Abstract
We determined whether single nucleotide polymorphisms (SNPs) in the glutathione S-transferase omega (GSTO) and arsenic(III)methyltransferase (AS3MT) genes were associated with concentrations of urinary arsenic metabolites among 900 individualswithout skin lesions in Bangladesh. Four SNPs were assessed in these genes. A pathway analysis evaluated the association between urinary arsenic metabolites and SNPs. GSTO1 rs4925 homozygous wild type was significantly associated with higher monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) urinary concentrations, whereas wild type AS3MT rs11191439 had significantly lower levels of AsIII and MMA. Genetic polymorphisms GSTO and As3MT modify arsenic metabolism as evidenced by altered urinary arsenic excretion.
Keywords: pathway analysis, arsenic metabolism, drinking water
Introduction
According to the International Agency for Research on Cancer (IARC), arsenic (As) is classified as a known human carcinogen (IARC, 1980). However, an individual’s ability to metabolize As can modify the risk associated with chronic arsenic exposure (Ahsan et al., 2007, Steinmaus et al., 2010). According to the classical pathway, As is metabolized in humans by oxidative-reduction methylation reactions where arsenate (AsV) is reduced to arsenite (AsIII) and then methylated to form monomethylarsonic acid (MMAV), which undergoes second reduction to form monomethylarsonous acid (MMAIII) which can be further methylated to form dimethylarsinic acid (DMAV) which can be further reduced to dimethylarsinous acid (DMAIII) (Challenger, 1945). Glutathione is a reducing agent whereas S-adenosylmethionine is the primary methyl donor in these reactions (Vahter, 2002).
The metabolism of As in humans is incomplete and all arsenic species (AsV, AsIII, MMA and DMA) are detected in urine. Urinary As profiles have been well documented in several populations and on average, the urinary As levels are composed of 10-30% inorganic As, 10-20% MMA, and 60-70% DMA (Vahter, 1999). Observational studies in populations chronically exposed to arsenic from drinking water have shown that there is large inter-individual variability in the distribution of urinary arsenic metabolites (Vahter, 1999, Loffredo et al., 2003, Steinmaus et al., 2005, Kile et al., 2009). Toxicological studies have shown that the toxicity of arsenic species differs with the trivalent monomethylated forms being the most toxic (Styblo et al., 2000). Epidemiological studies also suggest an individuals ability to methylate As influences susceptibility to As toxicity (Tseng, 2009). Specifically, individuals with higher %MMA or ratio of MMA-to-DMA have a greater risk for skin cancer (Styblo et al., 2000, Vega et al., 2001), bladder cancer (Tseng, 2009), hypertension (Chen et al., 2003), and peripheral vascular disease (Yu et al., 2000).
Understanding the factors associated with As metabolism is important to determine the characteristics that make one more susceptible to arsenic exposure. Age, gender, arsenic levels in drinking water, and urinary creatinine levels have been significantly associated with arsenic metabolism and urinary profiles (Ahsan et al., 2007, Lindberg et al., 2008), but it has also been shown that genetic differences influence As metabolism and urinary arsenic profiles (Lindberg et al., 2007, Marnell et al., 2003).
Several studies have examined the ability of genetic polymorphisms to modify arsenic metabolism. GSTO1 and GSTO2 are members of the glutathione s-transferase family which are involved in metabolizing xenobiotics such as arsenic. In vitro studies have determined that glutathione-S-transferase omega (GSTO1), which is identical to monomethyl arsenate MMAV reductase, is the rate-limiting enzyme involved in arsenic biotransformation (Zakharyan and Aposhian, 1999, Zakharyan et al., 2001) and catalyzes the reduction of AsV to AsIII and DMAV to DMAIII. GSTO2 encodes a protein that shares 64% amino acid identity with GSTO1 (Whitbread et al., 2003) and has also been shown to catalyzes the reduction of MMAV and DMAV (Schmuck et al., 2005). Additionally, it has been shown that the Met287Thr variant has higher enzyme (AS3MT) activity which may contribute to differences in arsenic metabolism, specifically higher levels of methylated arsenic compounds, and its toxicity (Wood et al., 2006).
While many studies have investigated factors associated with arsenic profiles and percentages of urinary metabolites, few studies have reported on excretion of arsenic using urinary arsenic concentrations. The main objective of this study was to determine whether single nucleotide polymorphisms (SNPs) in the glutathione S-transferase omega (GSTO) and arsenic(III)methyltransferase (AS3MT) genes were associated with the excretion of arsenic measured as urinary concentrations of urinary arsenic species, AsV, AsIII, MMA, and DMA. Given the complex relationship between urinary arsenic metabolites, we utilized a novel pathway analysis to simultaneously evaluate the association between the SNPs and all urinary arsenic metabolites amongst controls recruited in a large population-based case-control study of arsenic related skin lesions in an arsenic-endemic region of Bangladesh.
