Abstract
Exposure to traumatic stress is associated with increased risk for posttraumatic stress disorder (PTSD) and alterations of hypothalamic-pituitary-adrenocortical (HPA) function. Research linking traumatic stress with HPA function in PTSD has been inconsistent, however, in part due to (a) the inclusion of trauma-exposed individuals without PTSD (TE) in control groups and (b) a failure to consider comorbid major depressive disorder (MDD) and moderating variables. This meta-analysis of 47 studies (123 effect sizes, N=6,008 individuals) revealed that daily cortisol output was lower for PTSD (d=−.36, SE=.15, p=.008) and PTSD+MDD (d=−.65, SE=.25, p=.008) groups relative to no trauma controls (NTC); TE and NTC groups did not differ significantly from each other. Afternoon/evening cortisol was lower in TE (d=−.25, SE=.09, p=.007) and PTSD (d=−.27, SE=.12, p=.021) groups and higher in PTSD+MDD groups (d=.49, SE=.24, p=.041) relative to NTC. Post-DST cortisol levels were lower in PTSD (d=−.40, SE=.12, p<.001), PTSD+MDD (d=−.65, SE=.14, p<.001), and TE groups (d=−.53, SE=.14, p<.001) relative to NTC. HPA effect sizes were moderated by age, sex, time since index event, and developmental timing of trauma exposure. These findings suggest that enhanced HPA feedback function may be a marker of trauma-exposure rather than a specific mechanism of vulnerability for PTSD, whereas lower daily cortisol output may be associated with PTSD in particular.
Keywords: PTSD, Trauma, Comorbidity, Depression, Cortisol
Traumatic stress is characterized by the direct experience or witnessing of actual or threatened death or serious injury, or a threat to physical integrity, and responses that include intense fear, helplessness, or horror (American Psychiatric Association, 2000). Epidemiological studies indicate that 82% of individuals in the U.S. have experienced at least one traumatic event in their lifetime (Sledjeski, Speisman, & Dierker, 2008). Exposure to trauma dramatically increases vulnerability to a variety of psychiatric disorders, most commonly Posttraumatic Stress Disorder (PTSD) and Major Depressive Disorder (MDD). Estimates of the conditional risk for developing these disorders in the context of trauma vary widely and underscore their complex etiologies.
Approximately 8 to 18% of trauma-exposed individuals develop PTSD (e.g., Breslau et al., 1998; Kessler, Sonnega, Hughes, & Nelson, 1995; Sledjeski et al., 2008) and 7 to 19% develop MDD (e.g., MacMillan et al., 2001; Shalev et al., 1998). Moreover, rates of comorbid MDD among individuals meeting diagnostic criteria for PTSD have been reported to be as high as 37% (e.g., Breslau, Davis, Andreski, & Peterson, 1991). Examining differences in the way individuals with PTSD with or without MDD respond to stress and comparing them to individuals exposed to trauma (TE1) who do not develop PTSD (with or without MDD) may help identify possible mechanisms underlying vulnerability to these disorders. In addition, contrasting individuals with PTSD to those with PTSD+MDD may further our understanding of such comorbidity in relation to the stress response. Therefore, the goal of the current meta-analysis was to examine alterations of the stress response system in individuals diagnosed with PTSD or PTSD+MDD as compared to trauma-exposed (TE) individuals without PTSD or MDD, and never-traumatized controls (NTC).
Stress Response System
The hypothalamic-pituitary-adrenocortical (HPA; Stratakis & Chrousos, 1995) axis is one of three major systems activated as part of the stress response (Teicher, Andersen, Polcari, Anderson, & Navalta, 2002) and is a potential source of vulnerability to trauma-related psychopathology (e.g., Yehuda, 2002). The stress response system promotes adaptation, or allostasis, by allowing organisms to accommodate to changing conditions in their environment (McEwen & Seeman, 1999). Stress exposure triggers emotional responses, including activation in the limbic system that initiates HPA activity via connections with the hypothalamus. Neurons in the paraventricular nucleus of the hypothalamus then secrete corticotropin releasing hormone (CRH), which travels through the hypophyseal portal circulation and stimulates the anterior pituitary to release adrenocorticotropin hormone (ACTH). The ACTH signal, in turn, is carried through the peripheral circulation to the adrenal cortex where it triggers the production and release of cortisol, a glucocorticoid hormone responsible for a variety of regulatory functions in the central nervous system, metabolic system, and immune system (Sapolsky, Romero, & Munck, 2000). This entire process is referred to here as HPA feed-forward function. Elevated cortisol levels typically inhibit the HPA via negative feedback mechanisms in the pituitary, hypothalamus, and hippocampus (Jacobson & Sapolsky, 1991; Munck, Guyre, & Holbrook, 1984; Sapolsky, Krey, & McEwen, 1986). This latter process reflects HPA feedback function. Thus, the HPA axis plays an important role in both the stress response and the maintenance of homeostasis (Sapolsky, 1992).
Basal cortisol activity throughout the day represents an aggregation of circadian oscillations and superimposed activation related to stressful challenges. Basal cortisol levels can be measured from samples of saliva, blood, urine, or cerebrospinal fluid. Each of these measures of cortisol output offers unique temporal foci on diurnal HPA functioning, with saliva and blood samples reflecting activity within a 10-60 minute window, and urine samples reflecting activity over a 15-24 hour period (Baum & Grunberg, 1995). Diurnal rhythm of cortisol levels vary, typically increasing in the early morning, peaking approximately 15-30 minutes after awakening (Schmidt-Reinwald et al., 1999), diminishing over the course of the day, and reaching a nadir at the end of the activity phase (Bailey & Heitkemper, 1991). Thus, the measurement of cortisol may vary depending on the method used and time period assessed. Blood and saliva samples are appropriate for measuring cortisol activity when timing is important (e.g., morning/afternoon cortisol). In contrast, both urinary cortisol and an average of multiple saliva/blood samples over the day are appropriate for assessing daily output.
Although elevated cortisol levels are adaptive in the short-run, prolonged activation of the HPA-axis can have adverse effects (McEwen, 2003). This cumulative physiological wear and tear, termed allostatic load, can be caused by frequent stress, and the failure to (a) habituate to recurring stress, (b) inhibit allostatic processes following termination of stress, or (c) mount an adaptive response in some systems that can lead to the hyperactivation of others (McEwen, 1998). Cortisol is an important mediator of allostasis that contributes to allostatic load when it is not efficiently regulated.
One method of assessing cortisol regulation is to examine HPA basal activity and feedback function. These features indicate how effectively cortisol secretion is inhibited as well as patterns of output over the course of the day. Previous meta-analyses have examined basal cortisol levels (Klaassens, Giltay, Cuijpers, van Veen, & Zitman, in press; Meewisse, Reitsma, De Vries, Gersons, & Olff, 2007) and HPA feedback function (Klaassens et al., in press; Miller, Chen, & Zhou, 2007) in adults with and without PTSD. The present meta-analysis expands upon these reviews by addressing three important gaps in the literature. First we assessed features of HPA feedback function separately for PTSD and PTSD+MDD groups. Whereas prior reviews suggest that PTSD and MDD are each associated with very different patterns of cortisol activity (e.g., Kendall-Tackett, 2000), cortisol levels among individuals with PTSD and comorbid MDD have not been systematically evaluated with regard to HPA feedback function. Given high rates of comorbid MDD among individuals with PTSD (Breslau et al., 1991), this represents a critical gap in the literature. Second, we examined the impact of time of measurement on HPA basal activity effect sizes in addition to overall daily cortisol output. This is the first meta-analysis to address this question among TE and PTSD+MDD groups. Third, we examined the role of potential moderators of HPA basal activity and feedback function effect sizes, including age, sex, time since onset of focal trauma, and developmental timing of trauma exposure. This is the first meta-analytic review to evaluate the role of time since onset and developmental timing in TE and PTSD+MDD groups.
Whereas PTSD has been associated with lower basal cortisol activity, enhanced HPA feedback function, and a progressive sensitization of the HPA-axis, MDD has been associated with elevated basal cortisol activity, impaired HPA feedback function, and a progressive desensitization of the HPA-axis (Kendall-Tackett, 2000). Although considerable attention has focused on differences in HPA function between individuals with PTSD and MDD, less is known about the stress response in those with comorbid PTSD and MDD. The current meta-analytic review addressed three fundamental questions regarding HPA function in trauma-exposed individuals: (a) Do individuals with TE, PTSD, or PTSD+MDD differ from never-traumatized controls (NTC) with regard to basal cortisol activity and feedback functioning? (b) Do individuals with TE, PTSD, or PTSD+MDD differ from each other on these features of HPA function? (c) Are the relations between trauma-exposure and HPA function moderated by person (i.e., age, sex) and trauma (i.e., time since onset, developmental timing) features? Another relevant comparison group would be trauma-exposed individuals with MDD only (TE+MDD), but a paucity of studies examining HPA activity in this group precluded including them in the current analyses. Because of the conceptual importance of this group, however, we discuss available findings from studies of individuals with TE+MDD regarding HPA basal activity and feedback functioning.
Basal HPA activity
Morning cortisol levels
Patterns of HPA activity over the course of the day are influenced by internal factors, such as the suprachiasmatic nucleus in the hypothalamus that regulates circadian rhythms and the timing of the sleep-wake cycle (Weitzman, Czeisler, Zimmerman, & Moore-Ede, 1981), as well as cues from the environment. For example, the magnitude of the cortisol awakening response may be influenced by the experiences of the prior day (Adam, Hawkley, Kudielka, & Cacioppo, 2006). In addition, psychopathology may moderate the relations between external factors and basal HPA activity. For instance, social interactions are associated with steeper rates of decline in cortisol levels over the day in healthy controls but not in individuals with MDD (Stetler, Dickerson, & Miller, 2004). Indeed, a meta-analysis of depression and cortisol responses to stress revealed that depressed individuals (not necessarily in the context of trauma) had lower basal cortisol levels in the morning as compared to healthy controls (Burke, Davis, Otte, & Mohr, 2005). Studies examining basal cortisol activity in trauma-exposed individuals suggest that morning cortisol levels may be lower in TE individuals relative to NTC (Gunnar & Vazquez, 2001), whereas morning cortisol levels in TE+MDD (Juruena et al., 2006), PTSD, and PTSD+MDD groups (Meewisse et al., 2007; Miller et al., 2007) may not differ significantly from NTC.
Afternoon cortisol levels
Afternoon cortisol levels may be affected by exposure to stressful interpersonal interactions characterized by social-evaluative threat during the day. In particular, individuals whose social status is threatened may exhibit elevated afternoon cortisol levels, with shame possibly mediating this relation (Dickerson & Kemeny, 2004). Social withdrawal in response to feelings of loss elicits a flat diurnal profile that may reflect dysregulated circadian rhythms (Stetler, Dickerson, & Miller, 2004; Stetler & Miller, 2005). Elevated afternoon cortisol levels have been found in individuals with MDD both with (Juruena et al., 2006) or without trauma (Burke et al., 2005) relative to healthy controls. Previous studies have found similar afternoon cortisol levels in TE compared to NTC individuals (Gunnar & Vazquez, 2001), lower afternoon cortisol levels in PTSD individuals relative to NTC (e.g., Meewisse et al., 2007), and higher afternoon cortisol levels in PTSD+MDD individuals relative to NTC (Young & Breslau, 2004).
Daily cortisol output
Studies of daily cortisol output indicate chronic hypersecretion in individuals with MDD (trauma-exposure not indicated) (Plotsky, Owens, & Nemeroff, 1995) and increased daily output of cortisol in individuals with TE+MDD (Juruena et al., 2006; Kosten, Wahby, Giller, & Mason, 1990). A recent meta-analysis focusing on trauma exposure in adults found no differences in basal cortisol levels between TE and NTC groups or between TE and PTSD groups; however, this review did not examine the possible influence of the timing of measurement (Klaassens et al., in press). We predicted lower daily cortisol output in individuals with TE or PTSD relative to NTC (Gunnar & Vazquez, 2001; Miller et al., 2007). Due to inconsistencies in the literature, however, we were not able to make predictions regarding daily cortisol output for PTSD+MDD compared to NTC groups.
HPA Feedback Function
HPA feedback activity is measured by the rate at which cortisol levels decline following offset of a stressor and is influenced by a variety of factors, including functioning of the feedback arm of the HPA-axis and cognitive and affective processes (McEwen, 1998). The dexamethasone suppression test (DST) measures the extent to which administration of dexamethasone (DEX), a synthetic glucocorticoid, suppresses production of cortisol in the HPA axis. Post-DST cortisol levels are presumed to reflect the strength of negative feedback inhibition, with lower levels indicating stronger suppression.
