Abstract
Drosophila melanogaster has historically been the premier model system for understanding the molecular and genetic bases of complex behaviors. In the last decade technical advances, in the form of new genetic tools and electrophysiological and optical methods, have allowed investigators to begin to dissect the neuronal circuits that generate behavior in the adult. The blossoming of circuit analysis in this organism has also reinforced our appreciation of the inadequacy of wiring diagrams for specifying complex behavior. Neuromodulation and neuronal plasticity act to reconfigure circuits on both short and long time scales. These processes act on the connectome, providing context by integrating external and internal cues that are relevant for behavioral choices. New approaches in the fly are providing insight into these basic principles of circuit function.
Introduction
How many neuroscience talks have you attended that open with an image from the work of Ramon y Cajal? While it has become almost cliché (Oh no! Not the cerebellum again!) the reverence in which our field holds the work of this scientist reflects a deeply ingrained belief that understanding how neuronal circuits are wired will provide us with answers to how the brain generates behavior. Many of the ideas that we take for granted in this century, that neurons are independent cellular entities and that information transfer has a predictable directionality, were first compellingly expressed in his work. Ramon y Cajal’s ability to infer important principles of nervous system organization from observing the anatomy of a subsystem and knowing its function brilliantly laid the foundations for how we think about the brain.
As a starting point, the idea that behaviors emerge from collections of neurons that are wired together in a particular configuration is a very useful one. Identification of neurons that are required for a particular behavior provides a departure point for identifying other elements of the circuit and for gaining traction on processes that modulate the circuit’s connectivity. Genetic strategies in Drosophila developed in the last decade have taken this model organism from one that is primarily used for “gene discovery” to one that is also a powerful platform for “cell discovery”, giving it a prominent role in our quest for understanding the neural basis of behavior. Even more exciting is the ability to use this organism to gain insight into the ways in which experience, environment and behavioral state can modify identified networks. I will use examples from a subset of the behavioral networks that have been identified through analysis of genes involved in higher order behaviors in adult animals (learning, male courtship behavior and circadian rhythms). These networks illustrate the complexity of the problem and highlight the utility of the new approaches that are allowing Drosophila researchers to understand fundamental properties of behavioral circuits.
Genetic strategies for identifying behaviorally relevant neurons
In model systems where the nervous system has well-delimited anatomical landmarks that can be seen in a living preparation, the initial identification of neurons involved in a particular behavior can often be accomplished with lesion studies or direct recordings. In adult Drosophila, this has not usually been an option, largely due to issues of scale and accessibility. Instead, genetic methods for accessing behaviorally relevant neurons have been more effective.
For most of the best understood behaviors in the fly this begins with analysis of the expression patterns of genes that control those behaviors. The modern era of Drosophila behavioral genetics began in the 1960’s. Over the next decade, mutants that were defective for many behaviors, including learning [1], male courtship [2] and circadian rhythms [3], were isolated. Cloning of these genes (dunce (dnc), fruitless (fru) and period (per), respectively) and analysis of where they were expressed provided the first clues about the identity of the neurons that were responsible for those behaviors. A large number of the signal transduction proteins involved in olfactory learning turn out, like dnc, to be enriched in mushroom bodies (for review see [4]). Expression of Fru, a transcription factor that has sex-specific splicing, marks a complex sexually dimorphic network that likely underlies a number of social behaviors [5]. The nuclear shuttling of Per, a transcriptional regulator, defines the 150 cell circadian network in the central brain [6]. It is important to note, however, that while gene expression has been used successfully in these cases, not every behavior will be defined by an appropriate and specific mutant. There are now also many ways of doing anatomical screens for neurons that are required for a particular behavior using conditional and cell-specific genetic tools (reviewed in [7]). These methods operate in a relatively unbiased manner and only require a behavioral assay.