Methods
Study Population
A detailed description of participant selection and sample collection is published elsewhere (Breton et al., 2007). Briefly, a case-control study comprised of 900 cases and 900 controls was conducted in the Pabna region of Bangladesh from 2001-2003 to investigate the association between exposure to arsenic-contaminated drinking water and skin lesions. All participants were recruited for the study through the Dhaka Community Hospital and Pabna Community Clinic. Because this paper is investigating the effect of SNPs on the metabolism of arsenic and not a disease endpoint, the current analysis was limited to the 900 controls to eliminate any possible confounding by disease status. This restriction to controls ensures that the potential effects on arsenic metabolism are not due to the presence of disease. Participants were eliminated if they were missing data for any of the variables included in the path analysis, resulting in 842 controls. All protocols were approved by the Institutional Review Boards (IRB) at Harvard School of Public Health and Dhaka Community Hospital, and informed consent was obtained from each participant prior to the conduct of the study.
Sample Collection
At the time of enrollment, a water sample was collected from the tube well that each participant identified as their primary source of drinking water. The water samples were collected in 50mL polypropylene centrifuge tubes, and two drops of pure nitric acid were added. The water samples were stored at room temperature and analyzed for arsenic using Environmental Protection Agency (EPA) method 200.8 with inductively coupled plasma mass spectroscopy (ICP-MS) (Environmental Laboratory Services, North Syracuse, New York).
On the same day as the water sample collection, a spot urine sample (approximately 120 mL) was collected from each subject. Urine samples were placed in an icebox immediately upon collection, then transferred into 15-ml Falcon tubes, and then frozen at −20°C. Samples were shipped on dry ice to Taipei Medical University where they were analyzed for dimethylarsonic acid (DMA), monomethylarsonic acid (MMA), AsIII, and AsV. Total urinary arsenic was calculated by summing the concentrations of DMA, MMA, AsIII, and AsV. A detailed description of the laboratory procedures have been described elsewhere (McCarty et al., 2007). Urinary creatinine concentrations were quantified using the kinetic Jaffe Method using a Hitachi 7170S autoanalyzer (Tokyo, Japan).
Genotyping
DNA was extracted from whole blood using the Puregene DNA Isolation kit (Gentra Systems, Minneapolis, MN). The following SNPs were detected by the Taqman method using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) and were selected because they were functional (i.e. lead to an amino acid change) or were a tagging SNP: GSTO1 (Ala140Asp, rs4925), GSTO2 (Asn142Asp, rs156697), GSTO2 (rs2297235), and AS3MT (Met287Thr, rs11191439). As part of quality control, 10% of the samples were analyzed in duplicate and two readers evaluated the output. All selected SNPs passed the Hardy-Weinberg equilibrium chi-squared test with p-value > 0.05 (Rodriguez et al., 2009) and had a minor allele frequency (MAF) > 5%.
Statistical Analyses
The arsenic and creatinine variables (DMA, MMA, AsIII, AsV, water As, and creatinine) were natural log-transformed so these variables were more approximately normally distributed. Age and BMI were treated as continuous covariates and education and gender were dichotomous variable. The Wilcoxon rank sum test was used to compare the urinary arsenic concentrations for each metabolite by genotype while linear regression was used to determine the effect of each SNP on urinary arsenic concentrations while adjusting for water As levels, age, sex, body mass index (BMI), education, and urinary creatinine concentrations. We used the dominant genetic model where we combined the heterozygous and homozygous variants into one category due to the small number of variants for some SNPs.
Path analysis is an intuitive statistical method when the link between exposure and outcome are mediated by other variables. Path analysis is used to establish how well a statistical model accounts for existing correlations among variables (outcome and covariates) in observed data and is particularly well suited for analysis of variables with complex relationships. Path analysis was used to estimate the relationship among urinary arsenic metabolites (As3, As5, MMA, and DMA) and SNPs in the GSTO and AS3MT genes controlling for current As exposure.
This path analysis included 842 participants due to missing data on any of the included variables. In this path analysis the relationship between the urinary arsenic metabolites and SNPs was estimated using raw data, not covariance matrices, in Proc CALIS in SAS 9.1.3 (SAS Institute Inc, Cary, NC, USA). The paths incorporated factors that significantly (p<0.10) predicted the natural log of the DMA, MMA, AsIII, AsV variables. The process of selecting the model involved simultaneously fitting a series of linear regressions and selecting the model which best fit the observed covariances among the urinary arsenic metabolites and covariates. Ultimately, we chose the most biologically plausible model that conformed to standard path analysis goodness of fit indices. Fit is based on criteria described in ‘A Step by Step Approach to Using SAS for Factor Analysis and Structural Equation Modeling, 2007’ which included a chi-squared test (χ2 p-value>0.05), root mean square error of approximation (RMSEA <0.05), root mean square residuals (RMR <0.05), Bentler’s comparative fit index (CFI >0.9), Bentler and Bonnet’s Normed Fit Index (NFI>0.9), and Bentler and Bonnet’s Non-normed fit index (NNFI>0.9).
