Abstract
Inducing and experiencing emotions about others' mental and physical circumstances is thought to involve self‐relevant processing and personal memories of similar experiences. The hippocampus is important for self‐referential processing during recall and prospection; however, its contributions during social emotions have not been systematically investigated. We use event‐related averaging and Granger causal connectivity mapping to investigate hippocampal contributions during the processing of varieties of admiration and compassion pertaining to protagonists' mental versus physical circumstances [admiration for virtue (AV) versus for skill; compassion for social/psychological pain (CSP) versus for physical pain]. Data were collected using a multistep emotion‐induction paradigm that included psychosocial interviews, BOLD fMRI, and simultaneous psychophysiological recording. Given that mnemonic demands were equivalent among conditions, we tested whether: (1) the hippocampi would be recruited more strongly and for a longer duration during the processing of AV and CSP; and (2) connectivity between the hippocampi and cortical systems involved in visceral somatosensation/emotional feeling, social cognitive, and self‐related processing would be more extensive during AV and CSP. Results elucidate the hippocampus' facilitative role in inducing and sustaining appropriate emotional reactions, the importance of self‐related processing during social emotions, and corroborate the conception that varieties of emotional processing pertaining to others' mental and physical situations engage at least partially distinct neural mechanisms. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: admiration, compassion, social cognition, self‐processing, insula
INTRODUCTION
We often use our own self‐knowledge as a basis for understanding others and as a platform for evaluating and appropriately reacting to the emotional implications of others' situations [Amodio and Frith, 2006; Frith and Frith, 2006; Goldman and de Vignemont, 2009]. While much is known about the role of the hippocampus in self‐referential processing during recall and prospection [Muscatell et al., 2010], in the formation of emotional memories in social contexts [Eisenberger et al., 2007], and in the processing of emotional facial expressions [Critchley et al., 2000; Fusar‐Poli et al., 2009], less is known about the role of this structure in the processing of social emotions and feelings [Perry et al., 2011]. Emotions related to others' accomplishments and predicaments, such as admiration or compassion, along with their corresponding feelings, are inherently complex and often invoke personal memory as a source of information from which to evaluate the situation and respond [Haidt and Morris, 2009; Immordino‐Yang, 2010, 2011; Immordino‐Yang et al., 2009]. To elucidate the contribution of the hippocampus during the processing of these emotions, we examined its activation and connectivity during the induction and experience of positive (possessing a pleasurable quality) and negative (possessing a painful quality) social emotions of varying complexity.
Intense emotional responses are organized around the contents, context, and subjective interpretation of the situation being witnessed [Barrett and Kensinger, 2010]. Among the most cognitively complex and culturally shaped human emotions are those that pertain to other peoples' mental states, such as the admiration we feel for virtuous intentions or the compassion we feel for psychological distress. Appreciating and reacting to another person's mental state requires conjuring an empathic understanding of circumstances and qualities that may not be immediately apparent from a person's outward behavior or physical situation. By contrast, other social emotions are more directly induced and constitute relatively automatic responses to the immediate physical and cognitive situations or actions of other people, such as admiration for a skillful performance (AS) or compassion for physical pain (CPP) of a broken leg. Although both classes of emotions would involve building simulations and recalling personal memories, the simulations and memories necessary to appreciate a person's physical predicament or skill are likely less complex and abstract, and more heavily based in simple action simulations. In conceiving our experiment, we considered that the emotions about mental situations, by contrast, are less concrete and more dependent on perspective taking and reflection on one's own similar experiences, and would therefore involve increased complexity of self‐related and empathic processing.
Consistent with this distinction, an earlier fMRI analysis comparing these emotions revealed that feeling emotions about others' mental states induced more slowly rising maxima of activation in brain regions involved in emotion and empathy, the anterior insula, and anterior middle cingulate [Immordino‐Yang et al., 2009; Menon and Uddin, 2010; Panksepp, 1998; Singer, 2006]. Consistent with its presumed relative automaticity, CPP was induced more quickly both behaviorally and in the brain [Immordino‐Yang et al., 2009]. In addition to slower patterns of activation, emotions about others' mental states also more strongly recruited an inferior–posterior sector of the posteromedial cortex (PMC), a region encompassing portions of the posterior cingulate and precuneus that has been implicated in the default network and associated with processing of personal memory, self awareness, and emotional salience [Sestieri et al., 2011]. By contrast, emotions about others' physical circumstances preferentially activated a more anterior and superior posteromedial sector, known to be anatomically heavily interconnected with lateral parietal systems for representation of the musculoskeletal body [Parvizi et al., 2006]. The functional segregation of neural responses during processing of emotions pertaining to mental versus physical circumstances in the posteromedial cortices (PMC) suggests that emotions about mental states more heavily involve neural systems for memory, despite evidence that empathy for both physical and social pain share an overlapping substrate in somatosensory and pain processing in the anterior insula and anterior middle cingulate [Decety and Chaminade, 2003; Eisenberger and Lieberman, 2004; Panksepp, 2005].
Although these findings indirectly implicate memory processing during social emotions, they leave open the question of hippocampal involvement. Notably, the hippocampus has recently been associated with the brain's default mode and its associated episodic and autobiographical processes [Buckner et al., 2008]. Default mode activity in the hippocampus is thought to reflect retrieval rather than encoding [Huijbers et al., 2011] and to relate to the richness of self‐related and socioemotionally relevant processing supported by the default network. In accordance with this interpretation, resting medial temporal lobe activity and functional connectivity have been linked to individual differences in spontaneous episodic thoughts [Andrews‐Hanna et al., 2010], consolidation of recent experiences [Tambini et al., 2010], and long‐term recall for events [Wig et al., 2008]. These regions are also activated when subjects make decisions about themselves [Andrews‐Hanna et al., 2010], as well as when they mentalize about other people [Spreng and Grady, 2010; Spreng et al., 2009; St. Jacques et al., 2011]. Taken together, these findings support the idea that autobiographical memory, hypothetical simulation of socially relevant circumstances, and mentalizing about others share overlapping neural substrates [Buckner and Carroll, 2007], and centrally involve the hippocampus.