Studies examining cortisol suppression in patients with MDD typically have administered either 0.5 or 1.0 mg DEX, and have found abnormal feedback inhibition in the form of nonsuppression (also termed “early escape”) from the DST in approximately 40-60% of depressed patients (Carroll & Curtis, 1976; Carroll et al., 1980; see Ribeiro, Tandon, Grunhaus, & Greden, 1993 for a review). In these studies, nonsuppression was defined as a failure to suppress cortisol levels below 5.0μg/100 dL in response to 1.0mg DEX. Evidence concerning the efficiency of negative feedback mechanisms in TE+MDD groups is scant, but conforms to prior research on depression. Higher post-DST cortisol levels in individuals with TE+MDD have been reported in two studies (Juruena et al., 2006; Kosten et al., 1990).
Overall, evidence indicates that TE+MDD is associated with impaired negative feedback. Based on a recent meta-analysis (Klaassens et al., in press), we hypothesized that the TE group would show enhanced HPA negative feedback as reflected in lower post-DST cortisol levels relative to NTC. Results of DST studies administering 0.25 or 0.5 mg DEX in individuals with PTSD have shown lower post-DST cortisol levels than NTC (e.g., Griffin et al., 2005; Stein, Yehuda, Koverola, & Hanna, 1997; Yehuda et al., 1993; Yehuda et al., 2002), suggesting enhanced negative feedback or “super suppression.” A similar pattern appears to hold for individuals with PTSD+MDD (e.g., de Kloet et al., 2007; Yehuda et al., 1993). Therefore, we expected to find enhanced HPA negative feedback in both PTSD and PTSD+MDD groups.
Moderators of HPA Activity Following Trauma Exposure
Age
Patterns of basal HPA activity and feedback function change across development. A positive correlation has been found between age and diurnal cortisol secretion, with levels rising gradually during middle childhood and then more rapidly in adolescence (Walker, Walder, & Reynolds, 2001). Studies of HPA function in older adults reveal elevated basal levels (e.g., Deuschle et al., 1997). Studies of both psychological and biological challenge tests have found impaired HPA feedback function in older versus younger participants (Otte, Hart, Neylan, Marmar, Yaffe, & Mohr, 2005). Seeman and Robbins (1994, p. 233) observed that “age-related changes appear primarily in the re-setting of the HPA axis following a challenge,” and speculated that such a pattern could result from cumulative exposure to glucocorticoids. The current meta-analysis extends findings from previous reviews (Klaassens et al., in press) by examining age as a predictor of HPA basal activity and feedback functioning effect sizes in PTSD+MDD groups versus NTC.
Sex
Lifetime prevalence rates of both MDD and PTSD are twice as high in females as males (e.g., Breslau et al., 1998; Kessler et al., 1995). Although men are at greater risk for exposure to potentially traumatic events (Olff, Langeland, Draijer, & Gersons, 2007), the conditional risk of developing PTSD after trauma exposure is twice as high in women (e.g., Giaconia, Reinherz, Silverman, Pakiz, Frost, & Cohen, 1995). Among healthy controls, diurnal cortisol levels appear to be lower in women than in men (Van Cauter, Leproult, & Kupfer, 1996). Studies using biological challenge tests have found either no significant sex differences or decreased feedback sensitivity in females (e.g., Heuser et al., 1994; Otte et al., 2005). Regarding diurnal plasma cortisol levels, women with PTSD had significantly lower levels than healthy controls, whereas no significant differences have been found between men with and without PTSD (Meewisse et al., 2007). The current meta-analysis is the first quantitative review of sex differences in HPA basal activity and feedback function effect sizes in PTSD+MDD groups relative to NTC.
Time since onset of focal trauma
An inverse relation has been found between HPA activity and months since onset of chronic stress, such that morning cortisol levels, daily cortisol volume, and post-DEX cortisol levels decrease over time (Miller et al., 2007). A similar pattern appears to hold for traumatic stress, with studies showing decreases in cortisol levels following a focal event (Rasmusson et al., 2001; Yehuda, Halligan, & Grossman, 2001; although see Meewisse et al., 2007). Yehuda and colleagues (2004a) found a positive association between amount of cortisol suppression following low dose DST and the number of years since the most recent traumatic event.
Stressful life experiences may trigger initial increases in cortisol levels that precipitate a counter-regulatory response. Over time, however, these levels may diminish and eventually rebound to below normal (e.g., Hellhammer & Wade, 1993; Miller et al., 2007). This potentially adaptive regulatory response may involve a progressive strengthening of negative feedback inhibition (Yehuda, 2002). Traumatic life events - although initially associated with elevations in cortisol and catecholamines - may trigger a divergence in these measures over time culminating in the pattern of low cortisol and high noradrenaline levels typical of adults with chronic PTSD (Pervanidiou, 2008). The current meta-analytic review builds on prior work examining PTSD groups (Meewisse et al., 2007; Miller et al., 2007) by examining time elapsed since focal trauma as a potential predictor of effect sizes representing differences in HPA basal activity and feedback functioning between TE and PTSD+MDD groups relative to NTC.
Developmental timing of trauma exposure
The impact of a traumatic event on HPA-axis functioning may depend on the developmental epoch in which it occurs, with childhood being an important window of vulnerability to the effects of stress. Early exposure to trauma is associated with elevated risk for MDD (Kaufman & Charney, 2001; Pervanidou, 2008) and appears to impact diurnal cortisol rhythm (e.g., Carpenter et al., 2007; Heim et al., 2000). For example, young children who have experienced neglect have been found to have blunted early morning peak cortisol levels and an absence of typical decline over the course of the day (e.g., Carlson & Earls, 1997; Fisher, Gunnar, Chamerblain, & Reid, 2000). The current meta-analysis complements the recent meta-analytic review of trauma exposure during adulthood (Klaassens et al., in press) by examining developmental timing as a predictor of HPA basal activity and feedback function effect sizes.
Summary
The primary goals of the present review were to examine cortisol activity among trauma-exposed individuals without either PTSD or MDD (TE), and among individuals with PTSD and PTSD with comorbid MDD, in order to identify features of HPA function that are uniquely associated with PTSD. Using meta-analytic techniques, we estimated the magnitude of cortisol levels under basal and biological challenge conditions in individuals with TE, PTSD, and PTSD+MDD as compared to never-traumatized controls (NTC). Because too few studies were available for persons with TE+MDD, this group could not be included in the meta-analysis.
Prior qualitative reviews exist regarding HPA function in PTSD (e.g., de Kloet et al., 2006; Yehuda, 2002, 2006) and MDD (e.g., Heim et al., 2008) across multiple outcomes, diverse methodologies, and with sensitivity to comorbidity. Meta-analyses also have been conducted examining basal HPA activity and feedback functioning in depressed children and adolescents (Lopez-Duran, Kovacs, & George, 2009) and in individuals with PTSD and MDD (Klaassens et al., in press; Miller et al., 2007), and diurnal cortisol parameters in persons with PTSD and PTSD+MDD (Klaassens et al., in press; Meewisse et al., 2007). Building on this prior research, the current meta-analysis reviewed studies examining HPA basal activity and feedback functioning in trauma exposed individuals, distinguishing those with PTSD versus PTSD+MDD, and identifying factors that may have contributed to various inconsistencies in this literature.
We examined cortisol outcome variables (i.e., morning levels, afternoon/evening levels, daily output, post-DST levels) in separate analyses and identified the relations of TE, PTSD, and PTSD+MDD to these outcomes, which allowed us to balance the need for studies differing enough for comparisons to be fruitful yet similar enough to render comparisons meaningful. These meta-analytic models addressed three critical questions: (1) What alterations of cortisol secretion are associated with trauma exposure in general (i.e., common to both TE and PTSD groups) and which are specific to PTSD? (2) How does HPA feedback function in individuals with PTSD and those with PTSD and comorbid MDD? and (3) To what extent do HPA feedback function effect sizes in TE, PTSD, and PTSD+MDD individuals relative to NTC vary by age, sex, time since trauma, and developmental timing of trauma exposure?
Method
Selection of Studies
Articles for this meta-analysis were identified through searches of PsycINFO, Web of Knowledge, and PubMed databases and included all studies published through December 2011. Initial searches crossed keywords reflecting traumatic stress (abuse, accidents, assault, combat, loss, maltreatment, neglect, rape, refugees, terrorism, torture, trauma, veteran, and war) and trauma-related psychopathology (major depressive disorder, MDD, posttraumatic stress disorder, PTSD) with those reflecting tests and indicators of HPA function (adrenocortical, cortisol, dexamethasone, glucocorticoid, HPA). In addition, we searched reference sections of qualifying articles as well as recent reviews (de Kloet et al., 2006; Delahanty & Nugent, 2006; Heim et al., 2008; Meewisse et al., 2007; Pervanidou, 2008; Tarullo & Gunnar, 2006; Yehuda, 2002, 2006; Yehuda & LeDoux, 2007) for any studies not detected in our initial search.
To be included in this meta-analysis, a study had to (a) enroll participants exposed to traumatic events and diagnosed with current PTSD or PTSD+MDD, as defined in the Diagnostic and Statistical Manual (DSM-III, DSM-III-R, or DSM-IV; American Psychiatric Association, 1980, 1987, 1994) or trauma-exposed participants without current PTSD or MDD, (b) include a never-traumatized control (NTC) group, (c) measure basal cortisol levels or post-DST cortisol levels, (d) include sufficient data to compute effect sizes, and (e) be published in an English-language scientific journal or abstracted in Dissertation Abstracts International. Traumatic events were defined as circumstances in which an individual experienced or witnessed “events that involved actual or threatened death or serious injury, or a threat to the physical integrity of self or others” (American Psychiatric Association, 2000). Psychiatric groups consisted of individuals meeting DSM-IV (American Psychiatric Association, 2000) criteria for current PTSD or PTSD+MDD; those who only met criteria for lifetime, 12-month, or subthreshold diagnoses were excluded from analyses. In addition to including studies of basal HPA activity, the current meta-analysis reviewed studies employing the DST. When the results of a single study were reported in multiple articles, only effect sizes from the publication with the most participants were included.
Studies that included trauma-exposed participants with current MDD were excluded unless only a small minority (<10%) of TE participants met criteria for current MDD (Yehuda et al., 2005); for studies in which current MDD was not assessed, TE groups were included if they represented a sufficiently large sample size (n >70) for expected rates of MDD to exert minimal influence on cortisol outcomes (Boscarino, 1996; Pervanidou et al., 2007). One study included TE participants with current dysthymia (De Bellis et al., 1994); removing this study did not significantly alter results. Only a subset of articles assessed rates of lifetime psychopathology other than PTSD or MDD in NTC (n = 32; see Data Supplement 2), or TE (n = 12; Bremner et al., 2007; Carpenter et al., 2007; de Kloet et al., 2007; Golier et al., 2006, 2007; Newport et al., 2004; Pervanidou et al., 2007; Pfeffer et al., 2007; Yehuda et al., 2002; Yehuda, Golier, et al., 2004; Yehuda, Halligan, et al., 2004; Yehuda, Kahana, et al., 1995). Of these studies, four reported lifetime psychopathology in NTC groups (Carpenter et al., 2007; Pfeffer et al., 2007; Rasmusson et al., 2001; Yehuda, Golier, et al., 2004) and six reported lifetime psychopathology in TE groups (Bremner et al., 2007; Carpenter et al., 2007; de Kloet et al., 2007; Newport et al., 2004; Yehuda et al., 2002; Yehuda, Kahana, et al., 1995). We did not exclude these studies with NTC or TE groups reporting lifetime psychopathology from the meta-analytic models due to their small number.
The initial search yielded 249 articles that assessed participants for posttraumatic stress symptoms and measured cortisol levels (for details, see Data Supplement 1). Of these articles, 79 were excluded because they did not include a never-traumatized control group; another 63 studies were excluded because they assessed PTSD or MDD symptoms, but not disorders; 29 studies employed psychosocial or neuroendocrine challenges not examined in this meta-analysis; 24 did not examine PTSD and PTSD+MDD separately; 6 were excluded because they only assessed lifetime PTSD or combined participants with lifetime and current PTSD; 6 failed to report necessary statistics to compute effect sizes and requested information could not be obtained from corresponding authors; 3 reported data from larger studies already included in the meta-analysis; and 1 study combined participants with Depressive Disorder NOS and MDD.
Coded Variables
All studies meeting inclusion criteria were coded (by MCM) for methodological, trauma, and participant features. Independent coding of a randomly selected 20% of the studies was done by a second trained coder. Reliabilities were calculated using intraclass correlations (r1) for continuous variables and kappa for categorical variables (Orwin, 1994). Disparities were resolved by consensus.
