Abstract
Background
Improving the quality of mental health care requires integrating successful research interventions into “real-world” practice settings. Coordinated Anxiety Learning and Management (CALM) is a treatment-delivery model for anxiety disorders encountered in primary care. CALM offers cognitive behavioral therapy (CBT), medication, or both; non-expert care managers assisting primary care clinicians with adherence promotion and medication optimization; computer-assisted CBT delivery; and outcome monitoring. This study describes incremental benefits, costs, and net benefits of CALM versus usual care.
Methods
The CALM randomized, controlled effectiveness trial was conducted in 17 primary care clinics in 4 US cities from 2006 to 2009. Of 1,062 eligible patients, 1,004 English- or Spanish-speaking patients age 18–75 years with panic, generalized anxiety, social anxiety, and/or posttraumatic stress disorder with or without major depression were randomized. Anxiety-free days, quality-adjusted life years (QALYs), and expenditures for outpatient visits, emergency room visits, inpatient stays, and psychiatric medications were estimated based on blinded telephone assessments at baseline, 6, 12, and 18 months.
Results
Over 18 months, CALM participants, on average, experienced 57.1 more anxiety-free days [95% confidence interval (CI) 31–83] and $245 additional medical expenses (95% CI $ −733 to $1,223). The mean incremental net benefit of CALM versus usual care was positive when an anxiety-free day was valued ≥ $4. For QALYs based on the Short-Form Health Survey-12 and the EQ-5D the mean incremental net benefit was positive at ≥ $5,000.
Conclusions
Compared with usual care, CALM provides significant benefits with modest increases in health care expenditures.
INTRODUCTION
Anxiety disorders are prevalent, disabling, and costly (DuPont et al. 1996; Olfson et al. 1997; Mendlowicz & Stein, 2000; Stein & Heimberg, 2004; Kessler et al. 2005). Although effective treatments are available, relatively few patients receive them, especially in primary care settings, where the majority of anxious patients is seen (Stein et al. 2004; Stein et al. 2011). CALM is a flexible model for delivering evidence-based treatment for four anxiety disorders often encountered in primary care clinics: panic, generalized anxiety, social anxiety, and posttraumatic stress disorder.
Compared to usual care (UC), CALM resulted in greater improvement in anxiety and depression symptoms, functional disability, and quality of care from baseline to 18 months across all as well as each principal anxiety disorder (Roy-Byrne et al. 2010; Craske et al. 2011). The flexibility of treatment, targeting of multiple anxiety disorders, and clinical effectiveness across a range of patients and clinics suggest that the CALM model should be broadly applicable to primary care practices. However, even after clinical feasibility and effectiveness have been demonstrated, trade-offs between benefits and costs must be considered before disseminating a new treatment model. The current study reports estimates of benefits and costs for CALM versus UC.
When the cost-effectiveness of a new treatment is assessed, ideally, effects within and outside the health care system are taken into account (Russell et al. 1996). Outside the system, anxiety disorders can impact quality of life, employment, educational attainment, and more (Wittchen, 2002; Van Ameringen et al. 2003; Waghorn & Chant, 2005). However, because neither sufficient data on costs nor effects outside the health care system are available for CALM study participants, the current paper focuses on costs within the health care system.
Within the health care system, a new treatment will generate more costs if it requires additional medications, or more, or more expensive, health care visits. Most CALM treatment costs are incurred during a relatively short time, with the exception of minimal ongoing medication costs. In contrast, clinical effects from CALM continue to accumulate beyond the active treatment phase (Roy-Byrne et al. 2010). CALM benefits versus costs should therefore be evaluated beyond the end of treatment. The CALM RCT followed participants for 18 months. For the majority of participants, the 18 months included 12 months of data beyond the end of treatment, as 87% of CALM participants had completed treatment by six months.
METHOD
The CALM study is a randomized, controlled effectiveness trial of the CALM treatment model versus UC. The study and its methods are described in detail in (Sullivan et al. 2007; Roy-Byrne et al. 2010); they are summarized below. The study was approved by the institutional review boards of the Rand Corporation, University of Arkansas, University of California at San Diego, University of California at Los Angeles, and University of Washington.
Settings and subjects
Between June 2006 and April 2008, 1,004 primary care patients were enrolled in 17 clinics in four US cities. The clinics were located in Little Rock, Arkansas, Los Angeles County and San Diego, California, and Seattle, Washington. All participants gave written, informed consent.
Participants were between 18 and 75 years old and English- or Spanish-speaking. All met DSM-IV criteria for one or more of panic disorder (PD), generalized anxiety disorder (GAD), social anxiety disorder (SAD), and posttraumatic stress disorder (PTSD) based on the Mini International Neuropsychiatric Interview (Sheehan et al. 1998). At baseline, they also scored at least 8 (moderate anxiety symptoms on a scale ranging from 0–20) on the Overall Anxiety Severity and Impairment Scale (Campbell-Sills et al. 2009). Co-occurring major depression was permitted. After a baseline interview, participants were randomized to the CALM treatment model or UC.
CALM treatment model
The CALM treatment model offered patients the choice of cognitive behavioral therapy, anti-anxiety medication, or both. To enhance treatment decisions, the model included real-time, web-based clinical outcome monitoring (Unützer et al. 2002a) and a computer-assisted program to optimize CBT delivery by non-expert care managers (Craske et al. 2009). Care managers also assisted primary care clinicians with promoting treatment adherence and optimizing medications. Psychiatrists provided consultation as needed (Sullivan et al. 2007).
The CALM group obtained treatment for 3 to 12 months. Initially, participants received their preferred treatment for 10 to 12 weeks. Participants who were symptomatic and thought to benefit from additional treatment could then receive more of the same or the alternative modality for up to 3 more steps of treatment at 3-month intervals over a year. After treatment completion, participants were entered into continued care and received monthly follow-up telephone calls to reinforce CBT skills, medication adherence, or both.
Usual care
The UC group continued to be treated by their physician in the usual manner. Usual treatment could include medication, counseling (7 of 17 clinics had limited in-clinic mental health resources), or referral to a mental health specialist. After the eligibility diagnostic interview, the only contact between UC participants and study personnel was for assessment by telephone.
Assessments
An assessment battery was administered at baseline and 6, 12, and 18 months post baseline with centralized telephone surveys conducted by the RAND Survey Research Group. Interviewers were blinded to treatment assignment.
Clinical effectiveness measures
CALM’ s primary focus is anxiety. To capture changes in anxiety symptoms resulting from the CALM treatment model, we estimated the number of anxiety-free days (AFD). To capture potential additional benefits, such as improved depression symptoms or functioning in response to the collaborative care model employed by CALM, we also estimated QALYs.
We constructed AFDs with the 12-item Brief Symptom Inventory (BSI-12) subscales for anxiety and somatization (Derogatis, 1993), the main CALM outcome measure. Following (Lave et al. 1998), we first calculated for each BSI-12 score a value between 1 (‘ anxiety free’) and 0 (‘ fully symptomatic’). For BSI-12 scores ≤ 8, the day was considered anxiety-free; for scores ≥ 18 it was considered fully symptomatic; and for scores between 8 and 18, the day was considered anxiety-free proportionally (e.g., a score of 13 corresponds to ½ AFD), similar to criteria used for depression-free days (Simon et al. 2009). Next, we used linear interpolation to estimate the number of AFDs between baseline and the month-6 assessment by averaging the baseline and month-6 AFD values and multiplying the average with the number of days between the two assessments (Lave et al. 1998; Katon et al. 2002; Vannoy et al. 2010). We repeated this approach for the remaining assessment intervals and summed the resulting AFDs per participant.
