subjects and patients with agoraphobia and panic disorder. Arch. Gen. Psychiatry. 1986;43:1042–1054. doi: 10.1001/archpsyc.1986.01800110028005. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Sternberg DE, Redmond DE, Leckman JF, Maas JW, Roth RH. Presynaptic adrenergic receptor sensitivity in depression. The effect of long-term desipramine treatment. Arch. Gen. Psychiatry. 1981;38:1334–1340. doi: 10.1001/archpsyc.1981.01780370036004. [DOI] [PubMed] [Google Scholar]
- Charney DS, Woods SW, Krystal JH, Nagy LM, Heninger GR. Noradrenergic neuronal dysregulation in panic disorder: the effects of intravenous yohimbine and clonidine in panic disorder patients. Acta Psychiatr. Scand. 1992;86:273–282. doi: 10.1111/j.1600-0447.1992.tb03266.x. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chen AM, Perrin MH, Digruccio MR, Vaughan JM, Brar BK, Arias CM, Lewis KA, Rivier JE, Sawchenko PE, Vale WW. A soluble mouse brain splice variant of type 2alpha corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2620–2625. doi: 10.1073/pnas.0409583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Fu Y, Sharp BM. Chronic nicotine self-administration augments hypothalamic-pituitary-adrenal responses to mild acute stress. Neuropsychopharmacology. 2008;33:721–730. doi: 10.1038/sj.npp.1301466. [DOI] [PubMed] [Google Scholar]
- Chen H, Huang X, Guo X, Mailman RB, Park Y, Kamel F, Umbach DM, Xu Q, Hollenbeck A, Schatzkin A, Blair A. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74:878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherek DR. Effects of acute exposure to increased levels of background industrial noise on cigarette smoking behavior. Int. Arch. Occup. Environ. Health. 1985;56:23–30. doi: 10.1007/BF00380697. [DOI] [PubMed] [Google Scholar]
- Cheskin LJ, Hess JM, Henningfield J, Gorelick DA. Calorie restriction increases cigarette use in adult smokers. Psychopharmacology (Berl) 2005;179:430–436. doi: 10.1007/s00213-004-2037-x. [DOI] [PubMed] [Google Scholar]
- Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O'Donohue TL. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, Thorsell A, Heilig M. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacology (Berl) 2010;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Connolly GN, Alpert HR, Wayne GF, Koh H. Trends in nicotine yield in smoke and its relationship with design characteristics among popular US cigarette brands, 1997–2005. Tob. Control. 2007;16:e5. doi: 10.1136/tc.2006.019695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J, Jakupcak M, Rosenheck R, Fontana A, McFall M. Influence of PTSD symptom clusters on smoking status among help-seeking Iraq and Afghanistan veterans. Nicotine. Tob. Res. 2009;11:1189–1195. doi: 10.1093/ntr/ntp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine. Tob. Res. 2004;6:39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Costall B, Kelly ME, Naylor RJ, Onaivi ES. The actions of nicotine and cocaine in a mouse model of anxiety. Pharmacol. Biochem. Behav. 1989;33:197–203. doi: 10.1016/0091-3057(89)90450-4. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem. Pharmacol. 1997;54:743–753. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Gasparini F, van Heeke G, Markou A. Non-nicotinic neuropharmacological strategies for nicotine dependence: beyond bupropion. Drug Discov. Today. 2003a;8:1025–1034. doi: 10.1016/s1359-6446(03)02890-3. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol. Psychiatry. 2003b;54:49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 2002a;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol. Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur. J. Pharmacol. 2002b;436:197–205. doi: 10.1016/s0014-2999(01)01628-4. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the existence of monamine-containing neurons in the central nervous system. I. Demonstration of monamines in the cell bodies of brain stem neurons. Acta Physiol Scand. Suppl. 1964;232:1–55. [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol. Pharmacol. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J. Pharmacol. Exp. Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Dani JA, Balfour DJ. Historical and current perspective on tobacco use and nicotine addiction. Trends Neurosci. 2011;34:383–392. doi: 10.1016/j.tins.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Date Y, Mondal MS, Matsukura S, Ueta Y, Yamashita H, Kaiya H, Kangawa K, Nakazato M. Distribution of orexin/hypocretin in the rat median eminence and pituitary. Brain Res. Mol. Brain Res. 2000;76:1–6. doi: 10.1016/s0169-328x(99)00317-4. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc. Natl. Acad. Sci. U. S. A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. Seizures and bupropion: a review. J. Clin. Psychiatry. 1989;50:256–261. [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J. Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur. J. Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- De Biasi M, Dani JA. Reward, Addiction, Withdrawal to Nicotine. Annu. Rev. Neurosci. 2010;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998a;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998b;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J. Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wied D. Frontiers in Neuroendocrinology. London/New York: Oxford University Press; 1969. Effects of peptide hormones on behavior; pp. 97–140. [Google Scholar]
- De Wied D. Behavioral effects of neuropeptides related to ACTH MSH, beta LPH. Ann. N. Y. Acad. Sci. 1977;297:263–274. doi: 10.1111/j.1749-6632.1977.tb41859.x. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Pira L, Diana M, Gessa GL. Co-release of noradrenaline and dopamine in the prefrontal cortex after acute morphine and during morphine withdrawal. Psychopharmacology (Berl) 2002;160:220–224. doi: 10.1007/s00213-001-0985-y. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Avenevoli S, Merikangas KR, Flaherty BP, Stolar M. Association between psychiatric disorders and the progression of tobacco use behaviors. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:1159–1167. doi: 10.1097/00004583-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Dong J, Blier P. Modification of norepinephrine and serotonin, but not dopamine, neuron firing by sustained bupropion treatment. Psychopharmacology (Berl) 2001;155:52–57. doi: 10.1007/s002130000665. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rose C, Jacobs KS, Mielke MM, Sved AF. Differential effects of response-contingent and response-independent nicotine in rats. Eur. J. Pharmacol. 2000;402:231–240. doi: 10.1016/s0014-2999(00)00532-x. [DOI] [PubMed] [Google Scholar]
- Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res. 1999;842:473–477. doi: 10.1016/s0006-8993(99)01824-7. [DOI] [PubMed] [Google Scholar]
