Abstract
Background
the effects of serum vitamin D status on atopy, steroid requirement and functional responsiveness to corticosteroids in children vs. adults with asthma have not been studied systematically.
Objective
to explore age-specific effects of vitamin D in asthma.
Methods
serum vitamin D levels were examined in a prospective study of adults and children (102 normal controls and 103 asthmatics). Peripheral blood mononuclear cells (PBMC) were cultured for 3h +/−100nM dexamethasone (DEX) and the expression of corticosteroid-regulated genes was detected by real time PCR. Serum IgE levels were measured; information about asthmatics’ steroid requirement was collected.
Results
47.6% of asthmatics and 56.8% normal control subjects had deficient serum vitamin D levels (<20ng/ml) with mean ± SD of 20.7±9.8ng/ml and 19.2±7.7ng/ml, respectively. In multivariate regression models, a significant positive correlation between serum vitamin D and the expression of vitamin D regulated targets - cyp24a by PBMC (p=0.0084, pediatric asthma group only) and serum LL-37 levels (p=0.0006, pediatric; but p=0.0067 in adult asthma groups) was found. An inverse association between vitamin D and serum IgE levels was observed in the pediatric (p=0.006) asthma group. Serum vitamin D (p=0.05) as well as PBMC cyp24a expression (p=0.0312) demonstrated significant inverse relationship with daily ICS dose in the pediatric asthma group only. Cyp24a expression in PBMC correlated positively with in vitro suppression of TNFα (p=0.05) and IL-13 (p=0.0094) in PBMC by DEX only in the pediatric asthma group.
Conclusions
this study demonstrated significant associations between serum vitamin D status and steroid requirement and in vitro responsiveness to corticosteroids in the pediatric but not the adult asthma group. Vitamin D was also related to IgE levels in children but not in adults.
Clinical Implication
The results of this study suggest that vitamin D supplementation in children may enhance corticosteroid responses, control atopy and could thereby improve asthma control.
Keywords: vitamin D, asthma, corticosteroids
INTRODUCTION
The biomedical literature has demonstrated an enormous burst of interest in vitamin D over the past ten years. It has been reported that vitamin D insufficiency/deficiency is on the rise in the general population both in the US and globally.1–4 A number of epidemiologic investigations have shown an association between low serum vitamin D and various diseases.5–7 Several manuscripts have reported a potential role for vitamin D in asthma.8–12
The accumulated data showed an association of low serum vitamin D levels in asthmatics with increased airway obstruction and elevated corticosteroid requirement.13 Several recent studies have suggested that low serum vitamin D can influence the severity of asthma and/or atopy.14–16 The majority of these associations were reported for the pediatric asthma study groups. The study of Costa Rican children (age 6–14) with asthma by Brehm et al.14 reported an inverse association between serum vitamin D levels and total IgE and eosinophil count. Subjects with reduced serum vitamin D levels had increased airway hyperreactivity (AHR) and were more likely to be hospitalized for asthma complications and to use anti-inflammatory medications, including inhaled corticosteroids (ICS).14 In a follow up study using the samples from Childhood Asthma Management Program study Brehm et al.17 reported that children with insufficient serum vitamin D levels have higher odds of hospitalization or emergency department visit. It was shown that study subjects are likely to have severe asthma exacerbations if they have insufficient vitamin D levels despite using ICS.17 The study by Searing et al.10 reported that asthmatic children on ICS and on oral corticosteroids have significantly lower serum vitamin D levels. From the analysis of the results of the National Health and Nutrition examination Survey 2005–2006 in US children and adults it was reported that vitamin D deficiency is associated with higher levels of IgE sensitization in the pediatric and adolescent group (<21 years old).15 No associations between vitamin D levels and allergic sensitization were reported in adults.15 To our knowledge this is the only study that compared the relationship between vitamin D and allergic sensitization in adults and children. The nature of the age specific effects of vitamin D is unclear.
Recently an Institute of Medicine (IOM) Committee conducted a comprehensive review of the evidence for both skeletal and extraskeletal outcomes of vitamin D and calcium supplementation.18,19 The Committee concluded that available scientific evidence supports a key role for calcium and vitamin D in skeletal health. However, for other outcomes, including cancer, cardiovascular disease, diabetes, and autoimmune disorders, the evidence was inconsistent, inconclusive as to causality, and insufficient to inform nutritional requirements. It was stated that randomized clinical trial evidence for these extraskeletal outcomes was limited and generally uninformative. The Committee concluded that the prevalence of vitamin D inadequacy in North America has been overestimated as circulating levels of 20ng/ml were determined sufficient to support bone health in the majority of population. The IOM panel concluded that more research and reassessment of laboratory ranges for serum vitamin D should be done to avoid problems of both undertreatment and overtreatment.18,19
The goal of the current study was to examine the relationships between serum 25-hydroxyvitamin D levels, markers of activation of vitamin D receptor activated pathways, PBMC responsiveness to dexamethasone (DEX), and corticosteroid requirements in asthmatic patients and to explore whether these associations differed in adults and children. The knowledge about age specific relationships between the above mentioned markers is of great importance to justify potential benefits from vitamin D supplementation in different age groups of asthmatics.