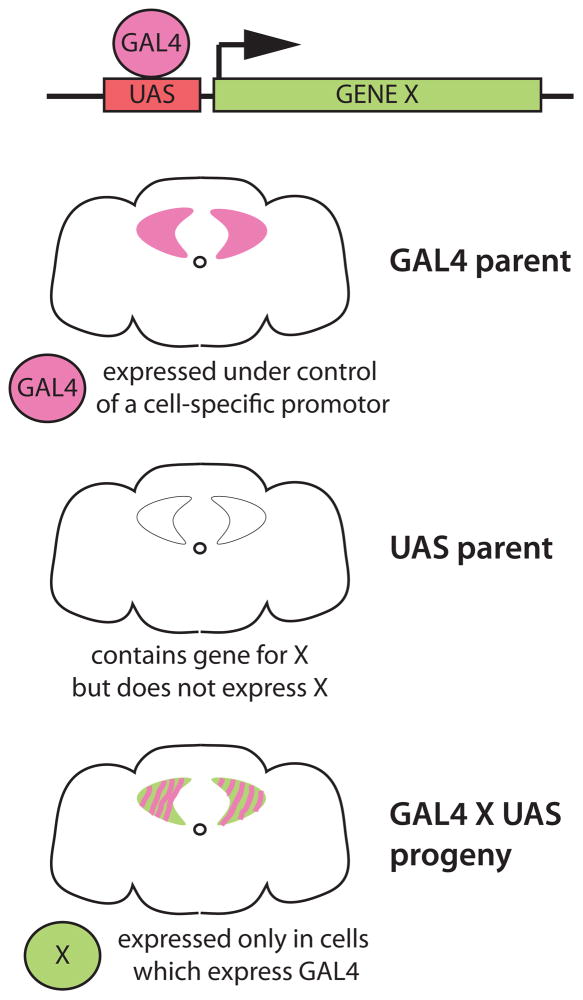
Knowing the expression pattern of a gene required for a particular behavior is an important first step, but to determine if those neurons are actually part of a behavioral circuit, some sort of experimental access is required. This access is usually achieved by use of enhancer trap-based binary expression systems such as the GAL4/UAS system (Figure 1). The concept, as first demonstrated by Brand and Perrimon [8], is simple: a cDNA for a transcriptional activator (GAL4) is inserted into the genome where it comes under control of local enhancer elements and is expressed in a cell-specific manner dictated by those elements. A second transgene, with a binding site for the transcriptional activator (UAS) upstream of the investigator’s gene of choice (“X” in Figure 1), is crossed into the stock and the experimental gene is expressed only in the cells that contain the activator. This system and other similar binary or ternary systems (reviewed in [9]) have been the workhorses of efforts in the fly to understand circuits because they allow the visualization and manipulation of specific populations of neurons in intact animals.
Figure 1.
The GAL4/UAS system for cell-specific transgene expression [8]. The yeast transcriptional activator GAL4 is typically expressed as a transgene under control of local DNA elements by either random insertion of its cDNA or by homologous recombination of its cDNA into a defined locus (e.g. the fru gene [38]), to capture that gene’s expression pattern. GAL4 can also be expressed under control of cloned promoter sequences (e.g. the pdf promoter which captures part of the clock circuit [18]). GAL4 lines are crossed to effector lines containing a transgene with a GAL4-binding UAS (upstream activation sequence) repeat upstream of the coding sequence of the gene of interest (in this example, X). Expression of X is dependent on GAL4, and only occurs in the progeny of the cross in cells that express GAL4. The Drosophila community has generated thousands of GAL4 lines, allowing specific genetic access to most of the nervous system. There are also variants of GAL4 that can further restrict expression patterns temporally and spatially, as well as several independent binary systems that can be used along side GAL4/UAS. A complete guide to these systems and the effector tools that can be used with them is provided in [9].
The ability to genetically access very small subsets of cells and to express transgenes can be used in a multitude of ways to both define and manipulate candidate neurons. An extensive catalog of neuronal effector transgenes and their uses can be found in [9]. Among the most commonly used effectors are fluorescent reporters like GFP that are used to mark cells to examine their anatomy or to allow them to be visualized for electrophysiological recordings. GAL4 can also be used to express reporters that are sensitive to elevation of intracellular second messengers such as calcium or cyclic nucleotides and monitor acute responses of neurons in an open preparation. There are also transgenes that alter the electrical activity of neurons, either chronically hyperpolarizing or depolarizing them, as well as transgenes that conditionally block neurotransmitter release. The newest frontier in effector transgenes involves activity modulators that are either light- or temperature-gated and can therefore be controlled with exquisite temporal specificity. The beauty of most of these effectors is that they can be used in intact animals to test the role of a particular set of neurons during specific phases of a behavior.