- Duman RS, Nestler EJ. Signal transduction pathways for catecholamine receptors. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res. Brain Res. Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Eaves M, Thatcher-Britton K, Rivier J, Vale W, Koob GF. Effects of corticotropin releasing factor on locomotor activity in hypophysectomized rats. Peptides. 1985;6:923–926. doi: 10.1016/0196-9781(85)90323-7. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- Elias GA, Gulick D, Wilkinson DS, Gould TJ. Nicotine and extinction of fear conditioning. Neuroscience. 2010;165:1063–1073. doi: 10.1016/j.neuroscience.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Farzin D, Mansouri N. Antidepressant-like effect of harmane and other beta-carbolines in the mouse forced swim test. Eur. Neuropsychopharmacol. 2006;16:324–328. doi: 10.1016/j.euroneuro.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Federal Trade Commission. "Tar," Nicotine, and Carbon Monoxide of the Smoke of 1294 Varieties of Domestic Cigarettes For the Year 1998: A Federal Trade Commission Report to Congress. 2000 [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and post-traumatic stress: a critical review of the empirical literature. Clin. Psychol. Rev. 2007;27:14–45. doi: 10.1016/j.cpr.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton HM, Liebman JM. Self-stimulation response decrement patterns differentiate clonidine, baclofen and dopamine antagonists from drugs causing performance deficit. Pharmacol. Biochem. Behav. 1982;17:1207–1212. doi: 10.1016/0091-3057(82)90122-8. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychol. Med. 2003;33:1357–1367. doi: 10.1017/s0033291703008596. [DOI] [PubMed] [Google Scholar]
- Ferris RM, Maxwell RA, Cooper BR, Soroko FE. Neurochemical and neuropharmacological investigations into the mechanisms of action of bupropion. HCl--a new atypical antidepressant agent. Adv. Biochem. Psychopharmacol. 1982;31:277–286. [PubMed] [Google Scholar]
- Fidler JA, West R. Self-perceived smoking motives and their correlates in a general population sample. Nicotine. Tob. Res. 2009;11:1182–1188. doi: 10.1093/ntr/ntp120. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Ouagazzal AM. Bimodal modulation by nicotine of anxiety in the social interaction test: role of the dorsal hippocampus. Behav. Neurosci. 1998;112:1423–1429. doi: 10.1037//0735-7044.112.6.1423. [DOI] [PubMed] [Google Scholar]
- Finkenauer R, Pomerleau CS, Snedecor SM, Pomerleau OF. Race differences in factors relating to smoking initiation. Addict. Behav. 2009;34:1056–1059. doi: 10.1016/j.addbeh.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory K, Hankin BL, Kloos B, Cheely C, Turecki G. Alcohol and cigarette use and misuse among Hurricane Katrina survivors: psychosocial risk and protective factors. Subst. Use. Misuse. 2009;44:1711–1724. doi: 10.3109/10826080902962128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le FB. Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology. 2010;35:1751–1760. doi: 10.1038/npp.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24:75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Wolf AP, Warner D, Cilento R, Zezulkova I. Neuropharmacological actions of cigarette smoke: brain monoamine oxidase B (MAO B) inhibition. J. Addict. Dis. 1998;17:23–34. doi: 10.1300/J069v17n01_03. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I, Wolf AP. Brain monoamine oxidase A inhibition in cigarette smokers. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JC, Schiele BC, Brozek J, Keys A. Observations on human behavior in experimental semistarvation and rehabilitation. 1948:28–45. doi: 10.1002/1097-4679(194801)4:1<28::aid-jclp2270040103>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Frederick SL, Reus VI, Ginsberg D, Hall SM, Munoz RF, Ellman G. Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biol. Psychiatry. 1998;43:525–530. doi: 10.1016/S0006-3223(97)00423-X. [DOI] [PubMed] [Google Scholar]
- Freis ED. Mental depression in hypertensive patients treated for long periods with large doses of reserpine. N. Engl. J. Med. 1954;251:1006–1008. doi: 10.1056/NEJM195412162512504. [DOI] [PubMed] [Google Scholar]
- Frenois F, Cador M, Caille S, Stinus L, Le MC. Neural correlates of the motivational and somatic components of naloxone-precipitated morphine withdrawal. Eur. J. Neurosci. 2002;16:1377–1389. doi: 10.1046/j.1460-9568.2002.02187.x. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J. Pharmacol. Exp. Ther. 1999;288:88–92. [PubMed] [Google Scholar]
- Fu Y, Matta SG, Valentine JD, Sharp BM. Adrenocorticotropin response and nicotine-induced norepinephrine secretion in the rat paraventricular nucleus are mediated through brainstem receptors. Endocrinology. 1997;138:1935–1943. doi: 10.1210/endo.138.5.5122. [DOI] [PubMed] [Google Scholar]
- Fuentealba JA, Forray MI, Gysling K. Chronic morphine treatment and withdrawal increase extracellular levels of norepinephrine in the rat bed nucleus of the stria terminalis. J. Neurochem. 2000;75:741–748. doi: 10.1046/j.1471-4159.2000.0750741.x. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, Meliska CJ, Williams CL, Jensen RA. Subjective correlates of cigarette-smoking-induced elevations of peripheral beta-endorphin and cortisol. Psychopharmacology (Berl) 1992;106:275–281. doi: 10.1007/BF02801984. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol. Biochem. Behav. 2008;90:475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Li TK, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol Clin. Exp. Res. 2003;27:787–794. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–1549. [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Gold MS, Pottash AC, Sweeney DR, Extein I, Annitto WJ. Opiate detoxification with lofexidine. Drug Alcohol Depend. 1981;8:307–315. doi: 10.1016/0376-8716(81)90040-5. [DOI] [PubMed] [Google Scholar]
- Gold MS, Redmond DE, Jr, Kleber HD. Clonidine blocks acute opiate-withdrawal symptoms. Lancet. 1978;2:599–602. doi: 10.1016/s0140-6736(78)92823-4. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Rodriguez-Puertas R, Meana JJ, Garcia-Sevilla JA, Guimon J. Neurotransmitter receptor-mediated activation of G-proteins in brains of suicide victims with mood disorders: selective supersensitivity of alpha(2A)-adrenoceptors. Mol. Psychiatry. 2002;7:755–767. doi: 10.1038/sj.mp.4001067. [DOI] [PubMed] [Google Scholar]
- Goodman E, Capitman J. Depressive symptoms and cigarette smoking among teens. Pediatrics. 2000;106:748–755. doi: 10.1542/peds.106.4.748. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Bunney WE., Jr Depressions following reserpine: a reevaluation. Semin. Psychiatry. 1971;3:435–448. [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Mol. Neurobiol. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol. Learn. Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav. Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Stead LF, Benowitz NL. Clonidine for smoking cessation. Cochrane. Database. Syst. Rev. 2004 doi: 10.1002/14651858.CD000058.pub2. CD000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor P, Feng Y, DeCarr LB, Cornfield LJ, McCaleb ML. Molecular characterization of a second mouse pancreatic polypeptide receptor and its inactivated human homologue. J. Biol. Chem. 1996;271:27776–27781. doi: 10.1074/jbc.271.44.27776. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, Frederick S, Triffleman E. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch. Gen. Psychiatry. 1998;55:683–690. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- Hauptmann N, Shih JC. 2-Naphthylamine, a compound found in cigarette smoke, decreases both monoamine oxidase A and B catalytic activity. Life Sci. 2001;68:1231–1241. doi: 10.1016/s0024-3205(00)01022-5. [DOI] [PubMed] [Google Scholar]