We estimated QALYs with scores from the Short-Form Health Survey-12 (SF-12) (Ware et al. 1996) and EQ-5D (Rabin & de Charro, 2001). We followed (Brazier & Roberts, 2004) to generate the preference-based index of health, SF-6D. The utility-based algorithm for estimating the measure from a 6-dimensional health state classification was modified to account for scoring of version 2 of the SF-12 (J.E. Brazier, PhD, written communication, April 2010). The algorithm for valuation of the EQ-5D used US population-based EQ-5D preference weights (Shaw et al. 2005). We calculated area under the curve to derive values over 18 months.
Health care cost measures
Participants reported health care use in response to survey questions developed for Partners in Care (Wells, 1999). Participants were asked to enumerate for the six months prior to each assessment the number of visits to primary care providers; medical specialists; psychiatrists; non-psychiatrist mental health providers (e.g., psychologist, psychotherapist); the emergency room; and hospitalizations. Participants were instructed to include CALM treatment visits in their counts. Participants were also asked about psychiatric medications used, including name, dosage, number of pills, and length of time taken.
The cost analysis focused on outpatient visits, ER visits, and psychiatric medication use. Hospitalization cost are presented as secondary information, because hospitalizations are relatively rare and require a large sample to examine differences between intervention and UC (Sturm et al. 1999). All costs are in 2009 US dollars. As recommended by the Medical Expenditure Panel Survey (MEPS), we adjusted cost to $2009 with the Personal Health Care Expenditure component of the National Health Expenditure Accounts (http://www.meps.ahrq.gov/mepsweb/about_meps/Price_Index.shtml).
To estimate cost in the absence of administrative data, we multiplied the number of visits reported by each participant at each assessment with average per-visit expenses from MEPS (Machlin & Carper, 2007b). MEPS expenses reflect payments by private insurance, Medicare, Medicaid, Workers Compensation, and individuals. We used separate average expenses for primary care providers; specialists other than psychiatrists; and psychiatrists. Because MEPS does not differentiate payments to non-psychiatrist mental health providers, we used the average primary care visit expense for them. To estimate ER cost, we multiplied the number of ER visits with the average MEPS ER visit expense. For consistency with outpatient expense estimates, the first author estimated average ER expenses with MEPS data File HC-085E: 2004 Emergency Room Visits. Hospital stay cost are based on MEPS expense estimates for inpatient stays (Machlin & Carper, 2007a). MEPS reports average per diem expenses by length of stay. Thus, we multiplied the number of nights for each stay with the corresponding per diem expense.
We based psychiatric medication cost on average wholesale prices in the 2009 Red Book edition. For each medication, we multiplied the number of pills participants reported having taken with its average Red Book price and then summed across all psychiatric medications by participant.
Cost-effectiveness measure
To compare CALM with UC, we estimated incremental costs and benefits and the incremental net benefit (INB). In recent years, the INB has become the preferred statistic for summarizing results of cost-effectiveness analyses (Nixon et al. 2010). The INB combines incremental costs and incremental benefits into a single, monetary measure (Stinnett & Mullahy, 1998) as follows:
where: λ = monetary value willing to pay per unit of benefit
μEffect CALM = CALM sample mean effect
μEffect UC = UC sample mean effect
μCost CALM = CALM sample mean cost
μCost UC = UC sample mean cost
In contrast to the incremental cost-effectiveness ratio (ICER), the INB is a sum and, as such, avoids some of the ICER’s inherent difficulties. For instance, two opposite cost-benefit results can have the same ICER value when a new intervention is either (a) clearly dominant, due to its lower cost and higher benefit, or (b) clearly inferior, due to its higher cost and lower benefit compared to UC. ICER confidence intervals can also include undefined values or be completely undefined (Willan & Lin, 2001). Because dollar values of an anxiety-free day or quality-adjusted life year have not been established, we estimated the INB for a range of monetary values.
Statistical analysis
We prepared data with SAS version 9.2 (SAS Institute, Cary, NC, USA) and analyzed them with StataSE 11 (StataCorp LP, College Station, TX, USA). Study participants were the unit of analysis. We conducted separate analyses based on original assignment, regardless of treatment received for: (1) participants with complete cost and effectiveness data; (2) participants with complete data using non-response weights; and (3) participants with complete and incomplete data using missing data imputation. We constructed non-response weights and imputed AFDs, QALYs, and health care expenditures to address potential bias due to nonparticipation at follow-up, loss to follow-up, and incomplete item-level data. Weights and imputations used baseline demographic characteristics, health care use, medical and psychiatric conditions, level of functioning and disability, and anxiety and depression symptom scores. We performed multiple imputations with Stata’s mi impute and mi estimate routines and 50 imputations. Twenty-two participants could not be included in the imputations due to missing baseline data.
The distribution of data can be of concern in cost-effectiveness analyses. Because true distributions are unknown, incorrect parametric assumptions about their form may lead to inappropriate inferences. To address this issue, we estimated mean INBs and their confidence intervals non-parametrically with the central limit theorem approach in (Nixon et al. 2010). However, a non-parametric approach is only appropriate if intervention and UC groups are similar at baseline. While this was the case in CALM, we nevertheless adjusted for site to be consistent with (Roy-Byrne et al. 2010) in assessing CALM clinical effects. To control for site, we estimated INBs with linear regression following (Hoch et al. 2002). To examine the influence of outliers, we also estimated INBs with median regression adjusted for site. Median regression is less sensitive to outliers than ordinary linear regression and appropriate when data are skewed (Koenker & Hallock, 2001). Median regression results represent the expected difference in INB medians between CALM and UC.
RESULTS
Complete cost and effectiveness data were available for 692 of the 1,004 participants (69%; 341 UC, 351 CALM). Five participants passed away during the study, 5.6% refused assessment after baseline, and the remaining participants lost to follow-up could not be contacted. Compared to participants with complete data, participants with missing information were younger, more likely to be Hispanic, have lower income, panic and multiple comorbid anxieties, higher disability, anxiety, and depression symptom scores, more ER visits, lower social support, and lower emotional functioning. We excluded two outliers.1
Table 1 provides baseline demographic and clinical characteristics and health care use for the cost-effectiveness sample. At baseline, there were no statistically significant differences between the CALM and UC groups. The sample included more women, was ethnically diverse, and represented a broad age range. It was a fairly ill group; more than half had at least 2 anxiety disorders, 2 chronic medical conditions, and comorbid major depression.
Table 1.