Based on the above, we hypothesized that the hippocampus would be recruited more strongly and for longer durations during the intense experience of emotions primarily concerning others' cumulative mental circumstances, as compared with during the experience of emotions concerning others' immediate physical situations and actions, reflecting the potential importance of this neural structure in calling up memories that may serve as a platform for formulating emotional simulations of others' mental states. To test this, in data obtained using an emotion induction and analysis technique reported in Immordino‐Yang et al. [ 2009], we investigated the strength and duration of hippocampal activation during the feeling of: (1) admiration for virtue (AV, positive, mental); (2) compassion for social/psychological pain (CSP, negative, mental); (3) admiration for skill (AS, positive, physical); and (4) compassion for physical pain (CPP, negative, physical).
We further hypothesized that the facilitative role of the hippocampus during the more complex emotions would involve more extensive interaction with key cortical systems known to be involved in affective and cognitive social processing and in processing related to the self. We used Granger causal modeling [Roebroeck et al., 2005], an effective connectivity technique, to investigate the directional connections between the right and left hippocampi and
-
a
anterior insular and anterior cingulate cortices involved in visceral somatosensation, emotional feeling and regulation, and in empathy for others' pain and emotion (affective processing);
-
b
lateral temporal and parietal cortices involved in social cognition and perspective‐taking, and ventral prefrontal cortices (PFC) involved in social emotion induction (social cognitive processing);
-
c
dorsal medial PFC (dmPFC) and PMC (an ensemble of mesial parietal, posterior cingulate, and retrosplenial cortices) involved in self‐related processing.
We chose Granger causal mapping (GCM) over alternative connectivity techniques such as dynamic causal modeling, because it allowed us to probe the direction of influence but did not require that we prespecify a detailed connectivity model. The direction of predominant Granger causal influence was not prespecified. Instead, the direction of predominant influence was treated as a separate analysis amounting to an exploration of the patterns of connectivity, given that the hypotheses were confirmed. Notably, patterns differed between the same regions across conditions, suggesting that the results cannot be explained by differences in hemodynamic properties of the regions probed.
MATERIALS AND METHODS
Participants
Data were obtained from 13 healthy volunteers (six women, seven men; mean age 30.3 years, SD 11.9; range 19–57 years); right‐handed native English speaking Americans born to monolingual English‐speaking parents (two ethnically Latino, 11 ethnically White; subjects were not screened by ethnicity but by home language and personal and family history), from the University of Southern California community, with no history of neurological or psychiatric illness, or use of psychotropic medication. As per the requirements of the Institutional Review Board of the USC, all participants had given written consent and were paid for taking part in the experiment.
Stimuli
Emotions were induced via exposure to a corpus of 50 true, documentary style narratives depicting real people (not actors) in various life circumstances. The corpus of narrative stimuli for this experiment was developed and piloted previously [see Immordino‐Yang et al., 2009, SI for details concerning stimulus development, piloting, and delivery]. The narratives depicted a gender balanced group of mentally competent protagonists ranging in age from early adolescence through late adulthood. Emotional narratives had been established by piloting to produce in participants equivalently strong emotional reactions corresponding to the following experimental conditions.
-
1
Admiration for virtue (AV); narratives depicted highly virtuous and morally elevated protagonists, such as people who have dedicated their lives to an important cause despite difficult obstacles;
-
2
Compassion for social pain (CSP); narratives depicted protagonists in social circumstances leading to states of grief, despair, social rejection, or other psychological pain;
-
3
Admiration for skill (AS); narratives depicted protagonists adeptly performing a rare or difficult feat, such as in athletics or music;
-
4
Compassion for physical pain (CPP); narratives depicted a protagonist sustaining an accidental bodily injury, such as in a sports mishap.
Control narratives depicted true‐life situations established by piloting to be equivalently engaging but less emotion provoking.
Mnemonic Properties of Narrative Stimuli
In developing the corpus of narratives, our primary intent was to create an experimental protocol that would go beyond emotion recognition to genuinely and strongly induce varieties of complex admiration and compassion in participants—a nontrivial accomplishment, especially in a scanner environment. Experimental narratives had been designed to be as equivalent as possible in their structure and presentation; however, to probe their mnemonic equivalence for the present study, we counted the number of critical facts presented in each narrative during the preparation interview, as judged from experimenter scripts. Mean number of facts presented in each narrative was 6.8, SD = 1.5. We found no significant differences between conditions (one way ANOVA F (4,45) = 0.949, P < 0.45).
Protocol
Using a multistep procedure first reported in Immordino‐Yang et al. [ 2009], narratives were first shared with participants during a 2‐h, one‐on‐one videotaped interview in which the experimenter discussed each narrative with the participant, prompting with the open‐ended question, “how does this person's situation make you feel?” Participants were not told the categories of emotion in the experiment; narratives were shared in pseudorandom order with no more than two narratives from the same category in a row. Following this interview, participants were scanned using fMRI and simultaneous psychophysiological recording. Each trial began with a 5‐sec segment presenting the crux of a previously learned narrative (video with one sentence of speech, also transcribed; the stimulus), followed by 13 sec of gray screen during which participants were asked to reflect on the narrative and rate the real‐time strength of their emotional reaction using button presses. A 2‐sec fixation separated trials. Stimuli were presented in four functional runs of ∼ 9 min each; each stimulus was presented twice during the experiment but never during the same run, for a total of 100 trials. Within runs, stimulus presentation was pseudorandom with no more than two stimuli from the same category in a row; order of runs was counterbalanced between participants. After the 1‐h scan, participants were again interviewed about their emotional reaction to each stimulus in the scanner.
