Abstract
Purpose
The objective of this study was to compare the biomechanical outcomes of a new method of anterior cruciate ligament (ACL) treatment, bio-enhanced ACL repair, with ACL reconstruction in a large animal model.
Methods
Twenty-four skeletally immature pigs underwent unilateral ACL transection and were randomly allocated to receive bio-enhanced ACL repair with a collagen-platelet composite, allograft (bone–patellar tendon– bone) reconstruction, or no further treatment (n = 8 for each group). The structural properties and anteroposterior laxity of the experimental and contralateral ACL-intact knees were measured 15 weeks postoperatively. All dependent variables were normalized to those of the contralateral knee and compared by use of generalized linear mixed models.
Results
After 15 weeks, bio-enhanced ACL repair and ACL reconstruction produced superior biomechanical outcomes to ACL transection. However, there were no significant differences between bio-enhanced ACL repair and ACL reconstruction for maximum load (P = .4745), maximum displacement (P = .4217), or linear stiffness (P = .6327). There were no significant differences between the 2 surgical techniques in anteroposterior laxity at 30° (P = .7947), 60° (P = .6270), or 90° (P = .9008).
Conclusions
Bio-enhanced ACL repair produced biomechanical results that were not different from ACL reconstruction in a skeletally immature, large animal model, although the variability associated with both procedures was large. Both procedures produced significantly improved results over ACL transection, showing that both were effective in this model.
Clinical Relevance
Bio-enhanced ACL repair may 1 day provide an alternative treatment option for ACL injury.
In recent years, substantial advances in the surgical treatment of the injured anterior cruciate ligament (ACL) have been made by combining methods of tissue engineering with primary suture repair.1–3 Recently, long-term clinical studies have shown that the current gold standard of treatment for ACL injury, ACL reconstruction with tendon graft, restores gross joint stability but only provides a slight reduction in post-traumatic osteoarthritis when compared with nonsurgical management.4–8 In addition, ACL reconstruction has a relatively high failure rate, particularly in younger patients.9 Finally, the options for ACL tears in pediatric patients with open physes are limited.10 Thus methods to improve the treatment of ACL injuries are of great interest.
Historically, suture repair was performed to treat a torn ACL, but this technique was soon abandoned because of high rates of failure and increased postoperative knee laxity.11,12 Recent studies suggest that clinical failure of suture repair may have been due to a premature loss of the blood clot between the torn ends of the ACL in the synovial environment and that the loss of this provisional scaffold in turn prevents healing.2,3 Identification of this mechanism led to the development of new suture repair techniques supplemented with tissue-engineered scaffolds that were designed to replace the natural provisional scaffold provided by the clot.2 ACL repair with a collagen scaffold alone without platelet-rich plasma13 or the use of platelet-rich plasma without a scaffold14 has been shown not to significantly improve the biomechanical properties of a repaired ACL; however, the combination of the 2 (a collagen-platelet composite) significantly improved healing over traditional suture repair.2 The addition of a collagen-platelet composite to an ACL graft has also been shown to improve biomechanical outcome after ACL reconstruction.15
The purpose of this study was to answer the question whether bio-enhanced ACL repair could produce equal biomechanical outcomes when compared with the current gold standard of treatment, ACL reconstruction. The primary hypothesis of this study was that there was no clinically relevant difference in biomechanical outcomes between bio-enhanced ACL repair and ACL reconstruction after 15 weeks of healing. The secondary hypothesis was that both bio-enhanced ACL repair and ACL reconstruction would have significantly improved biomechanical outcomes when compared with ACL transection alone. Biomechanical outcomes were defined by the structural properties of the bio-enhanced ACL repair or ACL reconstruction, including the yield and maximum failure load, yield and maximum failure displacement, linear stiffness, and anteroposterior (AP) knee laxity.16,17
METHODS
Study Design
The experimental protocol was approved by the Institutional Animal Care and Use Committee. The study was designed as an assessor-blinded, randomized, active-controlled, large animal trial comparing bio-enhanced ACL repair with ACL reconstruction. Both surgical treatments were compared with ACL transection as a negative control. According to an a priori sample size calculation, a total of 24 juvenile, female Yorkshire pigs (age [mean ± SD], 11.8 ± 0.4 weeks; body weight, 30 ± 1.1 kg) (8 per group) were used. At this age, the physes of a Yorkshire pig are still open (i.e., these animals were skeletally immature). A unilateral procedure was performed in all animals so that the contralateral knee would serve as an intact control. Biomechanical outcomes for all treated knees were normalized by the corresponding intact knee. Animals in the bio-enhanced ACL repair group were treated with suture stabilization of the knee by use of absorbable sutures and a collagen-platelet composite,18,19 whereas those in the ACL reconstruction group were treated with a bone–patellar tendon– bone allograft.15 Euthanasia with pentobarbital was performed after 15 weeks, because that time point is well beyond the nadir in strength that occurs between 6 and 9 weeks after repair and is in the time period at which tissue maturation and generation of biomechanical strength are increasing.20
Collagen Scaffold Production
The collagen scaffolds were manufactured in our laboratory as previously described.13,18,19 In brief, a collagen slurry was made by solubilizing sterilely harvested, bovine connective tissue. The slurry was adjusted to a collagen concentration of over 10 mg/mL. The slurry was frozen and lyophilized in a cylindrical mold to create a collagen scaffold measuring 30 mm × 22 mm in diameter. All scaffolds were stored in a vacuum at −80°C until use.