Baseline characteristics of cost-effectiveness sample a
| Characteristic | CALM (n = 349) | Usual Care (n =341) |
|---|---|---|
| n (%) | n (%) | |
| Female | 252 (72.2) | 243 (71.3) |
| Education | ||
| ▪ < High school | 18 (5.2) | 17 (5.0) |
| ▪ 12 years | 53 (15.2) | 58 (17.1) |
| ▪ > 12 years | 278 (79.7) | 265 (78.0) |
| Race/ethnicity | ||
| ▪ Black | 35 (10.0) | 44 (12.9) |
| ▪ Hispanic | 63 (18.1) | 51 (15.0) |
| ▪ White | 206 (59.0) | 205 (60.1) |
| ▪ Other b | 45 (12.9) | 41 (12.0) |
| Anxiety Disordersc | ||
| ▪ Panic | 149 (42.7) | 142 (41.6) |
| ▪ Generalized anxiety | 272 (77.9) | 255 (74.8) |
| ▪ Social phobia | 138 (39.5) | 124 (36.4) |
| ▪ Posttraumatic stress | 61 (17.5) | 58 (17.0) |
| Major depressive disorder | 219 (62.8) | 211 (61.9) |
| Chronic medical conditions | ||
| ▪ 0 | 86 (24.6) | 65 (19.1) |
| ▪ 1 | 66 (18.9) | 75 (22.0) |
| ▪ ≥ 2 | 197 (56.5) | 201 (58.9) |
| Type of health insurance c | ||
| ▪ Medicaid | 26 (7.5) | 34 (10.0) |
| ▪ Medicare | 40 (11.5) | 50 (14.7) |
| ▪ Other government insurance d | 13 (3.8) | 14 (4.1) |
| ▪ Private insurance | 262 (75.3) | 264 (77.7) |
| ▪ No insurance | 52 (14.9) | 35 (10.3) |
| Mean (S.D.) | Mean (S.D.) | |
| Age in years | 44.7 (12.8) | 45.6 (13.6) |
| Health care utilization in 6 months prior to baseline assessment | ||
| ▪ Primary care visits | 4.4 (4.4) | 4.3 (4.8) |
| ▪ Visits to medical specialists other than psychiatrists | 1.0 (2.2) | 1.1 (2.5) |
| ▪ Visits to psychiatrists | 0.3 (1.1) | 0.6 (2.5) |
| ▪ Visits to non-psychiatrist mental health providers | 1.2 (3.3) | 1.5 (3.8) |
| ▪ Emergency room visits | 1.1 (2.6) | 0.8 (1.7) |
| ▪ Nights in hospital | 0.3 (1.0) | 0.2 (0.7) |
There are no significant differences in any baseline characteristics between CALM and usual care participants at p < .05. Differences between CALM and UC participants were assessed with chi-square tests for categorical variables and t-tests for continuous variables. Some numbers do not add up to total number of participants because of missing data. Percentages may not add up to 100 due to rounding.
This category includes race/ethnicity endorsements other than black, Hispanic, or white.
Numbers may total more than 690, because participants can have more than 1 disorder or health insurance.
Other government insurance includes Veterans Administration benefits, TRICARE, county programs, or other government insurance, not otherwise specified.
At each follow-up, mean BSI-12 scores were statistically significantly lower for CALM than UC. The mean number of AFDs from baseline to 18-month follow-up was 57.1 days higher for the CALM treatment group (Table 2). Moreover, at each follow-up, mean EQ-5D and SF-6D scores were statistically significantly higher for CALM than UC by 0.04 to 0.05. Regardless of whether QALYs were measured with the EQ-5D or SF-6D, the CALM intervention added, on average, 0.05 QALYs between baseline and 18-month follow-up (Table 2).
Table 2.
Effectiveness: Baseline to month 6, 12, 18 assessments
| Effectiveness Measure | CALM (n = 349) | Usual Care (n = 341) | Difference |
|---|---|---|---|
| Mean (95% CI)a | Mean (95% CI)a | Mean (95% CI)a | |
|
| |||
| Anxiety-free days (BSI-12) | |||
| ▪ baseline to month 6 | 118.4 (111.8 – 124.9) | 104.5 ( 96.9 – 112.0) | 13.9 ( 3.9 – 23.8)** |
| ▪ month 6 to month 12 | 147.5 (140.9 – 154.0) | 120.1 (112.8 – 127.4) | 27.4 (17.6 – 37.1)*** |
| ▪ month 12 to month 18 | 143.4 (137.1 – 149.6) | 127.5 (120.2 – 134.7) | 15.9 ( 6.3 – 25.4)** |
| ▪ baseline to month 18 | 409.2 (392.3 – 426.1) | 352.1 (332.2 – 371.9) | 57.1 (31.1 – 83.2)*** |
|
| |||
| QALY (EQ-5D) | |||
| ▪ baseline to month 18 | 1.17 (1.14 – 1.19) | 1.11 (1.09 – 1.14) | 0.05 (0.01 – 0.09)** |
|
| |||
| QALY (SF-6D) | |||
| ▪ baseline to month 18 | 1.05 (1.04 – 1.07) | 1.00 (0.98 – 1.02) | 0.05 (0.03 – 0.08)*** |
Results are weighted for non-response.
p<.0001
p<.01
p<.05
The per-participant cost of visits to primary care providers, medical specialists, psychiatrists, other mental health providers, the ER, and psychiatric medications were, on average, $245 higher for CALM than UC (Table 3). This difference is mainly the result of additional primary care visits among the CALM group. From baseline to the 18-month assessment, the average number of visits to medical specialists, psychiatrists, mental health providers other than psychiatrists, and the ER are all lower for CALM than UC, but the differences are not statistically significant at conventional levels. In contrast, the average number of visits to primary care providers is significantly higher for CALM (5.0, 95% CI 3.3–6.6). These additional primary care visits mostly took place within 6 months after randomization, when CALM treatment participants attended CBT sessions and/or medication management visits. Such visits are included in primary care visit counts.
Table 3.
Health care utilization and costs: Baseline to month 6, 12, 18 assessments
| Utilization Measure | CALM (n = 349) | Usual Care (n = 341) | Difference |
|---|---|---|---|
| Mean (95% CI)a | Mean (95% CI)a | Mean (95% CI)a | |
|
| |||
| Primary care visits | |||
| ▪ baseline to month 6 | 8.87 ( 7.95 – 9.78) | 3.88 ( 3.38 – 4.38) | 4.98 ( 3.94 – 6.03)*** |
| ▪ month 6 to month 12 | 3.48 ( 2.92 – 4.04) | 3.37 ( 2.86 – 3.87) | 0.11 (−0.65 – 0.87) |