Identification of Valid fMRI Trials
To include in the results only those fMRI trials where the participants reported feeling strong emotion consistent with the experimental condition, data were sorted for inclusion/exclusion in three steps, using: (1) independent raters' analyses of participants' reactions to each narrative during the preparation interview; (2) participants' button press responses corresponding to subjective strength of emotion during each fMRI trial; and (3) raters' analyses of participants' recollections of their reactions to each narrative in the scanner, reported in the debriefing interviews. Psychophysiological data (heart and respiration rates) corresponding to valid fMRI trials were used to determine the time window of maximal emotion response to calculate the BOLD contrast (emotions > control) that would identify activated hippocampal voxels. For details concerning behavioral, psychophysiological, and GLM methods, see Immordino‐Yang et al., SI, 2009.
Image Acquisition and Processing
A Siemens 3 Tesla MAGNETON TIM Trio scanner with a 12‐channel matrix coil at the Dana and David Dornsife Neuroimaging Center at the University of Southern California was used to acquire the images. Functional scans were acquired using a T 2* weighted echo planar (EPI) sequence (TR = 2,000 ms, TE = 30 ms, and flip angle = 90°) with a voxel resolution of 3 mm × 3 mm × 4.5 mm. This acquisition rate has been shown sufficiently fast to support high sensitivity to connectivity effects using GCM, even for influences with moderate strength and delay [Formisano and Goebel, 2003; Menon et al., 1998; Roebroeck et al., 2005]. Thirty‐two transverse slices were acquired to cover the whole brain, including the brainstem. Functional data were acquired continuously for the duration of each run, with breaks between runs. Anatomical scans were acquired using a magnetization prepared rapid acquisition gradient (MPRAGE) sequence (TI = 900 ms, TR = 2,530 ms, TE = 7 ms, and flip angle = 7°) with an isotropic voxel resolution of 1 mm. Data analysis and image processing were conducted using BrainVoyager QX version 1.8 software (Brain Innovation, Maastricht, The Netherlands). After preprocessing and normalization, we used the psychophysiological data to identify the time window of maximal emotional response to include in the GLM contrast. To do this, the BOLD signal for each participant was estimated using a GLM that comprised nine independent regressors (boxcars) obtained from each individual TR of each trial type. Correcting for the 2‐sec expected delay in heart rate change, psychophysiological responses (heart rate and respiration increase) as well as behavioral piloting suggested that the maximal emotional response could be captured within the first 10 sec of the trial for all conditions. Estimating the hemodynamic delay at 6 sec, we therefore included TRs 4–8 (corresponding to BOLD collected 6–16 sec after trial onset) in the calculation of the GLM contrast [see Immordino‐Yang et al., SI, 2009 for details].
Region of Interest Definition and Calculation of Event‐Related Averages
The GLM contrast of all emotions versus control was used to identify the cluster of activated voxels in the region of interest (ROI). The GLM map was thresholded at q(FDR) < 0.05 [Benjamini and Hochberg, 1995; Genovese et al., 2002] and displayed on an average brain [Damasio, 2005b; Frank et al., 1997]. The functionally defined cluster within each hippocampus was then anatomically delineated to form a right and a left ROI. These ROIs were sequentially displayed individually on each participant's anatomical data and delimited to fall within the boundary of the hippocampal formation, as confirmed by a neuroanatomist (H. Damasio). Finally, BrainVoyager was used to produce event‐related averages (ERAs) of the z‐transformed signal for each emotion condition.
Analysis of Effective Connectivity
GCM is an “effective” connectivity technique that allows investigation of functional connections between brain areas and calculates the direction of influence [Friston, 2009; Roebroeck et al., 2005]. Based on the concept proposed by economist Clive Granger that temporal precedence discerns cause from effect [Granger, 1969; Granger and Joyeux, 1980], GCM treats the sequence of fMRI measurements at each voxel of the ROI as a vector of time series and tests whether the activity in a voxel x Granger‐causes activity in a voxel y, by testing whether activity over time in voxel x helps to explain the future time course of activation in voxel y, above and beyond the prediction power given by the past activation of voxel y alone.
GCM was used to test our hypotheses regarding the interactions between the hippocampus and cortical areas known to be critical for affective, social cognitive, and self‐related processing during the induction and experience of social emotions. GCM was performed using the GCM toolbox in BrainVoyager [Roebroeck et al., 2005]. We tested hypotheses concerning the existence of functional connection between the hippocampus and prespecified cortical regions without prespecifying the direction of influence; directionality is reported as a secondary exploratory analysis.
To compute GCMs for each hippocampal ROI, influence measures Fx→y, Fy→x, and Fxy were computed from the average 18‐sec time course of the voxels within the hippocampal ROI (as x) and the time courses of each voxel in the rest of the brain (as y). Maps showing directed influence from the hippocampal ROI to the other voxels (Ref2Vox) and maps showing voxels whose activity influences the activation in the hippocampal ROI (Vox2Ref) were computed. These maps were based on the computation of the influence difference term (Fx→y − Fy→x; Roebroeck et al., 2005) and were thresholded at q(FDR) = P < 0.01. Analyses of the computed maps involved inspection of each hypothesized region for significant connectivity; anatomical localization was confirmed by a neuroanatomist (H. Damasio).
RESULTS
Strength of Emotional Response
Participants rated “as honestly as possible” their strength of emotion to each narrative in the scanner. We find no differences between CSP, CPP, and AV; all produced equivalently high ratings of emotion in the scanner. AS was associated with slightly but significantly lower values of reported emotion, likely because of the decreased potency of these narratives upon multiple exposures (i.e., first in the interview and later in the scanner). However, these stimuli were still rated as substantially and significantly more emotional than control stimuli. See Table I for results, including tallies of trials disqualified either because the subject had a different emotional response than the one we had intended (3.4% of trials) or because they had failed to achieve an emotion to a particular trial in the scanner (12.4%). Overall, it was quite rare for a subject to report feelings such as jealousy to a stimulus meant to induce admiration or schadenfreude to a compassion stimulus. When this happened, the associated trials were excluded from further analysis.
Table IA and B.