In this study we examined age specific associations between vitamin D levels and atopy, steroid requirement and functional response to corticosteroids in pediatric (<18 years old) and adult (≥18 years old) asthma groups. In this study we used in vitro response of PBMC to DEX (TNFα and IL-13 mRNA suppression by DEX) as a read out of cellular functional response to corticosteroids. To our knowledge there are no studies to date that looked at atopy, steroid requirement and functional responses to corticosteroids in children vs adults with asthma in the same study in relationship to patients’ vitamin D status. Considering that cellular responses to vitamin D are influenced by genetics20 another novel aspect of this study was that we explored the relationship between serum vitamin D and PBMC cyp24a mRNA (a known target inducible by vitamin D) expression in PBMC, as a functional readout of vitamin D status. Vitamin D status was then analyzed in the context of laboratory biomarkers of corticosteroid response. A number of associations were found to be significant in the pediatric, but not the adult, asthma group, suggesting that future vitamin D oral supplementation studies should compare vitamin D response in children vs. adults.
METHODS
Subjects
25-hydroxyvitamin D levels and clinical features were analyzed in a total of 102 normal controls (51 adults, 51 children) and 103 asthmatics (50 adults, 53 children). Blood samples were collected between January and March of 2010. Approval was received from the National Jewish Health Institutional Review Board for the study.
Data Collection
Serum 25-hydroxyvitamin D levels (the inactive form of the vitamin D) was analyzed using the vitamin D, 25-hydroxy chemiluminescent immunoassay performed by DiaSorin Inc. This assay can measure both D2 and D3 derivates of 25-hydroxyvitamin D. Values were reported as ng/mL. 25-hydroxyvitamin D levels are the preferred marker of the body’s vitamin D status as this form has a longer half-life (2–3 weeks) than 1,25-dihydroxyvitamin D (4 hours).21 Total IgE and specific IgE testing was performed using Phadia ImmunoCAP method by Advanced Diagnostics Laboratories (Denver, CO) at National Jewish Health. Medication usage, vitamin supplementation and dosage were recorded.
Laboratory studies were performed on purified PBMCs collected from the patients recruited into the study. Human PBMCs were isolated by Ficoll-Hypaque® density gradient centrifugation. 2×106 freshly isolated PBMC were immediately preserved in RNA lysing solution (Qiagen). 2×106 PBMC were cultured in hormone-free medium with or without 100nM dexamethasone (DEX) for 3h and preserved in RLT buffer for RNA extraction. Total RNA was prepared (Qiagen), transcribed into cDNA, and analyzed by real-time PCR using the dual-labeled fluorigenic probe method on an ABI Prism 7300 Real Time PCR system (Applied Biosystems). Cyp24a, TNFα, IL-13 and 18s RNA expression were determined.
Plasma cathelicidin expression was determined in the serum samples from asthmatics using a human LL-37 ELISA kit (Hycult biotech, Netherlands) following manufacturer’s instructions.
Statistical Analysis
Population values for the variables examined are given in Table I. Multivariate linear regression modeling controlling for subjects’ BMI, gender, age, race and vitamin D supplementation was used to assess the relationship between serum vitamin D and cyp24a mRNA expression by PBMC and a number of clinical and laboratory variables that indicate patients steroid requirements and responsiveness to corticosteroids in vitro. Variables were considered statistically significant at p values less than 0.05 using two-sided tests. Statistical analysis was performed using JMP 8.0.1 software (SAS Institute Inc., Cary, NC).
Table I.
Patient Characteristics*
| Characteristic | Normal control | Asthmatics | ||
|---|---|---|---|---|
| Adults | Children | Adults | Children | |
| Age, years | 31 (24–39) | 12 (10–15) | 32.5 (22–46) | 10 (8.5–13) |
| Gender, M/F | 24/27 | 29/22 | 17/33 | 25/28 |
| Patient race, caucasian/other/african-american | 15/20/16 | 4/25/22 | 18/13/19 | 9/18/26 |
| BMI, kg/m2 | 27.8 (24.7–30.7) | 20.9 (18.4–24.2) | 29 (23.4–34.5) | 20 (16.6–24.2) |
| IgE, kU/ml | 58 (25–148) | 60 (24–182) | 122 (48–422) | 226 (89–423) |
| FEV1% | 100 (88–109) | 101 (90–112) | 88 (76–97) | 93 (77–105) |
| FEV1/FVC | 82 (77–84) | 85 (81–88) | 74 (69–81) | 82 (72–88) |
| Patients on ICS, Y/N | 32/18 | 41/12 | ||
| ICS dose, μg/day | 320 (228–500) | 360 (180–500) | ||
Values in the table are listed as median (IQR range).