Time of day (κ = 1.00 for morning studies, κ = .79 for afternoon studies)
Due to the strong influence of circadian rhythm on levels of cortisol, our analytic strategy took into account the time of day when cortisol samples were collected. Studies in which measurements of hormone levels were taken before 12 p.m. were coded as morning (a.m.) studies and those in which measurements were taken after 12 p.m. were coded as afternoon/evening (p.m.) studies. To examine diurnal cortisol activity, we ran separate analyses on morning and afternoon/evening studies. Only studies in which the time of day was the same for all participants were included in these analyses.
Participant Features
For each study, we coded the number of participants who were (a) NTC (inter-rater reliability: r1 = 1.00), (b) TE (r1 = 1.00), (c) PTSD (r1 = 1.00), and (d) PTSD+MDD (r1=1.00), the mean age of the participants (r1=1.00), and the sex composition of participants in the study (coded as percent male; r1=1.00).
Time since focal trauma (r1=.98)
To estimate the impact of time passed since trauma exposure, we coded the mean number of days since onset of the focal trauma. This was determined by (a) the mean time since onset reported by the article in question or by an article from the same study, (b) subtracting mean age at onset from the mean age of participants, (c) requesting mean time since onset from authors, (d) estimating mean time since onset from historical knowledge (e.g., in the case of participants exposed to combat in Vietnam we estimated time since onset as days between the date of article publication and the time when U.S. involvement in the war was at its peak – 1968 – consistent with Miller et al., 2007; for participants exposed to combat in the Croatian War of Independence, we estimated time since onset using 1991 - the height of the conflict and ethnic cleansing), (e) estimating mean time since onset from knowledge of developmental timing (e.g., in the case of participants exposed to childhood abuse where mean age at onset was not reported we subtracted 12 years from the mean age of participants), (f) estimating mean time since onset for veterans based on the mean year of their deployment, (g) estimating mean time since onset for the modal trauma when multiple focal traumas were reported, or (h) estimating mean time since onset from the midpoint when a range was reported or from the median duration. All values were log-10 transformed prior to use in meta-analytic models due to their nonnormal distributions.
Developmental timing of trauma exposure
For each study we coded whether participants experienced their focal traumatic event in childhood (r1=1.00), adulthood (r1=1.00), or if this information was not reported. Child onset was coded if more than 80% of participants experienced a traumatic event before age 18; adult onset was coded when more than 80% of participants experienced their focal traumatic event at age 18 or older.
Data Analytic Plan
Study-level effect sizes
We computed effect sizes (Cohen's d) for individual studies by subtracting the mean cortisol level of the NTC group from the psychopathology groups (i.e., PTSD or PTSD+MDD) or non-psychopathology group (i.e., TE) mean and dividing by the pooled standard deviation (Rosenthal, 1994), when these statistics were reported. Because studies with larger sample sizes provide more accurate estimates of true population parameters (Shadish & Haddock, 1994), each effect size was weighted by the inverse of its variance (Hedges' g) to adjust for possible sample size bias (Hedges & Olkin, 1985). When descriptive statistics were not reported and authors did not provide requested information, we computed d from inferential statistics (Hedges & Olkin, 1985; Rosenthal, 1994). Cohen's d may be conceptualized here as the magnitude of the difference between cortisol levels in psychiatric or TE groups versus NTC in standard deviation units. The use of d allows pooling of effect sizes across studies with variability in measurement (i.e., plasma/serum, saliva, urine) and methodological (e.g., sampling) characteristics. Effect sizes of 0.20 are considered small, 0.50 moderate, and 0.80 large (Cohen & Cohen, 1983).
Aggregate effect sizes
Similar to the approach used by Miller and colleagues (2007), we ran separate fixed-effects models for each dependent variable (HPA outcomes: morning levels, afternoon/evening levels, daily output, post-DST levels) stratified by subgroup (i.e., TE, PTSD, PTSD+MDD) to evaluate whether the aggregate effect size differed significantly from zero (Lipsey & Wilson, 2001). Fixed-effects procedures were used to test hypotheses regarding HPA function in a particular set of observed studies – those sampling trauma-exposed individuals with and without PTSD and/or MDD – rather than to generalize more broadly. The parameters examined in the current meta-analysis were not assumed to be randomly sampled from the population due to the relatively strict inclusion/exclusion criteria used here and the likely omission of relevant studies that differed systematically from those selected. In such cases, fixed effects models are appropriate (Hedges & Vevea, 1998) and generally confer increased statistical power (Cohn & Becker, 2003). Results should be interpreted with caution, however, and should be replicated in independent samples.
For each model, a heterogeneity coefficient (Cochran's Q) was calculated to determine whether significant between-study variability existed, which then might be explained by other factors (i.e., moderators). Coefficient Q is a chi-square distributed with k – 1 degrees of freedom, where k is the number of studies. Summary estimates for TE, PTSD, and PTSD+MDD groups were based on fixed-effects models using inverse variance weighting (see Table 1 and Figures 1 and 2). Differences in effect sizes between TE, PTSD, and PTSD+MDD groups were examined by group analysis with tests of interactions (Altman & Bland, 2003; Matthews & Altman, 1996).
Table 1. Summary of Meta-Analytic Findings across Studies and Cortisol Outcomes for TE, PTSD, and PTSD+MDD Groups versus NTC.
| Cortisol Outcomes | d | k | SE | 95% CI | p | Q | p | I2 |
|---|---|---|---|---|---|---|---|---|
| Morning (a.m.) | ||||||||
| TE | .01 | 18 | .03 | −.05, +.07 | .780 | 35.98 | .005 | 53 |
| PTSD | −.28 | 23 | .08 | −.43, −.13 | < .001 | 58. 96 | < .001 | 6 3 |
| PTSD+MDD | −.66 | 8 | .14 | −.94, −.39 | < .001 | 42.18 | < .001 | 83 |
| Afternoon/Evening (p.m.) | ||||||||
| TE | −.25 | 10 | .09 | −.44, −.07 | .007 | 31.36 | <.001 | 71 |
| PTSD | −.27 | 12 | .12 | −.50, −.04 | .021 | 110.00 | < .001 | 90 |
| PTSD+MDD | .49 | 4 | .24 | +.02, +.96 | .041 | 15.93 | < .001 | 81 |
| Daily output | ||||||||
| TE | −.13 | 6 | .15 | −.42, +.15 | .370 | 22.08 | <.001 | 73 |
| PTSD | −.36 | 9 | .13 | −.62, −.10 | .008 | 84.68 | < .001 | 91 |
| PTSD+MDD | −.65 | 4 | .25 | −1.13, −.17 | .008 | 23.39 | < .001 | 87 |
| Post-DST | ||||||||
| TE | −.53 | 8 | .14 | −.80, −.25 | <.001 | 11.49 | .119 | 39 |
| PTSD | −.40 | 13 | .12 | −.63, −.17 | <.001 | 89.75 | < .001 | 87 |
| PTSD+MDD | −.65 | 8 | .14 | −.93, −.38 | < .001 | 39.80 | < .001 | 82 |
Notes. PTSD = Post traumatic Stress Disorder; MDD = Major Depressive Disorder; TE = trauma-exposed without PTSD or MDD; NTC = never-traumatized controls; a.m. = morning (before 12 p.m.); p.m. = afternoon (after 12 p.m.); DST = dexamethasone suppression test; d = effect size; k = number of studies; SE = standard error; CI = confidence interval; Q = heterogeneity coefficient; I2 = percentage of total variation across studies due to heterogeneity
Figure 1.
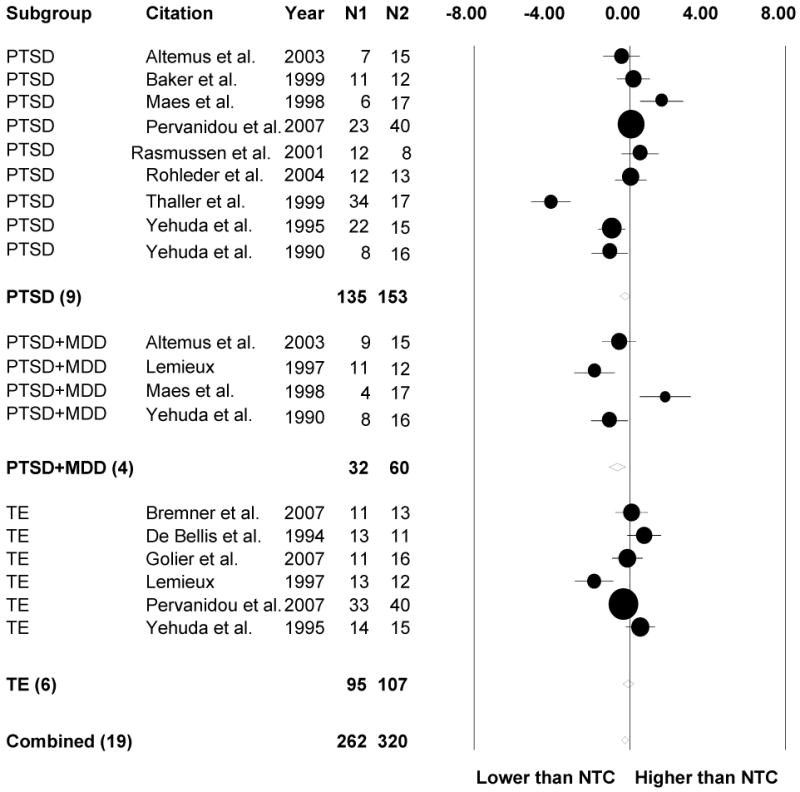
Standardized mean differences (with 95% CI) of daily cortisol output levels between psychiatric subgroups and no trauma controls (NTC). TE = trauma-exposed without PTSD or MDD; PTSD = Posttraumatic Stress Disorder; PTSD+MDD = PTSD and comorbid Major Depressive Disorder
Figure 2.
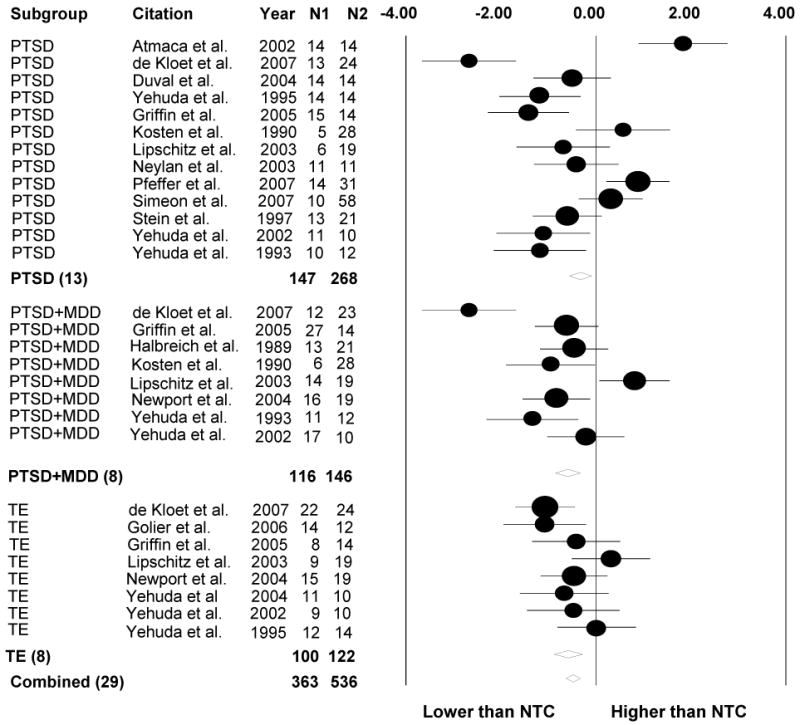
Standardized mean differences (with 95% CI) of post-DST cortisol levels between psychiatric subgroups and no trauma controls (NTC). TE = trauma-exposed without PTSD or MDD; PTSD = Posttraumatic Stress Disorder; PTSD+MDD = PTSD and comorbid Major Depressive Disorder; DST = Dexamethasone Suppression Test
Because the Q test is underpowered for small samples and overpowered for large samples, we also evaluated inconsistencies in results across studies by calculating the I2 statistic, which describes the percentage of total variation across studies due to heterogeneity rather than chance (Higgins, Thompson, Deeks, & Altman, 2003). We assessed the role of potential predictors using fixed-effects meta-regression for continuous variables (i.e., mean age, percentage male, time since onset of first/focal trauma) and fixed-effects meta-ANOVA for dichotomous variables (i.e., developmental timing of trauma exposure) even when significant heterogeneity was not detected, because heterogeneity estimates have limitations (Ioannidis, 2008) and moderators have been shown to exert significant influences on outcomes even under conditions of minimal between-study variability (Hall & Rosenthal, 1995). Forest plots were generated using meta-analysis software (Borenstein, Hedges, Higgins, & Rothstein, 2005) for daily cortisol output (Figure 1) and post-DST cortisol levels (Figure 2) showing study-level effect sizes and precision (circles) in addition to aggregate effect sizes (diamonds).