Summary of behavioral results. “A. Included trials” tallies the total number of trials included in the BOLD analyses for each condition as well as the mean number of trials included for each subject. “Strength” is the average button press value by condition (included trials only). Participants rated “as honestly as possible” the strength of emotion to each narrative using a 1–4 scale: 1 (low); 2 (moderate); 3 (strong); and 4 (overwhelming). Ratings for AS were lower than for the other emotions; differences between the other conditions are not significant. “B. Excluded trials” tallies the number of trials discarded due to “button press” (reflecting emotional strength during the scan session), pre‐ or post‐“interview” (reflecting emotional label given by the participant) and “other” reasons (including failure of the subject to press a button and technical failure). Tallies of included and excluded trials are out of 260 total trials for each condition. One subject had no valid AS trials, resulting in fewer exemplars and more excluded trials for this category. AV, admiration for virtue; AS, admiration for skill; CSP, compassion for social pain; CPP, compassion for physical pain
| Condition | A. Included trials | B. Excluded trials | ||||
|---|---|---|---|---|---|---|
| Tally | Strength (1–4) | Tally | ||||
| Total | Mean/subject (SD) | Mean (SD) | Button press | Interview | Other | |
| AV | 226 | 17.4 (2.5) | 2.66 (0.70) | 16 | 14 | 4 |
| AS | 176 | 13.5 (5.4) | 2.40 (0.68) | 72 | 3 | 9 |
| CSP | 226 | 17.4 (2.8) | 2.86 (0.73) | 12 | 18 | 4 |
| CPP | 227 | 17.5 (2.9) | 2.79 (0.77) | 29 | 0 | 4 |
Timing of Emotional Response (Induction)
Subjects had been instructed to respond with a button press as soon as they became aware of and could assess the strength of their emotion to each stimulus presentation in the scanner. On average, button presses in CSP, AV, and AS fell during the fourth TR of the nine TR trial, with no statistically significant difference in timing between conditions (F = 2.18, df = 2, P < 0.11). Responses to stimuli inducing CPP and the control condition occurred ∼ 2 sec (equivalent of 1 TR) earlier on average than responses to the other conditions (F = 12.22, df = 4, P < 0.001).
Time Course of Hippocampal Activation
Figure 1 shows the time course of activation by condition for voxels in the hippocampus that were activated for emotions relative to control. Time courses of activation for each emotion and for control were not appreciably different for voxels in the right versus the left hippocampus; therefore, we present one combined plot. As predicted, the more cognitively complex and elaborated social emotions of AV and CSP produced greater and more sustained hippocampal activation than did the simpler emotions of AS and CPP. Note that AV, arguably the most complex and nuanced emotion, peaked later than the other emotions, at ∼ 5 sec after CSP, and was sustained for the longest duration, ∼ 12 sec.
Figure 1.
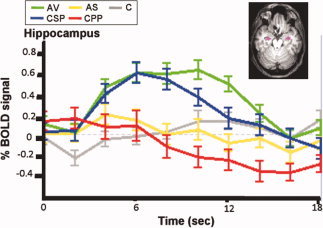
Event‐related averages for the time courses of admiration, compassion, and control in the hippocampus in voxels activated for emotions relative to control, with standard errors. Units are percent change in BOLD signal and time in seconds; time courses are not corrected for hemodynamic delay. The volume of interest is displayed in pink. Conditions: AV (green): admiration for virtue; CSP (blue): compassion for social pain; AS (yellow): admiration for skill; CPP (red): compassion for physical pain; C (gray): control. Note the rapid rise and sustained activation not only for AV (green) but also for CSP (blue).
Granger Causal Connectivity: Extent of Connection
During processing of AV and CSP, the results reveal extensive right and left hippocampal functional connectivity with systems involved in (1) visceral somatosensation, (2) social cognition, and (3) self‐related processes, more so than during processing of AS and CPP (see Fig. 2 and Table II). Specifically,
-
1
during AV and CSP, there was functional connectivity with the anterior insula and anterior middle cingulate; this was particularly extensive during AV. However, neither hippocampus showed functional connectivity with these regions during processing of AS or CPP;
-
2
the right hippocampus showed functional connectivity with the middle temporal gyrus for all emotions, with the superior temporal gyrus (STG) for AV and CSP, with the superior temporal sulcus (STS) for CSP and AS, and with the temporal‐parietal junction (TPJ) only for CSP. It also showed functional connectivity with the ventromedial prefrontal cortex during both admiration conditions, but with the ventrolateral prefrontal cortex during AV only. The left hippocampus showed extensive functional connectivity with the STS and STG along their lengths during the processing of AV and CSP, and with the temporal pole during processing of CSP. It showed no functional connectivity with lateral temporal regions during AS or CPP and no functional connectivity with the TPJ during any of the emotions. It showed functional connectivity with the ventrolateral PFC (vlPFC) for both AV and CSP and with the ventromedial cortices for CSP only;
-
3
the right hippocampus showed functional connectivity with the precuneus for AV, CSP, and AS; there was connectivity with the posterior cingulate only during AV and CSP and with the retrosplenial cortex during AV only. The right hippocampus showed connectivity with the dorsal medial prefrontal cortex during CSP. The left hippocampus showed connectivity with the precuneus during AV and CSP and with the posterior cingulate during AV only; no functional connectivity was detected between the left hippocampus and the retrosplenial cortex, and functional connectivity with the dorsal medial prefrontal cortex was detected only during AV.
Figure 2.