RESULTS
Subject Characteristics
Serum 25-hydroxyvitamin D levels and clinical features were analyzed in 103 asthmatics and 102 normal controls (Table I). The groups were balanced for age, gender and race distributions. Asthmatics had significantly lower FEV1% predicted and FEV1/FVC% ratio as well as significantly elevated serum IgE as compared to normal controls. Over 75% of asthma patients in this study were on inhaled corticosteroids. Blood samples were collected between January and March of 2010. This corresponds to winter season when little vitamin D can be produced via sun exposure in latitudes above 35° N.3
In this study, 47.6% of asthmatics and 56.8% of normal controls had deficient serum vitamin D levels (<20ng/ml) (mean ±SD of serum vitamin D were 20.7±9.8ng/ml and 19.2±7.7ng/ml, for the asthmatics and normal controls, respectively). The racial/ethnic breakdown of the study population and corresponding serum vitamin D levels are shown in Table II. Of note, adult African Americans (AA) had the lowest serum vitamin D levels as compared to other racial groups. Serum vitamin D levels of AA children were comparable to the serum vitamin D levels of children from both other racial groups (Table II). The proportions of adults and children (6–12 year old group and 13–18 years old group) with sufficient (>30ng/ml), insufficient (20–30ng/ml) and deficient (<20ng/ml) serum vitamin D levels are provided in the Table III.
Table II.
Serum vitamin D levels by race and age in asthmatics and normal controls, ng/ml (mean ± SD)
| Study groups | Caucasian | African-American | Other**** |
|---|---|---|---|
| Asthma, adults (≥18yrs) | 25.6±10.8 (n=18) | 12.5±6.2** (n=19) | 15.9±6.8* (n=13) |
| Asthma, children (<18yrs) | 27.6±10.0 (n=9) | 22.0±8.3 (n=26) | 22.6±9.2 (n=18) |
| Normal control, adults, (≥18yrs) | 19.8±8.4 (n=15) | 13.7±7.3*** (n=16) | 16.4±6.3 (n=20) |
| Normal control, children (<18yrs) | 24.8±7.0 (n=4) | 21.9±7.9 (n=22) | 21.3±6.1 (n=25) |
p<0.01 caucasian vs other,
p<0.0001 caucasian vs african-american,
p<0.05 caucasian vs african-american patients.
Other group consistent mainly of Hispanic/Latino ethnic category (100% for the asthma adults, 72% for the asthma children, 70% for the normal control adults and 100% for the normal control children groups, respectfully).
Table III.
Serum vitamin D levels in asthmatics and normal controls of different age groups
| Study groups | Serum vitamin D levels | ||
|---|---|---|---|
| Deficient (<20 ng/ml) | Insufficient (20–30 ng/ml) | Sufficient (>30 ng/ml) | |
| Asthma, adults (≥18yrs) | 28/50 (56%) | 16/50 (32%) | 6/50 (12%) |
| Asthma, children: total group (<18yrs) |
21/53 (40%) | 20/53 (38%) | 12/53 (22%) |
| 6–12yrs | 10/37 (27%)* | 15/37 (41%) | 12/37 (32%)** |
| 13–17yrs | 11/16 (69%) | 5/16 (31%) | 0/16 (0%) |
| Normal control, adults, (≥18yrs) | 35/51 (69%)*** | 12/51 (24%) | 4/51 (7%) |
| Normal control, children: total group (<18yrs) |
23/51 (45%) | 20/51 (39%) | 8/51 (16%) |
| 6–12yrs | 9/26 (35%) | 11/26 (42%) | 6/26 (23%) |
| 13–17yrs | 14/25 (56%) | 9/25 (36%) | 2/25 (8%) |
p=0.0064 as compared to asthma, children (>12 yrs)
p=0.0105 as compared to asthma, children (>12 yrs)
p=0.0273 as compared to normal control, children total group (<18 yrs)
The expression of vitamin D regulated genes in PBMC and sera from asthmatics
In this study, the expression of vitamin D regulated targets in sera (serum LL-37 levels) and by the PBMC (cyp24a) and their relationship to serum vitamin D levels was examined in pediatric (<18 years old) and adult (≥18 years old) asthma groups. Multivariate regression models were developed controlling for BMI, gender, race, age and vitamin D supplementation to explore the relationships between serum vitamin D and the expression of the vitamin D inducible targets, which reflect an intact vitamin D receptor activated cellular pathway.
A positive correlation between serum vitamin D levels and serum LL-37 levels was found in the pediatric asthma group (Rsq=0.34, p=0.0006) (Fig. 1A) and the adult asthma group (Rsq=0.35, p=0.0067) (Fig. 1B). However, serum vitamin D levels had a greater effect on serum LL-37 levels in the pediatric asthma group (+0.09 increase in log pg/ml of serum LL-37 for each ng/ml increase in serum vitamin D), as compared to adult asthma group (+0.04 increase in log pg/ml of serum LL-37 for each ng/ml increase in serum vitamin D) (Fig. 1). A positive association between serum vitamin D levels and cyp24a expression by PBMC was found only in the pediatric asthma group (Rsq=0.20, p=0.0084) (Fig. 1C), but not in the adult asthma group (Fig. 1D).
Figure 1.