Dependent data
To ensure that the assumption of independence was not violated in the meta-analytic models, we took the following precautions. Only one effect size per study was used to compute aggregate effect sizes. When studies provided more than one effect size due to multiple psychiatric groups, we selected the d based on the largest group for initial models examining HPA outcomes. When results for a study were reported in multiple articles, we computed effect sizes from the largest samples, because estimates based on larger samples are considered more accurate (Hedges & Vevea, 1998).
Descriptive Findings
Analyses were based on 47 independent studies from 50 published articles and 1 dissertation (see Data Supplement 2 for detailed study characteristics).2 Qualifying studies are marked with an asterisk in the reference section. Overall, 123 effect sizes were computed, with each study contributing an average of 2.4 effect sizes (range = 1-7, SD = 1.6). A total of 6,008 individuals were included across all studies. There were 2,521 TE individuals, 504 individuals with PTSD, 237 with comorbid PTSD+MDD, and 2,746 NTC. Computing the sampling error of d using the Hedges and Olkin (1985) formula allowed for unequal sample sizes across psychiatric and control groups.
Participants were 58% male; average age was 37.14 (range 9 to 71 years old). They experienced the following traumatic events: 19 studies (40%) included participants exposed to physical abuse, 17 (36%) experienced sexual abuse, 9 (19%) were emotionally abused, 19 (40%) involved combat/war experience, 3 (6%) were neglected, 3 (6%) involved refugee status, 8 (17%) were tortured, 12 (26%) experienced serious accidents/disasters, 12 (26%) involved separation or loss of a major relationship, 13 (28%) witnessed a trauma, and 5 (11%) involved receiving a life-threatening medical diagnosis. Basal cortisol activity was assessed using HPA outcomes including morning cortisol (k = 49), afternoon/evening cortisol (k = 26), and daily cortisol output (k = 19). Feedback function of the HPA-axis was assessed through post-DST cortisol (k =29). Regarding developmental timing, 20 studies examined childhood trauma, 14 examined trauma during adulthood, and 17 either did not assess or report data to determine developmental timing. Cortisol was assessed in plasma/serum (k = 33), saliva (k = 14), urine (k = 9) and cerebrospinal fluid (k = 1). Meta-ANOVA models examined the influence of measurement type on effect sizes for cortisol outcomes separately for TE, PTSD, and PTSD+MDD groups. For morning cortisol levels, effect sizes were significantly lower for saliva versus plasma samples in TE groups (d = −.27 versus .03, p = .016), and significantly higher for saliva versus plasma samples in PTSD+MDD groups (d = .25 versus −1.05, p < .001). For afternoon/evening cortisol levels, effect sizes were significantly lower for plasma versus saliva samples in TE (d = −.74 versus −.15, p = .018) and PTSD (d = −.64 versus −.01, p = .008) groups. Measurement type was not significantly associated with daily cortisol output or post-DST effect sizes. For subsequent meta-regression analyses of a.m. cortisol effect sizes (TE and PTSD+MDD groups) and p.m. cortisol effect sizes (TE and PTSD groups), measurement type was included as a covariate.
HPA basal activity
Fixed-effects models for HPA outcomes were run stratifying according to TE, PTSD, or PTSD+MDD groups (Table 1). Among TE groups, analyses revealed lower p.m. cortisol levels (d = −.25, SE = .09, p = .007) and no differences in a.m. or daily output cortisol levels relative to NTC. For PTSD groups, a.m. cortisol levels (d = −.28, SE = .08, p < .001), p.m. cortisol levels (d = −.27, SE = .12, p = .021), and daily cortisol output (d = −.36, SE = .13, p = .008) were all significantly lower than NTC. Analysis of basal HPA activity in individuals with PTSD+MDD revealed significantly lower a.m. cortisol (d = −.66, SE = .14, p < .001), higher p.m. cortisol (d = .49, SE = .24, p = .041), and lower daily cortisol output (d = −.65, SE = .25, p = .008) as compared to NTC.
Findings regarding HPA basal activity outcomes in TE, PTSD, and PTSD+MDD groups relative to NTC are presented in Figure 3 (see Figures 1 and 2 for forest plots for these HPA outcomes). Morning cortisol aggregate effect sizes for PTSD+MDD groups were significantly lower than those for PTSD (p = .009) and TE (p < .001) groups; morning cortisol aggregate effect sizes also were lower for PTSD as compared to TE individuals (p < .001). Regarding afternoon/evening cortisol, effect sizes for individuals with PTSD+MDD were significantly higher than those for PTSD (p = .005) and TE (p = .004) groups.3 No significant differences were observed in daily cortisol output effect sizes for the TE, PTSD, and PTSD+MDD groups.
Figure 3.
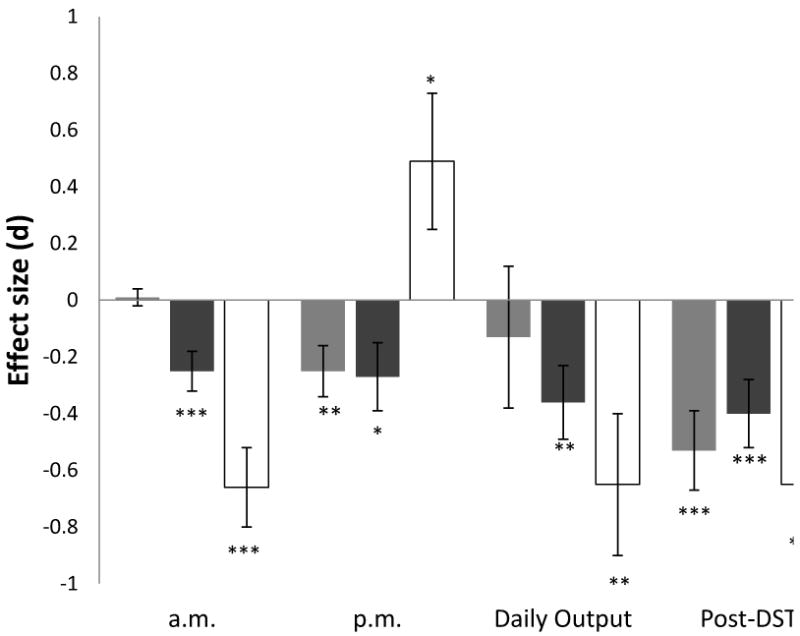
Mean (± SEM) cortisol effect size (d) for studies examining morning (a.m.), afternoon/evening (p.m.), daily output, and post-DST levels in TE, PTSD, and PTSD+MDD groups. TE = trauma-exposed without PTSD or MDD; PTSD = Posttraumatic Stress Disorder; PTSD+MDD = PTSD and comorbid Major Depressive Disorder; a.m. = morning (before 12 p.m.); p.m. = afternoon/evening (after 12 p.m.); DST = Dexamethasone Suppression Test. *p<.05; **p<.01;*** p<.001.
HPA feedback function
Analyses of feedback functioning revealed significantly lower post-DST cortisol levels in TE (d = −.53, SE = .14, p < .001), PTSD (d = −.40, SE = .12, p < .001) and PTSD+MDD (d = −.65, SE = .14, p < .001) groups relative to NTC, indicating enhanced suppression (see Figure 2). No significant differences were found in post-DST effect sizes among TE, PTSD, and PTSD+MDD groups.
Moderators of HPA function
Age
We examined relations between the mean age of participants and effect sizes for each HPA outcome using separate meta-regression models for TE, PTSD, and PTSD+MDD groups. For individuals with PTSD, mean age was negatively associated with post-DST cortisol (β = −.36, SE = .01, p < .001) effect sizes indicating that enhanced HPA suppression was linked with older ages. In individuals with PTSD+MDD, daily cortisol output effect sizes were positively associated with age (β = .67, SE = .04, p = .001) such that greater daily output was associated with older ages.
Sex
We examined relations between sex composition and effect sizes for each HPA outcome using separate meta-regression models for TE, PTSD, and PTSD+MDD groups. Percentage of males was negatively associated with p.m. cortisol effect sizes in TE groups (β = −.73, SE = .003, p < .001), after controlling for measurement type, with lower p.m. cortisol associated with a higher percent of males. Percentage of males was negatively associated with daily cortisol output among PTSD groups (β = −.45, SE = .004, p < .001), such that lower daily output was associated with having more males. Percentage of males was negatively associated with post-DST cortisol effect sizes among individuals with PTSD+MDD (β = −.39, SE = .003, p = .014), such that having more males was associated with lower post-DST cortisol effect sizes.
Time since onset of focal trauma
We examined relations between time elapsed since the focal trauma and effect sizes for each HPA outcome using separate meta-regression models for TE, PTSD, and PTSD+MDD groups. The mean time since onset across all studies was 17.6 years (SD = 13.2 years; range 1 month to 50 years). Time since trauma onset was negatively associated with p.m. cortisol effect sizes at the level of a trend (β = −.27, SE = .18, p = .055) in PTSD groups after controlling for measurement type, indicating that afternoon cortisol levels were lower for samples in which more time had elapsed since the focal trauma. Time since trauma onset also was negatively associated with daily output (β = −.23, SE = .12, p = .038) and post-DST effect sizes (β = −.48, SE = .28, p < .001) in PTSD groups, such that daily output and post-DST cortisol levels were lower for samples in which more time had elapsed since the focal trauma. Among individuals with PTSD+MDD, time since trauma onset was negatively related to daily output effect sizes (β = −.91, SE = .79, p < .001) and post-DST cortisol effect sizes (β = −.36, SE = .39, p = .024), indicating that daily output and post-DST cortisol levels were lower for samples in which more time had elapsed since the focal trauma.
Developmental timing
We examined relations between developmental timing and effect sizes for each HPA outcome using meta-ANOVA models for TE, PTSD, and PTSD+MDD groups. Trauma in childhood was examined in 14 studies, trauma during adulthood was examined in 20 studies, and 17 studies did not report sufficient information to make this determination. Developmental timing was not significantly associated with morning cortisol effect sizes for TE groups, controlling for measurement type, or for PTSD groups. However, results revealed that morning cortisol effect sizes were significantly lower for adult versus childhood trauma exposure (d = −1.40 versus −.01, p < .001) in individuals with PTSD+MDD. Developmental timing was not significantly associated with afternoon/evening cortisol or daily output effect sizes for TE or PTSD groups. There was insufficient data to examine the influence of developmental timing on p.m. cortisol or daily output effect sizes in individuals with PTSD+MDD. Developmental timing was not significantly associated with post-DST cortisol effect sizes for TE groups. Post-DST cortisol effect sizes were significantly lower for adult as compared to childhood trauma exposure for individuals with PTSD+MDD (d = −.96 versus −.04, respectively, p = .006) and at the level of a trend for individuals with PTSD (d = −.60 versus −.12, respectively, p = .053).
Finally, we examined whether relations between time since trauma onset and HPA outcomes remained after controlling for developmental timing. Preliminary analyses revealed no significant differences in time since onset between studies of child versus adult trauma exposure [t(26) = 1.51, p = .144]. Nevertheless, we explored whether including developmental timing as a covariate altered findings from meta-regression models examining the association between time since onset and cortisol effect sizes. Results of analyses of morning cortisol effect sizes were not changed. Developmental timing significantly predicted morning cortisol effect sizes for PTSD+MDD groups (β = .59, SE = .32, p < .001), controlling for time since onset, indicating that childhood trauma was associated with higher morning cortisol levels as compared to adult trauma exposure in individuals with PTSD+MDD.
For afternoon/evening cortisol, after controlling for developmental timing, time since trauma onset was negatively associated with effect sizes for TE groups (β = −.51, SE = .27, p= .010) and PTSD groups (β = −1.28, SE = .23, p = .006), indicating that p.m. cortisol levels were lower for samples in which more time had elapsed since the focal trauma. Results of analyses examining daily cortisol output were not altered when controlling for developmental timing. Finally, post-DST cortisol effect sizes were significantly associated with time since trauma onset (β = −.57, SE = .34, p < .001), but not developmental timing, for PTSD groups. For individuals with PTSD+MDD, results continued to show that post-DST effect sizes were lower for adult as compared to childhood trauma exposure (β = −.43, SE = .34, p = .010); the relation between time since trauma onset and post-DST effect sizes was no longer significant.