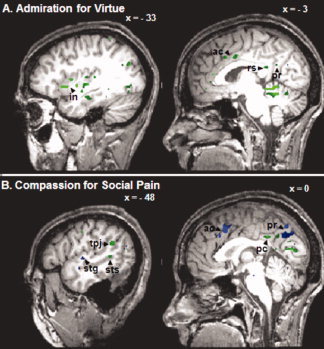
Granger causality maps depicting connectivity of the right hippocampus during admiration for virtue (A) and compassion for social pain (B). fMRI data are from 13 subjects, displayed on the brain of one subject. Talairach x coordinate of each sagittal slice is annotated. Images are thresholded using the false discovery rate statistic, q(FDR) < 0.01. Green indicates influence “toward” the hippocampus; blue indicates influence “from” the hippocampus. Note the extensive connectivity from the insula (in) associated with AV; note also the switch in direction of influence between the hippocampus and anterior cingulate (ac) for AV versus CSP. GCM results for the left hippocampus are described in the text. Retrosplenial cortex (rs); precuneus (pr); posterior cingulate cortex (pc); temporal‐parietal junction (tpj); superior temporal gyrus (stg); superior temporal sulcus (sts).
Table II.
Results from Granger causal connectivity analyses of the right and left hippocampus with the cortical areas involved in visceral somatosensation and emotional feeling; social cognition; and self‐related processing, during admiration for virtue (AV), compassion for social pain (CSP), admiration for skill (AS), and compassion for physical pain (CPP). Italics denote connectivity with the left hippocampus; nonitalics with the right hippocampus. Bold denotes direction of influence “toward” the hippocampus; nonbold denotes “from” the hippocampus. Talairach coordinates are in the format of x y z; negative x values correspond to the left hemisphere; coordinates are taken from regions showing Granger causal connectivity after thresholding using the false discovery rate statistic, q(FDR) < 0.01, corresponding to the following critical t‐statistics: ≥0.82 (AV), ≥1.17 (AS), ≥0.77 (CSP), and ≥0.91 (CPP)
| AV | CSP | AS | CPP | |
|---|---|---|---|---|
| Visceral somatosensation (emotional feeling) | ||||
| anterior insula | −33 16 −2, − 32 12 − 2, | −29 16 5, − 36 − 3 − 5 | ||
| −31 19 −8 | ||||
| anterior cingulate cortex | −1 4 35, − 2 − 3 34 | 0 16 40, 2 11 34 | ||
| Social cognitive processing | ||||
| temporal‐parietal junction | −47 −52 23 | |||
| middle temporal gyrus | 47 −28 −3 | 48 −32 2 | −60 −22 −11 | 54 −13 −18, |
| −54 −1 −20 | ||||
| superior temporal sulcus | −51 −33 −3, | 52 −22 −4, | 42 11 −20 | |
| 57 − 28 − 3 | −53 0 −8 | |||
| superior temporal gyrus | 48 − 19 13 | −51 −30 13, | ||
| −55 −44 4, | 48 −13 3 | |||
| −53 −1 19 | ||||
| temporal pole | −52 2 −1 | |||
| ventromedial PFC | −1 41 −5 | −3 47 −5 | −9 41 −8, | |
| 15 38 −5 | ||||
| ventrolateral PFC | −28 13 −10, −33 37 0 | −29 24 −7 | ||
| 29 43 0 | ||||
| Self‐related processing | ||||
| posteromedial cortices: | ||||
| posterior cingulate | 3 −34 31, 3 − 39 31 | 0 −41 31 | ||
| retrosplenium | 0 −38 23,−6 −53 16 | |||
| precuneus | 4 −40 40, 7 −61 44; | 2 −62 33 | −1 −61 46 | |
| 6 − 49 60, 9 − 52 35 | −3 −51 31, | |||
| −2 −47 46, 0 | ||||
| −57 32, 1 − 67 44 | ||||
| dorsomedial PFC | ||||
| − 1 42 31 | −3 51 9 |
Granger Causal Connectivity: Direction of Influence
The direction of influence between the hippocampi and the regions mentioned earlier varied:
-
1
relative to the visceral somatosensory systems, the influence during AV was predominantly toward the right hippocampus and from the left hippocampus; during CSP, the influence was predominantly from both the right and left hippocampus;
-
2
relative to social cognitive systems, the direction of influence was predominantly toward the hippocampus for AV and CSP, although some bidirectional functional connectivity with the left STG was apparent during AV for the left hippocampus;
-
2
relative to the self‐related systems in the PMC (mesial parietal and posterior cingulate cortices), the direction of influence was mixed for AV and CSP. In the dorsal medial prefrontal cortex, direction of influence was from the hippocampus toward these cortices.
DISCUSSION
Emotions about others' mental states are an important foundation for social interaction and behavior, as they are cornerstones of social responsibility and of important aspects of morality [Keltner and Haidt, 1999]. These emotions involve evaluating the psychological implications of others' situations, making inferences about others' mental qualities and experiences, and reacting appropriately to the contents of these inferences [Blakemore and Frith, 2004; Damasio, 2005a; Mitchell et al., 2005]. The processes leading to the induction and maintenance of these emotions are therefore complex and subjective and are likely to use reflections on one's own experiences [Ames et al., 2008], a process that has been connected to hippocampal activation [Perry et al., 2011]. Here, we show that the hippocampus, a structure with established roles in recall, prospection, and personal emotional experience [Eisenberger et al., 2007] also contributes to the processing of social emotions.
Together with anatomically related structures and by virtue of its cellular organization and anatomical placement, the hippocampus is involved in declarative memory, also referred to as explicit and relational memory [Squire and Zola‐Morgan, 1991]. Rather than a repository of permanent memory, it is involved in the encoding of perceptual representations and experiences and is also believed to play a role in the recall of established memories. The hippocampus functions to bind the distributed neocortical sites that together represent the record of a whole event so that, subsequently, a complete memory can be recovered from even a partial cue [Squire et al., 2004]. Although hippocampal damage does not preclude retrieval of previously formed memories, in normal health, the hippocampus is thought to facilitate memory retrieval. Most recently, this structure and its neighboring parahippocampal gyrus have been implicated in the brain's default mode, associated with self‐related processing, social processing with emotional and moral relevance, and simulation of hypothetical social scenarios with personal significance [Andrews‐Hanna et al., 2010]. In investigating the role of the hippocampus during the feeling of social emotions, we reasoned that emotions about others' mental situations (AV and CSP) are inherently more complex, requiring more extensive deliberations on inferred mental qualities than emotions about other's physical situations (AS and CPP), which are more immediate and apparent.