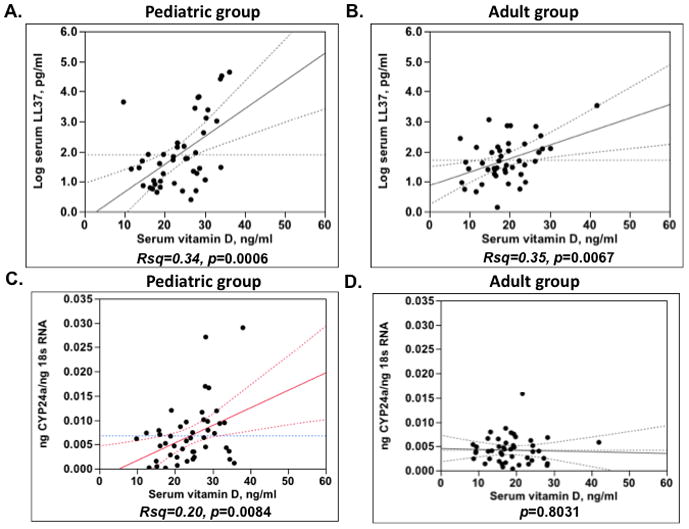
Associations between serum vitamin D levels and vitamin D regulated targets (serum LL-37 levels (A, C) and cyp24a expression (B, D)) in pediatric and adult asthma groups.
Relationship between serum IgE and serum vitamin D levels
In a linear regression model controlling for patient’s age, BMI, gender, race and vitamin D supplementation it was found that there is a significant inverse correlation between serum vitamin D levels and patients IgE levels in the pediatric group only (Rsq=0.24, p=0.006, −23 kU/L IgE decline for each ng/ml of serum vitamin D increase was observed) (Fig. 2A). No relationship between serum vitamin D levels and serum IgE was found in the adult asthma group (Fig. 2B). In an adjusted regression model it was found that asthmatic children with positive IgE levels to dust mites (n=13) had significantly lower serum vitamin D levels (serum vitamin D levels were 26.7±1.3ng/ml vs. 21.0±2.1ng/ml for the subjects with negative and positive IgE levels to D. farinae, p=0.0109; 27.3±1.3ng/ml vs. 20.5±2.1ng/ml for the subjects with negative and positive IgE to D. pteronyssinus, p=0.0037). No associations between positive IgE to cat, dog, Alternaria, tree mix, grass mix and weed mix and vitamin D levels was found.
Figure 2.
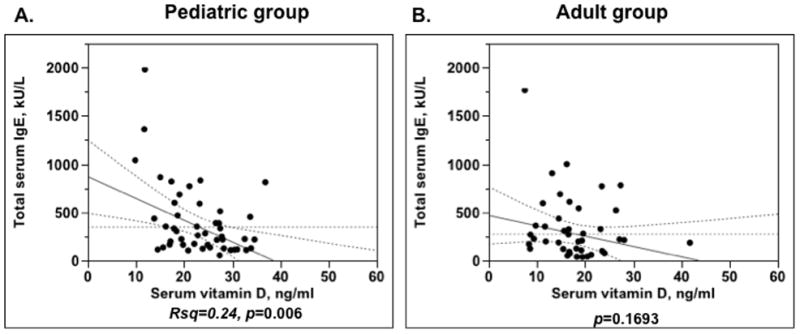
Significant inverse relationship between serum vitamin D levels and serum IgE levels in the pediatric asthma group (A), but not in the adult asthma group (B).
Relationship between serum vitamin D and asthma patients’ steroid requirement
In this study, in order to examine the relationship between serum vitamin D levels and patients’ steroid requirement the daily ICS dose for each patient was converted into the budesonide equivalents. A significant inverse association between serum vitamin D and ICS dose (Rsq=0.25, p=0.05, −27μg decrease in a daily ICS dose for each 1 ng/ml increase in serum vitamin D) was found only in the pediatric asthma group (Fig. 3A), but there was no association between serum vitamin D levels and ICS dose in the adult asthma group (Fig. 3B). Similarly, a significant inverse relationship between cyp24a expression by PBMC and patients daily ICS dose was observed in the pediatric asthma group (Rsq=0.16, p=0.0312) (Fig. 3C), but not the adult asthma group (Fig. 3D).
Figure 3.
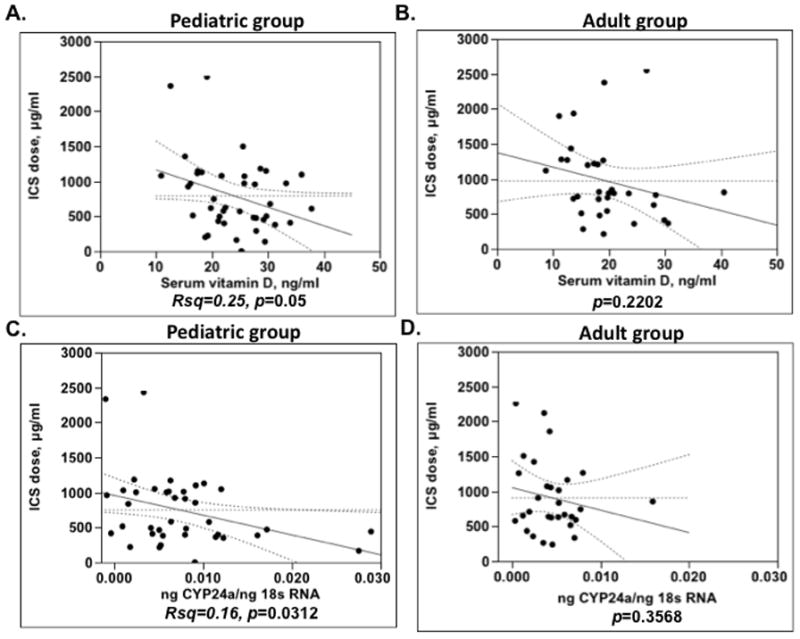
Pediatric asthma group (A, C) but not the adult asthma group (B, D) demonstrates significant inverse relationship between serum vitamin D levels, PBMC expression of the vitamin D regulated target cyp24a and daily ICS dose (in budesonide equivalents).