| ▪ month 12 to month 18 | 2.81 ( 2.48 – 3.14) | 2.92 ( 2.49 – 3.34) | −0.10 (−0.64 – 0.43) |
| ▪ baseline to month 18 | 15.15 (13.86 – 16.44) | 10.16 ( 9.13 – 11.20) | 4.99 (3.34 – 6.64)*** |
|
| |||
| Medical specialist visits | |||
| ▪ baseline to month 6 | 1.13 ( 0.89 – 1.36) | 1.11 ( 0.81 – 1.40) | −0.02 (−0.36 – 0.40) |
| ▪ month 6 to month 12 | 0.99 ( 0.75 – 1.24) | 1.18 ( 0.90 – 1.46) | −0.18 (−0.55 – 0.19) |
| ▪ month 12 to month 18 | 0.93 ( 0.67 – 1.18) | 1.21 ( 0.91 – 1.51) | −0.29 (−0.68 – 0.11) |
| ▪ baseline to month 18 | 3.05 ( 2.51 – 3.59) | 3.50 ( 2.83 – 4.16) | −0.45 (−1.30 – 0.41) |
|
| |||
| Psychiatrist visits | |||
| ▪ baseline to month 6 | 0.65 ( 0.39 – 0.91) | 0.70 ( 0.43 – 0.97) | −0.05 (−0.42 – 0.32) |
| ▪ month 6 to month 12 | 0.50 ( 0.23 – 0.78) | 0.67 ( 0.39 – 0.96) | −0.17 (−0.57 – 0.22) |
| ▪ month 12 to month 18 | 0.46 ( 0.16 – 0.77) | 0.88 ( 0.56 – 1.21) | −0.42 (−0.86 – 0.02) |
| ▪ baseline to month 18 | 1.61 ( 0.99 – 2.24) | 2.26 ( 1.60 – 2.92) | −0.64 (−1.55 – 0.26) |
|
| |||
| Non-psychiatrist mental health provider visits | |||
| ▪ baseline to month 6 | 2.27 ( 1.77 – 2.78) | 2.06 ( 1.48 – 2.65) | 0.21 (−0.56 – 0.99) |
| ▪ month 6 to month 12 | 1.53 ( 1.04 – 2.02) | 1.98 ( 1.38 – 2.59) | −0.45 (−1.23 – 0.32) |
| ▪ month 12 to month 18 | 1.06 ( 0.65 – 1.47) | 2.34 ( 1.61 – 3.04) | −1.27 (−2.09 – −0.44)** |
| ▪ baseline to month 18 | 4.87 ( 3.84 – 5.90) | 6.37 ( 4.76 – 7.99) | −1.51 (−3.42 – 0.41) |
|
| |||
| All outpatient visits | |||
| ▪ baseline to month 6 | 12.92 (11.80 – 14.04) | 7.75 ( 6.72 – 8.78) | 5.17 ( 3.64 – 69)*** |
| ▪ month 6 to month 12 | 6.50 ( 5.52 – 7.48) | 7.20 ( 6.12 – 8.28) | −0.70 (−2.15 – 76) |
| ▪ month 12 to month 18 | 5.26 ( 4.48 – 6.04) | 7.34 ( 6.18 – 8.49) | −2.08 (−3.47 – .69)** |
| ▪ baseline to month 18 | 24.68 (22.47 – 26.89) | 22.29 (19.54 – 25.04) | 2.39 (−1.13 – 91) |
|
| |||
| Emergency room visits | |||
| ▪ baseline to month 6 | 0.55 ( 0.39 – 0.70) | 0.65 ( 0.50 – 0.81) | −0.11 (−0.33 – 0.11) |
| ▪ month 6 to month 12 | 0.46 ( 0.34 – 0.58) | 0.48 ( 0.35 – 0.61) | −0.02 (−0.20 – 0.16) |
| ▪ month 12 to month 18 | 0.48 ( 0.35 – 0.61) | 0.50 ( 0.37 – 0.63) | −0.02 (−0.20 – 0.17) |
| ▪ baseline to month 18 | 1.48 ( 1.16 – 1.81) | 1.63 ( 1.32 – 1.94) | −0.15 (−0.60 – 0.30) |
|
| |||
| Nights in hospital | |||
| ▪ baseline to month 6 | 0.19 ( 0.07 – 0.32) | 0.66 (−0.08 – 1.39) | −0.46 (−1.20 – 0.28) |
| ▪ month 6 to month 12 | 0.42 ( 0.12 – 0.73) | 0.46 ( 0.08 – 0.84) | −0.04 (−0.52 – 0.45) |
| ▪ month 12 to month 18 | 0.27 ( 0.10 – 0.44) | 0.30 ( 0.10 – 0.50) | −0.03 (−0.29 – 0.23) |
| ▪ baseline to month 18 | 0.89 ( 0.45 – 1.32) | 1.41 ( 0.56 – 2.27) | −0.53 (−1.49 – 0.43) |
|
| |||
| Cost Measure (2009 US $) | CALM (n = 349) | Usual Care (n = 341) | Difference |
| Mean (95% CI)a | Mean (95% CI)a | Mean (95% CI)a | |
|
| |||
| Total outpatient visit, ER visit, psychiatric medication cost | |||
| ▪ baseline to month 6 | 3027.1(2776.3 – 3277.9) | 2478.2(2202.8 – 2753.6) | 548.9 (177.4 – 920.3)** |
| ▪ month 6 to month 12 | 2222.8(1969.9 – 2475.7) | 2342.1(2047.1 – 2637.1) | −119.3 (−507.2 – 268.6) |
| ▪ month 12 to month 18 | 2060.6(1810.1 – 2311.1) | 2245.4(1970.9 – 2519.8) | −184.8 (−555.4 – 185.8) |
| ▪ baseline to month 18 | 7310.5(6669.6 – 7951.4) | 7065.7(6325.0 – 7806.4) | 244.8 (−733.0 – 1222.6) |
|
| |||
| Primary care visit cost | |||
| ▪ baseline to month 6 | 1032.3 (925.7 – 1138.9) | 452.0 (393.6 – 510.4) | 580.3 (458.9 – 701.7)*** |
| ▪ month 6 to month 12 | 404.6 (338.3 – 471.0) | 392.0(333.0 – 451.1) | 12.6 (−76.1 – 101.3) |
| ▪ month 12 to month 18 | 327.4 (288.7 – 366.1) | 339.5(290.1 – 388.9) | −12.1 (−74.7 – 50.5) |
| ▪ baseline to month 18 | 1764.3(1614.2 – 1914.5) | 1183.5(1062.7 – 1304.3) | 580.8 (388.4 – 773.2)*** |
|
| |||
| Medical specialist visit cost | |||
| ▪ baseline to month 6 | 134.9 (106.4 – 163.4) | 132.7 ( 97.4 – 167.9) | 2.3 (−43.0 – 47.6) |
| ▪ month 6 to month 12 | 119.4 (89.5 – 149.2) | 141.0 (107.4 – 174.6) | −21.6 (−66.4 – 23.2) |
| ▪ month 12 to month 18 | 111.3 (80.7 – 141.8) | 145.4 (109.5 – 181.4) | −34.2 (−81.3 – 12.9) |
| ▪ baseline to month 18 | 365.6 (300.7 – 430.4) | 419.1 (339.7 – 498.5) | −53.5 (−155.9 – 48.8) |
|
| |||
| Psychiatrist visit cost | |||
| ▪ baseline to month 6 | 71.1 (43.1 – 99.2) | 76.6 (47.3 – 105.9) | −5.4 (−45.9 – 35.0) |
| ▪ month 6 to month 12 | 54.9 (24.9 – 85.0) | 73.8 (42.3 – 105.3) | −18.9 (−62.4 – 24.6) |
| ▪ month 12 to month 18 | 50.6 (17.3 – 83.9) | 96.8 (61.5 – 132.2) | −46.2 (−94.7 – 2.2) |
| ▪ baseline to month 18 | 176.7 (108.1 – 245.3) | 247.3 (174.9 – 319.7) | −70.6 (−170.1 – 28.9) |
|
| |||
| Non-psychiatrist mental health provider visit cost | |||
| ▪ baseline to month 6 | 265.1 (205.9 – 324.2) | 240.3 (171.9 – 308.8) | 24.7 (−65.6 – 115.0) |
| ▪ month 6 to month 12 | 178.2 (120.9 – 235.5) | 231.0 (161.0 – 301.1) | −52.8 (−143.1 – 37.5) |
| ▪ month 12 to month 18 | 123.3 (75.9 – 170.6) | 270.7 (187.2 – 354.2) | −147.4 (−243.3 – −51.6)** |
| ▪ baseline to month 18 | 566.5 (446.5 – 686.5) | 742.0 (554.0 – 930.0) | −175.5 (−398.2 – 47.3) |
|
| |||
| All outpatient visit cost | |||
| ▪ baseline to month 6 | 1503.4(1372.9 – 1634.0) | 901.6 (781.8 – 1021.4) | 601.8 (424.8 – 778.9)*** |
| ▪ month 6 to month 12 | 757.2 (643.5 – 870.9) | 837.9 (712.5 – 963.3) | −80.7 (−249.5 – 88.1) |