Our experiment probed the strength and duration of hippocampal activation during the processing of social emotions about others' painful versus rewarding mental and physical situations and used Granger causal connectivity analysis to investigate directional influence between the hippocampus and cortical regions involved in affective, social cognitive, and self‐related processing. Using a three‐step emotion induction and verification procedure described in Immordino‐Yang et al. [ 2009], we included for each participant only trials in which (1) the participant reported in the scanner feeling genuinely emotional and (2) the participant's pre‐ and post‐scan interviews revealed that the emotion experienced was the one we intended. We find that the magnitude and pattern of hippocampal contributions vary in accordance with the complexity of processing required and with the quality of the emotion experienced.
Consistent with our hypotheses, emotions about others' mental states were associated with greater and more sustained hippocampal activation, and more extensive connectivity, despite equivalent mnemonic demands associated with recalling the narratives in different conditions of the experiment. We find no differences in reported emotion strength between CSP, CPP, and AV, suggesting that differences in hippocampal involvement among these conditions cannot be attributed to differences in strength of emotional reaction. Reaction times in the scanner were equivalent for CSP and AV, suggesting that the time required for emotion induction cannot account for activation and connectivity differences between these conditions. Interestingly, responses to stimuli inducing CPP occurred ∼ 2 sec (equivalent of 1 TR) earlier on average than responses to CSP or AV stimuli; this was consistent with an earlier reported finding that CPP ramps up more quickly in the brain [Immordino‐Yang et al., 2009].
To probe the contributions of the hippocampus, we calculated the directional connectivity between the hippocampus and the anterior insula and anterior middle cingulate areas that play an important role as the “cortical somatosensory playground” subserving subjective emotional feelings [Craig, 2002; Damasio et al., 2000; Singer et al., 2004] and empathy for others' pain and emotion [Decety and Chaminade, 2003; Eisenberger and Lieberman, 2004; Panksepp, 2005; Singer et al., 2004]. We also probed connectivity to regions implicated in social cognition, including STS and STG, TPJ, and the ventromedial PFC (vmPFC), and vlPFC. Results from previous research have largely converged on the STS, STG, and the TPJ as comprising a neural system involved in drawing inferences about others' mind states and attributing beliefs to others [Mitchell, 2008; Young et al., 2007]. Activity in the vmPFC has been correlated with affective judgment and decision making [Clark et al., 2008; Northoff et al., 2006] and is thought to be involved in the induction of an appropriate social emotion. The vlPFC has been associated with emotion regulation and emotional introspection [Herwig et al., 2010; Wager et al., 2008].
Previously, we had reported significant changes in activation during both varieties of admiration and compassion in each of the regions associated with emotional feeling, social cognition, and self‐processing studied here [Immordino‐Yang et al., 2009]. However, the current study reveals that patterns of connectivity between the hippocampus and these regions differed between the emotions. We found that the hippocampus showed particularly strong and extensive connectivity during the processing of emotions pertaining to others' mental circumstances (AV and CSP), as hypothesized, and that patterns of directional influence differed between these two emotions. (See Fig. 3 for a graphic depiction of the results; although we report under “results,” the lateralization of these effects, lateralization differences were not hypothesized a priori, and we focus here on systems‐level connectivity.) There was hippocampal connectivity with the anterior insula and anterior cingulate, structures associated with visceral somatosensation, empathy and emotional feeling, only during AV and CSP, despite equivalent reported emotion strength during CPP, and despite our previously reported finding of significant BOLD activation during CPP in these regions. Further, although for conciseness we do not report connectivity results during the control condition, we note that these results are minimal, despite the equivalence of the self‐focused task in the interview, i.e., answering the question, “how does this person's story make you feel?”. This finding suggests that the self‐related processing we uncover is not likely due primarily to an artifact of our experimental protocol but to the emotions experienced. Together, these findings suggest a role for the hippocampus in facilitating the coordination of activity during social emotions in regions known to be involved in social and affective processing, and that this role may be particularly, prominent and may influence the emotional feeling state most directly, during emotions about others' minds.
Figure 3.
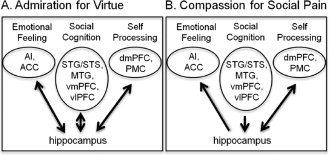
Schematic depicting the overall pattern of results obtained by the GCM for the AV and CSP conditions. The figure's arrows depict the direction of functional connectivity between the hippocampi and the cortical areas implicated in emotional feeling, social cognition, and self‐related processing.
Interestingly, although we did not set out to probe specific anatomical subdivisions within the hippocampus, it is notable that our functionally defined ROI was localized to the anterior rather than to the posterior sector of the structure. It is known that the anterior hippocampus receives projections from subcortical areas that include the hypothalamus and nucleus accumbens and reciprocally projects to the amygdala. Each of these structures is important for emotion processing. The localization of our ROI reinforces the emotion‐related role of the anterior hippocampus and extends it to complex social emotions.
The differences in the predominant direction of influence between AV and CSP may relate to the feeling and social cognitive demands of the two emotions. While CSP necessarily involves an empathic sharing of another person's psychologically painful circumstances, AV is a reaction to the accomplishments of the other person and therefore does not necessarily involve affective empathy. The driving role of the hippocampus toward the AI and ACC during CSP may reflect the importance of recalling the feeling of socially painful experiences as a reference point from which to build an empathic pain response. By contrast, processing leading to AV is relatively independent of direct reference to the protagonist's current emotion state; here, the quality of emotional feeling experienced in relation to the protagonists' accomplishments may provoke the search for relevant personal memories. Consistent with this interpretation, because of the known role of the TPJ in affective perspective‐taking [Moriguchi et al., 2006; Young et al., 2007], we had hypothesized connectivity between the hippocampus and this region during AV and CSP. Interestingly, we found this connectivity during CSP only.