Tools for determining connectivity between circuit components
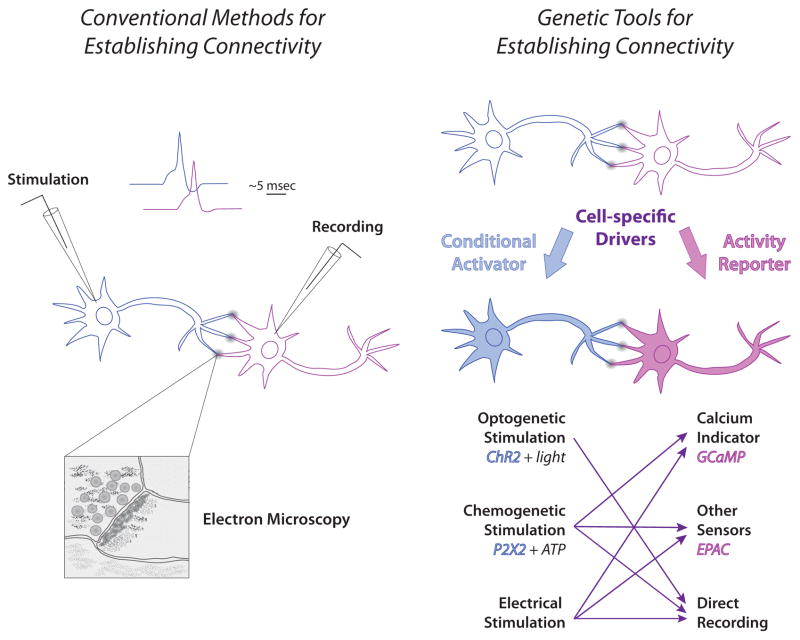
Demonstrating a role for several independent groups of neurons in the same behavior does not necessarily allow one to make conclusions about connectivity. For many recent “circuit” studies in the fly, the connectivity between elements has been inferred from proximity or genetic evidence. While in bigger brains this is an issue that can be directly addressed with electrodes, in adult flies it is only in recent years that the tools to ask these questions have become available (Figure 2). In some cases it has been possible to do dual recordings, for example within the olfactory system [10,11]. In other cases it has been easier to use a genetically encoded calcium sensor such as GCaMP to monitor activity in a putative postsynaptic neuron after stimulation of the presynaptic neuron or to use genetic methods to stimulate a presynaptic neuron while recording from a putative postsynaptic target.
Figure 2.
Methods for establishing functional connectivity. Traditional techniques for establishing connectivity are shown at left. Recording from a putative downstream neuron while electrically stimulating a presynaptic partner allows the investigator to determine if the two neurons are functionally connected, and via analysis of response latency, whether that connection is likely to be direct. In the example shown, the response in the downstream neuron occurs within 5 msec, which is typical for a monosynaptic connection (although temperature and other conditions can affect exact timing). Anatomical verification of a direct connection is accomplished using electron microscopy. Shown on the right are new methods for establishing functional connectivity. In Drosophila, direct recording can be done in some cases, but cell-specific expression systems can be used more generally to express transgenes which activate cells or report activation [9]. There are several methods for conditional activation of putative presynaptic neurons, including channel rhodopsin and the purine receptor P2X2. Responses in downstream cells after electrical or chemical stimulation can be monitored using a variety of genetically encoded fluorescent calcium sensors or reporters that respond to other consequences of presynaptic activity such as EPAC, which senses cyclic nucleotides. The time resolution of the current generation of reporters does not allow unambiguous determination of direct connectivity, and monosynaptic connection still needs to be verified anatomically with either electron microscopy or genetic techniques such as GRASP [39] to demonstrate cell-cell contact. Arrows indicate methods that can be used together. Optogenetic stimulation is not usually compatible with the current fluorescent reporters due to spectral overlap, but new variants are likely to address this incompatibility.
An elegant example using such strategies is found in work defining a sexually dimorphic circuit for sensing the male pheromone cis-vaccenyl acetate [12]. Photoactivatable GFP was used to identify candidate circuit elements by two-photon activation in putative synaptic regions and following diffusion of the activated GFP back to cell bodies. Antennal lobe projection neurons that receive input from pheromone-specific sensory neurons were shown to closely contact several groups of neurons in the lateral horn. These third order neurons were Fru+ and sexually dimorphic- one group appearing only in males. This male-specific group appeared to connect to a fourth order group of neurons that sends projections into the thoracic ganglion where motor neurons reside. The functional connectivity of the circuit was demonstrated by expressing GCaMP in Fru+ neurons and activating the olfactory sensory neurons with pheromone or the antennal lobe glomerulus with iontophoresed acetylcholine. Calcium elevation in the third order neurons was specific to activation of the pheromone pathway. Direct electrophysiological recording from this group of neurons and from fourth order neurons also demonstrated that they responded to chemical activation of the pheromone pathway and that the response was dependent on the projection neuron relay since laser ablation of that connection blocked the ability of pheromone to drive firing.