Results
Demographic information is presented in Table 1. The majority of participants were male and 40% had completed primary school or less. There was high variability in the water arsenic concentrations of the participants with an average arsenic concentration of 11.4 ug/L and a range from 0.5 to 1190 ug/L.
Table 1.
Characteristics of study population
| Characteristic | Controls n=896 |
|---|---|
| Age, mean (SDa) | 33.3 (12) |
| Body Mass Index (BMI), mean (SD) | 20.3 (3) |
| Water As level, ug/L, median (range) | 11.4 (0.5-1190) |
| missing | 24 |
| Years drinking from current well, median (range) | 8.0 (0-60) |
| missing | 5 |
| Urinary AsV, ug/L, median (range) | 0.97 (NDb- 269.2) |
| Urinary AsIII, ug/L, median (range) | 3.51 (ND-366.8) |
| Urinary MMA, ug/L, median (range) | 6.03 (ND-320.5) |
| Urinary DMA, ug/L, median (range) | 40.04 (ND-2135.6) |
| Gender, n (%) | |
| Male | 553 (62) |
| Female | 343 (38) |
| Education, n (%) | |
| Illiterate | 135 (15) |
| Able to write name | 219 (24) |
| Primary | 117 (13) |
| Middle school | 290 (32) |
| Secondary or more | 134 (15) |
| missing | 1 (1) |
| GSTO1-1, rs4925, n (%) | |
| HZ+Variant | 295 (33) |
| Wildtype | 577 (64) |
| missing | 24 (3) |
| GSTO2-1, rs156697, n (%) | |
| HZ+Variant | 437 (49) |
| Wildtype | 404 (45) |
| missing | 55 (6) |
| GSTO2-2, rs2297235, n (%) | |
| HZ+Variant | 279 (31) |
| Wildtype | 583 (65) |
| missing | 34 (4) |
| AS3MT, rs11191439, n (%) | |
| HZ+Variant | 97 (11) |
| Wildtype | 792 (88) |
| missing | 7 (1) |
The concentrations of creatinine-adjusted urinary arsenic species were compared for the homozygous wildtype genotype and the combined heterozygous and variant genotypes (Table 2). The homozygous wildtype for one of the SNPs in the GSTO2 gene (rs2297235) had statistically higher AsIII, MMA and DMA urinary concentrations, while the homozygous wildtype SNP in the AS3MT gene had lower concentrations of urinary AsIII and MMA.
Table 2.
Creatinine-adjusted urinary concentrations (μg/g) by genotype among controls
| AsV | AsIII | MMA | DMA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SNP | n | Median (IQRa) | p-value | Median (IQR) | p-value | Median (IQR) | p-value | Median (IQR) | p-value |
| GSTO1-1, rs4925 | |||||||||
| HZb+Variant | 295 | 1.97 (0.5, 7.1) | 0.75 | 5.61 (1.2, 15.7) | 0.24 | 11.55 (4.4, 26.9) | 0.05 | 72.33 (39.5, 156.4) | 0.12 |
| Wildtype | 577 | 2.12 (0.6, 6.9) | 6.90 (1.8, 18.5) | 13.40 (5.7, 30.9) | 82.61 (46.2, 154.9) | ||||
| GSTO2-1, rs156697 | |||||||||
| HZ+Variant | 437 | 2.16 (0.7, 7.2) | 0.45 | 6.17 (1.7, 16.4) | 0.20 | 12.61 (5.5, 28.4) | 0.37 | 75.82 (41.3, 149.3) | 0.14 |
| Wildtype | 404 | 2.00 (0.5, 6.9) | 7.10 (2.0, 19.5) | 13.70 (5.9, 29.4) | 85.28 (47.2, 160.6) | ||||
| GSTO2-2, rs2297235 | |||||||||
| HZ+Variant | 279 | 1.98 (0.5, 7.1) | 0.67 | 5.38 (1.0, 15.3) | 0.03 | 10.88 (4.9, 25.6) | 0.03 | 70.25 (39.5, 146.0) | 0.02 |
| Wildtype | 583 | 2.11 (0.6, 7.2) | 7.32 (1.9, 18.9) | 13.78 (5.8, 31.3) | 83.62 (47.1, 158.9) | ||||
| AS3MT, rs11191439 | |||||||||
| HZ+Variant | 97 | 2.29 (0.5, 8.3) | 0.86 | 8.70 (4.3, 22.3) | 0.01 | 18.08 (6.6, 34.6) | 0.01 | 77.54 (41.9, 167.1) | 0.82 |
| Wildtype | 792 | 2.04 (0.6, 6.8) | 6.32 (1.2, 16.2) | 12.27 (5.2, 27.4) | 80.55 (44.0, 152.9) | ||||
Note: Wilcoxon rank sum test was used to compare the urinary concentrations by genotype.