| ▪ month 12 to month 18 | 612.5 (523.0 – 702.0) | 852.5 (718.4 – 986.5) | −240.0 (−400.9 – −79.0)** |
| ▪ baseline to month 18 | 2873.1(2617.3 – 3128.9) | 2591.9(2273.6 – 2910.3) | 281.2 (−126.6 – 688.9) |
|
| |||
| Emergency Room visit cost | |||
| ▪ baseline to month 6 | 418.4 (301.4 – 535.5) | 502.4 (380.9 – 623.9) | −84.0 (−252.3 – 84.4) |
| ▪ month 6 to month 12 | 353.0 (257.8 – 448.2) | 368.4 (268.7 – 468.0) | −15.4 (−152.9 – 122.1) |
| ▪ month 12 to month 18 | 368.0 (267.3 – 468.8) | 382.5 (282.0 – −483.0) | −14.5 (−156.6 – 127.6) |
| ▪ baseline to month 18 | 1139.5 (887.9 – 1391.0) | 1253.3 (1016.8 – 1489.8) | −113.9 (−458.8 – 231.1) |
|
| |||
| Psychiatric medication cost | |||
| ▪ baseline to month 6 | 1105.2 (947.8 – 1262.7) | 1074.2 (901.6 – 1246.8) | 31.0 (−202.0 – 264.0) |
| ▪ month 6 to month 12 | 1112.7 (944.1 – 1281.3) | 1135.8 (940.7 – 1331.0) | −23.2 (−280.6 – 234.2) |
| ▪ month 12 to month 18 | 1080.0 (894.0 – 1266.1) | 1010.4 (849.6 – 1171.2) | 69.7 (−175.8 – 315.2) |
| ▪ baseline to month 18 | 3298.0(2839.8 – 3756.1) | 3220.5 (2752.6 – 3688.3) | 77.5 (−576.1 – 731.1) |
|
| |||
| Nights in hospital cost | |||
| ▪ baseline to month 6 | 531.6 (249.2 – 814.0) | 1526.8 (27.1 – 3026.6) | −995.2 (−2521.0 – 530.5) |
| ▪ month 6 to month 12 | 1053.4 (409.8 – 1696.9) | 1095.6 (313.4 – 1877.9) | −42.3 (−1053.5 – 986.9) |
| ▪ month 12 to month 18 | 692.4 (324.9 – 1059.9) | 808.4 (385.9 – 1230.9) | 116.0 (−675.0 – 443.0) |
| ▪ baseline to month 18 | 2277.3 (356.3 – 3198.4) | 3430.9 (1655.3 – 5206.5) | −1153.5 (−3151.4 – 844.4) |
Results are weighted for non-response.
p<.0001
p<.01
p<.05
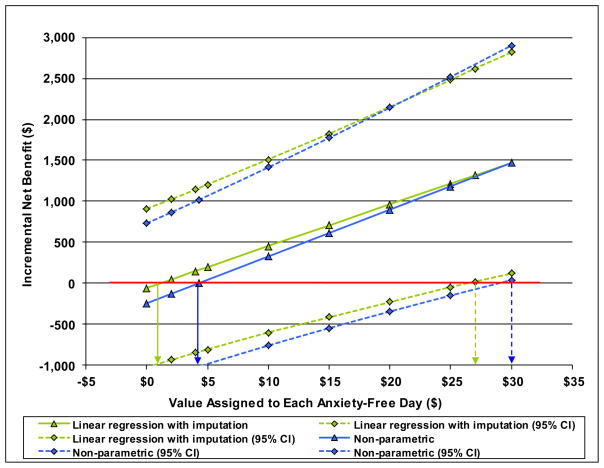
The INB for anxiety-free days of CALM versus UC represents the monetary value of the additional mean AFDs experienced by CALM participants minus their additional mean costs for outpatient and ER visits and psychiatric medications. Figure 1 depicts how this INB varies depending on the value assigned to an AFD according to (a) non parametric and (b) linear regression estimates with imputed data. When an AFD is valued $0, the INB of CALM is negative in the amount of the CALM added cost of about $245 over 18 months. As an AFD is valued increasingly more highly, the INB becomes positive; that is, the value of added days free of anxiety exceeds the additional cost of CALM. According to the non-parametric results, an AFD has to be worth $4 to reach a positive INB, but at $4, the 95% confidence interval includes negative INBs. This confidence interval includes only positive values when an AFD is valued $30. For the linear regression results with imputation, the INB becomes positive when an AFD is valued ≥ $2 and the 95% CI includes only positive values at ≥ $27. The results obtained with the other estimation approaches are qualitatively similar to those in Figure 1 (Table 4).
Figure 1.
Incremental Net Benefit of CALM compared to Usual Care for anxiety-free days. The Incremental Net Benefit varies with the $ value assigned to each additional anxiety-free day.
Table 4.
Incremental Net Benefit (INB) Estimates by Estimation Approach
| Estimation Approach | |||||
|---|---|---|---|---|---|
|
| |||||
| INB Measure | Non- Parametric with Non- Response Weight (n = 690) | Linear Regression (n = 690) | Linear Regression with Non- Response Weight (n = 690) | Linear Regression with Imputation (n = 982) | Median Regression with Imputation (n = 982) |
|
| |||||
| Anxiety-Free Days | |||||
| INB positive at | $4 | $3 | $4 | $2 | $10 |
| INB 95% CI positive at | $30 | $30 | $29 | $27 | $20 |
|
| |||||
| QALY (EQ-5D) | |||||
| INB positive at | $5,000 | $3,000 | $5,000 | $2,500 | $10,000 |
| INB 95% CI positive at | $60,000 | $55,000 | $60,000 | $90,000 | $80,000 |
|
| |||||
| QALY (SF-6D) | |||||
| INB positive at | $5,000 | $2,500 | $5,000 | $2,500 | $15,000 |
| INB 95% CI positive at | $35,000 | $30,000 | $35,000 | $30,000 | $70,000 |
The INB estimates for QALYs are also quite similar across estimation approaches (Table 4). One exception is the somewhat wider EQ-5D QALY confidence interval obtained with linear regression and imputation. For both QALY measures, a QALY has to be worth between $2,500 and $5,000 to reach a positive INB. To reach positive 95% CIs, a QALY has to be worth ≥ $90,000 for the EQ-5D and ≥ $35,000 for the SF-6D.
DISCUSSION
For depression treatment in primary care, over 40 studies have documented the effectiveness of collaborative care, that is, care-manager assisted chronic disease management programs (e.g., (Katon et al. 1995; Katon et al. 1996; Katon et al. 1999; Katzelnick et al. 2000; Simon et al. 2000; Wells et al. 2000; Rost et al. 2002; Unützer et al. 2002b). Although anxiety disorders are more prevalent than depression (Kessler et al. 1994) and equally as disabling and costly (Greenberg et al. 1999; Kessler, 2000; Mendlowicz & Stein, 2000; Stein & Kean, 2000; Stein & Heimberg, 2004), collaborative care for the treatment of anxiety disorders in primary care has been examined in only three prior studies (Roy-Byrne et al. 2001; Rollman et al. 2005; Roy-Byrne et al. 2005). These studies focused on panic or generalized anxiety disorder. Thus, CALM is the first RCT to provide estimates of benefits and costs of a collaborative care treatment model in primary care settings for patients with multiple anxiety disorders.