Platelet Concentrate
Preoperatively, autologous blood was drawn into a syringe containing 10% acid-citrate-dextrose (Harvest Technologies, Plymouth, MA) and centrifuged (mean relative centrifugal force, 150g; 6 minutes). The platelet-rich buffy coat was harvested and the platelets isolated with a second spin (mean relative centrifugal force, 500g; 8 minutes). Platelets were counted and resuspended in the harvested plasma to produce a 5-fold (5.09 ± 0.4) concentration relative to the systemic platelet count. The platelet concentrate was used within 1 hour of procurement.
Surgical Procedure
ACL Transection
All animals underwent ACL transection. With the animal under general anesthesia, a medial arthrotomy was made and the fat pad was partially resected to expose the ACL. The ACL was transected in its center portion, at the junction of the proximal and middle third of its length, to mimic the location of a midsubstance tear. The ligament was cut with a scalpel, and any fibers that could not be reached with the blade were torn with a curved mosquito hemostat. All knees showed anterior tibiofemoral sub-luxation after complete ACL transection. The knee was irrigated with 500 mL of 0.09% saline solution. For the animals randomized to the ACL transection–only group, the incision was closed in layers. All animals were kept under anesthesia for 1 hour to allow the platelet-rich plasma to clot in the repair group and to avoid differential treatment in the ACL transection and reconstruction groups.
Bio-enhanced ACL Repair
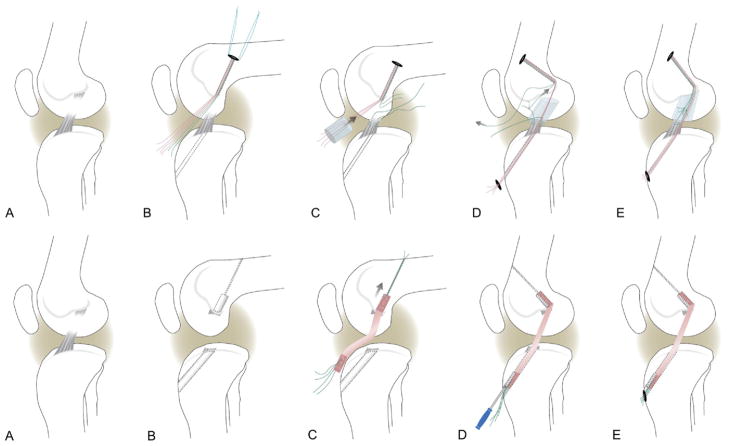
For the animals randomized to the bio-enhanced ACL repair group, tunnels in the femur (4.5 mm) and tibia (2.4 mm) were created in the standard positions for ACL reconstruction, with the tibial tunnel exiting in the center of the tibial attachment and the femoral tunnel placed in the center of the femoral ACL attachment site. A drill guide (ACUFEX Director; Smith & Nephew, Andover, MA) and a 6-mm-offset femoral aimer (ACUFEX; Smith & Nephew) were used to place 2.4-mm guidewires. The femoral guidewire was overdrilled with an EndoButton drill (4.5 mm; Smith & Nephew), and an EndoButton (Smith & Nephew) armed with 3 No. 1 Vicryl sutures (Ethicon, Somerville, NJ) was placed through the femoral tunnel and engaged on the femoral cortex. A Kessler suture using No. 1 Vicryl was placed in the tibial stump of the ACL to repair the transected ligament.13,18,19 Vicryl is an absorbable suture that completely dissolves in approximately 63 days (Ethicon). Two of the EndoButton sutures were threaded through the collagen scaffold, passed through the tibial tunnel, and tied over a button with the knee in full extension. The scaffold was saturated in situ with 3 mL of the platelet concentrate. The remaining suture from the femoral tunnel was tied to the suture in the tibial ACL stump to reduce the ACL and secure it. The collagen-platelet composite was observed for 10 minutes to ensure that clotting occurred before the incisions were closed in layers with absorbable sutures (Fig 1A).
Figure 1.