Relationship between the expression of vitamin D regulated targets by PBMC and cellular response to corticosteroids in vitro
A significant positive correlation between cyp24a expression (a vitamin D inducible gene) by PBMC and the degree of TNFα and IL-13 suppression by DEX in PBMC in vitro was found in the cells from the pediatric asthma group (Rsq=0.19, p=0.05; Rsq=0.36, p=0.0094 for TNFα and IL-13 suppression by DEX, respectively) (Fig. 4A,C), but was not observed in the adult asthma group (Fig. 4B,D).
Figure 4.
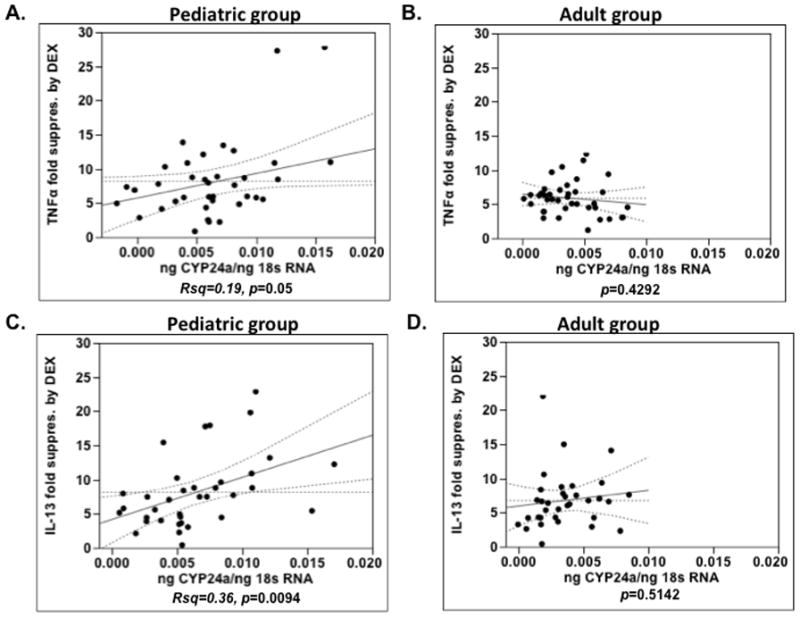
A significant positive correlation between cyp24a expression by PBMC and the degree of TNFα and IL-13 suppression by DEX in PBMC of pediatric asthma group (A, C), but not adult asthma group (B, D).
Can the relationships between serum vitamin D in the pediatric group be driven by significantly higher proportion of patients with sufficient serum vitamin D levels?
The results of this study demonstrate higher prevalence of vitamin D insufficiency/deficiency in adults then children (Table III). Reported associations were reexamined limiting serum vitamin D levels to <30 ng/ml. The associations between serum vitamin D and serum LL-37 levels and between serum vitamin D and cyp24a expression by PBMC were no longer significant in the pediatric asthma group that had serum vitamin D levels <30ng/ml. The association between serum vitamin D and serum LL-37 levels remained significant in the adult asthma group with serum vitamin D levels <30ng/ml.
If restricted to serum vitamin D levels<30ng/ml the following relationships remained significant in the pediatric asthma group: between serum vitamin D and serum IgE (Rsq=0.38, p=0.001); between serum vitamin D and total ICS dose (Rsq=0.36, p=0.011); between cyp24 expression by PBMC and total ICS dose (Rsq=0.16, p=0.05); between cyp24 expression by PBMC and IL-13 suppression by DEX in PBMC (Rsq=0.43, p=0.012). The relationship between cyp24 expression by PBMC and TNFα suppression by DEX in PBMC was no longer significant in the pediatric asthma group if the analysis was restricted to subjects with serum vitamin D levels <30ng/ml. As before, all of these relationships remained not significant in the adult asthma group if the analysis was restricted only to the subjects with insufficient/deficient serum vitamin D levels (<30ng/ml).
Is there difference in the reported associations in children 6–12 years old and >12 years old?
The data presented in Table III suggests that the prevalence of vitamin D insufficiency/deficiency in children older then 12 years old is similar to adults. We further analyzed the relationships between serum vitamin D/PBMC expression of cyp24a and vitamin D response markers, steroid requirements and in vitro response of PBMC to corticosteroids in children with asthma that are 6–12 years old and >12 years old (Table IV). We found that associations between serum vitamin D and serum LL-37 levels, between serum vitamin D levels and serum IgE levels were still significant for the 6–12 years age group and trended to be significant in the group >12 years old. Similarly, the association between PBMC expression of cyp24a with IL-13 suppression by DEX in PBMC trended to be significant in both age groups of children. In contrast, the associations between serum vitamin D levels and cyp24a expression by PBMC were only found significant in the age group of 6–12 years old children. As well, the relationship between serum vitamin D levels/cyp24a expression by PBMC and total daily ICS dose trended to be significant only in the younger pediatric age group (6–12 years old). This was also true for the relationship between cyp24a expression by PBMC and TNFα suppression by DEX in PBMC.
