Abstract
The Hmong are a distinct ethnic group from Laos. Little is known about how opiate addicted Hmong respond to methadone maintenance treatment. Therefore, opium addicted Hmong (exclusive route of administration: smoking) attending an urban methadone maintenance program in Minneapolis, Minnesota were matched by gender and date of admission with predominately heroin addicted non-Hmong (predominant route of administration: injection) attending the same program and both groups were evaluated for 1-year treatment retention, stabilization dose of methadone, and urine drug screen results. Hmong had greater 1-year treatment retention (79.8%) than non-Hmong (63.5%; p<0.01). In both groups, methadone dose was significantly associated with retention (p=0.005). However, Hmong required lower doses of methadone for stabilization (mean 49.0 mg versus 77.1 mg; p<0.0001). For both groups, positive urine drug screens were associated with stopping treatment. Further research to determine levels of tolerance, psychosocial, and pharmacogenetic factors contributing to differences methadone treatment outcome and dosing in Hmong may provide further insight into opiate addiction and its treatment.
Keywords: Methadone, opiate dependence, treatment outcome, ethnicity, Hmong
1. Introduction
The Hmong are an ethnic minority from the mountains of Laos. They have been linked historically and linguistically to southern China but emigrated to Laos in the mid 18th century. The Hmong are a clan-based agrarian society and are known for their centuries old practice of opium cultivation (Lee, 2005). Most traditional Hmong households in Laos have been involved in opium production and opium has played an important therapeutic role in the practice of Hmong medicine. While contact with opium through cultivation and/or traditional medical practice is widespread for Laotian Hmong, the number of Hmong who have used opium is unknown. Estimates from the 1960’s and 1970’s indicate that 8-12% of Hmong opium farmers were addicted to opium (Westermeyer, 1981). With intensive opium eradication strategies this number has decreased, with current prevalence of opium addiction in Laos estimated at 1% with household prevalence reaching 15% in some villages (United Nations Office on Drugs and Crime, 2005). Current Hmong-specific prevalences are unknown.
Formalized treatment outcome research for opium addicted Hmong in Laos has been limited by low sample sizes and non-uniformity of treatment approaches (e.g., herbal remedies, detoxification, Buddhist monastery, “reeducation” camps) (Westermeyer, 1982; World Health Organization, 2002a). Estimates indicate a poor long-term treatment outcome for abstinence-based treatment, with 80-100% of patients returning to opium use after discharge (Westermeyer, 1982; World Health Organization, 2002b; World Health Organization, 2002a). Pharmacotherapy for opiate addiction in Laos is limited and mostly consists of detoxification rather than maintenance. Therefore, evaluation of treatment outcome in Hmong may best be conducted in settings with a more established treatment infrastructure such as the United States.
Following the end of the Vietnam War, communist persecution of Hmong in Laos became untenable and an exodus began. Since the mid-1970’s more than 180,000 Hmong have arrived in the United States with major concentrations in Minnesota, California, and Wisconsin. Among these immigrants, an estimated 2-5% are addicted to opium (Westermeyer, 1995). The Twin Cities of Minneapolis and St. Paul are home to the largest urban Hmong population in the world. Of the 60,000 Minnesotan Hmong, more than 200 are currently enrolled in methadone maintenance treatment for opium addiction.