(A) Schema of bio-enhanced ACL repair. (A) Defect model. (B) Femoral and tibial tunnels (dashed lines) and EndoButton pulled through femoral tunnel and placed on femoral cortex. The EndoButton is loaded with 3 sutures, resulting in 6 free-ending strands (4 red and 2 green). (C) A Kessler suture is placed in the tibial ACL stump, and a collagen scaffold is threaded onto 4 strands (red), pushed into the notch, and saturated with 3 mL of platelet-rich plasma. (D) The 4 suture strands running through the scaffold (red) are passed through the tibial tunnel, while the remaining suture (green) is tied to the tibial Kessler suture, using it as a pulley to reduce and stabilize the tibial ACL stump. (E) The transtibial sutures (red) are tightened and tied over an extracortical button. The free ends of the ACL suture pulley (green) are knotted to secure the reduced ACL in the collagen-platelet composite. (B) Schema of ACL reconstruction. (A) Defect model. (B) ACL remnants are removed, and tibial and femoral tunnels (9 mm and 8 mm) are placed by use of aimers, guidewires, and cannulated drills (as described in the text). (C) A bone–patellar tendon– bone allograft is placed in the femoral tunnel and secured with an interference screw. (D) The distal end of the graft is passed through the tibial tunnel and tightened by manual tension under joint cycling. The tightened graft is secured distally with a second interference screw. (E) Analogous to the bio-enhanced ACL repair, the same type of extracortical button is used as a secondary tibial fixation.
ACL Reconstruction
The animals in the reconstruction group underwent a standard ACL reconstruction procedure with bone–patellar tendon– bone allograft as previously described.15 The knees for allograft procurement were harvested from age-, weight-, and gender-matched animals and stored at −20°C until use. The knees were thawed for 18 hours at room temperature, and the bone–patellar tendon– bone allografts were harvested by sterile technique. The entire patellar tendon was used for the tendinous portion of the allograft. The patellar bone plug was sized to 7 mm in diameter and 15 mm in length. The tibial bone plug was sized to 6 × 10 mm and the bone folded over on the tendinous portion of the graft to adjust the total graft length to 55 to 60 mm. The allografts were rinsed in an antibiotic solution (5% PenStrep; Mediatech, Manassas, VA) and wrapped in moist gauze until implantation.
For the reconstruction, 2.4-mm guidewires were placed as described for bio-enhanced ACL repair and overdrilled with cannulated drills to create a 25 × 8 –mm tunnel in the center of the femoral ACL insertion and a 9-mm tunnel transtibially with the exit site in the center of the ACL tibial insertion. The graft was passed into the femoral tunnel and secured with a 6 × 20 –mm interference screw (BioSure; Smith & Nephew). The tibial bone plug was then passed retrograde through the tibial tunnel and held under firm tension with retention sutures in the tibial block while the knee was cycled to seat the graft. The graft was fixed in full extension with a second 6 × 20 –mm interference screw. The Vicryl retention sutures were tied over an extracortical button on the tibia for secondary fixation (Fig 1B). The arthrotomy was closed in layers in the same fashion as in the repair group.
After 15 weeks of healing, all animals were euthanized by an intravenous injection of a pentobarbital (Fatal Plus; Vortech, Dearborn, MI) and both hindlimbs were harvested by coxofemoral disarticulation. All limbs were wrapped in saline solution–soaked towels, frozen, and stored at −20°C.
Physical Examination
A physical examination, consisting of measurements of knee range of motion with a goniometer, as well as a thigh circumference measurement 5 cm above the proximal pole of the patella by use of a tape measure, was performed on anesthetized animals in a supine position preoperatively and before limb harvest on the treated and intact knees.
Biomechanical Testing
Biomechanical testing was performed following a previously published protocol.15 The knees were thawed overnight at room temperature, and the soft tissues surrounding the tibia and femur were removed, leaving the joint capsule intact. The specimens were potted in Schedule 40 polyvinyl chloride pipe tubes by use of urethane potting compound (Smooth On, Easton, PA). The tibia and femur were oriented such that their long axes and tubes were parallel. All testing was done with an MTS 810 servohydraulic load frame (MTS Systems, Eden Prairie, MN). All mechanical testing evaluators were blinded as to treatment group during the testing process.
AP laxity testing was performed with the knee flexion angle set at 30°, 60°, and 90° by application of fully reversed, sinusoidal AP directed shear loads of ±40 N at 0.0833 (1/12) Hz for 12 cycles as previously described.13,18,19 During the AP laxity tests, axial rotation was locked in the neutral position whereas the varus-valgus angulation and the coronal-plane translations were left unconstrained. Data for load and displacement were collected at 20 Hz.