For circuits in which an external sensory signal cannot be used to initiate activation, another method for demonstration of connectivity needs to be used. One approach is to express the ATP-gated P2X2 receptor, a non-specific cation channel, in the presumptive presynaptic cell and perform direct recording in the putative target cell [11,13]. Application of ATP, which causes firing of the presynaptic cell, is read out as activation the postsynaptic cell if the presynaptic cell is excitatory. Inhibitory connections may be more difficult to see with this system; in antennal lobe single cell inhibitory connections were not well detected, although inhibition that occurred via multiple connections was measurable [11]. It is likely that other combinations of genetically encoded effectors and sensors will provide even more ways to probe circuit connectivity in the future.
Beyond the wiring diagram
Although the field is currently assessing synaptic connections one neuron pair at a time, it is likely that there will soon be a full map of the connectivity of the D. melanogaster brain [14] analogous to the one available for several decades for the C. elegans nervous system [15]. As appealing as this is, it has been clear for about the same number of decades that anatomical circuits can be functionally reconfigured by both plastic and neuromodulatory processes. While first demonstrated convincingly in small invertebrate networks like the crustacean stomatogastric ganglion [16], neuromodulation and plasticity are now known to be crucial in both vertebrates and invertebrates for state transitions of multifunctional networks, e.g. those responsible for sleep/wake cycles, olfactory processing, feeding [17]. The ability of a sensorimotor circuit to adjust its output by assessing the context in which the animal is performing the behavior has obvious survival value: it is bad to fall sleep when you are flying, and interactions between the circuits that drive these two behaviors should prevent this from happening. While connectivity maps will provide incredibly useful tools, they cannot not be used to simply read out a full picture of the function of a system since they are static and cannot capture the almost infinite variations in context that an animal experiences.
With its rich behavioral tradition, work in Drosophila has provided numerous paradigms in which the role of internal state or experience can be shown to affect behavior. Knowledge about the basic circuitry of these behaviors provides a starting point for understanding how modulation sculpts behavior. An excellent example is circadian rhythms. All animals have a clock that helps to schedule basic functions: feeding, sex, sleep, locomotion etc. In Drosophila, the central brain clock consists of ca. 150 neurons that contain an oscillating biochemical machinery that keeps time. The clock circuit is, however, not simply a vessel for the molecular machine; the electrical connectivity of the network is important to its function [18]. Moreover, the role of individual components of the core clock network change over the course of the day to shape the animal’s behavior in constant conditions. Small ventrolateral neurons (sLNvs) that express the neuromodulatory peptide Pigment Dispersing Factor (PDF) control morning activity, while a PDF- sLNv working with dorsal lateral neurons specifies evening activity [19,20]. Not surprisingly, the clock is also responsive to alterations in external conditions such light or temperature. Large PDF+ LNvs have been shown to be the important sensor for light-mediated arousal at night [21,22]. Their responsiveness to neurotransmitters varies as a function of time of day and light history [23]. Dorsal neurons of the DN1 group integrate light and temperature and can contribute to both morning and evening activity depending on specific environmental conditions [24]. While complicated, the ability to manipulate individual components of the clock circuit and examine behavioral impact, cellular activity and functional connectivity in a variety of conditions makes this a very attractive system for understanding the complexities of circuit modulation.
Synaptic plasticity is also an important regulator of circuit connectivity. Work using an aversive associative olfactory learning paradigm that pairs an odor with electric shock in Drosophila has demonstrated functional circuit consequences of learning in the form changes in calcium dynamics, measured with genetically encoded sensors, at multiple levels of the circuit (for review see [25]). The anatomical location of the changes in calcium response to the paired odor changes over time and correlates with temporally distinct forms of memory. Immediately after conditioning, antennal lobe glomeruli show altered responses to the paired odor. This “memory trace” dissipates over time to be replaced with enhanced calcium responses in neurons involved in intermediate forms of memory and eventually to areas associated with long-term memory in the mushroom bodies. Implicit in these experiments is the idea that learning has altered the connectivity of the circuit such that there is a facilitation of excitation at specific nodes in the circuit that reflects memory. Aversive memory formation is dependent on dopamine, which is believed to act as a neuromodulator, signaling the electric shock [26,27].