Table IV.
Relationship between serum vitamin D/cyp24a expression by PBMC and vitamin D/corticosteroid response markers in asthmatic children
| Predictor | Outcome | Asthma, children | ||
|---|---|---|---|---|
| 6–12yrs, n=37 | 13–17yrs, n=16 | |||
| Rsq | p value | p value | ||
| Serum vitamin D, ng/ml | Log serum LL-37, pg/ml | 0.39 | 0.006 | 0.10 |
| cyp24a expression by PBMC (ng cyp24a/ng 18s RNA) | 0.23 | 0.016 | 0.86 | |
| IgE, U/ml | 0.21 | 0.023 | 0.056 | |
| ICS dose, μg | 0.11 | 0.72 | ||
| cyp24a expression by PBMC (ng cyp24a/ng 18s RNA) | ICS dose, μg | 0.067 | 0.21 | |
| TNFα fold suppression by DEX in PBMC | 0.069 | 0.28 | ||
| IL-13 fold suppression by DEX in PBMC | 0.13 | 0.052 | ||
However, the associations between serum vitamin D and serum LL-37 levels, serum vitamin D levels and serum IgE levels; PBMC expression of cyp24a and IL-13 fold suppression by DEX in PBMC remained significant or trended to be significant in both pediatric age groups (Table IV).
DISCUSSION
The current prospective study in asthmatics assessed the age-specific relationship between serum vitamin D and atopy, steroid requirement and cellular responsiveness to in vitro treatment with corticosteroids. Importantly, this study also assessed, for the first time, the relationship between PBMC expression of vitamin D inducible cyp24a mRNA, as a functional readout of vitamin D receptor pathway activation, with clinical and laboratory variables designed to evaluate corticosteroid requirements and response to corticosteroids. These relationships were analyzed separately in the adult and pediatric asthma groups. A number of associations were found to be significant in the pediatric, but not the adult, asthma group, suggesting that future vitamin D supplementation studies in asthmatics should include pediatric populations as a target group. Thus, future findings from oral vitamin D supplementation in adult asthmatics may not necessarily be generalized to children with asthma.
In this study, a significant positive correlation between serum vitamin D and the expression of vitamin D regulated targets - cyp24a expression by PBMC (pediatric asthma group only) and serum LL-37 levels (pediatric and adult asthma groups) was observed. Serum vitamin D levels had a greater effect on serum LL-37 levels in the pediatric asthma group, as compared to the adult asthma group. Significant inverse correlation between serum IgE and serum vitamin D levels was observed in the pediatric asthma group only. Serum vitamin D and cyp24a expression by PBMC demonstrated significant inverse association with patients’ daily ICS dose in the pediatric asthma group only. The degree of in vitro TNFα and IL-13 suppression by DEX in PBMC from children with asthma positively correlated with the expression of vitamin D regulated cyp24a by these cells.
The majority of subjects in the current study had serum vitamin D levels <30ng/ml. 47.6% of asthmatics and 56.8% normal control subjects had deficient serum vitamin D levels (<20ng/ml) with mean ± SD of 20.7±9.8ng/ml and 19.2±7.7ng/ml, respectively. Overall, the prevalence of vitamin D insufficiency/deficiency in subjects with asthma in our study population appeared similar to normal control subjects that participated in this study. Given this data the contribution of vitamin D insufficiency as a risk factor for the development of asthma requires further evaluation.
We recently reported on associations between a number of clinical variables and serum vitamin D levels in pediatric group of asthmatics.10 The retrospective nature of the data collection made it difficult to create an efficient multivariate model to examine the strength of the correlations noted in univariate analysis. All of these limitations were accounted for in the current prospective study. Race, BMI, age and supplement use (multivitamins, vitamin D supplements and/or calcium supplements) were identified as significant confounding factors that affect subjects’ serum vitamin D levels. The multivariate regression models accounting for these variables in this prospective study, continued to show significant relationships between ICS dose and vitamin D levels in children.
To our knowledge, this is the first study that concurrently compared the relationship between steroid requirement and serum vitamin D levels both in children and adults with asthma. Previously several pediatric studies reported that subjects with deficient serum vitamin D levels are more likely to use anti-inflammatory medications14 and are likely to have asthma exacerbations despite using ICS.17 The study by Searing et al.10 in unadjusted linear regression model demonstrated that children with asthma that are on ICS or oral corticosteroids have significantly lower serum vitamin D levels. The study by Sutherland et al.9 reported that reduced vitamin D levels in adults with asthma are associated with impaired lung function, increased AHR and reduced in vitro response to corticosteroids in PBMC. We are not aware of a study that examined the relationship between serum vitamin D levels and ICS dose in adults with asthma. Importantly, our study demonstrated significant inverse association between patients ICS dose and serum vitamin D levels. Patients with the lowest serum vitamin D levels required the use of significantly higher doses of ICS to control their asthma. One explanation for our findings is that lower vitamin D levels contribute to increasing asthma severity resulting in a concomitant need to escalate pharmacologic intervention with ICS. We recently demonstrated that in vitro vitamin D has steroid sparing effects on cellular in vitro response to corticosteroids10 in normal subjects, thus presenting the novel concept that vitamin D pathways demonstrate cross talk with glucocorticoid pathways. This is clinically important because vitamin D insufficiency could promote the need for higher doses of glucocorticoids to achieve treatment effects.