As previously reported (Roy-Byrne et al. 2010; Craske et al. 2011), compared to usual care, CALM showed clinical benefits for patients with PD, GAD, SAD, and PTSD over the 18-month study. As described here, CALM, also resulted in 57 additional anxiety-free days over the 18 months. This average is below estimates reported earlier for primary care PD patients during a one-year follow-up (Katon et al. 2002; Katon et al. 2006). The third study (Rollman et al. 2005) did not report AFD estimates. One reason for the discrepancy may be measurement-based. In contrast to CALM, the panic disorder studies derived anxiety-free days from ASI not BSI-12 scores. For panic disorder, the ASI has been shown to have a larger effect size than other anxiety self-report measures (Hazen et al. 1996). Further, CALM enrolled participants who used alcohol or marijuana. Such patients may be more treatment resistant, which may result in fewer AFDs.
The average difference in combined costs for outpatient visits, ER visits, and psychiatric medications between CALM and UC was $245 during the 18 months. These additional costs of CALM are below the $473 incremental outpatient cost reported by (Katon et al. 2006) for primary care patients with PD. The earlier study was able to include additional cost categories, collected health care use data differently, but also used a narrower cost measure than the study reported here. The other extant PD study reported $325 fewer outpatient cost for the intervention group (Katon et al. 2002). In this latter study, diagnostic tests and non-mental health medications contributed considerably to the lower cost for the intervention group. The CALM study did not have cost data for either category.
The incremental net benefit of CALM reflects the trade-off between its clinical benefits and additional health care costs compared to usual care. When an anxiety-free day is valued ≥ $4, the additional cost of CALM is offset by the additional AFDs CALM affords. This result compares favorably with the $8.40 reported by one PD study (Katon et al. 2006), but is slightly higher than the -$4.00 reported by the other PD study (Katon et al. 2002).
To our knowledge, there is no agreement on how to value a day free of anxiety. Primary care patients who have been treated for depression, on average, were willing to pay about $10 (in 2000 US dollars) per depression-free day (Unützer et al. 2003). If patients with anxiety disorders value a day free of anxiety similarly, the CALM treatment model provides a worthwhile benefit.
A figure of $50,000 per quality-adjusted life-year gained is commonly referenced in the literature as a threshold for considering a new intervention (Grosse, 2008). Thus, at point estimates of between $2,500 and $5,000 per QALY gained, the CALM treatment model has potential to provide value to patients.
LIMITATIONS
Several study limitations need to be noted. First, costs and benefits are based on the first 18 months after randomization. Studies of collaborative care for depression in primary care indicate that clinical benefits and reductions in general medical costs may continue considerably beyond 18 months (Simon et al. 2009). Hence, if the results from depression studies extend to collaborative care treatment for anxiety disorders in primary care, the incremental benefits and cost reported for CALM are conservative.
Second, our cost estimates were derived from self-reported health care and medication use. If there is a systematic difference in reporting health care visits and medication use between CALM and UC participants, the incremental net benefit of CALM could be biased. A priori, we have no reason to expect differential reporting between the two groups.
Third, due to data limitations, the cost estimates do not cover medical procedures and non-psychiatric medications. Because CALM reduced somatic anxiety symptoms, CALM participants likely underwent fewer medical procedures during follow-up than UC participants. In this case, the incremental cost of CALM may be overestimated, resulting in conservative cost-effectiveness estimates.
Fourth, data limitations also prevented us from distinguishing between primary care and Anxiety Clinical Specialist (ACS) visits. ACS visits, which are central to the CALM treatment model, are typically cheaper than primary care visits, as they are provided mostly by social workers and nurses. The reported cost estimates for CALM could, therefore, be higher than its actual cost, producing again conservative cost-effectiveness estimates.
Fifth, benefits realized outside the health care system, such as improved productivity at work or at home, are not incorporated for lack of data. If such benefits were included, the INB may become positive at a lower monetary value per AFD than reported here.
Lastly, the results are based on data from 70% of participants in the baseline sample. It is unknown whether the benefit-cost trade-off is different for the remaining participants and whether a difference would change the results. Because our estimates remained qualitatively the same when we addressed missing data with weights or multiple imputations, we are hopeful the reported results are stable.
CONCLUSION
Persons with anxiety disorders are most often treated in primary care settings. Despite the high prevalence of anxiety disorders (Kessler et al. 2005) and an increase in the proportion of individuals seeking help (Wang et al. 2005), patient care is not necessarily evidence-based. Quality improvement interventions within those settings are, therefore, much needed. Patients with anxiety disorders whose care was provided with the CALM treatment model, on average, experienced greater improvement in anxiety and depression symptoms, functional disability, and quality of care during 18 months of follow-up than patients in UC (Roy-Byrne et al. 2010). Importantly, these benefits were achieved with modest increases in health care expenditures. Thus, CALM holds promise for improving the lives of patients with anxiety disorders seen in primary care clinics.
It has been well documented that under the current reimbursement system, financial barriers preclude the integration of mental health services into primary care (Butler et al. 2008). Organizational barriers pose further challenges to the successful integration of mental health services into primary care. Whether private insurers and other payers will use research findings to make decisions about covering evidence-based treatments and how much to pay for them remain open questions. Until these challenges are addressed, mental health care in the U.S. will continue to fail millions of patients in need of effective care.
Acknowledgments
This work was supported by grants U01 MH057858 and K24 MH065324 (Dr. Roy-Byrne), U01 MH058915 (Dr. Craske), U01 MH070022 (Dr. Sullivan), U01 MH070018 (Dr. Sherbourne), and U01 MH057835 and K24 MH64122 (Dr. Stein) from the National Institute of Mental Health.
The CALM study’s oversight was managed by the National Institute of Mental Health data and safety monitoring board, which has a rotating panel of members. The National Institute of Mental Health had no other involvement with the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Bernadette Benjamin, MS (RAND Corporation, Santa Monica, California); Daniela Golinelli, PhD (RAND Corporation, Santa Monica, California); and Imara I. West, MPH (University of Washington, Seattle, Washington) prepared some of the data used in the analysis.
In addition to the second through sixth author of this manuscript, the following individuals conducted the CALM randomized controlled trial (in alphabetical order): Alexander Bystritsky, MD (University of California, Los Angeles, California), Laura Campbell-Sills, PhD (University of California, San Diego, California), Denise A. Chavira, PhD (University of California, San Diego, California), Mark J. Edlund, MD, PhD (University of Arkansas for Medical Sciences, Little Rock, Arkansas), Daniela Golinelli, PhD (RAND Corporation, Santa Monica, California), Ariel J. Lang, PhD, MPH (VA San Diego Healthcare System and University of California, San Diego, California), Raphael D. Rose, PhD (University of California, Los Angeles, California), and Stacy Shaw Welch, PhD (University of Washington, Seattle, Washington). Kristin Bumgardner, BS (University of Washington, Seattle, Washington) was responsible for the central coordination of the CALM study.
Footnotes
One participant reported 194 outpatient and ER visits for 18 months, including 90 primary care visits for a 6-month time period. The other participant reported 162 outpatient and ER visits for the 18 months.