The structural properties of the ligament or graft constructs were determined by a tensile test to failure as previously described.13,18,19 Before failure testing, the joint capsule, menisci, collateral ligaments, and posterior cruciate ligament were dissected from the joint, with the femur–ACL scar mass–tibia complex being left intact. During this dissection, all knees were grossly assessed for repair tissue integrity and the presence of remaining suture material. For failure testing, the knee flexion angle was initially set at 30°. The tibia was mounted to the base of the MTS system by use of a sliding X-Y platform with the femur unconstrained to rotation. Before the tensile test started, the femur was lowered until the load across the joint surface was +5 N of compression. A ramp at 20 mm/min was performed, and the load-displacement data were recorded at 100 Hz. After testing of the knee to failure, the load-displacement tracing of the failure test was used to determine the yield load and yield displacement, as well as maximum load and maximum displacement. Yield was defined as the point where the load-displacement curve became nonlinear. Linear stiffness was represented by the slope of the load-displacement curve between the points corresponding to 20% and 80% of the yield load.13,15,18,21
Statistical Methods
The sample size for this study was based on an a priori power calculation. Using biomechanical data from earlier studies,13–15,19 we wanted our study to be powered to detect a 20% difference in maximum displacement, maximum load, and linear stiffness between ACL repair and reconstruction with an SD of 10% with an α (P value) of 5% and a minimum power of 95%. With these parameters, the minimum required sample size was 8 animals per group, or 24 in total.
All analyses were performed by use of intent to treat. Biomechanical outcomes are given as relative values (experimental/intact) for tensile testing and as a difference (experimental – intact) for AP laxity. Statistical assessment was performed in 2 steps. First, surgical treatment was compared with ACL transection and had to show significantly improved results to proceed to step 2, the comparison of bio-enhanced ACL repair with ACL reconstruction. All outcomes were tested in generalized mixed models, an analysis-of-variance framework that accounts for both within-animal comparisons (normal v contralateral knees) and across-animal comparisons (ACL reconstruction v repair v transection). Results for physical examination were assessed as absolute change in range of motion or thigh circumference between day 0 and 15 weeks by use of generalized mixed models. An α of 5% was considered significant, and Holm adjustments were used to account for multiple comparisons. Results are given as mean values with 95% confidence intervals (95% CIs) in parentheses. Statistical testing was done by use of SAS software (SAS Institute, Cary, NC).
RESULTS
Animal Welfare
All animals recovered well from surgery. Full weight-bearing status was achieved within 48 to 72 hours for all groups. All animals reached the 15-week time point with no infections or other complications.
Physical Examination
Preoperatively, there were no differences in maximum flexion angle (P = .268), minimum extension angle (P = .460), or thigh circumference (P = .118) among knees allocated to ACL transection, ACL reconstruction, and bio-enhanced ACL repair (Table 1).
Table 1.
Outcomes for Physical Examination for Operated Knees (Not Normalized by Intact)
| Thigh Circumference (cm)* [Mean (95% CI)]
|
Flexion (°) [Mean (95% CI)]
|
Extension (°) [Mean (95% CI)]
|
||||
|---|---|---|---|---|---|---|
| Preoperatively | 15 wk | Preoperatively | 15 wk | Preoperatively | 15 wk | |
| ACL reconstruction | 22.7 (21.5–24.0) | 26.8 (25.4–28.1)† | 31.5 (27.5–35.0) | 30.6 (27.9–33.3) | 148.0 (144.7–154.1) | 148.8 (146.8–150.7) |
| Enhanced ACL repair | 21.6 (21.0–22.5) | 28.9 (27.7–30.7)†,‡ | 34.3 (32.2–35.6) | 30.7 (29.4–35.0) | 145.0 (140.0–149.0) | 142.1 (139.6–149.3) |
| ACL transection | 21.3 (19.5–23.2) | 27.2 (24.4–28.0)† | 31.7 (27.4–36.0) | 34.4 (30.6–36.0) | 148.3 (145.6–151.0) | 150.0 (141.4–156.9) |
| P value across groups | .1180 | .035†/.974‡ | .2679 | .1465 | .4596 | .5268 |
Five centimeters proximal to patella.
Significant difference between ACL reconstruction and repair (P = .035 after adjustment for multiple testing).
No significant difference between ACL repair and transection (P = .9743 after adjustment for multiple testing).
From these preoperative values to the postoperative assessment at 15 weeks, the animals in the ACL reconstruction group had a significantly lower increase (P = .035) in thigh circumference (21.3%; 95% CI, −7% to 49%) than those animals in the bio-enhanced ACL repair group (34.0%; 95% CI, 1% to 67%). The mean change in range of motion from preoperative to postoperative values was not significantly different among any of the 3 groups for maximum flexion angle (P = .147) or minimum extension angle (P = .527) and was less than 3° for all groups in both flexion and extension (Table 1).