There is also a role for the animal’s internal and external state in learning and retrieval of associative memory. Appetitive olfactory conditioning, generated by pairing food with an odor, exhibits state-dependence at multiple levels. Animals have to be hungry to be effectively trained, and are only able to recall the memory of the paired odor in that state. Interestingly, dopamine is also critical for in this behavior, but in this case it appears to be a gate for motivational state [28]. A small number of dopaminergic neurons that innervate the mushroom body suppress retrieval of the memory unless the animal is hungry. Hunger releases the memory by generating a neuropeptide F-dependent suppression of those dopaminergic neurons. The involvement of dopamine in both appetitive and aversive memory (as well as arousal [29,30], motor control [31,32], sexual receptivity [33] and drug responsiveness [34]) suggests that this neuromodulator interacts with multiple circuits (for review see [35]).
As the mapping of behavioral circuits has progressed and the electrophysiological and optical methods available become more sophisticated, the cellular mechanisms of context-dependence are starting to be addressed directly in behaving animals. Work in the visual system has shown that the animal’s behavioral state has profound influence on the processing of sensory information. Both walking [36] and flying [37] increase the sensitivity of the visual system to stimuli, presumably allowing the animal to compensate for its own movement in space. How motor systems interact with early sensory processing is unknown, but it is clear that this level of systems analysis is approachable in the fly. It is not unreasonable to expect that a fully integrated picture of neural processing, from genes to behavior, is achievable.
Conclusions
Drosophila provides a powerful platform for gene discovery, and many of the genetic pathways that are known to be important for human disease were first described and studied in the fly. In the last decade, genetic tools for the manipulation of subsets of neurons with both exquisite spatial and temporal resolution have made it possible to begin to identify neural circuits for many behaviors, though only a few of the many examples are mentioned here. Parallel and complementary technological advances in optical imaging and electrophysiology are allowing investigators to begin to address the cellular mechanisms of circuit modulation. With the rich behavioral repertoire of this animal and the increasingly sophisticated methods for manipulating and recording from neurons in the adult brain, the fly is a system that has the potential to allow a complete (gene to action) understanding of behavior in all its astonishing complexity.
Highlights.
behavioral mutants can be used to identify candidate circuit components
binary expression systems allow precise manipulation of specific neurons
Drosophila has a wide variety of tools for manipulation of neuronal activity in behaving animals
functional connectivity between circuit elements can be demonstrated with genetic tools
modulation of connectivity in a circuit is a general feature of behavior
Acknowledgments
This work was supported by National Institutes of Health grant R01 MH067284 to LCG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci U S A. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall JC. Courtship among males due to a male-sterile mutation in Drosophila melanogaster. Behav Genet. 1978;8:125–141. doi: 10.1007/BF01066870. [DOI] [PubMed] [Google Scholar]
- 3.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busto GU, Cervantes-Sandoval I, Davis RL. Olfactory learning in Drosophila. Physiology (Bethesda) 2010;25:338–346. doi: 10.1152/physiol.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Yu JY, Kanai MI, Demir E, Jefferis GS, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. An anatomical tour de force that defines 100 groups of Fru+ neurons (some of which are sexually dimorphic) and their putative directionality of information flow. This distributed network has the potential to integrate information from multiple sensory modalities. [DOI] [PubMed] [Google Scholar]
- 6.Zerr DM, Hall JC, Rosbash M, Siwicki KK. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci. 1990;10:2749–2762. doi: 10.1523/JNEUROSCI.10-08-02749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Simpson JH. Mapping and manipulating neural circuits in the fly brain. Adv Genet. 2009;65:79–143. doi: 10.1016/S0065-2660(09)65003-3. This review provides a comprehensive accounting of the experimental strategies available in Drosophila for mapping neural circuits. A how-to manual for the fly behaviorist. [DOI] [PubMed] [Google Scholar]
- 8.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 9**.Venken KJ, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72:202–230. doi: 10.1016/j.neuron.2011.09.021. This review provides an excellent overview and catalog of the genetic tools available for manipulation of the nervous system in Drosophila. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazama H, Wilson RI. Origins of correlated activity in an olfactory circuit. Nat Neurosci. 2009;12:1136–1144. doi: 10.1038/nn.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Zhang W, Qiao W, Hu A, Wang Z. Functional connectivity and selective odor responses of excitatory local interneurons in Drosophila antennal lobe. Neuron. 2010;67:1021–1033. doi: 10.1016/j.neuron.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 12*.Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. A combination of cell tracing with a photoactivatable GFP, calcium imaging and electrophysiology are used to define a compact circuit which senses an important male pheromone and connects to motor output centers. [DOI] [PubMed] [Google Scholar]