Discussion
The primary aim of the current meta-analytic review of 47 independent studies was to determine whether trauma-exposed individuals with PTSD, PTSD+MDD, or neither (TE) differed from never traumatized controls (NTC) and from each other regarding HPA basal activity and feedback functioning. We were particularly interested in identifying features of HPA function that distinguished trauma-exposed individuals who developed PTSD from those who did not, and whether individuals with PTSD versus those with PTSD+MDD could be distinguished by patterns of HPA function. Finally, we examined whether the relations between trauma-exposure and HPA function were moderated by person and trauma features.
HPA basal activity
Meta-analytic models revealed that morning cortisol levels were lower in PTSD and PTSD+MDD groups relative to NTC. This finding contrasts with the results of earlier meta-analyses showing no significant differences in morning cortisol levels between adults with PTSD and healthy controls (Meewisse et al., 2007; Miller et al., 2007). Our analyses of morning cortisol levels, however, included over twice as many studies (i.e., 33 versus 15) as had been available at the time of the earlier meta-analyses (e.g., Meewisse et al., 2007), and therefore we might have had greater power to detect small effect sizes.
Enhanced HPA negative feedback is associated with diminished basal cortisol activity in individuals with PTSD (Yehuda et al., 1996). Interestingly, we found that TE individuals (without PTSD or MDD) exhibited enhanced negative feedback but did not differ significantly from NTC in their morning cortisol levels. The reason for this apparent decoupling of HPA feedback function from morning cortisol levels among TE individuals remains unclear. Given that social interactions exert a regulatory effect on cortisol circadian rhythm (e.g., Dickerson & Kemeny, 2004; Stetler & Miller, 2005), we speculate that TE individuals might not experience the same level of disruptions of social behavior observed in trauma-related MDD (e.g., social withdrawal) and PTSD (e.g., diminished interest or participation in significant activities, feelings of detachment or estrangement from others).
Consistent with prior research (Meewisse et al., 2007), afternoon/evening cortisol levels were significantly lower in PTSD groups relative to NTC. Contrary to expectation, however, a similar pattern was observed in TE individuals. Lower p.m. cortisol levels may be related to the enhanced suppression of cortisol secretion in trauma exposed individuals without PTSD or MDD. As anticipated, p.m. cortisol levels were significantly higher in PTSD+MDD groups compared to NTC. This finding parallels results of a study examining basal cortisol activity in TE+MDD individuals (Juruena et al., 2006). We speculate that the presence of comorbid MDD in individuals with PTSD may be associated with alterations in sensitivity to social-evaluative threat, which has been linked to increased cortisol responses and recovery times (Dickerson & Kemeny, 2004).
Consistent with expectation, the meta-analytic models revealed lower daily cortisol output in individuals with PTSD (without MDD) relative to NTC. Interestingly, daily cortisol output also was lower in PTSD+MDD groups relative to NTC, despite elevated p.m. cortisol levels. This pattern of results in those with PTSD+MDD is consistent with our prediction of a flattened diurnal cortisol slope for this group. TE individuals did not differ significantly from NTC in daily cortisol output, however, despite lower p.m. cortisol levels. In addition, daily cortisol output effect sizes in TE, PTSD, and PTSD+MDD groups did not differ from each other. These findings contrast with those from a recent meta-analysis that detected no significant differences in daily cortisol output among TE, PTSD, and NTC groups (Klaassens et al., in press). Whereas the current review attempted to capture patterns of cortisol secretion over the course of the day by examining morning, afternoon/evening, and daily output effect sizes separately, Klaassens and colleagues (in press) assessed basal HPA activity without controlling for the time of measurement. Chronobiological studies such as those conducted with PTSD and MDD groups (Yehuda et al., 1996) are needed to elucidate patterns of circadian cortisol secretion in TE individuals.
HPA feedback function
In line with expectation that the pattern of HPA feedback function would be characterized by enhanced negative feedback in TE (e.g., Klaassens et al., in press), PTSD, and PTSD+MDD groups (Miller et al., 2007), we found that these groups showed lower post-DST cortisol levels compared to NTC. No significant differences were detected in post-DST effect sizes among the three trauma-exposed groups. Neurobiological explanations of why only some trauma-exposed individuals develop PTSD have emphasized the “failure of mechanisms involved in recovery and restitution of physiological homeostasis, possibly resulting from individualistic predisposition” (Yehuda & LeDoux, 2007, p. 19). If enhanced negative feedback explains HPA abnormalities in individuals with PTSD (Yehuda et al., 1995) and is associated with increased risk for the development of PTSD, then we might not expect to find it among trauma exposed individuals who do not develop PTSD. The current meta-analysis, however, found enhanced HPA negative feedback among TE individuals as well, and the post-DST cortisol effect sizes in TE individuals did not differ significantly from those with PTSD or PTSD+MDD.
Two alternative explanations are possible for these findings. First, enhanced HPA feedback function may not be associated with risk for PTSD in particular, but rather may be linked with trauma-exposure in general. Second, enhanced HPA feedback function may be associated with increased risk for PTSD among TE individuals. A recent prospective study examining post-DST cortisol levels in the aftermath of trauma found that most, but not all, individuals who developed PTSD showed enhanced negative feedback in response to DEX challenge (McFarlane, Barton, Yehuda, & Wittert, 2011). That enhanced HPA negative feedback is only reported in some individuals who develop PTSD, and characterizes some trauma-exposed individuals who do not develop PTSD, suggests that such enhanced negative feedback likely is not a specific vulnerability to PTSD (Pitman et al., 2006). These competing hypotheses could be addressed by prospective studies following TE individuals to determine whether they are at increased risk of developing PTSD as a function of enhanced HPA feedback.
Moderators of HPA function
Age
Studies examining the relation of age to HPA function in healthy individuals generally have found elevated basal activity and impaired feedback functioning in older samples (Otte et al., 2005). The current meta-analysis investigated age as a possible moderator of HPA function in TE, PTSD, and PTSD+MDD groups. Meta-regression models revealed that mean age of participants was negatively associated post-DST cortisol effect sizes in PTSD groups. That is, among individuals with PTSD, lower post-DST levels were associated with older ages. For individuals with PTSD+MDD, mean age was positively associated with daily cortisol output effect sizes, such that greater daily cortisol output was associated with older ages. Interestingly, mean age was not significantly associated with HPA basal activity or feedback function in TE individuals. Taken together, these findings suggest that enhanced HPA negative feedback may characterize older individuals with PTSD. Additionally, older individuals with PTSD+MDD appear to exhibit greater daily cortisol output, and therefore may be at increased risk for physical health problems associated with hypercortisolism (e.g., McEwen, 2007).
Sex
Elevated rates of MDD and PTSD in women relative to men (e.g., Breslau et al., 1998), coupled with evidence of sex differences in HPA function (e.g., Van Cauter et al., 1996), suggest that alterations of the stress response may confer increased vulnerability to psychopathology in women. The current meta-analysis examined whether differences in HPA function for TE, PTSD, and PTSD+MDD groups relative to NTC varied by sex. Meta-regression models revealed that the percentage of males in study samples was negatively associated with afternoon/evening cortisol effect sizes in TE groups, indicating that women may exhibit more elevated p.m. cortisol output than men. Percentage of males also was negatively associated with post-DST cortisol effect sizes in PTSD+MDD groups; that is, among those with PTSD+MDD men appear to show enhanced HPA feedback function relative to women. In addition, among PTSD groups, having more males was associated with lower daily cortisol output. Thus, although we anticipated that sex differences in cortisol outcomes might partially explain increased vulnerability to PTSD among women, these results indicate that HPA alterations linked to psychopathology are found in both men (i.e., lower daily cortisol output, lower post-DST cortisol levels) and women (i.e., elevated afternoon cortisol levels).
Time since onset of trauma
Prior reviews of this literature have reached different conclusions regarding the relation between time since the onset of the trauma and HPA function. Whereas one review showed that the amount of time elapsed since the onset of chronic or traumatic stress was inversely associated with HPA basal activity and feedback function in individuals with PTSD (e.g., Miller et al., 2007), another reported no significant relation of years since trauma to cortisol levels in PTSD groups relative to controls (Meewisse et al., 2007). The current meta-analysis explored the relation of time since the trauma to HPA function in TE and PTSD+MDD groups in addition to PTSD groups. Meta-regression analyses revealed that the time elapsed since focal trauma was associated with lower p.m. cortisol, daily output, and post-DST cortisol effect sizes in PTSD groups, and lower daily cortisol output and post-DST effect sizes in PTSD+MDD groups. When controlling for developmental timing, the time elapsed since focal trauma also was negatively associated with p.m. cortisol for TE groups, but time since onset was no longer significantly associated with post-DST cortisol for individuals with PTSD+MDD.
This pattern of HPA activity is consistent with theories positing that the effect of stress on cortisol secretion is time-dependent; that is, hypersecretion may characterize the short-term response, whereas hyposecretion may develop in the long-term (Fries et al., 2005; Hellhammer & Wade, 1993; Miller et al., 2002). Interestingly, the apparent strengthening of HPA feedback function and diminishing daily cortisol output over time was not observed in TE individuals. Thus, these time-dependent processes may be involved particularly in the onset and maintenance of PTSD in response to trauma. Longitudinal studies are needed to replicate the findings of the current meta-analysis, which were based on cross-sectional studies of group differences.
Developmental timing
Exposure to traumatic events during childhood is associated with increased risk for stress-related psychopathology (e.g., Kaufman & Charney, 2001) and may be linked to alterations of stress response systems including the HPA-axis. Previous studies have found alterations in diurnal cortisol secretion associated with early adversity (e.g., Carlson & Early, 1997; Carpenter et al., 2007; Fisher et al., 2000; Heim et al., 2000). In contrast, a recent meta-analysis reported no differences in basal cortisol levels between TE and PTSD groups exposed to trauma during adulthood and NTC (Klaassens et al., in press). Qualitative reviews have shown that maltreated children with internalizing problems exhibit increased basal HPA activity, particularly elevated morning cortisol secretion, whereas adults who were maltreated in childhood tend to exhibit decreased basal HPA activity (Tarullo & Gunnar, 2006). The current meta-analysis expanded upon this literature by showing that among individuals with PTSD+MDD, trauma exposure during childhood was associated with higher morning cortisol and post-DST effect sizes relative to trauma exposure during adulthood, controlling for the time elapsed since the focal traumatic event.
Comorbid PTSD+MDD
Expanding upon a previous review that included only 4 studies of individuals with comorbid PTSD+MDD (Meewisse et al., 2007), the current meta-analysis included effect sizes from 16 studies (24 effect sizes) of PTSD+MDD groups. Results revealed lower a.m. and higher p.m. cortisol levels in individuals with PTSD+MDD relative to NTC, as well as compared to those with PTSD only. Although PTSD+MDD and PTSD only groups had a similar pattern of enhanced HPA feedback function, their HPA outcomes were differentially associated with the various moderators tested (i.e., mean age of participants, percentage of males, time since trauma onset, and developmental timing). Mean age was negatively associated with post-DST effect sizes in PTSD only groups and positively associated with daily cortisol output effect sizes in PTSD+MDD groups. Higher percentage of males was associated with lower post-DST cortisol effect sizes among individuals with PTSD+MDD but not those with PTSD only. Time since trauma onset was associated with p.m. cortisol effect sizes in PTSD only but not PTSD+MDD groups. Finally, developmental timing of the trauma was significantly associated with morning cortisol and post-DST cortisol effect sizes for those with PTSD+MDD but not for individuals with PTSD only.
Some researchers have argued that “the bulk of psychopathology in the aftermath of trauma is best conceptualized as a general traumatic stress factor” (O'Donnell et al., 2004, p. 1395), with symptoms of PTSD and MDD reflecting shared vulnerability and predictors (Breslau et al., 2000). Results of the current meta-analysis, however, revealed unique HPA features of PTSD versus PTSD+MDD in addition to unique predictors of effect sizes. Thus, the emergence of PTSD only and PTSD+MDD may be governed by both shared and distinct vulnerability factors. This hypothesis is consistent with the findings of a recent confirmatory factor analysis revealing that PTSD and MDD are distinguishable constructs in the aftermath of trauma, although both load on a higher order emotional numbing/dysphoria construct (Grant, Beck, Marques, Palyo, & Clapp, 2008).