Gross Assessment
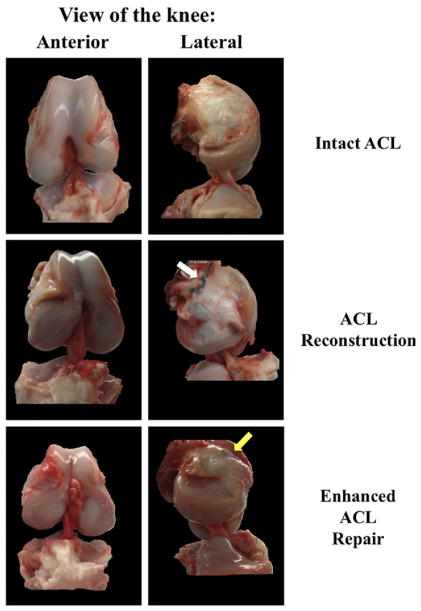
No residual suture material was found in any of the knees in the bio-enhanced suture repair group during macroscopic assessment before tensile testing (Fig 2). One of the ACL grafts appeared to be completely absorbed when the joint was opened. There were no macroscopic signs of weight-bearing cartilage injury or meniscus damage in either the bio-enhanced repair or reconstruction group.
Figure 2.
Gross assessment of bio-enhanced ACL repair and reconstruction compared with intact ACL. The white arrow shows the sutures used to pull the graft into the femoral tunnel. The yellow arrow shows the EndoButton used for the bio-enhanced repair.
Surgical Treatment Versus ACL Transection (Negative Control)
Knees with an untreated ACL transection had 140% (95% CI, 105% to 175%) higher displacement to yield and 124% (95% CI, 96% to 152%) higher and maximum displacement when compared with the intact ACLs. Untreated ACL transection resulted in repair tissue with only 11% (95% CI, 6% to 16%) of the yield load and 10% (95% CI, 7% to 14%) of the maximum load of the intact ACLs. The untreated ACL transections had a linear stiffness that was only 11% (95% CI, 8% to 15%) of intact ACLs.
For AP laxity testing at 30° and 60°, the laxity values of both bio-enhanced ACL repair and bone–patellar tendon–bone ACL reconstruction were significantly less than that of ACL transection (P = .015 and P = .003, respectively). However, there was no significant difference in AP laxity at 90° of flexion (P = .332).
All endpoints in tensile testing showed significantly superior results for surgical treatments than for ACL transection (yield displacement, P = .008; maximum displacement, P = .004; yield load, P = .031; maximum load, P = .038; stiffness, P = .026).
Bio-enhanced ACL Repair Versus ACL Reconstruction
AP Laxity
There was no statistically significant difference in AP laxity between bio-enhanced ACL repair and ACL reconstruction at 30° (P = .795), 60° (P = .627), or 90° (P = .901) of flexion (Table 2).
Table 2.
Results From AP Laxity Testing
| Knee Flexion Difference (Experimental – Control Knee) (95% CI) (mm)
|
|||
|---|---|---|---|
| 30° | 60° | 90° | |
| ACL reconstruction | 6.7 (5.0–8.4) | 10.9 (8.9–12.8) | 8.5 (6.9–10.1) |
| Enhanced ACL repair | 6.4 (4.8–8.0) | 10.3 (9.1–11.6) | 8.3 (7.1–9.5) |
| ACL transection | 9.5 (7.4–11.6)* | 13.9 (12.3–15.4)* | 9.2 (8.1–10.3) |
NOTE. Laxity testing was performed within the shear load limits of ±40 N. Surgical treatments produced significantly improved results compared with untreated ACL transection at 30° and 60° of flexion.
P < .005.
Tensile Testing
The yield and maximum loads between the bio-enhanced ACL repair and ACL reconstruction constructs were similar after 15 weeks of healing. Comparisons found no significant difference in yield load (P = .760) or maximum load (P = .475). The yield and maximum loads of the bio-enhanced ACL repairs were 23% (95% CI, 13% to 33%) and 24% (95% CI, 13% to 35%) of the contralateral intact ACL, whereas the ACL reconstructions averaged 23% (95% CI, 12% to 34%) and 21% (95% CI, 11% to 31%) of the intact ACL (Table 3).
Table 3.