IQR: Interquartile range defined as the range from the 25th percentile to the 75th percentile
HZ: Heterozygous
Our findings were similar after adjusting for water arsenic levels, age, sex, BMI, education, and urinary creatinine levels (Table 3). The results of the linear regression models show that the heterozygous and homozygous variant genotypes in the GSTO genes were associated with lower urinary arsenic concentrations, specifically MMA, DMA, and total As, indicating an overall decrease in urinary arsenic excretion. Conversely, the heterozygous and homozygous variant Met287Thr had higher urinary arsenic concentrations, specifically AsIII and MMA, compared to the wildtype.
Table 3.
Univariate effect estimates of related factors on urinary arsenic metabolite concentrations among controls only
| LN(AsV) | LN(AsIII) | LN(MMA) | LN(DMA) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | n | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| Ln Water As level, μg/L | 872 | 0.27 | 0.05 | <0.0001 | 0.47 | 0.07 | <0.0001 | 0.40 | 0.04 | <0.0001 | 0.26 | 0.02 | <0.0001 |
| Age, years | 896 | 0.0009 | 0.01 | 0.93 | 0.04 | 0.01 | 0.0008 | 0.01 | 0.007 | 0.04 | 0.01 | 0.003 | 0.005 |
| Sex | |||||||||||||
| Female | 553 | −0.23 | 0.23 | 0.32 | 0.37 | 0.30 | 0.21 | −0.22 | 0.17 | 0.19 | 0.16 | 0.08 | 0.06 |
| Male | 343 | Ref | Ref | Ref | Ref | ||||||||
| BMI | 896 | −0.05 | 0.03 | 0.16 | −0.03 | 0.04 | 0.51 | −0.06 | 0.02 | 0.02 | −0.005 | 0.01 | 0.71 |
| Education | 0.04 | <0.0001 | 0.08 | 0.002 | |||||||||
| Illiterate | 135 | 0.77 | 0.41 | 0.06 | 1.90 | 0.53 | 0.0003 | 0.68 | 0.29 | 0.02 | 0.51 | 0.15 | 0.0006 |
| Able to write name | 219 | 1.16 | 0.37 | 0.002 | 1.46 | 0.47 | 0.002 | 0.37 | 0.26 | 0.16 | 0.39 | 0.13 | 0.004 |
| Primary | 117 | 0.74 | 0.43 | 0.08 | −0.09 | 0.55 | 0.87 | −0.07 | 0.31 | 0.82 | 0.17 | 0.15 | 0.26 |
| Middle School | 290 | 0.63 | 0.35 | 0.08 | 0.51 | 0.45 | 0.26 | 0.26 | 0.25 | 0.31 | 0.16 | 0.13 | 0.21 |
| Secondary or more | 134 | Ref | Ref | Ref | Ref | ||||||||
| Urinary creatinine levels, mg/dL | 896 | 0.005 | 0.002 | 0.02 | 0.02 | 0.003 | <0.0001 | 0.01 | 0.001 | <0.0001 | 0.01 | 0.0007 | <0.0001 |
| Drinking from current well, years | 891 | 0.02 | 0.01 | 0.19 | 0.04 | 0.02 | 0.03 | −0.00007 | 0.01 | 0.99 | 0.002 | 0.005 | 0.72 |
LN: natural logarithm; SE: standard error; β: percent change in the urinary metabolite
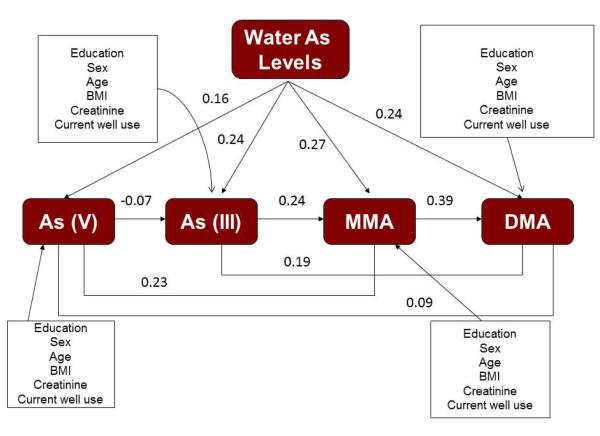
In addition to investigating the effect of the SNPs on each urinary arsenic metabolite separately, path analysis was used to investigate the relationship between the SNPs and the urinary metabolites in one model. The path analysis tested Model 1 shown in Figure 1 (does not include SNPs) and Model 2 in Figure 2 (includes SNPs). Similar to the regression models, each urinary arsenic metabolite was modeled with demographic variables (e.g. sex, age, bmi, and education), water arsenic concentrations, urinary creatinine levels, and the relevant SNPs as predictors. Additionally, the urinary arsenic concentrations of the other metabolites were included as predictors (e.g. MMA concentration was a predictor of DMA concentration). The standardized path coefficients for the main effects of the urinary metabolites in Model 1 are shown in Figure 1 and are all statistically significant (p<0.05). Water arsenic levels were significantly associated with each of the urinary metabolites with the standardized path coefficients ranging from 0.16 to 0.27, showing the greatest effect on MMA. Additionally, Model 2 (Figure 2) shows the significant relationships between the SNPs and the urinary metabolites in the path analysis are consistent with those found in the linear regression models. In Figure 2, all standardized path coefficients for the included SNPs are significant at p<0.05, except the interaction between GSTO and water As for DMA which has a p-value=0.08. Unlike the regression models, the path analysis allows us to show the significant associations among the urinary metabolites as well.