HPA markers of vulnerability to PTSD
Daily cortisol output was lower in both PTSD and PTSD+MDD groups relative to NTC; TE and NTC groups were not significantly different from each other. Thus, reduced HPA basal activity may be associated with vulnerability to PTSD. Hellhammer and Wade (1993) proposed that trauma exposure may trigger a cascade of events among individuals at risk for PTSD beginning with chronic stress-related CRH hypersecretion associated with recurrent memories and threat-appraisals of novel situations (Baum, Cohen, & Hall, 1993), followed by an adaptive down-regulation of pituitary CRH receptors, culminating in cortisol hyposecretion after a return to normal levels of CRH secretion. Decreased basal cortisol activity in the aftermath of trauma may interfere with stress recovery processes. In support of this hypothesis, a double-blind, randomized control study examining recovery from the potentially traumatic experience of septic shock found a lower incidence of PTSD in patients who were administered a stress-dosage of hydrocortisone (synthetic cortisol) compared to patients who were given saline (Schelling, Briegel, Roozendaal, Stoll, Rothenhausler, & Kapfhammer, 2001). Animal studies have shown that early treatment with high-dose corticosterone immediately following psychogenic stress reduced prevalence of PTSD-like behaviors (e.g., cue-induced freezing) relative to a saline control condition (Cohen, Matar, Buskila, Kaplan, & Zohar, 2008). Finally, prospective studies of trauma exposure in humans generally have found that lower cortisol levels shortly after a traumatic event are associated with increased risk for PTSD (Delahanty, Raimonde, & Spoonster, 2000; Ehring, Ehlers, Cleare, & Glucksman, 2008; McFarlane, Atchison, & Yehuda, 1997; although see Bonne et al., 2003; Shalev, Videlock, Peleg, Segman, Pitman, & Yehuda, 2008).
Reduced basal cortisol activity following trauma may represent an adaptation to anticipated threat insofar as it allows the HPA-axis to mount a rapid response to trauma-related cues, unfettered by ongoing negative feedback that would result from elevated circulating cortisol levels. Lower daily cortisol output also may serve as a means of increasing preparedness for real or perceived threats to the physical self by reducing inhibition of inflammatory processes (e.g., Raison & Miller, 2003). However, in the long-run, a pattern of reduced cortisol secretion may place individuals with PTSD at greater risk for a variety of health conditions via a failure to inhibit immune responses, sympathetic nervous system (SNS) activity, and short-term CRH secretion (Heim et al., 2000; Raison & Miller, 2003). For example, a lack of cortisol-mediated inhibition of SNS activation in the aftermath of a traumatic event may lead to hypersecretion of norepinephrine (NE), an ‘overconsolidation’ of trauma-related memories, elevated and sustained hyperarousal related to ‘re-experiencing’ symptoms, and, ultimately, the development of PTSD (Pitman, 1989; Yehuda & Harvey, 1997). Over time, individuals may become increasingly sensitized to threats via disruptions of neural fear and anxiety networks and a progressive divergence of HPA and SNS activity reflected in decreasing levels of evening cortisol and increasing concentrations of NE (Pervanidou, 2008).
Given that most individuals exposed to trauma will not develop PTSD (e.g., Kessler et al., 1995), researchers have sought to identify pre-trauma risk factors that interact with exposure to traumatic events to trigger the onset of PTSD. Some individuals may exhibit a relatively stable pattern of reduced HPA basal activity that places them at greater risk for developing PTSD when confronted with a traumatic event. For example, children of parents with PTSD (high risk) are more likely to develop PTSD themselves following trauma-exposure than children of trauma survivors without PTSD and children of never-traumatized parents. These high-risk children also exhibit patterns of reduced cortisol secretion over the course of the day (Yehuda et al., 2007).
Reduced daily cortisol output may represent a relatively stable, pre-existing vulnerability factor for PTSD, possibly connected to early adversity; in contrast, lower post-DST cortisol levels may worsen over time as a result of exposure to the traumatic event and its sequelae (e.g., Yehuda, 2002). The current meta-analysis showed that reduced daily cortisol output was characteristic of PTSD and PTSD+MDD groups, but not TE groups, and that daily cortisol output and post-DST cortisol levels were inversely related to the time since trauma exposure for individuals with PTSD. These findings are consistent with evidence that prior trauma exposure is associated with both lower cortisol secretion immediately following trauma exposure and higher risk for subsequent PTSD (Resnick, Yehuda, Pitman, & Foy, 1995).
Reduced daily cortisol output may represent both a relatively stable and a dynamic vulnerability factor for PTSD; that is, some individuals may be ‘pre-sensitized’ to the effects of trauma (e.g., based on genetic liability or early adversity), whereas others may become increasingly sensitized with each trauma exposure. Although results of the current meta-analysis indicate that HPA basal activity may diminish over time following trauma-exposure in individuals with PTSD or PTSD+MDD, longitudinal high-risk studies are needed to determine whether HPA hypoactivity represents a relatively stable preexisting vulnerability that increases risk for PTSD. Based on the findings regarding TE groups, we speculate that the maintenance of normative diurnal cortisol secretion in the aftermath of trauma, despite enhanced HPA negative feedback, may be protective. Future studies should examine psychosocial factors (e.g., coping factors and social support) potentially associated with this profile of HPA secretion.
Limitations and Future Directions
Limitations of the current meta-analysis should be noted. First, our analyses were based on cross-sectional studies and therefore could not address whether observed HPA characteristics that distinguished TE, PTSD, and PTSD+MDD groups from never traumatized controls were pre-existing vulnerability factors (i.e., trait markers), emerging vulnerability factors (i.e., scar markers), or state-like concomitants of trauma (i.e., state markers). Prospective studies that examine individuals before, during, and after exposure to traumatic events are needed to identify vulnerability and protective factors for trauma-related psychopathology. This could be accomplished by selecting children who are at increased risk based on parental PTSD and/or MDD, but who have not yet been exposed to trauma or had an episode of either PTSD or MDD, and following them across salient developmental transitions associated with increased stress (e.g., puberty, school changes, emerging adulthood).
Second, due to a paucity of relevant studies, we were not able to compute aggregate effect sizes for individuals with TE+MDD. To disentangle correlates and risk factors associated with individual and comorbid disorders, more research is needed that examines HPA function in individuals exposed to trauma who develop MDD only. The current meta-analysis revealed unique HPA features and predictors associated with PTSD versus PTSD+MDD. Future studies should investigate HPA function in PTSD either excluding individuals with comorbid MDD or recruiting large enough samples to allow assessment of the unique characteristics and correlates of PTSD versus MDD in traumatized individuals.
Third, despite its widespread use, the DST has several drawbacks. Levels of circulating glucocorticoids resulting from DEX administration may be much higher than levels of endogenous cortisol typically elicited in response to psychosocial stressors (Burke et al., 2005), thereby reducing ecological validity. Moreover, DEX does not readily cross the blood-brain barrier, preferentially targeting the pituitary and causing only partial depletion of circulating cortisol in the brain (de Kloet et al., 1998). Hence, the DST does not directly influence corticolimbic structures. For these reasons, psychosocial stressor paradigms such as the Trier Social Stress Test (TSST; Kirschbaum et al., 1993) may provide a better window into processes associated with stress response and recovery.
Fourth, a dearth of studies employing the DEX/CRH challenge to examine the impact of trauma on negative feedback inhibition prohibited the computation of aggregate effect sizes. Future research should use this paradigm because it is a more sensitive assessment than the DST (Holsboer, von Bardeleben, Wiedemann, Muller, & Stalla, 1987), particularly for examining HPA function in individuals with MDD (Heuser et al., 1994). The recent meta-analysis by Klaassens and colleagues (in press) included two studies that utilized the DEX/CRH challenge and found no difference between PTSD and NTC groups. The current meta-analysis also did not include challenge studies administering metyrapone – a drug that inhibits an enzyme involved in the synthesis of cortisol. Results of two studies using this paradigm have failed to demonstrate enhanced HPA feedback function in patients with PTSD (Kanter et al., 2001; Kellner et al., 2003), thus highlighting the need for more research in this area.
We were not able to control for lifetime psychiatric diagnoses in TE and NTC groups because this information was not provided in most studies. Some studies have found that alterations in HPA function may persist after recovery from depressive episodes and may be associated with risk for recurrence (e.g., Appelhof et al., 2006; Rao et al, 2010; Zobel et al., 2001). Future studies including TE and NTC groups without lifetime psychopathology are needed to address this concern (e.g., Klaassens et al., 2009). Finally, we were unable to disentangle the relative influence of time since onset of focal trauma and chronicity of posttraumatic stress symptoms for the PTSD and PTSD+MDD groups because this information was rarely reported.
Summary and Conclusions
Results of this meta-analytic review revealed reliable differences in HPA outcomes among TE, PTSD, and PTSD+MDD groups relative to NTC. Lower daily cortisol output and enhanced HPA feedback function were found in PTSD and PTSD+MDD groups. Comorbid PTSD+MDD was associated with unique HPA function features (e.g., elevated p.m. cortisol levels relative to NTC) compared to PTSD alone. Reduced HPA basal activity distinguished PTSD and PTSD+MDD groups from TE groups, although TE individuals showed enhanced HPA feedback function similar to both PTSD groups.
In addition, HPA function was differentially moderated by mean age and sex composition of samples, time elapsed since the index trauma, and the timing of trauma exposure for TE, PTSD, and PTSD+MDD groups. TE individuals exhibit similar daily cortisol output to NTC, whereas those with PTSD or PTSD+MDD show reduced daily cortisol output relative to NTC. Importantly, TE individuals did not differ from PTSD and PTSD+MDD groups regarding HPA negative feedback function. Thus, whereas considerable attention has focused on the role of enhanced HPA negative feedback as a possible marker of risk for PTSD, the present findings suggest that this alteration may not distinguish trauma-exposed individuals who will go on to develop PTSD from those who will not. Finally, the current meta-analytic review showed that reduced daily cortisol output is a potential risk factor for PTSD and is not simply a marker of trauma exposure. Due to the debilitating nature of PTSD and its impact on disease outcomes, the identification of early risk factors and refinement of integrative psychoneuroenocrine models represents a critical avenue for future research.
Supplementary Material
Highlights.
Daily cortisol output was lower for PTSD and PTSD+MDD groups relative to NTC
No significant differences between TE and NTC groups in daily cortisol output
Afternoon cortisol was lower in PTSD groups relative to NTC
Afternoon cortisol was higher in PTSD+MDD groups relative to NTC
Post-DST cortisol levels were lower in PTSD, PTSD+MDD, TE groups relative to NTC
Acknowledgments
Matthew Morris was supported in part by an Individual NRSA Fellowship (1F31 MH084425-01A1), Elizabeth Munsterberg Koppitz Graduate Student Fellowship, Vanderbilt Institute for Clinical and Translational Research grant support (1 UL1 RR024975 from NCRR/NIH), and a grant (R01MH068391) and training grant (T32MH18921) from the National Institute of Mental Health. Bruce E. Compas was supported by a National Institute of Mental Health Grant (R01HM069940). Judy Garber was supported in part by National Institute of Mental Health Grants R01MH64735, RC1MH088329, and by the Vanderbilt CTSA grant UL1RR024975-01 from NCRR/NIH during completion of this work. We thank Sandra Jo Wilson and David Cole for their helpful comments on early drafts of this article. We also thank the corresponding authors of previous articles who provided additional information as requested. Correspondence regarding this article should be sent to Matthew Morris, 1005 Dr. D.B. Todd, Jr., Boulevard, Meharry Medical College, Nashville, TN 37208. E-mail: mmorris@mmc.edu.
Footnotes
TE = trauma-exposed individuals without PTSD or PTSD+MDD
The effect size generated from this dissertation study for analysis of daily cortisol output did not represent an outlier.
We re-ran analyses excluding studies that had TE participants with a history of PTSD (Bremner et al., 2007; Carpenter et al., 2007; Newport et al., 2004; Yehuda, Kahana, et al., 1995) and NTC participants with a history of PTSD (Pfeffer et al., 2007). For afternoon/evening cortisol, PTSD groups now showed marginally lower levels (d=−.24, SE=.12, p=.052) and TE groups no longer showed lower levels relative to NTC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Matthew C. Morris, Email: mmorris@mmc.edu, Meharry Medical College.
Bruce E. Compas, Vanderbilt University
Judy Garber, Vanderbilt University.
References
References marked with an asterisk indicate studies included in the meta-analysis.