Results From Tensile Testing
| Displacement [% on Intact ACL (95% CI)]
|
Load [% on Intact ACL (95% CI)]
|
Linear Stiffness [% on Intact ACL (95% CI)] | |||
|---|---|---|---|---|---|
| Yield | Maximum | Yield | Maximum | ||
| ACL reconstruction | 85 (55–115) | 79 (52–107) | 23 (12–34) | 21 (11–31) | 27 (19–36) |
| Enhanced ACL repair | 98 (72–125) | 84 (71–96) | 23 (13–33) | 24 (13–35) | 32 (21–43) |
| ACL transection | 140 (105–175)* | 124 (96–152)* | 11 (6–16)* | 10 (7–14)* | 11 (8–15)* |
NOTE. Tensile testing was performed at 20 mm/min. Surgical treatments produced significantly improved results compared with ACL transection in the regression model.
P < .05.
Statistically, there was no significant difference in yield displacement (P = .225) or maximum displacement (P = .422) between the 2 treatment groups. Yield displacement was 98% (95% CI, 72% to 125%) of the contralateral intact ACL after bio-enhanced ACL repair and 85% (95% CI, 55% to 115%) after ACL reconstruction. Maximum displacement was 84% (95% CI, 71% to 96%) and 79% (95% CI, 52% to 107%) after repair and reconstruction, respectively (Table 3).
For ACL linear stiffness, there was no significant difference between bio-enhanced ACL repair and ACL reconstruction (P = .633). The linear stiffness of the bio-enhanced ACL repair tissue was 32% (95% CI, 21% to 43%) of intact, whereas that of the ACL graft was 27% (95% CI, 19% to 36%) (Table 3).
DISCUSSION
It was the primary objective of this study to compare the functional outcome of bio-enhanced ACL repair, a method under development, with ACL reconstruction, the current standard of care for patients with ACL injuries. The secondary objective was to ensure that both treatments were effective by comparing them with a control group of animals with an untreated ACL transection. In this study we found that the structural properties of the ligament after bio-enhanced ACL repair were not significantly different from the grafts after ACL reconstruction. In addition, both treatments offered improvement in AP knee laxity, ACL linear stiffness, and displacements to yield and failure over ACL transection.
The rationale behind bio-enhanced ACL repair is to augment a normal suture repair with a bioactive collagen-platelet composite. Earlier studies have identified the lack of clot formation in the knee as a key reason for the high rate of failure of primary suture repair.2,3 The collagen-platelet composite serves both as a scaffold for cell-based tissue remodeling and as a source of anabolic growth factors, thus stimulating healing. Using collagen or platelets alone led to significantly worse biomechanical outcomes in prior in vivo experiments.14,22 It has also been shown that the addition of a collagen-platelet composite can also significantly improve biomechanical outcomes after ACL reconstruction in a porcine model,15 but again, the addition of platelets alone has no beneficial effect on ACL reconstruction in human trials or in animal models.23 These results for bio-enhanced ACL reconstruction support our proposed technique. However, we used conventional ACL reconstruction in this study because we wanted to use a clinically accepted positive control with which to compare bio-enhanced ACL repair, rather than another experimental technique.
No significant differences were observed between ACL reconstruction and bio-enhanced ACL repair for any of the measured biomechanical outcomes. From prior studies, it is also known that the type of suture repair that we use in this model is not effective in improving AP laxity unless both collagen and platelet-rich plasma are combined.13,14,21 For this study, AP laxity of bio-enhanced ACL repair was not significantly different from ACL reconstruction at any knee flexion angle. However, whereas surgical treatment produced significantly less AP laxity than ACL transection at 30° and 60°, there was no difference at 90°. When one is interpreting these results, it should be noted that AP laxity testing was performed with the joint capsule and the menisci in situ. These secondary stabilizers may play more of a role in AP stability with the porcine knee in deeper flexion.24–28
None of the animals in this study had postoperative flexion or extension contractures develop. In addition, all thigh circumferences increased over time, consistent with what would be expected for a juvenile, growing animal; however, the ACL reconstruction group had a significantly lower rate of increase of almost 50% of circumference. This could possibly be because of increased pain in this group because of the larger tunnels required for graft implantation; the persistence of a subclinical effusion in reaction to the allograft material, which could potentially limit leg usage in the postoperative period; or loss of the pro-prioceptive function of the ACL when the ligament is removed before reconstruction. Further work to determine the cause of this observation is required but may point to other advantages of repair over reconstruction.