Figure 1.
Arsenic metabolism pathway illustrating the relationships between water arsenic levels and urinary arsenic metabolites while adjusting for covariates (Model 1). The standardized path coefficients (r) are listed for each path and effects of the SNPs. Only the SNPs that were significant at the 0.05 level remained in the path analysis.
Figure 2.
Arsenic metabolism pathway illustrating the relationships between water arsenic levels and urinary arsenic metabolites, including the main effects of genetic SNPs and covariates (Model 2). The standardized path coefficients (r) are listed for each path and effects of the SNPs. Only the SNPs that were significant at the 0.05 level remained in the path analysis.
The goodness of fit indices are listed in Table 4 and indicate that Model 2 (with SNPs) fits the data quite well. Specifically, a non-significant model chi-square p-value for Model 2 (p=0.62) indicates that the proposed model fits the data better than a model that does not include SNPs (chi-square p-value=0.009 for Model 1). Additional goodness of fit indices include the normed fit index (NFI), non-normed fit index (NNFI), and the comparative fit index (CFI), and all these indices were greater than 0.99 indicating that both models provided a good fit to the data. However, the AIC statistic is smaller for Model 2 (-12.9514 vs 0.2478), which again supports a better fit with the SNPs included in the model.
Table 4.
Effect of genetic SNPs on urinary arsenic metabolite concentrations among controls only*
| LN(AsV) | LN(AsIII) | LN(MMA) | LN(DMA) | LN(Total As) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
|
GSTO1-1 (Ala140Asp) |
|||||||||||||||
| HZ+Variants | −0.06 | 0.24 | 0.80 | −0.22 | 0.29 | 0.44 | −0.44 | 0.16 | 0.005 | −0.19 | 0.07 | 0.005 | −0.18 | 0.06 | 0.005 |
| Wildtype | ref | ref | ref | ref | ref | ||||||||||
| GSTO2-1 | |||||||||||||||
| HZ+Variants | 0.23 | 0.24 | 0.34 | 0.06 | 0.28 | 0.82 | −0.10 | 0.14 | 0.50 | −0.14 | 0.07 | 0.03 | −0.12 | 0.06 | 0.06 |
| Wildtype | ref | ref | ref | ref | ref | ||||||||||
| GSTO2-2 | |||||||||||||||
| HZ+Variants | −0.10 | 0.25 | 0.70 | −0.37 | 0.29 | 0.21 | −0.40 | 0.15 | 0.009 | −0.23 | 0.07 | 0.0009 | −0.21 | 0.06 | 0.001 |
| Wildtype | ref | ref | ref | ref | ref | ||||||||||
| AS3MT | |||||||||||||||
| HZ+Variants | −0.20 | 0.37 | 0.59 | 1.13 | 0.44 | 0.01 | 0.66 | 0.23 | 0.005 | 0.12 | 0.10 | 0.24 | 0.18 | 0.10 | 0.06 |
| Wildtype | ref | ref | ref | ref | ref | ||||||||||
Adjusted for water arsenic levels, age, sex, bmi, education, urinary creatinine levels and years of drinking from current well.
LN: natural logarithm; SE: standard error; HZ: heterozygous; β: percent change in the urinary metabolites compared to the wildtype
Both pathway models explained 4%, 22%, 40% (39% for Model 1), and 67% of the variability in AsV, AsIII, MMA and DMA, respectively (Table 5). In addition to calculating the total variability explained, we were able to calculate both the direct and indirect effects of each variable on an outcome. We demonstrate this in Table 6 for Model 2 where we calculate the direct and indirect effects of the urinary arsenic metabolites on DMA. As expected, MMA had the greatest effect on DMA, and AsV had the smallest. While the direct effects were larger, the indirect effects were notable, especially for those further down the pathway from DMA. The indirect effects indicate that the urinary metabolites should be considered simultaneously as they may impact each other through intermediates. Water had the greatest direct effect on urinary MMA concentrations compared to the other metabolites which is consistent with the previous findings that MMA (V) reductase is the rate-limiting enzyme in arsenic biotransformation.
Table 5.