- Heilig M, Wahlestedt C, Ekman R, Widerlov E. Antidepressant drugs increase the concentration of neuropeptide Y (NPY)-like immunoreactivity in the rat brain. Eur. J. Pharmacol. 1988;147:465–467. doi: 10.1016/0014-2999(88)90182-3. [DOI] [PubMed] [Google Scholar]
- Heilig M, Zachrisson O, Thorsell A, Ehnvall A, Mottagui-Tabar S, Sjogren M, Asberg M, Ekman R, Wahlestedt C, Agren H. Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. J. Psychiatr. Res. 2004;38:113–121. doi: 10.1016/s0022-3956(03)00101-8. [DOI] [PubMed] [Google Scholar]
- Helton DR, Modlin DL, Tizzano JP, Rasmussen K. Nicotine withdrawal: a behavioral assessment using schedule controlled responding, locomotor activity, and sensorimotor reactivity. Psychopharmacology (Berl) 1993;113:205–210. doi: 10.1007/BF02245698. [DOI] [PubMed] [Google Scholar]
- Herraiz T, Chaparro C. Human monoamine oxidase is inhibited by tobacco smoke: beta-carboline alkaloids act as potent and reversible inhibitors. Biochem. Biophys. Res. Commun. 2005;326:378–386. doi: 10.1016/j.bbrc.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Moc K, Koob GF. Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacology. 2003;28:1463–1471. doi: 10.1038/sj.npp.1300175. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Koob GF. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin. Exp. Res. 1997;21:784–791. [PubMed] [Google Scholar]
- Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, Minneman KP, Ruffolo RR., Jr International Union of Pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol. Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- Holahan MR, Kalin NH, Kelley AE. Microinfusion of corticotropin-releasing factor into the nucleus accumbens shell results in increased behavioral arousal and oral motor activity. Psychopharmacology (Berl) 1997;130:189–196. doi: 10.1007/s002130050228. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol. Sci. 2003 Nov.24(11):580–588. doi: 10.1016/j.tips.2003.09.011. 2003. 24, 580–588. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Arch. Gen. Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch. Gen. Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am. J. Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane. Database. Syst. Rev. 2007 doi: 10.1002/14651858.CD000031.pub3. CD000031. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. N. Engl. J. Med. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Husum H, Mikkelsen JD, Hogg S, Mathe AA, Mork A. Involvement of hippocampal neuropeptide Y in mediating the chronic actions of lithium, electroconvulsive stimulation and citalopram. Neuropharmacology. 2000;39:1463–1473. doi: 10.1016/s0028-3908(00)00009-5. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs) Int. Clin. Psychopharmacol. 1994;9(Suppl 1):19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- Ikard FF, Green DE, Horn D. A Scale to Differentiate between Types of Smoking as Related to the Management of Affect. 1969:649–659. [Google Scholar]
- Irvine EE, Cheeta S, File SE. Time-course of changes in the social interaction test of anxiety following acute and chronic administration of nicotine. Behav. Pharmacol. 1999;10:691–697. doi: 10.1097/00008877-199911000-00016. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Tolerance to nicotine's effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacol. Biochem. Behav. 2001;68:319–325. doi: 10.1016/s0091-3057(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Ishida H, Shirayama Y, Iwata M, Katayama S, Yamamoto A, Kawahara R, Nakagome K. Infusion of neuropeptide Y into CA3 region of hippocampus produces antidepressant-like effect via Y1 receptor. Hippocampus. 2007;17:271–280. doi: 10.1002/hipo.20264. [DOI] [PubMed] [Google Scholar]
- Isola R, Vogelsberg V, Wemlinger TA, Neff NH, Hadjiconstantinou M. Nicotine abstinence in the mouse. Brain Res. 1999;850:189–196. doi: 10.1016/s0006-8993(99)02131-9. [DOI] [PubMed] [Google Scholar]
- Jain MR, Horvath TL, Kalra PS, Kalra SP. Evidence that NPY Y1 receptors are involved in stimulation of feeding by orexins (hypocretins) in sated rats. Regul. Pept. 2000;87:19–24. doi: 10.1016/s0167-0115(99)00102-0. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ. Why people smoke. BMJ. 2004;328:277–279. doi: 10.1136/bmj.328.7434.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaszberenyi M, Bujdoso E, Pataki I, Telegdy G. Effects of orexins on the hypothalamic-pituitary-adrenal system. J. Neuroendocrinol. 2000;12:1174–1178. doi: 10.1046/j.1365-2826.2000.00572.x. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA. 2000;284:2348–2351. doi: 10.1001/jama.284.18.2348. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Hollander JA, Kenny PJ. Decreased brain reward function during nicotine withdrawal in C57BL6 mice: evidence from intracranial self-stimulation (ICSS) studies. Pharmacol. Biochem. Behav. 2008;90:409–415. doi: 10.1016/j.pbb.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JA, Lineberry CG, Ascher JA, Davidson J, Khayrallah MA, Feighner JP, Stark P. A 102-center prospective study of seizure in association with bupropion. J. Clin. Psychiatry. 1991;52:450–456. [PubMed] [Google Scholar]
- Johren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P. Preproorexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology. 2001;142:3324–3331. doi: 10.1210/endo.142.8.8299. [DOI] [PubMed] [Google Scholar]
- Jonkman S, Henry B, Semenova S, Markou A. Mild anxiogenic effects of nicotine withdrawal in mice. Eur. J. Pharmacol. 2005;516:40–45. doi: 10.1016/j.ejphar.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Jonkman S, Risbrough VB, Geyer MA, Markou A. Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacology. 2008;33:2131–2138. doi: 10.1038/sj.npp.1301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hatsukami DK, Smith SS, Fiore MC, Allen S, Jensen J, Baker TB. Characterization of tobacco withdrawal symptoms: transdermal nicotine reduces hunger and weight gain. Psychopharmacology (Berl) 1996;128:130–138. doi: 10.1007/s002130050118. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N. Engl. J. Med. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Joseph S, Yule W, Williams R, Hodgkinson P. Increased substance use in survivors of the Herald of Free Enterprise disaster. Br. J. Med. Psychol. 1993;66(Pt 2):185–191. doi: 10.1111/j.2044-8341.1993.tb01740.x. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS. NPY: A novel on/off switch for control of appetite and reproduction. In: Michel MC, editor. Neuropeptide Y and related peptides. Berlin: Springer; 2004. pp. 221–249. [Google Scholar]