Given the role of the hippocampus in autobiographical memory, and consequently in creating continuity of self [Damasio, 1998], and given that social emotions are thought to recruit personal experiences as a source of information about the emotional implications of others' situations [Perry et al., 2011], we probed connections between the hippocampus and other brain systems involved in self‐related processing, including the dmPFC and the PMC. Functionally, these areas are part of the default network [Hagmann et al., 2008; Raichle et al., 2001], a system that shows greater activation during rest and that is relatively suppressed when attention is focused on external stimuli [Greicius et al., 2003]. The dmPFC has been implicated in judgments about psychological traits related to the self and close others [Blakemore and Frith, 2004; Kitayama and Park, 2010; Mitchell et al., 2005]. Activation in the PMC has been related to self‐awareness [Buckner et al., 2008], personal salience [Seeley et al., 2007], and autobiographical self [Damasio and Meyer, 2009] and has been consistently implicated in episodic memory [Wagner et al., 2005] and in tasks involving moral judgment [Greene et al., 2001], daydreaming [Christoff et al., 2009], and more recently in social emotion [Immordino‐Yang et al., 2009]. Self‐involvement had previously been shown to modulate hippocampal connectivity to the PMC [Muscatell et al., 2010]. Our finding of bidirectional connectivity between the hippocampus and these cortical areas during the processing of AV and CSP supports the importance of self‐processing as a basis from which to experience complex emotions about others' minds. Further, it corroborates the finding reported earlier [Immordino‐Yang et al., 2009] that varieties of emotional processing pertaining to others' mental and physical situations engage distinct, although partially overlapping, neural systems.
From a clinical perspective, our results could help to explain why patients with dementias resulting from degeneration of the hippocampus and associated medial temporal structures often have a deficit in social emotions, especially compassion, as reported by their families [Calabria et al., 2009; Fernandez‐Duque et al., 2010; Wittenberg et al., 2008]. In these patients, the degradation of hippocampal and medial temporal structures [Dickerson and Sperling, 2008] may preclude sufficient access to personal social memories in real‐time processing and thereby interfere with emotion processing.
The above activation and connectivity results, together with the results of another study demonstrating stronger hippocampal recruitment during emotional mentalizing about others judged to be more similar to one's self [Perry et al., 2011], suggest that the prospective and retrospective functions of the hippocampus may play an important facilitative role in the processing of social emotions, in particular those pertaining to others' mental states. This novel finding suggests that complex emotions may engage ongoing recall and/or prospective simulation as a means to generate and sustain the emotion. Future studies should attempt to disambiguate the potential retrospective and prospective contributions of the hippocampus during emotional processing, especially in relation to others' social and psychological situations.
Acknowledgements
The authors thank Antonio and Hanna Damasio for their discussion of the findings and for their comments on an earlier version of the manuscript.
REFERENCES
- Ames DL, Jenkins AC, Banaji MR, Mitchell JP ( 2008): Taking another person's perspective increases self‐referential neural processing. Psychol Sci 19: 642–644. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD ( 2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7: 268–277. [DOI] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Huang C, Buckner RL ( 2010): Evidence for the default network's role in spontaneous cognition. J Neurophysiol 104: 322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Kensinger EA ( 2010): Context is routinely encoded during emotion perception. Psychol Sci 21: 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y ( 1995): Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soc Series B: Methodological 57: 289–300. [Google Scholar]
- Blakemore SJ, Frith U ( 2004): How does the brain deal with the social world?. Neuroreport 15: 119–128. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC ( 2007): Self‐projection and the brain. Trends Cogn Sci 11: 49–57. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL ( 2008): The brain's default network—Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Calabria M, Cotelli M, Adenzato M, Zanetti O, Minuissi C ( 2009): Empathy and emotion recognition in semantic dementia:a case report. Brain Cogn 70: 247–252. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW ( 2009): Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci 106: 8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW ( 2008): Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision‐making. Brain 131: 1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, Bullmore E, Williams S, Van Amelsvoort T, Robertson D, David A, Murphy D ( 2000): Explicit and implicit neural mechanisms for processing of social information from facial expressions: A functional magnetic resonance imaging study. Hum Brain Mapp 9: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR ( 1998): Emotion in the perspective of an integrated nervous system. Brain Res Rev 26: 83–86. [DOI] [PubMed] [Google Scholar]
- Damasio AR ( 2005a): The neurobiological grounding of human values In: Changeux J‐PP, Damasio AR, Singer W, Christian Y, editors. Neurobiology of Human Values. Berlin, Heidelberg: Springer‐Verlag; pp 47–56. [Google Scholar]
- Damasio H ( 2005b): Human Brain Anatomy in Computerized Images, 2nd ed New York: Oxford University Press. [Google Scholar]
- Damasio AR, Meyer K ( 2009): Consciousness: An overview of the phenomenon and of its possible neural basis In: Laureys S, Tononi G, editors. The Neurology of Consciousness. London: Elsevier; pp 3–14. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD ( 2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- Decety J, Chaminade T ( 2003): Neural correlates of feeling sympathy. Neuropsychologia 41: 127–138. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA ( 2008): Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer's disease: Insights from funtional MRI studies. Neuropsychologia 46: 1624–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD ( 2004): Why rejection hurts: A common neural alarm system for physical and social pain. Trends Cogn Sci 8: 294–300. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Gable SL, Lieberman MD ( 2007): Functional magnetic resonance imaging responses relate to differences in real‐world social experience. Emotion 7: 745–754. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Duque D, Hodges SD, Baird JA, Black SE ( 2010): Empathy in frontotemporal dementia and Alzheimer's disease. J Clin Exp Neuropsychol 32: 289–298. [DOI] [PubMed] [Google Scholar]
- Formisano E, Goebel R ( 2003): Tracking cognitive processes with functional MRI mental chronometry. Curr Opin Neurobiol 13: 174–181. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ ( 1997): Brainvox: An interactive, multimodal visualization and analysis system for neuroanatomical imaging. NeuroImage 5: 13–30. [DOI] [PubMed] [Google Scholar]