Model Fit Indices (SAS Proc CALIS), N=837
| Index (criterion for “good fit”) | Model 1 | Model 2 |
|---|---|---|
| Chi-squared test p-value (>.05) | 0.0126 | 0.6908 |
| RMSEA (<.05) | 0.0337 | 0.0000 |
| RMR (<.05) | 0.0346 | 0.0265 |
| CFI (>0.9) | 0.9955 | 1.0000 |
| NFI (>0.9) | 0.9911 | 0.9977 |
| NNFI (>0.9) | 0.9707 | 1.0077 |
| AIC (smaller is better) | −0.7746 | −13.7504 |
| SBC (smaller is better) | −76.4517 | −65.7785 |
|
R-squared values for DMA, MMA, As(III), As(V) |
0.67, 0.39, 0.22, 0.04 | 0.67 0.40, 0.22, 0.04 |
Model 1: no genetic SNPs in the model
Model 2: significant genetic SNPs included in model
Independence Model Chi-square=3513.2, df=105, p-value<0.001
Table 6.
The direct and indirect effects of urinary arsenic metabolites on DMA.
| Effect of | Indirecta | Direct | Total |
|---|---|---|---|
| MMA | 0.39 | 0.39 | |
| As(III) | 0.23*0.39=0.09 | 0.19 | 0.28 |
| As(V) | (−0.07*0.23*0.39) + (0.23*0.39)+(−0.07*0.19)=0.07 |
0.10 | 0.17 |
Indirect effects are calculated by multiplying the partial correlation coefficients for each path and summing them together.
Discussion
While the physiological role of GSTO1 enzyme is not fully understood, the function of the rs4925 variant (Ala140Asp) has been characterized and thioltransferase activity has been shown to be significantly lower for this variant compared to the wildtype. In contrast, no difference in the kinetic parameters of MMAV reductase activity was observed between the variant and wildtype (Tanaka-Kagawa et al., 2003). Our findings show that this variant is associated with decreased excretion of arsenic measured as lower urinary arsenic concentrations, indicating that thioltransferase activity may play a role in arsenic metabolism or this variant may be highly correlated with another SNP that is involved in the metabolism of arsenic.
Similar to other Asian populations (Fujihara et al., 2008), the frequency of the Met287Thr variant in the AS3MT gene in this study population was very low (0.25%), but the combined heterozygous and variant group had significantly higher concentrations of urinary AsIII and MMA. This finding is consistent with another study that found increased arsenic methylation (higher %MMA) among males living in Chile with the polymorphism (Hernandez et al., 2008b, Hernandez et al., 2008a). Unlike our study, Hernandez et al. did not find significant differences in urinary AsIII levels.
In addition to investigating the main effect of the SNPs, we also investigated whether there was effect modification by gender. While the stratified results appeared different for males and females, the interaction terms were not statistically significant indicating that the gender difference was not significant. While seafood may contain organic arsenicals, our analytical approach focused on urinary metabolites that are not related to seafood consumption (Hsueh et al., 2002). Smoking status was also not included in the analysis because it was strongly correlated with gender as women in Bangladesh do not smoke. While we excluded individuals with arsenic-related skin lesions from this analysis we were unable to exclude other-arsenic related diseases because individual medical histories only collected information on broad categories of chronic diseases. Also, we were unable to evaluate heritability in this study even though it is plausible that some participants were related to each other given our recruitment in small rural villages because we did not collect information on participant’s genealogy.
There are several strengths of this study. Namely, it is a large population based study which was confined to controls to eliminate potential confounding by disease status. However, these controls were only defined as not having arsenic-induced skin lesions, and they may have had other diseases which could influence metabolic processes. The path analysis is a statistically efficient method for evaluating the relationship between highly collinear variables and allows us to calculate both direct and indirect effects of independent variables. Although the fit indices for the model indicated that the model fit the data quite well, there may be additional variables that were not included in the model. For example, there may be other SNPs in these investigated genes or other genes that are involved in the metabolism of arsenic or other environmental and health factors that may explain more of the variability in the urinary arsenic concentrations.
Unlike the linear regression models, the use of path analysis allows us to include several correlated urinary arsenic metabolites in the same model. While the effect estimates resulting from the different analyses cannot be directly compared, the results are consistent showing that the same significant associations are observed between the genetic polymorphisms and the urinary metabolites for both statistical methods.
Conclusions
Single nucleotide polymorphism (SNPs) in the glutathione-s transferase Omega genes (GSTO) and arsenic methyltransferase (AS3MT) genes are associated with urinary arsenic metabolite concentrations among individuals not exhibiting skin lesions. These genes play a role in the metabolism and excretion of arsenic which may lead to increased risk of arsenic-related diseases. While this study shows significant findings, the results cannot be generalized to individuals with arsenic-related or other chronic health conditions.
Acknowledgements
The authors thank all our colleagues and research staff at Dhaka Community Hospital and Pabna Community Clinic in Bangladesh. We also acknowledge Li Su, Rihong Zhai, and Chau-Chyun Sheu for their laboratory assistance.