- 13.Hu A, Zhang W, Wang Z. Functional feedback from mushroom bodies to antennal lobes in the Drosophila olfactory pathway. Proc Natl Acad Sci U S A. 2010;107:10262–10267. doi: 10.1073/pnas.0914912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chklovskii DB, Vitaladevuni S, Scheffer LK. Semi-automated reconstruction of neural circuits using electron microscopy. Curr Opin Neurobiol. 2010;20:667–675. doi: 10.1016/j.conb.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 15.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London B, Biological Sciences. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 16.Marder E. Mechanisms underlying neurotransmitter modulation of a neuronal circuit. Trends in Neurosciences. 1984;7:48–53. [Google Scholar]
- 17.Nadim F, Brezina V, Destexhe A, Linster C. State dependence of network output: modeling and experiments. J Neurosci. 2008;28:11806–11813. doi: 10.1523/JNEUROSCI.3796-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila free-running rhythms require intercellular communication. PLoS Biol. 2003;1:E13. doi: 10.1371/journal.pbio.0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 20.Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 21.Shang Y, Griffith LC, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci U S A. 2008;105:19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol. 2008;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Shang Y, Haynes P, Pirez N, Harrington KI, Guo F, Pollack J, Hong P, Griffith LC, Rosbash M. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat Neurosci. 2011;14:889–895. doi: 10.1038/nn.2860. The effects of dopamine on sleep are modulated by light. A genetically encoded cyclic nucleotide sensor is used to show that the responsiveness of large LNvs, the main arousal-promoting light sensors at night, are also gated by light. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P. Light and temperature control the contribution of specific DN1 neurons to Drosophila circadian behavior. Curr Biol. 2010;20:600–605. doi: 10.1016/j.cub.2010.02.044. Cell-specific rescue in a per mutant is used to dissect the role of DN1s in morning and evening activity. These cells can promote activity at both times of day, but their contributions are differnetially gated by temperature and light. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aso Y, Siwanowicz I, Bracker L, Ito K, Kitamoto T, Tanimoto H. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenbock G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. Appetitive olfactory conditioning is shown to be state-dependent both in its formation and its recall. Hunger-related release of neuropeptide F inhibits dopaminergic neurons that block memory retrieval when the animal is in the fed state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Kong EC, Woo K, Li H, Lebestky T, Mayer N, Sniffen MR, Heberlein U, Bainton RJ, Hirsh J, Wolf FW. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS One. 2010;5:e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neckameyer WS. Dopamine modulates female sexual receptivity in Drosophila melanogaster. J Neurogenet. 1998;12:101–114. doi: 10.3109/01677069809167259. [DOI] [PubMed] [Google Scholar]
- 34.Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 35.Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 2010;33:457–464. doi: 10.1016/j.tins.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Chiappe ME, Seelig JD, Reiser MB, Jayaraman V. Walking modulates speed sensitivity in Drosophila motion vision. Curr Biol. 2010;20:1470–1475. doi: 10.1016/j.cub.2010.06.072. Two-photon calcium imaging is used to address the effects of locomotion on visual processing in tethered animals walking on a ball. Walking speed correlates with the speed of processing, allowing the animal to adjust to its own movement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Maimon G, Straw AD, Dickinson MH. Active flight increases the gain of visual motion processing in Drosophila. Nat Neurosci. 2010;13:393–399. doi: 10.1038/nn.2492. Whole cell patch clamp of visual system neurons in a tethered fly in a flight arena demonstrate that the gain of vertical system visual neurons is altered by flight. [DOI] [PubMed] [Google Scholar]
- 38.Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 39*.Feinberg EH, Vanhoven MK, Bedesky A, Wang G, Fetter RD, Shen K, Bargmann CI. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. Reconstitution of a split GFP by plasma membrane expression of the halves in putative synaptic partners allows visualization of cells which make direct contact with one another. GFP fragments which also contain synaptic localization signals limit the reconstitution to synaptic contacts. [DOI] [PubMed] [Google Scholar]