Since 1964, methadone maintenance has become the most commonly used medication to treat opiate addiction (National Institutes of Health, 1998). Methadone’s long-acting properties and full mu-opioid agonism reduce many pharmacologically and behaviorally reinforcing effects of short-acting opiates. This results in general improvements in illicit drug use, criminality, and quality of life (Ball & Ross, 1991). One of the best predictors of achieving these positive clinical outcomes is retention in treatment (Simpson & Sells, 1982). In the multisite Treatment Outcome Prospective Study (TOPS), the average 1-year treatment retention in methadone maintenance was 34% (Hubbard et al., 1989). The more recent Drug Abuse Treatment Outcome Study (DATOS) confirmed the variability in treatment retention (15%-76%) noted in TOPS, but indicates that with wider adoption of evidence-based treatment standards, treatment has improved with an average of 50% of all patients retained for 1-year (Simpson et al., 1997). Treatment response to methadone is also dose dependent, with patients taking 60mg-120mg of methadone daily having better treatment outcome than those taking less than 60 mg daily (National Institutes of Health, 1998; Mattick et al., 2003; Caplehorn & Bell, 1991; Johnson et al., 2000; Strain et al., 1999). In addition to methadone dose, other positive predictors of treatment retention include age older than 35 years, lower frequency of daily drug injections at admission, counseling session attendance, rapport with counselor, and desire for help (Marsch et al., 2005; Simpson et al., 1997; Kelly et al., 2011). Studies evaluating ethnicity as a predictor of treatment outcome in methadone patients have not found this to be a significant influence although Asian, let alone Hmong, populations were not specifically included in the analyses (Deck & Carlson, 2005; Marsch et al., 2005).
Because methadone treatment data on Hmong are lacking, we conducted the current retrospective chart-review study to evaluate treatment outcome (measured as 1-year retention in treatment and urine drug screen results) and dose requirement in Hmong compared to non-Hmong attending a single urban methadone maintenance program.
2. Methods
We conducted a retrospective chart review to compare 1-year retention in treatment, stabilization dose of methadone, and urine drug screen results between Hmong and non-Hmong patients enrolled in the Hennepin Faculty Associates (HFA) Addiction Medicine Program. This study was approved by the Human Subjects Research Committee of the Hennepin County Medical Center and, as a chart review study, was exempted from consent requirements.
In June 1994, the Hennepin Faculty Associates Addiction Medicine Program opened a methadone maintenance clinic to serve the needs of the opiate-addicted population of the greater Twin Cities community. Based in the Department of Medicine of the Hennepin County Medical Center in downtown Minneapolis, the HFA program is an academically affiliated non-profit clinic serving as a safety-net resource.
All admissions from clinic inception through March 31, 2005 were reviewed (n=1411). Patients who transferred from other methadone programs were excluded; otherwise all patients receiving at least one dose of methadone were eligible for inclusion. In instances where a subject had multiple admissions to the HFA program, the earliest admission was chosen for review. To ensure that the two groups had the same fraction of males and were treated contemporaneously, each Hmong patient (n=104) was matched with a non-Hmong patient (n=104) based on gender and date of admission (in most cases matched admission dates were no more than one week apart). However, age matching could not be performed as the Hmong tended to be older than the non-Hmong patients. Therefore, analyses (described below) did not use the matched pairs. Charts were reviewed for date of admission, date of and reason for discharge (or retention through April 1, 2006 if they were still in the program on that date), stabilization dose of methadone (defined as the dose received on the majority of days during the first year or the highest dose achieved for patients retained less than one year), and urine drug screen results. Specimens from random and clinically indicated urine drug screens were analyzed by a commercial laboratory (Hennepin County Medical Center or Hennepin Faculty Associates) using a standard commercial immunoassay kit capable of detecting the presence of methadone, methadone metabolites, amphetamine, cocaine, benzoylecgonine, barbiturates, benzodiazepines, opiates, and alcohol. Urine drug screen results were categorized as negative, positive for opiates, positive for non-opiate drugs, and positive for both opiates and non-opiates. Cannabinoids were not routinely tested for and therefore were not included in the analysis.