Declaration of Interest This work was supported by the United States National Institute of Health (NIH) grant T32 ES07069, and National Institute of Environmental Health Sciences (NIEHS) grants # ES R01011622, ES P42016454, and ES 00002.
References
- AHSAN H, CHEN Y, KIBRIYA MG, SLAVKOVICH V, PARVEZ F, JASMINE F, GAMBLE MV, GRAZIANO JH. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol Biomarkers Prev. 2007;16:1270–8. doi: 10.1158/1055-9965.EPI-06-0676. [DOI] [PubMed] [Google Scholar]
- BRETON CV, ZHOU W, KILE ML, HOUSEMAN EA, QUAMRUZZAMAN Q, RAHMAN M, MAHIUDDIN G, CHRISTIANI DC. Susceptibility to arsenic-induced skin lesions from polymorphisms in base excision repair genes. Carcinogenesis. 2007;28:1520–5. doi: 10.1093/carcin/bgm063. [DOI] [PubMed] [Google Scholar]
- CHALLENGER F. Biological Methylation. Chemical Reviews. 1945;36:315–361. [Google Scholar]
- CHEN YC, GUO YL, SU HJ, HSUEH YM, SMITH TJ, RYAN LM, LEE MS, CHAO SC, LEE JY, CHRISTIANI DC. Arsenic methylation and skin cancer risk in southwestern Taiwan. J Occup Environ Med. 2003;45:241–8. doi: 10.1097/01.jom.0000058336.05741.e8. [DOI] [PubMed] [Google Scholar]
- FUJIHARA J, SOEJIMA M, KODA Y, KUNITO T, TAKESHITA H. Asian specific low mutation frequencies of the M287T polymorphism in the human arsenic (+3 oxidation state) methyltransferase (AS3MT) gene. Mutat Res. 2008;654:158–61. doi: 10.1016/j.mrgentox.2008.06.001. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ A, XAMENA N, SEKARAN C, TOKUNAGA H, SAMPAYO-REYES A, QUINTEROS D, CREUS A, MARCOS R. High arsenic metabolic efficiency in AS3MT287Thr allele carriers. Pharmacogenet Genomics. 2008a;18:349–55. doi: 10.1097/FPC.0b013e3282f7f46b. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ A, XAMENA N, SURRALLES J, SEKARAN C, TOKUNAGA H, QUINTEROS D, CREUS A, MARCOS R. Role of the Met(287)Thr polymorphism in the AS3MT gene on the metabolic arsenic profile. Mutat Res. 2008b;637:80–92. doi: 10.1016/j.mrfmmm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- HSUEH YM, HSU MK, CHIOU HY, YANG MH, HUANG CC, CHEN CJ. Urinary arsenic speciation in subjects with or without restriction from seafood dietary intake. Toxicol Lett. 2002;133:83–91. doi: 10.1016/s0378-4274(02)00087-5. [DOI] [PubMed] [Google Scholar]
- INTERNATIONAL AGENCY FOR RESEARCH ON CANCER (IARC) Arsenic and Arsenic Compounds. Lyon: 1980. (IARC monographs 23). [Google Scholar]
- KILE ML, HOFFMAN E, HSUEH YM, AFROZ S, QUAMRUZZAMAN Q, RAHMAN M, MAHIUDDIN G, RYAN L, CHRISTIANI DC. Variability in biomarkers of arsenic exposure and metabolism in adults over time. Environ Health Perspect. 2009;117:455–60. doi: 10.1289/ehp.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDBERG AL, EKSTROM EC, NERMELL B, RAHMAN M, LONNERDAL B, PERSSON LA, VAHTER M. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ Res. 2008;106:110–20. doi: 10.1016/j.envres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- LINDBERG AL, KUMAR R, GOESSLER W, THIRUMARAN R, GURZAU E, KOPPOVA K, RUDNAI P, LEONARDI G, FLETCHER T, VAHTER M. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environ Health Perspect. 2007;115:1081–6. doi: 10.1289/ehp.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOFFREDO CA, APOSHIAN HV, CEBRIAN ME, YAMAUCHI H, SILBERGELD EK. Variability in human metabolism of arsenic. Environ Res. 2003;92:85–91. doi: 10.1016/s0013-9351(02)00081-6. [DOI] [PubMed] [Google Scholar]
- MARNELL LL, GARCIA-VARGAS GG, CHOWDHURY UK, ZAKHARYAN RA, WALSH B, AVRAM MD, KOPPLIN MJ, CEBRIAN ME, SILBERGELD EK, APOSHIAN HV. Polymorphisms in the human monomethylarsonic acid (MMA V) reductase/hGSTO1 gene and changes in urinary arsenic profiles. Chem Res Toxicol. 2003;16:1507–13. doi: 10.1021/tx034149a. [DOI] [PubMed] [Google Scholar]