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Altemus M, Cloitre M, Dhabhar FS. Enhanced cellular immune response in women with PTSD related to childhood abuse. The American Journal of Psychiatry. 2003;160:1705–1707. doi: 10.1176/appi.ajp.160.9.1705. [DOI] [PubMed] [Google Scholar]
- Altman DG, Bland JM. Interaction revisited: The difference between two estimates. British Medical Journal. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd. Washington D.C.; 1980. Author. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd rev. Washington D.C.; 1987. Author. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington D.C.; 1994. Author. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev) Washington D.C.; 2000. Author. [Google Scholar]
- Appelhof BC, Huyser J, Verweij M, Brouwer JP, van Dyck R, Fliers E, Hoogendijk WJG, Tijssen JGP, Wiersinga WM, Schene AH. Glucocorticoids and relapse of major depression (dexamathasone/corticotropin-releasing hormone test in relation to relapse of major depression) Biological Psychiatry. 2006;59:696–701. doi: 10.1016/j.biopsych.2005.09.008. [DOI] [PubMed] [Google Scholar]
- *Atmaca M, Kuloglu M, Tezcan E, Onal S, Ustundag B. Neopterin levels and dexamethasone suppression test in posttraumatic stress disorder. European Archives of Psychiatry and Clinical Neuroscience. 2002;252:161–165. doi: 10.1007/s00406-002-0374-5. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Heitkemper MM. Morningness-eveningness and early-morning salivary cortisol levels. Biological Psychology. 1991;32:181–192. doi: 10.1016/0301-0511(91)90009-6. [DOI] [PubMed] [Google Scholar]
- *Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, Bednarik L, Geracioti TD., Jr Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. The American Journal of Psychiatry. 2005;162:992–994. doi: 10.1176/appi.ajp.162.5.992. [DOI] [PubMed] [Google Scholar]
- *Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD., Jr Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- *Bauer M, Priebe S, Graf KJ, Kurten I, Baumgartner A. Psychological and endocrine abnormalities in refugees from East Germany: Part II. Serum levels of cortisol, prolactin, luteinizing hormone, follicle stimulating hormone, and testosterone. Psychiatry Research. 1994;51:75–85. doi: 10.1016/0165-1781(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Baum A, Cohen L, Hall M. Control and intrusive memories as possible determinants of chronic stress. Psychosomatic Medicine. 1993;55:274–286. doi: 10.1097/00006842-199305000-00005. [DOI] [PubMed] [Google Scholar]
- Baum A, Grunberg N. Measurement of stress hormones. In: Cohen S, Kessler RC, Underwood LG, editors. Measuring stress: A guide for health and social scientists. New York: Oxford University Press; 1995. pp. 193–212. [Google Scholar]
- Bonne O, Brandes D, Segman R, Pitman PK, Yehuda R, Shalev AY. Prospective evaluation of plasma cortisol in recent trauma survivors with posttraumatic stress disorder. Psychiatry Research. 2003;119:171–175. doi: 10.1016/s0165-1781(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis (Version 1) Englewood, NJ: 2000. [Google Scholar]
- *Boscarino JA. Posttraumatic stress disorder, exposure to combat, and lower plasma cortisol among Vietnam veterans: Findings and clinical implications. Journal of Consulting and Clinical Psychology. 1996;64:191–201. doi: 10.1037//0022-006x.64.1.191. [DOI] [PubMed] [Google Scholar]
- *Bremner D, Vermetten E, Kelley ME. Cortisol, dehydroepiandrosterone, and estriadol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. The Journal of Nervous and Mental Disease. 2007;195:919–927. doi: 10.1097/NMD.0b013e3181594ca0. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Archives of General Psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: The posttraumatic stress disorder – major depression connection. Biological Psychiatry. 2000;48:902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community. Archives of General Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- *Carmassi C, Da Pozzo E, Martini C, Paggini R, Tonini S, Mundo F, Cesari D, Amendola L, Ciapparelli A, Dell'Osso L. Serum cortisol and DHEA-S levels in PTSD patients versus controls: Correlations to panic-agoraphobic spectrum symptoms. European Neuropsychopharmacology. 2008;18:S485–S486. [Google Scholar]
- *Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC. Neuroendocrine identification of depressed patients. Australian and New Zealand Journal of Psychiatry. 1976;10:13–20. doi: 10.3109/00048677609159480. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Feinberg M, Greden JF, Haskett RF, James NM, Steiner M, et al. Diagnosis of endogenous depression: Comparison of clinical, research and neuroendocrine criteria. Journal of Affective Disorders. 1980;2:177–194. doi: 10.1016/0165-0327(80)90004-x. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum; 1983. [Google Scholar]
- Cohen H, Matar MA, Buskila D, Kaplan Z, Zohar J. Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biological Psychiatry. 2008;64:708–717. doi: 10.1016/j.biopsych.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Cohn LD, Becker BJ. How meta-analysis increases statistical power. Psychological Methods. 2003;8:243–253. doi: 10.1037/1082-989X.8.3.243. [DOI] [PubMed] [Google Scholar]
- *De Bellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, Trickett PK, Putnam FW. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. Journal of Clinical Endocrinology and Metabolism. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HGM. Assessment of HPA-axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review. Journal of Psychiatric Research. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- *de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lentjes EGWM, Westenberg HGM. Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2007;32:215–226. doi: 10.1016/j.psyneuen.2006.12.009. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocrine Reviews. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Nugent NR. Predicting PTSD prospectively based on prior trauma history and immediate biological responses. Annals of the New York Academy of Sciences. 2006;1071:27–40. doi: 10.1196/annals.1364.003. [DOI] [PubMed] [Google Scholar]
- Delahanty D, Raimonde A, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biological Psychiatry. 2000;48:940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Gotthardt U, Schweiger U, Weber B, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sciences. 1997;61:2239–2246. doi: 10.1016/s0024-3205(97)00926-0. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- *Duval F, Crocq MA, Guillon MS, Mokrani MC, Monreal J, Bailey P, Macher JP. Increased adrenocorticotropin suppression following dexamethasone administration in sexually abused adolescents with posttraumatic stress disorder. Psychoneuroendocrinology. 2004;29:1281–1289. doi: 10.1016/j.psyneuen.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ehring T, Ehlers A, Cleare A, Glucksman E. Do acute psychological and psychobiological responses to trauma predict subsequent symptom severities and PTSD and depression? Psychiatry Research. 2008;161:67–75. doi: 10.1016/j.psychres.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: Impact on children's behavior, neuroendocrine activity, and foster parent functioning. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Giaconia RM, Reinherz HZ, Silverman AB, Pakiz B, Frost AK, Cohen E. Traumas and posttraumatic-stress-disorder in a community population of older adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:1369–1380. doi: 10.1097/00004583-199510000-00023. [DOI] [PubMed] [Google Scholar]
- *Golier JA, Schmeidler J, Legge J, Yehuda R. Enhanced cortisol suppression to dexamethasone associated with Gulf War deployment. Psychoneuroendocrinology. 2006;31:1181–1189. doi: 10.1016/j.psyneuen.2006.08.005. [DOI] [PubMed] [Google Scholar]
- *Golier JA, Schmeidler J, Legge J, Yehuda R. Twenty-four hour plasma cortisol and adrenocorticotropic hormone in Gulf War veterans: Relationships to posttraumatic stress disorder and health symptoms. Biological Psychiatry. 2007;62:1175–1178. doi: 10.1016/j.biopsych.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Grant DM, Beck G, Marques L, Palyo SA, Clapp JD. The structure of distress following trauma: Posttraumatic stress disorder, major depressive disorder, and generalized anxiety disorder. Journal of Abnormal Psychology. 2008;117:662–672. doi: 10.1037/a0012591. [DOI] [PubMed] [Google Scholar]
- *Griffin MG, Resick PA, Yehuda R. Enhanced cortisol suppression following dexamethasone administration in domestic violence survivors. American Journal of Psychiatry. 2005;162:1192–1199. doi: 10.1176/appi.ajp.162.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Grossman R, Yehuda R, Boisoneau D, Schmeidler J, Giller EL., Jr Prolactin response to low-dose dexamethasone challenge in combat-exposed veterans with and without posttraumatic stress disorder and normal controls. Biological Psychiatry. 1996;40:1100–1105. doi: 10.1016/S0006-3223(95)00600-1. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- *Halbreich U, Olympia J, Carson S, Glogowski J, Yeh CM, Axelrod S, Desu MM. Hypothalamo-pituitary-adrenal activity in endogenously depressed post-traumatic stress disorder patients. Psychoneuroendocrinology. 1989;14:365–370. doi: 10.1016/0306-4530(89)90006-1. [DOI] [PubMed] [Google Scholar]
- Hall J, Rosenthal R. Interpreting and evaluating meta-analysis. Evaluation & the Health Profession. 1995;18:393–407. doi: 10.1177/016327879501800404. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- Hedges LV, Vevea JL. Fixed and random-effects models in meta-analysis. Psychological Methods. 1998;3:486–504. [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wade S. Endocrine correlates of stress vulnerability. Psychotherapy and Psychosomatics. 1993;60:8–17. doi: 10.1159/000288675. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. Journal of Psychiatric Research. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F, von Bardeleben U, Wiedemann K, Muller OA, Stalla GK. Serial assessment of corticotropin-releasing hormone response after dexamethasone in depression: Implications for pathophysiology of DST nonsuppression. Biological Psychiatry. 1987;22:228–234. doi: 10.1016/0006-3223(87)90237-x. [DOI] [PubMed] [Google Scholar]
- *Inslicht SS, Marmar CR, Neylan TC, Metzler TJ, Hart SL, Otte C, McCaslin SE, Larkin GL, Hyman KB, Baum A. Increased cortisol in women with intimate partner violence-related posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:825–838. doi: 10.1016/j.psyneuen.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Ioannidis J. Interpretation of tests of heterogeneity and bias in meta-analysis. Journal of Evaluation in Clinical Practice. 2008;14:951–957. doi: 10.1111/j.1365-2753.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrine Reviews. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- *Jensen CF, Keller TW, Peskind ER, McFall ME, Veith RC, Martin D, Wilkinson CW, Raskind MA. Behavioral and neuroendocrine responses to sodium lactate infusion in subjects with posttraumatic stress disorder. The American Journal of Psychiatry. 1997;154:266–268. doi: 10.1176/ajp.154.2.266. [DOI] [PubMed] [Google Scholar]
- Juruena MF, Cleare AJ, Papadopoulos AS, Poon L, Lightman S, Pariante CM. Different responses to dexamethasone and prednisolone in the same depressed patients. Psychopharmacology. 2006;189:225–235. doi: 10.1007/s00213-006-0555-4. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Effects of early stress on brain structure and function: Implications for understanding the relationship between child maltreatment and depression. Development and Psychopathology. 2001;13:451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- *Kellner M, Yassouridis A, Hubner R, Baker DG, Wiedemann K. Endocrine and cardiovascular responses to corticotropin-releasing hormone in patients with posttraumatic stress disorder: A role for atrial natruiretic peptide? Neuropsychobiology. 2003;47:102–108. doi: 10.1159/000070018. [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett KA. Physiological correlates of childhood abuse: Chronic hyperarousal in PTSD, depression, and irritable bowel syndrome. Child Abuse & Neglect. 2000;24:799–810. doi: 10.1016/s0145-2134(00)00136-8. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, Zitman FG. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: A meta-analysis. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2011.07.003. in press. [DOI] [PubMed] [Google Scholar]
- *Klaassens ER, Giltay EJ, van Veen T, Veen G, Zitman FG. Trauma exposure in relation to basal salivary cortisol and the hormone response to the dexamethasone/CRH test in male railway employees without lifetime psychopathology. Psychoneuroendocrinology. 2010;35:878–886. doi: 10.1016/j.psyneuen.2009.11.012. [DOI] [PubMed] [Google Scholar]
- *Klaassens ER, van Noorden MS, Giltay EJ, van Pelt J, van Veen T, Zitman FG. Effects of childhood trauma on HPA-axis reactivity in women free of lifetime psychopathology. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33:889–894. doi: 10.1016/j.pnpbp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- *Klaassens ER, van Veen T, Giltay EJ, Rinne T, van Pelt J, Zitman FG. Trauma exposure and hypothalamic-pituitary-adrenal axis functioning in mentally healthy Dutch peacekeeping veterans, 10-25 years after deployment. Journal of Traumatic Stress. 2010;23:124–131. doi: 10.1002/jts.20491. [DOI] [PubMed] [Google Scholar]
- *Kosten TR, Wahby V, Giller E, Jr, Mason J. The dexamethasone suppression test and thyrotropin-releasing hormone stimulation test in posttraumatic stress disorder. Biological Psychiatry. 1990;28:657–664. doi: 10.1016/0006-3223(90)90452-8. [DOI] [PubMed] [Google Scholar]
- *Lemieux AM. Abuse-related posttraumatic stress disorder: Challenge to the norepinephrine-to-cortisol hypothesis (Doctoral dissertation, University of Wisconsin, Madison) Dissertation Abstracts International. 1998;59(2B):0908. [Google Scholar]
- Lipsey M, Wilson D. Practical Meta-Analysis. SAGE Publications; Thousand Oaks: 2001. [Google Scholar]