- Kane JK, Parker SL, Li MD. Hypothalamic orexin-A binding sites are downregulated by chronic nicotine treatment in the rat. Neurosci. Lett. 2001;298:1–4. doi: 10.1016/s0304-3940(00)01730-4. [DOI] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000;141:3623–3629. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- Karteris E, Machado RJ, Chen J, Zervou S, Hillhouse EW, Randeva HS. Food deprivation differentially modulates orexin receptor expression and signaling in rat hypothalamus and adrenal cortex. Am. J. Physiol Endocrinol. Metab. 2005;288:E1089–E1100. doi: 10.1152/ajpendo.00351.2004. [DOI] [PubMed] [Google Scholar]
- Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci. Biobehav. Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: an update. Pharmacol. Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Dove S, Javors M, Morilak DA. Behavioral reactivity to stress: amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol. Biochem. Behav. 2002;71:407–417. doi: 10.1016/s0091-3057(01)00683-9. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: characteristics of depressed smokers and effects of nicotine replacement. J. Consult Clin. Psychol. 1996;64:791–798. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Strasburger CJ. 'Normal' cigarette smoking increases free cortisol in habitual smokers. Life Sci. 1992;50:435–442. doi: 10.1016/0024-3205(92)90378-3. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, Eisen SA, True W, Tsuang M. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch. Gen. Psychiatry. 2005;62:1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drug addiction: the yin and yang of hedonic homeostasis. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Ann. N. Y. Acad. Sci. 1999;897:27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci. Biobehav. Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. Mol. Endocrinol. 1998;12:1077–1085. doi: 10.1210/mend.12.8.0145. [DOI] [PubMed] [Google Scholar]
- Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, Yamashita H. Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport. 2000;11:1977–1980. doi: 10.1097/00001756-200006260-00034. [DOI] [PubMed] [Google Scholar]
- Lader D. Smoking-related Behaviour and Attitudes, 2007. London: Office of National Statistics; 2007. [Google Scholar]
- Lambe EK, Liu RJ, Aghajanian GK. Schizophrenia, hypocretin (orexin), and the thalamocortical activating system. Schizophr. Bull. 2007;33:1284–1290. doi: 10.1093/schbul/sbm088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang WJ, Latiff AA, Mcqueen A, Singer G. Self administration of nicotine with and without a food delivery schedule. Pharmacol. Biochem. Behav. 1977;7:65–70. doi: 10.1016/0091-3057(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Langer SZ. Presynaptic regulation of the release of catecholamines. Pharmacol. Rev. 1980;32:337–362. [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC. Public Health. 2009;9:285. doi: 10.1186/1471-2458-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RM, Cruz FC, Planeta CS. Exposure to acute restraint stress reinstates nicotine-induced place preference in rats. Behav. Pharmacol. 2009;20:109–113. doi: 10.1097/FBP.0b013e3283242f41. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA. Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl) 2010;209:203–212. doi: 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman JM, Hall N, Prowse J. Effects of various catecholamine receptor antagonists, muscle relaxation and physical hindrance on shuttlebox self-stimulation. Pharmacol. Biochem. Behav. 1982;16:785–790. doi: 10.1016/0091-3057(82)90235-0. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lin Y, de Vaca SC, Carr KD, Stone EA. Role of alpha(1)-adrenoceptors of the locus coeruleus in self-stimulation of the medial forebrain bundle. Neuropsychopharmacology. 2007;32:835–841. doi: 10.1038/sj.npp.1301145. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O. Tetrabenazine (nitoman) in the treatment of psychoses. With a discussion on the central mode of action of tetrabenazine and reserpine. Acta Psychiatr. Scand. 1963;39(Suppl.):170–109. [PubMed] [Google Scholar]
- Lopez M, Senaris R, Gallego R, Garcia-Caballero T, Lago F, Seoane L, Casanueva F, Dieguez C. Orexin receptors are expressed in the adrenal medulla of the rat. Endocrinology. 1999;140:5991–5994. doi: 10.1210/endo.140.12.7287. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. U. S. A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg JM, Terenius L, Hokfelt T, Goldstein M. High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci. Lett. 1983;42:167–172. doi: 10.1016/0304-3940(83)90401-9. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Brown MC, Nerio N, Aimiuwu O, Tran B, Anghel A, Friedman TC. Repeated stress alters the ability of nicotine to activate the hypothalamic-pituitary-adrenal axis. J. Neurochem. 2006;99:1321–1327. doi: 10.1111/j.1471-4159.2006.04217.x. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Berrendero F. Endogenous cannabinoid and opioid systems and their role in nicotine addiction. Curr. Drug Targets. 2010;11:440–449. doi: 10.2174/138945010790980358. [DOI] [PubMed] [Google Scholar]
- Maley BE, Seybold VS. Distribution of [3H]quinuclidinyl benzilate, [3H]nicotine, and [125I]alpha-bungarotoxin binding sites in the nucleus tractus solitarii of the cat. J. Comp Neurol. 1993;327:194–204. doi: 10.1002/cne.903270203. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB. Rodent model of nicotine abstinence syndrome. Pharmacol. Biochem. Behav. 1992;43:779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Katz ES. Elevation of glucocorticoids is necessary but not sufficient for the escalation of cocaine self-administration by chronic electric footshock stress in rats. Neuropsychopharmacology. 2007;32:367–376. doi: 10.1038/sj.npp.1301077. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34:1743–1752. doi: 10.1038/npp.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Markou A. Metabotropic glutamate receptor antagonists: novel therapeutics for nicotine dependence and depression? Biol. Psychiatry. 2007;61:17–22. doi: 10.1016/j.biopsych.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Massachusetts Department of Public Health. Change in nicotine yields 1998–2004. 2010 [Google Scholar]
- Mathew RJ, Weinman ML, Mirabi M. Physical symptoms of depression. Br. J. Psychiatry. 1981;139:293–296. [PubMed] [Google Scholar]
- Matta SG, Beyer HS, McAllen KM, Sharp BM. Nicotine elevates rat plasma ACTH by a central mechanism. J. Pharmacol. Exp. Ther. 1987;243:217–226. [PubMed] [Google Scholar]