The physiological effects of active form of the vitamin D (1,25(OH)2D3) are mediated through the vitamin D receptor (VDR). The binding of the biologically active form of vitamin D to the VDR enhances the interaction of VDR with the retinoid X receptor (RXR). The vitamin D bound VDR-RXR heterodimers interacts with vitamin D response elements (VDREs) in the promoters of vitamin D regulated genes. The levels of 1,25(OH)2D3 are tightly controlled by a feedback regulation that is designed to rapidly metabolize its excess. According to previous studies, the gene encoding 25-dihydroxyvitamin D3 24-hydroxylase, CYP24A, is the strongest known 1,25(OH)2D3-responsive gene; it has multiple VDREs in its promoter. CYP24A belongs to the cytochrome P450 (CYP) family, which encodes a variety of enzymes that are needed in the oxidative metabolism of many endogenous and exogenous compounds. Cyp24a converts the active form of the vitamin D into its inactive form.6
Considering the high likelihood that vitamin D responses are subject to genetic influence,20 we not only examined the relationship between serum vitamin D and a number of clinical and laboratory variables, but studied their relationships to a vitamin D inducible gene, i.e. cyp24a mRNA expression, as a functional readout of vitamin D cellular response. In this study, we demonstrate that cyp24a expression by PBMC correlates significantly with serum vitamin D levels in the pediatric asthma group. A significant positive correlation was found both for serum vitamin D and cyp24 PBMC mRNA expression and serum levels LL-37, another highly vitamin D inducible target. Importantly, both serum vitamin D and cyp24a mRNA expression by PBMC showed inverse correlation with total ICS requirements by the pediatric asthma group in the current study. The functional impact of these associations were supported by the observation that vitamin D induced cyp24a mRNA positively correlated with TNFα and IL-13 mRNA fold suppression in PBMC in vitro by DEX in the pediatric asthma group.
No information about cyp24a polymorphisms were collected in this study, although it has been reported that cyp24a may influence serum vitamin D levels20. Future studies examining cyp24a as a diagnostic or clinical marker in relationship to vitamin D mediated responses should take this into consideration.
Serum LL-37 was used as another vitamin D regulated target in this study. We did not examine the relationship between serum LL-37 levels and atopy markers, PBMC steroid response and patients steroid requirement as serum LL-37 is known to be produced by multiple cell types and a number of vitamin D-independent pathways control LL-37 production by the cells.22 Of interest, the association between serum vitamin D and serum LL-37 levels remained significant in the adult asthma group with serum vitamin D levels <30ng/ml. The study by Bhan et al.23 also recently reported significant relationship between serum vitamin D and serum LL-37 levels in normal adults, but only to serum levels of vitamin D <32ng/ml, above this level of serum vitamin D no relationship to serum LL-37 levels was observed. Importantly, Bhan et al.23 reported that significant change in LL-37 levels was only seen in subjects with the greatest increase in serum vitamin D levels after vitamin D supplementation; as well, these subjects appeared to be the most deficient for their serum vitamin D levels prior to supplementation with vitamin D.
The regression models performed in this study showed a relationship between the in vitro responsiveness to corticosteroids by PBMC and PBMC expression of the vitamin D regulated cyp24a mRNA, but not serum vitamin D. A significant positive correlation between cyp24a expression by PBMC and the degree of TNFα and IL-13 suppression by DEX in PBMC in vitro was found in the pediatric asthma group. As for serum vitamin D, a significant positive correlation was found only between serum vitamin D levels and the degree of TNFα suppression by DEX when the total asthma group was analyzed (Rsq=0.14, p=0.0256) (data not shown). This relationship was no longer significant if the data was sub-grouped for the pediatric and adult asthma groups. No relationship between serum vitamin D levels and the degree of IL-13 suppression by DEX was found. This data suggests that measuring cyp24a expression by PBMC may provide further insights over measuring serum vitamin D for the assessment of patients functional responsiveness to vitamin D and evaluation of patients PBMC in vitro responsiveness to corticosteroids. This data emphasized that variations in the genes regulating steps in the VDR-activated pathways could obscure the relationship between serum vitamin D levels and the outcomes of interest measured. As well, it points out that while evaluating epidemiologic role of vitamin D it might be worth examining not only patients’ serum vitamin D levels but also the cellular responsiveness to vitamin D based on the expression of genes in the VDR-activated pathways.
The results of this study demonstrate higher prevalence of vitamin D insufficiency/deficiency in adults then children (Table III). Importantly, prevalence of vitamin D insufficiency/deficiency in children older then 12 years old is similar to adults. Since all samples in this study were collected during winter/early spring when skin production of vitamin D is minimal, the findings emphasize the importance of nutrition and life style factors that determine higher serum vitamin D levels in children. No information about subjects’ socioeconomic status was collected in this study.