- MCCARTY KM, CHEN YC, QUAMRUZZAMAN Q, RAHMAN M, MAHIUDDIN G, HSUEH YM, SU L, SMITH T, RYAN L, CHRISTIANI DC. Arsenic methylation, GSTT1, GSTM1, GSTP1 polymorphisms, and skin lesions. Environ Health Perspect. 2007;115:341–5. doi: 10.1289/ehp.9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ S, GAUNT TR, DAY IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol. 2009;169:505–14. doi: 10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMUCK EM, BOARD PG, WHITBREAD AK, TETLOW N, CAVANAUGH JA, BLACKBURN AC, MASOUMI A. Characterization of the monomethylarsonate reductase and dehydroascorbate reductase activities of Omega class glutathione transferase variants: implications for arsenic metabolism and the age-at-onset of Alzheimer’s and Parkinson’s diseases. Pharmacogenet Genomics. 2005;15:493–501. doi: 10.1097/01.fpc.0000165725.81559.e3. [DOI] [PubMed] [Google Scholar]
- STEINMAUS C, YUAN Y, KALMAN D, ATALLAH R, SMITH AH. Intraindividual variability in arsenic methylation in a U.S. population. Cancer Epidemiol Biomarkers Prev. 2005;14:919–24. doi: 10.1158/1055-9965.EPI-04-0277. [DOI] [PubMed] [Google Scholar]
- STEINMAUS C, YUAN Y, KALMAN D, REY OA, SKIBOLA CF, DAUPHINE D, BASU A, PORTER KE, HUBBARD A, BATES MN, SMITH MT, SMITH AH. Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicol Appl Pharmacol. 2010;247:138–45. doi: 10.1016/j.taap.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STYBLO M, DEL RAZO LM, VEGA L, GERMOLEC DR, LECLUYSE EL, HAMILTON GA, REED W, WANG C, CULLEN WR, THOMAS DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74:289–99. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- TANAKA-KAGAWA T, JINNO H, HASEGAWA T, MAKINO Y, SEKO Y, HANIOKA N, ANDO M. Functional characterization of two variant human GSTO 1-1s (Ala140Asp and Thr217Asn) Biochem Biophys Res Commun. 2003;301:516–20. doi: 10.1016/s0006-291x(02)03066-8. [DOI] [PubMed] [Google Scholar]
- TSENG CH. A review on environmental factors regulating arsenic methylation in humans. Toxicol Appl Pharmacol. 2009;235:338–50. doi: 10.1016/j.taap.2008.12.016. [DOI] [PubMed] [Google Scholar]
- VAHTER M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog. 1999;82(Pt 1):69–88. doi: 10.1177/003685049908200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAHTER M. Mechanisms of arsenic biotransformation. Toxicology. 2002;181-182:211–7. doi: 10.1016/s0300-483x(02)00285-8. [DOI] [PubMed] [Google Scholar]
- VEGA L, STYBLO M, PATTERSON R, CULLEN W, WANG C, GERMOLEC D. Differential effects of trivalent and pentavalent arsenicals on cell proliferation and cytokine secretion in normal human epidermal keratinocytes. Toxicol Appl Pharmacol. 2001;172:225–32. doi: 10.1006/taap.2001.9152. [DOI] [PubMed] [Google Scholar]
- WHITBREAD AK, TETLOW N, EYRE HJ, SUTHERLAND GR, BOARD PG. Characterization of the human Omega class glutathione transferase genes and associated polymorphisms. Pharmacogenetics. 2003;13:131–44. doi: 10.1097/00008571-200303000-00003. [DOI] [PubMed] [Google Scholar]
- WOOD TC, SALAVAGIONNE OE, MUKHERJEE B, WANG L, KLUMPP AF, THOMAE BA, ECKLOFF BW, SCHAID DJ, WIEBEN ED, WEINSHILBOUM RM. Human arsenic methyltransferase (AS3MT) pharmacogenetics: gene resequencing and functional genomics studies. J Biol Chem. 2006;281:7364–73. doi: 10.1074/jbc.M512227200. [DOI] [PubMed] [Google Scholar]
- YU RC, HSU KH, CHEN CJ, FROINES JR. Arsenic methylation capacity and skin cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1259–62. [PubMed] [Google Scholar]
- ZAKHARYAN RA, APOSHIAN HV. Enzymatic reduction of arsenic compounds in mammalian systems: the rate-limiting enzyme of rabbit liver arsenic biotransformation is MMA(V) reductase. Chem Res Toxicol. 1999;12:1278–83. doi: 10.1021/tx9901231. [DOI] [PubMed] [Google Scholar]
- ZAKHARYAN RA, SAMPAYO-REYES A, HEALY SM, TSAPRAILIS G, BOARD PG, LIEBLER DC, APOSHIAN HV. Human monomethylarsonic acid (MMA(V)) reductase is a member of the glutathione-S-transferase superfamily. Chem Res Toxicol. 2001;14:1051–7. doi: 10.1021/tx010052h. [DOI] [PubMed] [Google Scholar]