- Matta SG, Foster CA, Sharp BM. Nicotine stimulates the expression of cFos protein in the parvocellular paraventricular nucleus and brainstem catecholaminergic regions. Endocrinology. 1993a;132:2149–2156. doi: 10.1210/endo.132.5.8386611. [DOI] [PubMed] [Google Scholar]
- Matta SG, Foster CA, Sharp BM. Selective administration of nicotine into catecholaminergic regions of rat brainstem stimulates adrenocorticotropin secretion. Endocrinology. 1993b;133:2935–2942. doi: 10.1210/endo.133.6.8243321. [DOI] [PubMed] [Google Scholar]
- Matta SG, Fu Y, Valentine JD, Sharp BM. Response of the hypothalamo-pituitary-adrenal axis to nicotine. Psychoneuroendocrinology. 1998;23:103–113. doi: 10.1016/s0306-4530(97)00079-6. [DOI] [PubMed] [Google Scholar]
- Matta SG, Singh J, Sharp BM. Catecholamines mediate nicotine-induced adrenocorticotropin secretion via alpha-adrenergic receptors. Endocrinology. 1990;127:1646–1655. doi: 10.1210/endo-127-4-1646. [DOI] [PubMed] [Google Scholar]
- McEwen A, West R, McRobbie H. Motives for smoking and their correlates in clients attending Stop Smoking treatment services. Nicotine. Tob. Res. 2008;10:843–850. doi: 10.1080/14622200802027248. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Goletiani N, Sholar MB, Siegel AJ, Mello NK. Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology. 2008;33:749–760. doi: 10.1038/sj.npp.1301455. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J. Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology (Berl) 2007;190:171–180. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Moore RY, Card JP. Noradrenaline-containing neuron systems. In: Bjorklund A, Hokfelt T, editors. Handbook of chemical neuroanatomy. Amsterdam: Elsevier; 1984. pp. 123–156. [Google Scholar]
- Moore TJ, Furberg CD. Varenicline and suicide. Risk of psychiatric side effects with varenicline. BMJ. 2009;339:b4964. doi: 10.1136/bmj.b4964. [DOI] [PubMed] [Google Scholar]
- Morens DM, Grandinetti A, Reed D, White LR, Ross GW. Cigarette smoking and protection from Parkinson's disease: false association or etiologic clue? Neurology. 1995;45:1041–1051. doi: 10.1212/wnl.45.6.1041. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Immediate-early genes: ten years on. Trends Neurosci. 1995;18:66–67. [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Mullins D, Kirby D, Hwa J, Guzzi M, Rivier J, Parker E. Identification of potent and selective neuropeptide Y Y(1) receptor agonists with orexigenic activity in vivo. Mol. Pharmacol. 2001;60:534–540. [PubMed] [Google Scholar]
- Muzzin P, Revelli JP, Kuhne F, Gocayne JD, McCombie WR, Venter JC, Giacobino JP, Fraser CM. An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J. Biol. Chem. 1991;266:24053–24058. [PubMed] [Google Scholar]
- Myers WD, Ng KT, Singer G. Intravenous self-administration of acetaldehyde in the rat as a function of schedule, food deprivation and photoperiod. Pharmacol. Biochem. Behav. 1982;17:807–811. doi: 10.1016/0091-3057(82)90364-1. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Nandi A, Galea S, Ahern J, Vlahov D. Probable cigarette dependence, PTSD, and depression after an urban disaster: results from a population survey of New York City residents 4 months after September 11, 2001. Psychiatry. 2005;68:299–310. doi: 10.1521/psyc.2005.68.4.299. [DOI] [PubMed] [Google Scholar]
- Nelson JC. A review of the efficacy of serotonergic and noradrenergic reuptake inhibitors for treatment of major depression. Biol. Psychiatry. 1999;46:1301–1308. doi: 10.1016/s0006-3223(99)00173-0. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychol. Addict. Behav. 2001;15:13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- Nichter M, Nichter M, Vuckovic N, Quintero G, Ritenbaugh C. Smoking experimentation and initiation among adolescent girls: qualitative and quantitative findings. Tob. Control. 1997;6:285–295. doi: 10.1136/tc.6.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijsen MJ, Croiset G, Diamant M, de WD, Wiegant VM. CRH signalling in the bed nucleus of the stria terminalis is involved in stress-induced cardiac vagal activation in conscious rats. Neuropsychopharmacology. 2001;24:1–10. doi: 10.1016/S0893-133X(00)00167-6. [DOI] [PubMed] [Google Scholar]
- O'Connor EC, Parker D, Rollema H, Mead AN. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology (Berl) 2010;208:365–376. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Koob GF. 'Nicotine deprivation effect' in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol. Biochem. Behav. 2007;86:346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol. Biochem. Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Ouagazzal AM, Kenny PJ, File SE. Modulation of behaviour on trials 1 and 2 in the elevated plus-maze test of anxiety after systemic and hippocampal administration of nicotine. Psychopharmacology (Berl) 1999;144:54–60. doi: 10.1007/s002130050976. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kopin IJ, Goldstein DS. Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol. 1995;16:89–150. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- Panagis G, Hildebrand BE, Svensson TH, Nomikos GG. Selective c-fos induction and decreased dopamine release in the central nucleus of amygdala in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Synapse. 2000;35:15–25. doi: 10.1002/(SICI)1098-2396(200001)35:1<15::AID-SYN3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Individual differences in stress and arousal during cigarette smoking. Psychopharmacology (Berl) 1994;115:389–396. doi: 10.1007/BF02245082. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Stress modulation over the day in cigarette smokers. Addiction. 1995;90:233–244. doi: 10.1046/j.1360-0443.1995.9022339.x. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Does cigarette smoking cause stress? Am. Psychol. 1999;54:817–820. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- Parslow RA, Jorm AF. Tobacco use after experiencing a major natural disaster: analysis of a longitudinal study of 2063 young adults. Addiction. 2006;101:1044–1050. doi: 10.1111/j.1360-0443.2006.01481.x. [DOI] [PubMed] [Google Scholar]
- Pasumarthi RK, Reznikov LR, Fadel J. Activation of orexin neurons by acute nicotine. Eur. J. Pharmacol. 2006;535:172–176. doi: 10.1016/j.ejphar.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology. 2005;30:119–128. doi: 10.1038/sj.npp.1300524. [DOI] [PubMed] [Google Scholar]
- Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G. Depression, anxiety, and smoking initiation: a prospective study over 3 years. Am. J. Public Health. 1998;88:1518–1522. doi: 10.2105/ajph.88.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, Collins AC. An autoradiographic analysis of alterations in nicotinic cholinergic receptors following 1 week of corticosterone supplementation. Neuroendocrinology. 1993;57:262–271. doi: 10.1159/000126368. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Grun EU, Collins AC. Chronic corticosterone administration modulates nicotine sensitivity and brain nicotinic receptor binding in C3H mice. Psychopharmacology (Berl) 1990;101:310–316. doi: 10.1007/BF02244047. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Grun EU, Collins AC. Tolerance to nicotine following chronic treatment by injections: a potential role for corticosterone. Psychopharmacology (Berl) 1992;108:33–39. doi: 10.1007/BF02245282. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J. Pharmacol. Exp. Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Robinson SF, van de Kamp JL, Collins AC. Chronic nicotine and mecamylamine treatment increase brain nicotinic receptor binding without changing alpha 4 or beta 2 mRNA levels. J. Pharmacol. Exp. Ther. 1996;278:361–369. [PubMed] [Google Scholar]
- Pauly JR, Ullman EA, Collins AC. Adrenocortical hormone regulation of nicotine sensitivity in mice. Physiol Behav. 1988;44:109–116. doi: 10.1016/0031-9384(88)90353-8. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE. Increased desire to smoke during acute stress. Br. J. Addict. 1992;87:1037–1040. doi: 10.1111/j.1360-0443.1992.tb03121.x. [DOI] [PubMed] [Google Scholar]
- Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu. Rev. Pharmacol. Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le MM, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc. Natl. Acad. Sci. U. S. A. 1991;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Marinelli M, Jodogne C, Deroche V, Rouge-Pont F, Maccari S, Le MM, Simon H. Inhibition of corticosterone synthesis by Metyrapone decreases cocaine-induced locomotion and relapse of cocaine self-administration. Brain Res. 1994;658:259–264. doi: 10.1016/s0006-8993(09)90034-8. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fleming MF, Bittrich AA, Brown JL, Leitzke CJ, Zehner ME, Fiore MC, Baker TB. Psychiatric disorders in smokers seeking treatment for tobacco dependence: relations with tobacco dependence and cessation. J. Consult Clin. Psychol. 2010;78:13–23. doi: 10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–2756. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Flores A, Maldonado R, Berrendero F. Hypocretin/Orexin Signaling in the Hypothalamic Paraventricular Nucleus is Essential for the Expression of Nicotine Withdrawal. Biol. Psychiatry. 2012;71:214–223. doi: 10.1016/j.biopsych.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martin-Garcia E, de LL, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J. Neurosci. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le PM, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, La Greca AM. Childhood depressive symptoms and adolescent cigarette use: a six-year longitudinal study controlling for peer relations correlates. Health Psychol. 2009;28:283–291. doi: 10.1037/a0013949. [DOI] [PubMed] [Google Scholar]
- Prochazka AV, Petty TL, Nett L, Silvers GW, Sachs DP, Rennard SI, Daughton DM, Grimm RH, Jr, Heim C. Transdermal clonidine reduced some withdrawal symptoms but did not increase smoking cessation. Arch. Intern. Med. 1992;152:2065–2069. [PubMed] [Google Scholar]
- Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D. A randomized trial of nortriptyline for smoking cessation. Arch. Intern. Med. 1998;158:2035–2039. doi: 10.1001/archinte.158.18.2035. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Reader SC, Alaghband-Zadeh J, Daly JR, Robertson WR. Negative rate-sensitive feedback effects on adrenocorticotrophin secretion by cortisol in normal subjects. J. Endocrinol. 1982;92:443–448. doi: 10.1677/joe.0.0920443. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Carvajal C, Kask A, Dumont Y, Quirion R. Neuropeptide Y and its receptor subtypes in the central nervous system: emphasis on their role in animal models of psychiatric disorders. In: Michel MC, editor. Neuropeptide Y and related peptides. Berlin: Springer; 2004. pp. 101–136. [Google Scholar]
- Redrobe JP, Dumont Y, Fournier A, Quirion R. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacology. 2002;26:615–624. doi: 10.1016/S0893-133X(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Reijneveld SA, Crone MR, Verhulst FC, Verloove-Vanhorick SP. The effect of a severe disaster on the mental health of adolescents: a controlled study. Lancet. 2003;362:691–696. doi: 10.1016/S0140-6736(03)14231-6. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol. Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Robinson SF, Grun EU, Pauly JR, Collins AC. Changes in sensitivity to nicotine and brain nicotinic receptors following chronic nicotine and corticosterone treatments in mice. Pharmacol. Biochem. Behav. 1996;54:587–593. doi: 10.1016/0091-3057(95)02281-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol. Sci. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rose JE, Ananda S, Jarvik ME. Cigarette smoking during anxiety-provoking and monotonous tasks. Addict. Behav. 1983;8:353–359. doi: 10.1016/0306-4603(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Ruffolo RR, Jr, Hieble JP. Alpha-adrenoceptors. Pharmacol. Ther. 1994;61:1–64. doi: 10.1016/0163-7258(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Russell SH, Small CJ, Dakin CL, Abbott CR, Morgan DG, Ghatei MA, Bloom SR. The central effects of orexin-A in the hypothalamic-pituitary-adrenal axis in vivo and in vitro in male rats. J. Neuroendocrinol. 2001;13:561–566. doi: 10.1046/j.1365-2826.2001.00672.x. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav. Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Rylkova D, Boissoneault J, Isaac S, Prado M, Shah HP, Bruijnzeel AW. Effects of NPY and the specific Y1 receptor agonist [D-His(26)]-NPY on the deficit in brain reward function and somatic signs associated with nicotine withdrawal in rats. Neuropeptides. 2008;42:215–227. doi: 10.1016/j.npep.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sallee FR, Sethuraman G, Sine L, Liu H. Yohimbine challenge in children with anxiety disorders. Am. J. Psychiatry. 2000;157:1236–1242. doi: 10.1176/appi.ajp.157.8.1236. [DOI] [PubMed] [Google Scholar]
- Samson WK, Bagley SL, Ferguson AV, White MM. Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. Am. J. Physiol Regul. Integr. Comp Physiol. 2007;292:R382–R387. doi: 10.1152/ajpregu.00496.2006. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Do glucocorticoid concentrations rise with age in the rat? Neurobiol. Aging. 1992;13:171–174. doi: 10.1016/0197-4580(92)90025-s. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am. J. Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Schweimer J, Fendt M, Schnitzler HU. Effects of clonidine injections into the bed nucleus of the stria terminalis on fear and anxiety behavior in rats. Eur. J. Pharmacol. 2005;507:117–124. doi: 10.1016/j.ejphar.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. The alpha2 adrenergic receptor antagonist idazoxan, but not the serotonin-2A receptor antagonist M100907, partially attenuated reward deficits associated with nicotine, but not amphetamine, withdrawal in rats. Eur. Neuropsychopharmacol. 2010;20:731–746. doi: 10.1016/j.euroneuro.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology (Berl) 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2003;168:170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, Gnys M, Evoniuk G, DeVeaugh-Geiss J. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl) 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu. Rev. Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J, Potter EK, Biden T, Selbie LA, Herzog H. Neuropeptide Y and regulation of the cardiovascular system. J. Hypertens. Suppl. 1994;12:S41–S45. [PubMed] [Google Scholar]