Clinical Trial Registration: clinicaltrials.gov Identifier: NCT00347269
DECLARATION OF INTEREST
Dr Roy-Byrne reported receiving research grant support from the National Institutes of Health; having served as a paid member of advisory boards for Jazz Pharmaceuticals and Solvay Pharmaceuticals (1 meeting for each); having received honoraria for CME-sponsored speaking from the American Psychiatric Association, Anxiety Disorders Association of America, CME LLC, CMP Media, Current Medical Directions, Imedex, Massachusetts General Hospital Academy, and PRIMEDIA Healthcare; and serving as editor in chief for Journal Watch Psychiatry (Massachusetts Medical Society), Depression and Anxiety (Wiley-Liss Inc), and UpToDate in Psychiatry (UpToDate Inc). Dr Roy-Byrne reported also serving as an expert witness on multiple legal cases related to anxiety; none involving pharmaceutical companies or specific psychopharmacology issues.
Dr Craske reported receiving research grant support from the National Institutes of Health and having received honoraria for sponsored speaking from the Anxiety Disorders Association of America.
Dr Stein reported receiving or having received research support from the US Department of Defense, Eli Lilly, GlaxoSmithKline, Hoffmann-La Roche, National Institutes of Health, and the US Veterans Affairs Research Program; and is currently or has been a paid consultant for AstraZeneca, Avera Pharmaceuticals, BrainCells Inc, Bristol-Myers Squibb, Comprehensive NeuroScience, Eli Lilly, Forest Laboratories, GlaxoSmithKline, Hoffmann-La Roche, Jazz Pharmaceuticals, Johnson & Johnson, Mindsite, Pfizer, Sepracor, and Transcept Pharmaceuticals. Dr Stein is paid for editorial work on the journal Depression and Anxiety and UpToDate in Psychiatry (UpToDate Inc).
No other author reported financial disclosures or conflicts of interest.
Contributor Information
Jutta M. Joesch, Department of Psychiatry & Behavioral Sciences, University of Washington School of Medicine and Harborview Center for Healthcare Improvement for Addictions, Mental Illness, and Medically Vulnerable Populations (CHAMMP), Seattle, Washington.
Cathy D. Sherbourne, Rand Corporation, Santa Monica, California.
Greer Sullivan, Department of Psychiatry and VA South Central Mental Illness Research, Education, and Clinical Center, University of Arkansas for Medical Sciences, Little Rock, Arkansas.
Murray B. Stein, Departments of Psychiatry and Family and Preventive Medicine, University of California, La Jolla, California.
Michelle G. Craske, Departments of Psychology and Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, California.
Peter Roy-Byrne, Department of Psychiatry & Behavioral Sciences, University of Washington School of Medicine and Harborview Center for Healthcare Improvement for Addictions, Mental Illness, and Medically Vulnerable Populations (CHAMMP), Seattle, Washington.
References
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Medical Care. 2004;42:851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- Butler M, Kane RL, McAlpine D, Kathol RG, Fu SS, Hagedorn H, Wilt TJ. Integration of mental health/substance abuse and primary care. Evidence Report/Technology Assessment (Full Rep) 2008:1–362. [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Norman SB, Craske MG, Sullivan G, Lang AJ, Chavira DA, Bystritsky A, Sherbourne CD, Roy-Byrne P, Stein MB. Validation of a brief measure of anxiety-related severity and impairment: the Overall Anxiety Severity and Impairment Scale (OASIS) Journal of Affective Disorders. 2009;112:92–101. doi: 10.1016/j.jad.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Rose RD, Lang AJ, Welch SS, Campbell-Sills L, Sullivan G, Sherbourne CD, Bystritsky A, Stein MB, Roy-Byrne PP. Computer-assisted delivery of cognitive behavioral therapy for anxiety disorders in primary-care settings. Depression and Anxiety. 2009;26:235–242. doi: 10.1002/da.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Sullivan G, Sherbourne CD, Bystritsky A, Rose RD, Lang AJ, Welch SS, Campbell-Sills L, Golinelli D, Roy-Byrne P. Disorder specific impact of CALM treatment for anxiety disorders in primary care. Archives of General Psychiatry. 2011;68:378–388. doi: 10.1001/archgenpsychiatry.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. Brief Symptom Inventory (BSI) Administration, Scoring, and Procedures Manual. 3. NCS Person, Inc; Minneapolis: 1993. [Google Scholar]
- DuPont RL, Rice DP, Miller SL, Shiraki SS, Rowland CR, Harwood HJ. Economic costs of anxiety disorders. Anxiety. 1996;2:167–172. doi: 10.1002/(SICI)1522-7154(1996)2:4<167::AID-ANXI2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, Ballenger JC, Fyer AJ. The economic burden of anxiety disorders in the 1990s. Journal of Clinical Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- Grosse SD. Assessing cost-effectiveness in healthcare: History of the $50,000 per QALY threshold. Expert Review of Pharmacoeconomics & Outcomes Research. 2008;8:165–178. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- Hazen AL, Walker JR, Eldridge GD. Anxiety sensitivity and treatment outcome in panic disorder. Anxiety. 1996;2:34–39. doi: 10.1002/(SICI)1522-7154(1996)2:1<34::AID-ANXI5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Economics. 2002;11:415–430. doi: 10.1002/hec.678. [DOI] [PubMed] [Google Scholar]
- Katon W, Robinson P, Von Korff M, Lin E, Bush T, Ludman E, Simon G, Walker E. A multifaceted intervention to improve treatment of depression in primary care. Archives of General Psychiatry. 1996;53:924–932. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- Katon W, Roy-Byrne PP, Russo J, Cowley D. Cost-effectiveness and cost offset of a collaborative care intervention for primary care patients with panic disorder. Archives of General Psychiatry. 2002;59:1098–1104. doi: 10.1001/archpsyc.59.12.1098. [DOI] [PubMed] [Google Scholar]
- Katon W, Russo J, Sherbourne CD, Stein MB, Craske MG, Fan M-Y, Roy-Byrne P. Incremental cost-effectiveness of a collaborative care intervention for panic disorder. Psychological Medicine. 2006;36:353–363. doi: 10.1017/S0033291705006896. [DOI] [PubMed] [Google Scholar]
- Katon W, Von Korff M, Lin E, Simon G, Walker E, Unützer J, Bush T, Russo J, Ludman E. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Archives of General Psychiatry. 1999;56:1109–1115. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- Katon W, von Korff M, Lin EH, Walker ED, Simon GE, Bush TM, Robinson P, Russo J. Collaborative management to achieve treatment guidelines. Impact on depression in primary care. JAMA. 1995;273:1026–1031. [PubMed] [Google Scholar]
- Katzelnick DJ, Simon GE, Pearson SD, Manning WG, Helstad CP, Henk HJ, Cole SM, Lin EH, Taylor LH, Kobak KA. Randomized trial of a depression management program in high utilizers of medical care. Archives of Family Medicine. 2000;9:345–351. doi: 10.1001/archfami.9.4.345. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. Journal of Clinical Psychiatry. 2000;61(suppl 5):4–12. discussion 13–14. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Koenker R, Hallock KF. Quantile regression: an introduction. Journal of Economic Perspectives. 2001;15:143–156. [Google Scholar]
- Lave JR, Frank RG, Schulberg HC, Kamlet MS. Cost-effectiveness of treatments for major depression in primary care practice. Archives of General Psychiatry. 1998;55:645–651. doi: 10.1001/archpsyc.55.7.645. [DOI] [PubMed] [Google Scholar]
- Machlin SR, Carper K. Expenses for Hospital Inpatient Stays, 2004. Statistical Brief #164. Agency for Healthcare Research and Quality; Rockville, MD: 2007a. [PubMed] [Google Scholar]