It should be noted that the animals used in this study were skeletally immature. In earlier studies it was shown that young age is associated with increased cellular migration and proliferation, translating into better biomechanical outcomes in a porcine model at 3 months.19,29–31 Thus the results for bio-enhanced ACL repair might be less striking in adults. However, cellular invasion of the graft is thought to be essential for successful long-term function of the graft; thus both methods may change in efficacy with age. An important point that steered us toward the use of skeletally immature animals is the current, particularly high need for improved ACL treatment options in pediatric patients. ACL reconstruction is still avoided in many skeletally immature patients because of fear of growth disturbances, leading to secondary cartilage and meniscus injury in these conservatively treated patients.10 Extra-articular stabilization is a valuable proposition, but there are only limited long-term data and it is not an anatomic reconstruction of the knee kinematics.10,32 The technique presented herein could be an effective option for skeletally immature patients leading to an anatomic repair without affecting the growth plate.33
Bio-enhanced ACL repair has several potential advantages over ACL reconstruction if equivalent efficacy can be shown. Bio-enhanced ACL repair obviates the need for graft harvest and can thus be a less invasive procedure. In addition, bio-enhanced ACL repair offers the opportunity to retain the insertion sites of the ligament, as well as the proprioceptive function of the ligament.2,3,34 Finally, whereas bio-enhanced ACL repair shows promise in this in vivo study, there are multiple ways in which this basic technique can be improved. The deliberate use, and controlled release, of selected growth factors is 1 potential way to improve tissue healing, as is the improvement of the collagen scaffold and suture technique. Further work to refine and improve this technique may now be warranted with this proof-of-principle study.
Our study has potential shortcomings. First, although results at 15 weeks have been proven to be likely predictors of long-term outcome,20 the long-term outcomes of these procedures remain unknown. Second, an animal model cannot fully reproduce the human situation. For example, we could not control postoperative rehabilitation in the animals. Likewise, the ACL injury was simulated with sharp transection in the midsubstance. It is possible that a frayed disruption would heal differently. Third, allografts were used in this study because harvesting the patellar tendon can compromise the extensor mechanism of the porcine knee. In addition, the allograft used was a complete patellar tendon rather than the middle third of the tendon, which may result in a relatively stronger time 0 graft than would be used clinically. Finally, the animals were adolescents, and whether these results will translate to older individuals remains to be seen.29,30
CONCLUSIONS
Bio-enhanced ACL repair produced biomechanical results that were not different from ACL reconstruction in a skeletally immature, large animal model, although the variability associated with both procedures was large. Both procedures produced significantly improved results over ACL transection, showing that both were effective in this model.
Acknowledgments
Supported by National Institutes of Health grants R01-AR054099, R01-AR056834, and R01-AR052772.
The authors are greatly indebted to Elise Magarian and Sophia L. Harrison for assistance during animal surgeries and to David Paller and Alison Biercevicz for assistance with mechanical testing. They are also grateful to Arthur Nedder, Kathryn Mullen, Kimberlie Hauser, and Mark Kelly for their veterinary expertise and care.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article.
References
- 1.Murray MM. Current status and potential of primary ACL repair. Clin Sports Med. 2009;28:51–61. doi: 10.1016/j.csm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vavken P, Murray MM. Translational studies in anterior cruciate ligament repair. Tissue Eng Part B Rev. 2010;16:5–11. doi: 10.1089/ten.teb.2009.0147. [DOI] [PubMed] [Google Scholar]
- 3.Vavken P, Murray MM. The potential for primary repair of the ACL. Sports Med Arthrosc. 2011;19:44–49. doi: 10.1097/JSA.0b013e3182095e5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linko E, Harilainen A, Malmivaara A, Seitsalo S. Surgical versus conservative interventions for anterior cruciate ligament ruptures in adults. Cochrane Database Syst Rev. 2005;2:CD001356. doi: 10.1002/14651858.CD001356.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Lind M, Menhert F, Pedersen AB. The first results from the Danish ACL reconstruction registry: Epidemiologic and 2 year follow-up results from 5,818 knee ligament reconstructions. Knee Surg Sports Traumatol Arthrosc. 2009;17:117–124. doi: 10.1007/s00167-008-0654-3. [DOI] [PubMed] [Google Scholar]