- Shoaib M, Shippenberg TS. Adrenalectomy attenuates nicotine-induced dopamine release and locomotor activity in rats. Psychopharmacology (Berl) 1996;128:343–350. doi: 10.1007/s002130050143. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q. J. Stud. Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Singer G, Simpson F, Lang WJ. Schedule induced self injections of nicotine with recovered body weight. Pharmacol. Biochem. Behav. 1978;9:387–389. doi: 10.1016/0091-3057(78)90302-7. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol. Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011 doi: 10.1503/cmaj.110218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E, Shah HP, Davenport JJ, Geier JE, Yavarovich KR, Yamada H, Sabarinath SN, Derendorf H, Pauly JR, Gold MS, Bruijnzeel AW. Tobacco smoke exposure induces nicotine dependence in rats. Psychopharmacology (Berl) 2010;208:143–158. doi: 10.1007/s00213-009-1716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Amit Z, Splawinsky J. Conditioned place preference induced by intraventricular infusions of acetaldehyde. Alcohol. 1984;1:193–195. doi: 10.1016/0741-8329(84)90097-1. [DOI] [PubMed] [Google Scholar]
- Song C, Earley B, Leonard BE. The effects of central administration of neuropeptide Y on behavior, neurotransmitter, and immune functions in the olfactory bulbectomized rat model of depression. Brain Behav. Immun. 1996;10:1–16. doi: 10.1006/brbi.1996.0001. [DOI] [PubMed] [Google Scholar]
- Sonntag H, Wittchen HU, Hofler M, Kessler RC, Stein MB. Are social fears and DSM-IV social anxiety disorder associated with smoking and nicotine dependence in adolescents and young adults? Eur. Psychiatry. 2000;15:67–74. doi: 10.1016/s0924-9338(00)00209-1. [DOI] [PubMed] [Google Scholar]
- Sorensen G, Lindberg C, Wortwein G, Bolwig TG, Woldbye DP. Differential roles for neuropeptide Y Y1 and Y5 receptors in anxiety and sedation. J. Neurosci. Res. 2004;77:723–729. doi: 10.1002/jnr.20200. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, Morgan CA, III, Nicolaou AL, Nagy LM, Johnson DR, Heninger GR, Charney DS. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch. Gen. Psychiatry. 1997;54:749–758. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, Heninger GR, Charney DS. Abnormal noradrenergic function in posttraumatic stress disorder. Arch. Gen. Psychiatry. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC. Functional evidence for subsensitivity of noradrenergic alpha 2 receptors after chronic desipramine treatment. Life Sci. 1980;27:1863–1867. doi: 10.1016/0024-3205(80)90431-2. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Entsuah R, Rudolph RL. Comparative efficacy between venlafaxine and SSRIs: a pooled analysis of patients with depression. Biol. Psychiatry. 2002;52:1166–1174. doi: 10.1016/s0006-3223(02)01425-7. [DOI] [PubMed] [Google Scholar]
- Steckler T, Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol. Psychiatry. 1999;46:1480–1508. doi: 10.1016/s0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Ussher M. Smoking, cortisol and nicotine. Int. J. Psychophysiol. 2006;59:228–235. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Stogner KA, Holmes PV. Neuropeptide-Y exerts antidepressant-like effects in the forced swim test in rats. Eur. J. Pharmacol. 2000;387:R9–R10. doi: 10.1016/s0014-2999(99)00800-6. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Fink R, Jarvik ME. Acute and chronic tolerance to nicotine measured by activity in rats. Psychopharmacologia. 1973;30:329–342. doi: 10.1007/BF00429192. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Swan GE, Ward MM, Carmelli D, Jack LM. Differential rates of relapse in subgroups of male and female smokers. J. Clin. Epidemiol. 1993;46:1041–1053. doi: 10.1016/0895-4356(93)90172-w. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Perry KW, Schoepp DD. The mGlu2/3 receptor agonist, LY354740, blocks immobilization-induced increases in noradrenaline and dopamine release in the rat medial prefrontal cortex. J. Neurochem. 2004;88:194–202. doi: 10.1046/j.1471-4159.2003.02125.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Talhout R, Opperhuizen A, van Amsterdam JG. Role of acetaldehyde in tobacco smoke addiction. Eur. Neuropsychopharmacol. 2007;17:627–636. doi: 10.1016/j.euroneuro.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Heilig M. Behavioral effects of neuropeptide Y. In: Michel MC, editor. Neuropeptide Y and related peptides. Berlin: Springer; 2004. pp. 251–282. [Google Scholar]
- Thiele TE, Marsh DJ, Ste ML, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Heilig M. Diverse functions of neuropeptide Y revealed using genetically modified animals. Neuropeptides. 2002;36:182–193. doi: 10.1054/npep.2002.0897. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Repunte-Canonigo V, O'Dell LE, Chen SA, King AR, Lekic D, Koob GF, Sanna PP. Viral vector-induced amygdala NPY overexpression reverses increased alcohol intake caused by repeated deprivations in Wistar rats. Brain. 2007;130:1330–1337. doi: 10.1093/brain/awm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin-releasing factor on ethanol intake in Wistar rats: interaction with chronic ethanol exposure. Behav. Brain Res. 2005;161:133–140. doi: 10.1016/j.bbr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Totsuka Y, Ushiyama H, Ishihara J, Sinha R, Goto S, Sugimura T, Wakabayashi K. Quantification of the co-mutagenic beta-carbolines, norharman and harman, in cigarette smoke condensates and cooked foods. Cancer Lett. 1999;143:139–143. doi: 10.1016/s0304-3835(99)00143-3. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol. Rev. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- Ussher M, West R, Evans P, Steptoe A, McEwen A, Clow A, Hucklebridge F. Reduction in cortisol after smoking cessation among users of nicotine patches. Psychosom. Med. 2006;68:299–306. doi: 10.1097/01.psy.0000204926.27215.a1. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van der Velden PG, Grievink L, Olff M, Gersons BP, Kleber RJ. Smoking as a risk factor for mental health disturbances after a disaster: a prospective comparative study. J. Clin. Psychiatry. 2007;68:87–92. doi: 10.4088/jcp.v68n0112. [DOI] [PubMed] [Google Scholar]