When pediatric group was further subgrouped into two age groups, i.e. 6–12 years olf and >12 years old ) we found that some of the reported associations remained significant only in the younger age group (6–12 years old) (Table IV). Therefore it is possible that in the number of the reported associations in children the significance of the association is driven by the greater proportion of serum vitamin D values >30ng/ml in the 6–12 year old age group.
The fact that the associations between serum vitamin D and serum LL-37 levels, serum vitamin D levels and serum IgE levels; PBMC expression of cyp24a and IL-13 fold suppression by DEX in PBMC remained significant or trended to be significant in both pediatric age groups (i.e. 6–12 years old and >12 years old), perhaps points to the potential plasticity of the vitamin D-mediated responses in children as LL-37 and atopy markers are more responsive to serum vitamin D levels in children then adults. Based on this data we can speculate that vitamin D supplementation in children with asthma could reduce atopy, and boost protection from seasonal respiratory viruses (serum LL-37 levels).
Several recent studies have suggested that low serum vitamin D can influence the severity of asthma and/or atopy,14–16 particularly in the pediatric group. From an analysis of the NHANES 2005–2006 data, it was reported that vitamin D deficiency is associated with higher levels of IgE sensitization in children and adolescents15 as compared with adults. In a retrospective analysis of the associations between asthma phenotypes and serum vitamin D levels in the cohort of patients from Perth, Australia it was noted that serum vitamin D levels at age 6 were significant predictors of subsequent atopy/asthma-associated phenotypes at age 14.24 In a non-selected community setting, children (particularly boys) with inadequate vitamin D were determined to be at increased risk of developing atopy, and subsequently bronchial hyperresponsiveness and asthma. 22 A recent prospective study demonstrated that the control of newly diagnosed asthma in children can be enhanced by vitamin D oral supplementation.16 In our study a significant inverse correlation between serum IgE levels and serum vitamin D was observed in the pediatric asthma group, but not in the adults, also suggesting that vitamin D supplementation may be beneficial in the pediatric group to downregulate allergic responses.
In the current study, we also made the novel finding that the associations between serum vitamin D and vitamin D regulated targets, and patient’s steroid response in vitro and steroid requirements were mainly significant in the pediatric, but not adult asthma group. The basis for these differences in children vs adults are unknown but may have clinical significance as it suggests children may respond better than adults to oral vitamin D supplementation. As stated15 since most allergies start in childhood, vitamin D insufficiency in childhood may influence initiation of allergy. On contrary, adult vitamin D status has no relationship to the patients’ IgE levels, as it is no longer reflective of the patients’ vitamin D status in childhood at the time of allergic sensitization. Further studies are needed to determine whether the vitamin D receptor and the molecules activated following receptor binding function differently in children vs adults.
Acknowledgments
We thank Maureen Sandoval for help in preparing this manuscript.
We would like to thank Dr. James Murphy and Dr. Doug Everett from the Division of Biostatistics at National Jewish Health for his consultations and assistance with statistical data analysis.
This work was supported in part by a research grant from Diasorin as well as NIH grants AI070140 and HL37260. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, National Heart, Lung, and Blood Institute or the National Institutes of Health.
ABBREVIATIONS
- AHR
airway hyperreactivity
- Cyp24a
Cytochrome P450, family 24, subfamily a
- DEX
Dexamethasone
- ICS
Inhaled corticosteroids
- PBMC
Peripheral blood mononuclear cells
- SD
Standard deviation
- VDR
Vitamin D receptor
Footnotes
Disclosure of potential conflict of interest: The authors have declared no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S–64S. doi: 10.1093/ajcn/88.2.558S. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and Associations of 25-Hydroxyvitamin D Deficiency in US Children: NHANES 2001–2004. Pediatrics. 2009 doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011 doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 6.Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9:941–55. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 7.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404–12. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- 8.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125:995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camargo CA, Jr, Clark S, Kaplan MS, Lieberman P, Wood RA. Regional differences in EpiPen prescriptions in the United States: the potential role of vitamin D. J Allergy Clin Immunol. 2007;120:131–6. doi: 10.1016/j.jaci.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 12.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginde AA, Sutherland ER. Vitamin D in asthma: panacea or true promise? J Allergy Clin Immunol. 2010;126:59–60. doi: 10.1016/j.jaci.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 14.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML. Vitamin D levels and food and environmental allergies in the United States: Results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2011;127:1195–202. doi: 10.1016/j.jaci.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majak P, Olszowiec-Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294–6. doi: 10.1016/j.jaci.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010;126:52–8. e5. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietary Reference Intakes for Calcium and Vitamin D. Institute of Medicine of the National Academies; 2010. pp. 1–4. [Google Scholar]
- 19.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 22.Gallo RL, Murakami M, Ohtake T, Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy Clin Immunol. 2002;110(6):823–31. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- 23.Bhan I, Camargo CA, Jr, Wenger J, Ricciardi C, Ye J, Borregaard N, Thadhani R. Circulating levels of 25-hydroxyvitamin D and human cathelicidin in healthy adults. J Allergy Clin Immunol. 2011;127(5):1302–4. doi: 10.1016/j.jaci.2010.12.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollams EM, Hart PH, Holt BJ, Serralha M, Parsons F, de Klerk NH, et al. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J. 2011 doi: 10.1183/09031936.00029011. [DOI] [PubMed] [Google Scholar]