- 6.Lidén M, Sernert N, Rostgård-Christensen L, Kartus C, Ejerhed L. Osteoarthritic changes after anterior cruciate ligament reconstruction using bone–patellar tendon– bone or hamstring tendon autografts: A retrospective, 7-year radiographic and clinical follow-up study. Arthroscopy. 2008;24:899–908. doi: 10.1016/j.arthro.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 7.Meunier A, Odensten M, Good L. Long-term results after primary repair or non-surgical treatment of anterior cruciate ligament rupture: A randomized study with a 15-year follow-up. Scand J Med Sci Sports. 2007;17:230–237. doi: 10.1111/j.1600-0838.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 8.Aït Si Selmi T, Fithian D, Neyret P. The evolution of osteoarthritis in 103 patients with ACL reconstruction at 17 years follow-up. Knee. 2006;13:353–358. doi: 10.1016/j.knee.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: A case-control study. Am J Sports Med. 2009;37:2362–2367. doi: 10.1177/0363546509340633. [DOI] [PubMed] [Google Scholar]
- 10.Vavken P, Murray M. Treating anterior cruciate ligament tears in skeletally immature patients. Arthroscopy. 2011;27:704–716. doi: 10.1016/j.arthro.2010.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odensten M, Lysholm J, Gillquist J. Suture of fresh ruptures of the anterior cruciate ligament. A 5-year follow-up. Acta Orthop Scand. 1984;55:270–272. doi: 10.3109/17453678408992354. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures. A long-term follow-up study. Am J Sports Med. 1990;18:354–358. doi: 10.1177/036354659001800404. [DOI] [PubMed] [Google Scholar]
- 13.Fleming BC, Magarian EM, Harrison SL, Paller DJ, Murray MM. Collagen scaffold supplementation does not improve the functional properties of the repaired anterior cruciate ligament. J Orthop Res. 2010;28:703–709. doi: 10.1002/jor.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: An in vivo study. J Orthop Res. 2009;27:639–645. doi: 10.1002/jor.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cipriani A, Girlanda F, Barbui C. Superiority, equivalence or non-inferiority? Epidemiol Psichiatr Soc. 2009;18:311–313. doi: 10.1017/s1121189x00000269. [DOI] [PubMed] [Google Scholar]
- 17.Lesaffre E. Superiority, equivalence, and non-inferiority trials. Bull NYU Hosp Jt Dis. 2008;66:150–154. [PubMed] [Google Scholar]
- 18.Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament using a collagen-platelet composite. Arthroscopy. 2010;26(suppl):S49–S57. doi: 10.1016/j.arthro.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92:2039–2049. doi: 10.2106/JBJS.I.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37:2401–2410. doi: 10.1177/0363546509339915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26:1500–1505. doi: 10.1002/jor.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 23.Vavken P, Sadoghi P, Murray M. The effect of platelet concentrates on graft maturation and graft-bone interface healing in anterior cruciate ligament reconstruction in human patients: A systematic review of controlled trials. Arthroscopy. 2011;27:1573–1583. doi: 10.1016/j.arthro.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen CR, Wong EK, Livesay GA, Sakane M, Fu FH, Woo SL. Importance of the medial meniscus in the anterior cruciate ligament-deficient knee. J Orthop Res. 2000;18:109–115. doi: 10.1002/jor.1100180116. [DOI] [PubMed] [Google Scholar]
- 25.Battaglia MJ, II, Lenhoff MW, Ehteshami JR, et al. Medial collateral ligament injuries and subsequent load on the anterior cruciate ligament: A biomechanical evaluation in a cadaveric model. Am J Sports Med. 2009;37:305–311. doi: 10.1177/0363546508324969. [DOI] [PubMed] [Google Scholar]
- 26.Papageorgiou CD, Gil JE, Kanamori A, Fenwick JA, Woo SL, Fu FH. The biomechanical interdependence between the anterior cruciate ligament replacement graft and the medial meniscus. Am J Sports Med. 2001;29:226–231. doi: 10.1177/03635465010290021801. [DOI] [PubMed] [Google Scholar]
- 27.Sakane M, Livesay GA, Fox RJ, Rudy TW, Runco TJ, Woo SL. Relative contribution of the ACL, MCL, and bony contact to the anterior stability of the knee. Knee Surg Sports Traumatol Arthrosc. 1999;7:93–97. doi: 10.1007/s001670050128. [DOI] [PubMed] [Google Scholar]
- 28.Shoemaker SC, Markolf KL. The role of the meniscus in the anterior-posterior stability of the loaded anterior cruciate-deficient knee. Effects of partial versus total excision. J Bone Joint Surg Am. 1986;68:71–79. [PubMed] [Google Scholar]
- 29.Mastrangelo AN, Haus BM, Vavken P, Palmer MP, Machan JT, Murray MM. Immature animals have higher cellular density in the healing anterior cruciate ligament than adolescent or adult animals. J Orthop Res. 2010 doi: 10.1002/jor.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mastrangelo AN, Magarian EM, Palmer MP, Vavken P, Murray MM. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2010;28:644–651. doi: 10.1002/jor.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vavken P, Saad FA, Murray MM. Age dependence of expression of growth factor receptors in porcine ACL fibroblasts. J Orthop Res. 2010;28:1107–1112. doi: 10.1002/jor.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kocher M, Garg S, Micheli L. Physeal sparing reconstruction of the anterior cruciate ligament in skeletally immature prepubescent children and adolescents. Surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1, pt 2):283–293. doi: 10.2106/JBJS.F.00441. [DOI] [PubMed] [Google Scholar]
- 33.Vavken P, Peterson C, Fleming BC, Machan JT, Murray MM. Effects of suture choice on biomechanics and physeal status after bio-enhanced ACL repair in skeletally immature patients—A large animal study. Arthroscopy. doi: 10.1016/j.arthro.2012.07.006. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82:1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]