- *Lipshitz DS, Rasmusson AM, Yehuda R, Wang S, Anyan W, Gueoguieva R, Grilo CM, Fehon DC, Southwick SM. Salivary cortisol responses to dexamethasone in adolescents with posttraumatic stress disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:1310–1317. doi: 10.1097/01.chi.0000084832.67701.0d. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Luecken LJ, Appelhans BM. Early parental loss and salivary cortisol in young adulthood: The moderating role of family environment. Development and Psychopathology. 2006;18:295–308. doi: 10.1017/S0954579406060160. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, Duku EK, Walsh CA, Wong MYY, Beardslee WR. Childhood abuse and lifetime psychopathology in a community sample. The American Journal of Psychiatry. 2001;158:1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- *Maes M, Lin A, Bonaccorso S, van Hunsel F, Van Gastel A, Delmeire L, Biondi M, Bosmans E, Kenis G, Sharpe S. Increased 24-hour urinary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiatrica Scandinavica. 1998;98:328–335. doi: 10.1111/j.1600-0447.1998.tb10092.x. [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG. Statistics notes. Interaction 2: Compare effect sizes not P values. British Medical Journal. 1996;313:808. doi: 10.1136/bmj.313.7060.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biological Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation. Physiological Review. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McFarlane AC, Atchison M, Yehuda R. The acute stress response following motor vehicle accidents and its relation to PTSD. Annals of the New York Academy of Sciences. 1997;821:437–441. doi: 10.1111/j.1749-6632.1997.tb48299.x. [DOI] [PubMed] [Google Scholar]
- McFarlane AC, Barton CA, Yehuda R, Wittert G. Cortisol response to acute trauma and risk of posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36:720–727. doi: 10.1016/j.psyneuen.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, De Vries GJ, Gersons BPR, Olff M. Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. British Journal of Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their pharmacological actions. Endocrine Reviews. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- *Newport DJ, Heim C, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biological Psychiatry. 2004;55:10–20. doi: 10.1016/s0006-3223(03)00692-9. [DOI] [PubMed] [Google Scholar]
- *Neylan TC, Schuff N, Lenoci M, Yehuda R, Weiner MW, Marmar CR. Cortisol levels are positively correlated with hippocampal N-acetylaspartate. Biological Psychiatry. 2003;54:1118–1121. doi: 10.1016/S0006-3223(03)01974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Olff M, Guzelcan T, de Vries GJ, Assies J, Gersons BPR. HPA- and HPT-axis alterations in chronic posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:1220–1230. doi: 10.1016/j.psyneuen.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BPR. Gender differences in posttraumatic stress disorder. Psychological Bulletin. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- Orwin RG. Evaluating coding decisions. In: Cooper H, Hedges LV, editors. The handbook of research synthesis. New York: Russell Sage Foundation; 1994. pp. 139–162. [Google Scholar]
- Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: Importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Otte C, Neylan TC, Pole N, Metzler T, Best S, Henn-Haase C, Yehuda R, Marmar CR. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biological Psychiatry. 2005;57:27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Pervanidou P. Biology of post-traumatic stress disorder in childhood and adolescence. Journal of Neuroendocrinology. 2008;20:632–638. doi: 10.1111/j.1365-2826.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- *Pervanidou P, Kolaitis G, Charitaki S, Lazaropoulou C, Papassotirou I, Hindmarsh P, Bakoula C, Tsiantis J, Chrousos GP. The natural history of neuroendocrine changes in pediatric posttraumatic stress disorder (PTSD) after motor vehicle accidents: Progressive divergence of noradrenaline and cortisol concentrations over time. Biological Psychiatry. 2007;62:1095–1102. doi: 10.1016/j.biopsych.2007.02.008. [DOI] [PubMed] [Google Scholar]
- *Pfeffer CR, Altemus M, Heo M, Jiang H. Salivary cortisol and psychopathology in children bereaved by the September 11, 2001 terror attacks. Biological Psychiatry. 2007;61:957–965. doi: 10.1016/j.biopsych.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Posttraumatic stress disorder, hormones, and memory. Biological Psychiatry. 1989;26:645–652. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Pitman R, Gilbertson M, Gurvits T, May F, Lasko N, Metzger L, Shenton M, Yehuda R, Orr S. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Annals of the New York Academy of Sciences. 2006;1071:242–254. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky P, Owens MJ, Nemeroff CB. Neuropeptide alterations in affective disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 971–981. [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosomatic Medicine. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen CL, Poland RE. Longitudinal course of adolescent depression: Neuroendocrine and psychosocial predictors. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:141–151. doi: 10.1097/00004583-201002000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Rasmusson AM, Lipschitz DS, Wang S, Hu S, Vojvoda D, Bremner JD, Southwick SM, Charney DS. Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder. Biological Psychiatry. 2001;50:965–977. doi: 10.1016/s0006-3223(01)01264-1. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Yehuda R, Pitman RK, Foy DW. Effect of previous trauma on acut plasma cortisol following rape. American Journal of Psychiatry. 1995;152:1675–1677. doi: 10.1176/ajp.152.11.1675. [DOI] [PubMed] [Google Scholar]
- Ribeiro SC, Tandon R, Grunhaus L, Greden JF. The DST as a predictor of outcome in depression: A meta-analysis. American Journal of Psychiatry. 1993;150:1618–1629. doi: 10.1176/ajp.150.11.1618. [DOI] [PubMed] [Google Scholar]
- *Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-infammatry cytokine production in Bosnian War refugees with posttraumatic stress disorder. Biological Psychiatry. 2004;55:745–751. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV, editors. The handbook of research synthesis. New York: Russell Sage Foundation; 1994. pp. 231–244. [Google Scholar]
- Sapolsky RM. Stress, the Aging Brain, and the Mechanisms of Neuron Death. Cambridge; London: MIT Press; 1992. [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocrine Reviews. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glococorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schelling G, Briegel J, Roozendaal B, Stoll C, Rothenhausler HB, Kapfhammer HP. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biological Psychiatry. 2001;50:978–985. doi: 10.1016/s0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schurmeyer TH, Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sciences. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- *Seedat S, Stein MB, Kennedy CM, Hauger RL. Plasma cortisol and neuropeptide Y in female victims of intimate partner violence. Psychoneuroendocrinology. 2003;28:796–808. doi: 10.1016/s0306-4530(02)00086-0. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Robbins RJ. Aging and hypothalamic-pituitary-adrenal response to challenge in humans. Endocrine Reviews. 1994;15:233–261. doi: 10.1210/edrv-15-2-233. [DOI] [PubMed] [Google Scholar]
- Shadish WR, Haddock CK. Combining estimates of effect size. In: Cooper H, Hedges LV, editors. The handbook of research synthesis. New York: Russell Sage Foundation; 1994. pp. 261–281. [Google Scholar]
- Shalev AY, Freedman S, Peri T, Brandes D, Sahar T, Orr SP, Pitman RK. Prospective study of posttraumatic stress disorder and depression following trauma. The American Journal of Psychiatry. 1998;155:630–637. doi: 10.1176/ajp.155.5.630. [DOI] [PubMed] [Google Scholar]
- Shalev A, Videlock E, Peleg T, Segman R, Pitman R, Yehuda R. Stress hormones and post-traumatic stress disorder in civilian trauma victims: A longitudinal study Part I: HPA axis responses. International Journal of Neuropsychopharmacology. 2008;11:365–372. doi: 10.1017/S1461145707008127. [DOI] [PubMed] [Google Scholar]
- *Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J, Smith LM. Hypothalamic-pituitary-adrenal axis function in dissociative disorders, post-traumatic stress disorder, and healthy volunteers. Biological Psychiatry. 2007;61:966–973. doi: 10.1016/j.biopsych.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledjeski EM, Speisman B, Dierker LC. Does the number of lifetime traumas explain the relationship between PTSD and chronic medical conditions? Answers from the National Comorbidity Survey – Replication (NCS-R) Journal of Behavioral Medicine. 2008;31:341–349. doi: 10.1007/s10865-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Smith MA, Davidson J, Ritchie JC, Kudler H, Lipper S, Chappell P, Nemeroff CB. The corticotropin-releasing hormone test in patients with posttraumatic stress disorder. Biological Psychiatry. 1989;26:349–355. doi: 10.1016/0006-3223(89)90050-4. [DOI] [PubMed] [Google Scholar]
- *Spivak B, Maayan R, Mester R, Weizman A. Plasma testosterone levels in patients with combat-related posttraumatic stress disorder. Neuropsychobiology. 2003;47:57–60. doi: 10.1159/000070009. [DOI] [PubMed] [Google Scholar]
- *Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biological Psychiatry. 1997;42:680–686. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- Stetler C, Dickerson SS, Miller GE. Uncoupling of social zeitgebers and diurnal cortisol secretion in clinical depression. Psychoneuroendocrinology. 2004;29:1250–1259. doi: 10.1016/j.psyneuen.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Stetler CA, Miller GE. Blunted cortisol response to awakening in mild to moderate depression: Regulatory influences of sleep patterns and social contacts. Journal of Abnormal Psychology. 2005;114:697–705. doi: 10.1037/0021-843X.114.4.697. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Annals of the New York Academy of Sciences. 1995;771:1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatric Clinics of North America. 2002;25:397–426. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- *Thaller V, Vrkljan M, Hotujac L, Thakore J. The potential role of hypocortisolism in the pathophysiology of PTSD and psoriasis. Collegium Anthropologicum. 1999;23:611–619. [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. Journal of Clinical Endocrinology and Metabolism. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- *Vidovic A, Vilibic M, Sabioncello A, Gotovac K, Rabatic S, Folnegovic-Smalc V, Dekaris D. Circulating lymphocyte subsets, natural killer cell cytotoxicity, and components of hypothalamaic-pituitary-adrenal axis in Croatian War veterans with posttraumatic stress disorder: Cross-sectional study. Croatian Medical Journal. 2007;48:198–206. [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Development and Psychopathology. 2001;13:721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Czeisler CA, Zimmerman JC, Moore-Ede MC. Biological rhythms in man: Relationship of sleep-wake, cortisol, growth hormone, and temperature during temporal isolation. Advances in Biochemistry and Psychopharmacology. 1981;28:475–499. [PubMed] [Google Scholar]
- *Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatric Clinics of North America. 2002;25:341–368. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their implications. Annals of the New York Academy of Sciences. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- *Yehuda R, Boisoneau D, Lowy MT, Giller EL., Jr Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Archives of General Psychiatry. 1995;52:583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- *Yehuda R, Golier JA, Harvey PD, Stavitsky K, Kaufman S, Grossman RQ, Tischler L. Relationship between cortisol and age-related memory impairments in Holocaust survivors with PTSD. Psychoneuroendocrinology. 2005;30:678–687. doi: 10.1016/j.psyneuen.2005.02.007. [DOI] [PubMed] [Google Scholar]
- *Yehuda R, Golier JA, Yang RK, Tischler L. Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biological Psychiatry. 2004b;55:1110–1116. doi: 10.1016/j.biopsych.2004.02.010. [DOI] [PubMed] [Google Scholar]
- *Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM. Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology. 2004a;29:389–404. doi: 10.1016/s0306-4530(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for PTSD: Relationship to intergenerational effects of trauma, parental PTSD and cortisol excretion. Developmental Psychopathology. 2001;27:733. doi: 10.1017/s0954579401003170. [DOI] [PubMed] [Google Scholar]
- *Yehuda R, Halligan SL, Grossman R, Golier JA, Wong C. The cortisol and glucocorticoid receptor response to low dose dexamethasone administration in aging combat veterans and Holocaust survivors with and without posttraumatic stress disorder. Biological Psychiatry. 2002;52:393–403. doi: 10.1016/s0006-3223(02)01357-4. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Harvey H. Relevance of neuroendocrine alterations in PTSD to cognitive impairments of trauma survivors. In: Read D, Lindsay S, editors. Recollections of Trauma: Scientific Research and Clinical Practice. New York: Plenum Press; 1997. pp. 221–252. [Google Scholar]
- *Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL. Low urinary cortisol excretion in holocaust survivors with posttraumatic stress disorder. The American Journal of Psychiatry. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- *Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. The American Journal of Psychiatry. 1993;150:83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- *Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. The Journal of Nervous and Mental Disease. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Seckly JR, Grossman RA, Morris A, Bierer LM. Parental posttraumatic stress disorder as a vulnerability factor for low cortisol trait in offspring of Holocaust survivors. Archives of General Psychiatry. 2007;64:1040–1048. doi: 10.1001/archpsyc.64.9.1040. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biological Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Saliva cortisol in posttraumatic stress disorder in a community epidemiologic study. Biological Psychiatry. 2004;56:205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Young EA, Haskett RF, Grunhaus L, Pande A, Weinberg VM, Waston SJ, Akil H. Increased evening activation of the hypothalamic-pituitary-adrenal axis in depressed patients. Archives of General Psychiatry. 1994;51:701–707. doi: 10.1001/archpsyc.1994.03950090033005. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Sonntag A, Uhr M, Holsboer F, Ising M. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression: A prospective study. Journal of Psychiatric Research. 2001;35:83–94. doi: 10.1016/s0022-3956(01)00013-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