- Friston K ( 2009): Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol 7: 220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U ( 2006): The neural basis of mentalizing. Neuron 50: 531–534. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P ( 2009): Functional atlas of emotional faces processing: A voxel‐based meta‐analysis of 105 functional magnetic resonance imaging studies. J Psychiatr Neurosci 34: 418–432. [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Goldman A, de Vignemont F ( 2009): Is social cognition embodied? Trends Cogn Sci 13: 154–159. [DOI] [PubMed] [Google Scholar]
- Granger CWJ ( 1969): Investigating causal relations by econometric models and cross‐spectral methods. Econometrica 37: 424–438. [Google Scholar]
- Granger CWJ, Joyeux R ( 1980): An introduction to long‐memory time series models and fractional differencing. J Time Series Anal 1: 15–30. [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD ( 2001): An fMRI investigation of emotional engagement in moral judgment. Science 293: 2105–2108. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V ( 2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O ( 2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6: e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidt J, Morris JP ( 2009): Finding the self in self‐transcendent emotions. Proc Natl Acad Sci 106: 7687–7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Kaffenberger T, Jancke L, Bruhl AB ( 2010): Self‐related awareness and emotion regulation. Neuroimage 50: 734–741. [DOI] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CMA, Cabeza R, Daselaar SM ( 2011): The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS ONE 6: e17463. doi:10.1371/journal.pone.0017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino‐Yang MH ( 2010): Toward a microdevelopmental, interdisciplinary approach to social emotion. Emotion Rev 2: 217–220. [Google Scholar]
- Immordino‐Yang M.H. ( 2011). Me, my “self” and you: Neuropsychological relations between social emotion, self awareness, and morality. Emotion Review, 3: 313–315. [Google Scholar]
- Immordino‐Yang MH, McColl A, Damasio H, Damasio A ( 2009): Neural correlates of admiration and compassion. Proc Natl Acad Sci 106: 8021–8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner D, Haidt J ( 1999): Social functions of emotions at four levels of analysis. Cogn Emotion 13: 505–521. [Google Scholar]
- Kitayama S, Park J ( 2010): Cultural neuroscience of the self: Understanding the social grounding of the brain. Social Cogn Affective Neurosci 5: 111–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ ( 2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon RS, Gati JS, Goodyear B, Luknowsky DC, Thomas CG ( 1998): Spatial and temporal resolution of functional magnetic resonance imaging. Biochem Cell Biol 76: 560–571. [DOI] [PubMed] [Google Scholar]
- Mitchell JP ( 2008): Contributions of functional neuroimaging to the study of social cognition. Curr Directions Psychol Sci 17: 142–146. [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN ( 2005): The link between social cognition and self‐referential thought in the medial prefrontal cortex. J Cogn Neurosci 17: 1306–1315. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Onishi T, Lane RD, Maeda M, Mori T, Nemoto K, Matsuda H, Komaki G ( 2006): Impaired self‐awareness and theory of mind: an fMRI study of mentalizing in alexithymia. Neuroimage 32: 1472–1482. [DOI] [PubMed] [Google Scholar]
- Muscatell K, Addis D, Kensinger E ( 2010): Self‐involvement modulates the effective connectivity of the autobiographical memory network. SCAN 5: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J ( 2006): Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. NeuroImage 31: 440–457. [DOI] [PubMed] [Google Scholar]
- Panksepp J ( 1998): Affective Neuroscience: The foundation of Human and Animal Emotions. New York: Oxford University Press. [Google Scholar]
- Panksepp J ( 2005): Why does separation distress hurt? Comment on MacDonald and Leary (2005). Psychol Bull 131: 224–230. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio AR ( 2006): Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci U S A 103: 1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D, Hendler T, Shamay‐Tsoory SG ( 2011): Projecting memories: The role of the hippocampus in emotional mentalizing. Neuroimage 54: 1669–1676. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R ( 2005): Mapping directed influences over the brain using Granger causality and fMRI. Neuroimage 25: 230–242. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD ( 2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL ( 2011): Episodic memory retrieval, parietal cortex, and the default mode network: Functional and topographic analyses. J Neurosci 31: 4407–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T ( 2006): The neuronal basis and ontogeny of empathy and mind reading: Review of literature and implications for future research. Neurosci Biobehav Rev 30: 855–863. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD ( 2004): Empathy for pain involves the affective but not sensory components of pain. Science 303: 1157–1162. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL ( 2010): Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci 22: 1112–1123. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS ( 2009): The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta‐analysis. J Cogn Neurosci 21: 489–510. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola‐Morgan S ( 1991): The medial temporal lobe memory system. Science 253: 1380–1386. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE ( 2004): The medial temporal lobe. Annu Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- St. Jacques PL, Conway MA, Lowder MW, Cabeza R ( 2011): Watching my mind unfold versus yours: An fMRI study using a novel camera technology to examine neural differences in self‐projection of self versus other perspectives. J Cogn Neurosci 23: 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L ( 2010): Enhanced brain correlations during rest are related to memory for recent experiences. Neuron 65: 280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN ( 2008): Prefrontal‐subcortical pathways mediating successful emotion regulation. Neuron 59: 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL ( 2005): Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9: 445–453. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Wolford GL, Petersen SE, Kelley WM ( 2008): Medial temporal lobe BOLD activity at rest predicts individual differences in memory ability in healthy young adults. Proc Nat Acad Sci 105: 18555–18560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg D, Possin K, Rascovsky K, Rankin KP, Miller BL, Kramer JH ( 2008): The early neuropsychological and behavioral characteristics of frontotemporal dementia. Neuropsychol Rev 18: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Cushman F, Hauser M, Saxe R ( 2007): The neural basis of the interaction between theory of mind and moral judgment. Proc Natl Acad Sci 104: 8235–8240. [DOI] [PMC free article] [PubMed] [Google Scholar]