- Van der Velden PG, Kleber RJ, Koenen KC. Smoking predicts posttraumatic stress symptoms among rescue workers: a prospective study of ambulance personnel involved in the Enschede Fireworks Disaster. Drug Alcohol Depend. 2008;94:267–271. doi: 10.1016/j.drugalcdep.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahov D, Galea S, Ahern J, Resnick H, Boscarino JA, Gold J, Bucuvalas M, Kilpatrick D. Consumption of cigarettes, alcohol, and marijuana among New York City residents six months after the September 11 terrorist attacks. Am. J. Drug Alcohol Abuse. 2004a;30:385–407. doi: 10.1081/ada-120037384. [DOI] [PubMed] [Google Scholar]
- Vlahov D, Galea S, Ahern J, Resnick H, Kilpatrick D. Sustained increased consumption of cigarettes, alcohol, and marijuana among Manhattan residents after september 11, 2001. Am. J. Public Health. 2004b;94:253–254. doi: 10.2105/ajph.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagena EJ, Knipschild P, Zeegers MP. Should nortriptyline be used as a first-line aid to help smokers quit? Results from a systematic review and meta-analysis. Addiction. 2005;100:317–326. doi: 10.1111/j.1360-0443.2005.00998.x. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Blendy JA, Kellar KJ, Heilig M, Widerlov E, Ekman R. Electroconvulsive shocks increase the concentration of neocortical and hippocampal neuropeptide Y (NPY)-like immunoreactivity in the rat. Brain Res. 1990;507:65–68. doi: 10.1016/0006-8993(90)90523-e. [DOI] [PubMed] [Google Scholar]
- Walter A, Mai JK, Jimenez-Hartel W. Mapping of neuropeptide Y-like immunoreactivity in the human forebrain. Brain Res. Bull. 1990;24:297–311. doi: 10.1016/0361-9230(90)90084-d. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of noradrenergic system within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Psychopharmacology (Berl) 2003;170:80–88. doi: 10.1007/s00213-003-1504-0. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J. Pharmacol. Exp. Ther. 2000;292:1053–1064. [PubMed] [Google Scholar]
- Weiner ED, Mallat AM, Papolos DF, Lachman HM. Acute lithium treatment enhances neuropeptide Y gene expression in rat hippocampus. Brain Res. Mol. Brain Res. 1992;12:209–214. doi: 10.1016/0169-328x(92)90086-q. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, Bailey WH. Behavioral depression produced by an uncontrollable stressor: Relationship to norepinephrine, dopamine, and serotonin levels in various regions of rat brain. 1981:167–205. [Google Scholar]
- Weiss JW, Palmer PH, Chou CP, Mouttapa M, Johnson CA. Association between psychological factors and adolescent smoking in seven cities in China. Int. J. Behav. Med. 2008;15:149–156. doi: 10.1080/10705500801929825. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Smoking, nicotine and human performance. Pharmacol. Ther. 1983;21:189–208. doi: 10.1016/0163-7258(83)90072-4. [DOI] [PubMed] [Google Scholar]
- Widdowson PS, Ordway GA, Halaris AE. Reduced neuropeptide Y concentrations in suicide brain. J. Neurochem. 1992;59:73–80. doi: 10.1111/j.1471-4159.1992.tb08877.x. [DOI] [PubMed] [Google Scholar]
- Widerlov E, Lindstrom LH, Wahlestedt C, Ekman R. Neuropeptide Y and peptide YY as possible cerebrospinal fluid markers for major depression and schizophrenia, respectively. J. Psychiatr. Res. 1988;22:69–79. doi: 10.1016/0022-3956(88)90030-1. [DOI] [PubMed] [Google Scholar]
- Wilkins JN, Carlson HE, Van Vunakis H, Hill MA, Gritz E, Jarvik ME. Nicotine from cigarette smoking increases circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers. Psychopharmacology (Berl) 1982;78:305–308. doi: 10.1007/BF00433730. [DOI] [PubMed] [Google Scholar]
- Wills TA, Sandy JM, Yaeger AM. Stress and smoking in adolescence: a test of directional hypotheses. Health Psychol. 2002;21:122–130. [PubMed] [Google Scholar]
- Winternitz WW, Quillen D. Acute hormonal response to cigarette smoking. J. Clin. Pharmacol. 1977;17:389–397. doi: 10.1002/j.1552-4604.1977.tb04621.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr. Opin. Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Woldbye DP, Klemp K, Madsen TM. Neuropeptide Y attenuates naloxone-precipitated morphine withdrawal via Y5-like receptors. J. Pharmacol. Exp. Ther. 1998;284:633–636. [PubMed] [Google Scholar]
- Woldbye DP, Ulrichsen J, Haugbol S, Bolwig TG. Ethanol withdrawal in rats is attenuated by intracerebroventricular administration of neuropeptide Y. Alcohol Alcohol. 2002;37:318–321. doi: 10.1093/alcalc/37.4.318. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic, 2009: implementing smoke-free environments. 2009
- World Health Organization. Systematic review of the link between tobacco and poverty. 2011
- Wu LT, Anthony JC. Tobacco smoking and depressed mood in late childhood and early adolescence. Am. J. Public Health. 1999;89:1837–1840. doi: 10.2105/ajph.89.12.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Bruijnzeel AW. Stimulation of alpha2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology. 2011;60:303–311. doi: 10.1016/j.neuropharm.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999;849:248–252. doi: 10.1016/s0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- Yannielli PC, Harrington ME. Neuropeptide Y in the mammalian circadian system: effects on light-induced circadian responses. Peptides. 2001;22:547–556. doi: 10.1016/s0196-9781(01)00356-4. [DOI] [PubMed] [Google Scholar]
- Yates SL, Bencherif M, Fluhler EN, Lippiello PM. Up-regulation of nicotinic acetylcholine receptors following chronic exposure of rats to mainstream cigarette smoke or alpha 4 beta 2 receptors to nicotine. Biochem. Pharmacol. 1995;50:2001–2008. doi: 10.1016/0006-2952(95)02100-0. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Wydra K, Filip M. Effects of serotonin (5-HT)(2) receptor ligands on depression-like behavior during nicotine withdrawal. Neuropharmacology. 2010;58:1140–1146. doi: 10.1016/j.neuropharm.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Zarcone V. Narcolepsy. N. Engl. J. Med. 1973;288:1156–1166. doi: 10.1056/NEJM197305312882205. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Piotrovskaya VR. Melatonin treatment attenuates symptoms of acute nicotine withdrawal in humans. Pharmacol. Biochem. Behav. 2000;67:131–135. doi: 10.1016/s0091-3057(00)00302-6. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;58:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski MA, Slominski AT. Emerging role of alternative splicing of CRF1 receptor in CRF signaling. Acta Biochim. Pol. 2010;57:1–13. [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J. Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Gibson LE, Vujanovic AA, Gregor K, Bernstein A, Kahler C, Legues CW, Brown RA, Feldner MT. Impact of Posttraumatic Stress Disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine. Tob. Res. 2008;10:1415–1427. doi: 10.1080/14622200802238951. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, Steeves D. Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine. Tob. Res. 2009;11:323–331. doi: 10.1093/ntr/ntn037. [DOI] [PMC free article] [PubMed] [Google Scholar]