- Machlin SR, Carper K. Statistical Brief #166. Agency for Healthcare Research and Quality; Rockville, MD: 2007b. Expenses for Office-Based Physician Visits by Specialty, 2004. [Google Scholar]
- Mendlowicz MV, Stein MB. Quality of life in individuals with anxiety disorders. American Journal of Psychiatry. 2000;157:669–682. doi: 10.1176/appi.ajp.157.5.669. [DOI] [PubMed] [Google Scholar]
- Nixon RM, Wonderling D, Grieve RD. Non-parametric methods for cost-effectiveness analysis: the central limit theorem and the bootstrap compared. Health Economics. 2010;19:316–333. doi: 10.1002/hec.1477. [DOI] [PubMed] [Google Scholar]
- Olfson M, Fireman B, Weissman MM, Leon AC, Sheehan DV, Kathol RG, Hoven C, Farber L. Mental disorders and disability among patients in a primary care group practice. American Journal of Psychiatry. 1997;154:1734–1740. doi: 10.1176/ajp.154.12.1734. [DOI] [PubMed] [Google Scholar]
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Annals of Medicine. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- Rollman BL, Belnap BH, Mazumdar S, Houck PR, Zhu F, Gardner W, Reynolds CF, 3rd, Schulberg HC, Shear MK. A randomized trial to improve the quality of treatment for panic and generalized anxiety disorders in primary care. Archives of General Psychiatry. 2005;62:1332–1341. doi: 10.1001/archpsyc.62.12.1332. [DOI] [PubMed] [Google Scholar]
- Rost K, Nutting P, Smith JL, Elliott CE, Dickinson M. Managing depression as a chronic disease: a randomised trial of ongoing treatment in primary care. BMJ. 2002;325:934–937. doi: 10.1136/bmj.325.7370.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne P, Craske MG, Sullivan G, Rose RD, Edlund MJ, Lang AJ, Bystritsky A, Welch SS, Chavira DA, Golinelli D, Campbell-Sills L, Sherbourne CD, Stein MB. Delivery of evidence-based treatment for multiple anxiety disorders in primary care: a randomized controlled trial. JAMA. 2010;303:1921–1928. doi: 10.1001/jama.2010.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne P, Katon W, Cowley D, Russo J. A randomized effectiveness trial of collaborative care for patients with panic disorder in primary care. Archives of General Psychiatry. 2001;58:869–876. doi: 10.1001/archpsyc.58.9.869. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Craske MG, Stein MB, Sullivan G, Bystritsky A, Katon W, Golinelli D, Sherbourne CD. A randomized effectiveness trial of cognitive-behavioral therapy and medication for primary care panic disorder. Archives of General Psychiatry. 2005;62:290–298. doi: 10.1001/archpsyc.62.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172–1177. [PubMed] [Google Scholar]
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Medical Care. 2005;43:203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar G. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59 (suppl 20):22–33. [PubMed] [Google Scholar]
- Simon GE, Ludman E, Rutter CM. Incremental benefit and cost of telephone care management and telephone psychotherapy for depression in primary care. Archives of General Psychiatry. 2009;66:1081–1089. doi: 10.1001/archgenpsychiatry.2009.123. [DOI] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Rutter CM, Wagner E. Randomized trial of monitoring, feedback, and management of care by telephone to improve treatment of depression in primary care. BMJ. 2000;320:550–554. doi: 10.1136/bmj.320.7234.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Heimberg RG. Well-being and life satisfaction in generalized anxiety disorder: comparison to major depressive disorder in a community sample. Journal of Affective Disorders. 2004;79:161–166. doi: 10.1016/S0165-0327(02)00457-3. [DOI] [PubMed] [Google Scholar]
- Stein MB, Kean YM. Disability and quality of life in social phobia: epidemiologic findings. American Journal of Psychiatry. 2000;157:1606–1613. doi: 10.1176/appi.ajp.157.10.1606. [DOI] [PubMed] [Google Scholar]
- Stein MB, Sherbourne CD, Craske MG, Means-Christensen A, Bystritsky A, Katon W, Sullivan G, Roy-Byrne PP. Quality of care for primary care patients with anxiety disorders. American Journal of Psychiatry. 2004;161:2230–2237. doi: 10.1176/appi.ajp.161.12.2230. [DOI] [PubMed] [Google Scholar]
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Medical Decision Making. 1998;18:S68–S80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- Sturm R, Unützer J, Katon W. Effectiveness research and implications for study design: sample size and statistical power. General Hospital Psychiatry. 1999;21:277–283. doi: 10.1016/s0163-8343(99)00024-9. [DOI] [PubMed] [Google Scholar]
- Sullivan G, Craske MG, Sherbourne CD, Edlund MJ, Rose RD, Golinelli D, Chavira DA, Bystritsky A, Stein MB, Roy-Byrne PP. Design of the Coordinated Anxiety Learning and Management (CALM) study: innovations in collaborative care for anxiety disorders. General Hospital Psychiatry. 2007;29:379–387. doi: 10.1016/j.genhosppsych.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unützer J, Choi Y, Cook IA, Oishi S. A web-based data management system to improve care for depression in a multicenter clinical trial. Psychiatric Services. 2002a;53:671–673. 678. doi: 10.1176/ps.53.6.671. [DOI] [PubMed] [Google Scholar]
- Unützer J, Katon W, Callahan CM, Williams JWJ, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EHB, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C. Collaborative care management of late-life depression in the primary care setting. JAMA. 2002b;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- Unützer J, Katon WJ, Russo J, Simon G, von Korff M, Lin E, Walker E, Ludman E, Bush T. Willingness to pay for depression treatment in primary care. Psychiatric Services. 2003;54:340–345. doi: 10.1176/ps.54.3.340. [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Mancini C, Farvolden P. The impact of anxiety disorders on educational achievement. Journal of Anxiety Disorders. 2003;17:561–571. doi: 10.1016/s0887-6185(02)00228-1. [DOI] [PubMed] [Google Scholar]
- Vannoy SD, Arean P, Unützer J. Advantages of using estimated depression-free days for evaluating treatment efficacy. Psychiatric Services. 2010;61:160–163. doi: 10.1176/appi.ps.61.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghorn GR, Chant DC. Employment restrictions among persons with ICD-10 anxiety disorders: characteristics from a population survey. Journal of Anxiety Disorders. 2005;19:642–657. doi: 10.1016/j.janxdis.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC. Twelve-month use of mental health services in the United States. Results from the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:629–640. doi: 10.1001/archpsyc.62.6.629. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–223. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Wells KB. The design of partners in care: evaluating the cost-effectiveness of improving care for depression in primary care. Social Psychiatry and Psychiatric Epidemiology. 1999;34:20–29. doi: 10.1007/s001270050107. [DOI] [PubMed] [Google Scholar]
- Wells KB, Sherbourne CD, Schoenbaum M, Duan N, Meredith L, Unützer J, Miranda J, Carney M, Rubenstein L. Impact of disseminating quality improvement programs for depression in managed primary care: A randomized controlled trial. JAMA. 2000;283:212–220. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]
- Willan AR, Lin DY. Incremental net benefit in randomized clinical trials. Statistics in Medicine. 2001;20:1563–1574. doi: 10.1002/sim.789. [DOI] [PubMed] [Google Scholar]
- Wittchen HU. Generalized anxiety disorder: prevalence, burden, and cost to society. Depression and Anxiety. 2002;16:162–171. doi: 10.1002/da.10065. [DOI] [PubMed] [Google Scholar]