Hmong and non-Hmong groups were compared according to age and stabilization dose using a two-sample t-test. Urine drug screen data were aggregated by quarter for each person. Differences between Hmong and non-Hmong and changes over the 4 quarters of follow-up were tested, separately for opiates and non-opiate drugs, using generalized linear mixed models (GLMM), specifically a logistic regression conditional on person and a person-specific random effect (a 4-variate normal with AR(1) correlation structure). For each person and quarter, the outcome was a pair of numbers, that person’s total number of urine tests and total number of positive tests in that quarter. A variant analysis added subject age as a continuous covariate. An alternative analysis weighted each person in each quarter to account for subjects who were no longer in treatment, using weights proportional to the reciprocal of the probability of having data in that quarter (estimated using logistic regression, depending on group, age, sex, stable dose and, for non-Hmong, ethnicity). Results of the weighted analysis were identical to those presented here, to the table’s accuracy. Other alternative analyses used generalized estimating equations (GEE) with an AR(1) working correlation, both weighted and unweighted. These results very similar to those presented here and are not shown. Treatment retention of Hmong and non-Hmong at each follow-up time were estimated using the Kaplan-Meier procedure and compared using the log-rank test. The association of retention with urine drug screen results was analyzed using Cox regression with time-varying covariates describing fraction of positive tests for opiates and for non-opiate drugs. For each person, these were the logits of their estimated probabilities of testing positive in each quarter, from the GLMM analysis. For all retention analyses, patients transferring to another methadone program were censored; all other patients who left the clinic without returning prior to 365 days were considered to have dropped out of treatment.
3. Results
3.1 Patient characteristics
Non-Hmong patients were matched to a Hmong patient by gender and date of admission. The Hmong were significantly older in age than non-Hmong (Table 1), making age matching difficult.
Table 1.
Subject Characteristics
| Hmong (n=104) |
Non-Hmong (n=104) | Significance | |
|---|---|---|---|
| Male (%) | 78 (75%) | 78 (75%) | Identical by matching |
|
Mean age in years
(range, SD) |
49.6 (24-88, 14.0) |
41.0 (20-58, 8.3) |
P<0.0001 |
| Ethnicity (%) | Hmong 104 (100%) |
Caucasian 52 (50%) | |
| African American 40 (38%) | |||
| Native American 4 (4%) | |||
| Hispanic White 3 (3%) | |||
| Hispanic Black 2 (2%) | |||
| Asian 2 (2%) | |||
| Mixed 1 (1%) |
The mean (SD) stabilization dose of methadone for all patients was 63 (25.8) mg. The Hmong patients were stabilized on a significantly lower methadone dose than non-Hmong patients: 49.0 (17.4) mg versus 77.1 (25.1) mg (p<0.0001). The groups did not differ in methadone dose when adjusted for body weight (Hmong average 0.95 mg/kg, SD 0.43; non-Hmong average 0.98 mg/kg, SD 0.37).
3.2 Urine drug screen
Table 2 shows comparisons of estimated fraction of positive urine drug screens in each quarter of treatment. The chance of an opiate-positive drug screen decreased after the first quarter of treatment (main effect of quarters, X2=24.50 on 3 df, p<0.0001), but Hmong did not differ significantly from non-Hmong (main effect of ethnicity X2=0.73 on 1 df, p=0.39). The Hmong group’s fraction of positive tests started somewhat higher in the first quarter of treatment (0.37 vs. 0.26) but the two groups had similar fractions of positive tests thereafter; the two groups’ time paths did not differ significantly (interaction of ethnicity and quarter X2=1.51 on 3 df, p=0.21). Comparing Hmong vs. non-Hmong in the first quarter alone gave P = 0.046, but this is not significant after adjusting the significance threshold for multiple comparisons (one comparison for each of the four quarters). Regarding non-opiate drug screens, Hmong had far fewer positive screens in all quarters (main effect of ethnicity X2=87.34, p<0.0001 overall and in each quarter individually), but there was no effect of time in treatment (X2=0.65, p=0.58 and X2=0.49, p=0.69, for the main effect of quarters and the interaction, respectively). In analyses adjusting for age, age was not significant (P > 0.14 for both opiates and non-opiate drugs) and tests comparing ethnicities and quarters of treatment were nearly unchanged after adjusting for age (data not shown).
Table 2.
Urine test results: Fractions using opiates and other drugs, Hmong and non-Hmong, by quarter
| Fraction using opiates | Fraction using other drugs | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quarter | Ethnicity | Estimate | P* | Lower 95% CL |
Upper 95% CL |
Estimate | P* | Lower 95% CL |
Upper 95% CL |
| 1 | Hmong | 0.37 | 0.046 | 0.29 | 0.46 | 0.01 | <0.000 1 |
0.01 | 0.02 |
|
Non-
Hmong |
0.26 | 0.19 | 0.34 | 0.24 | 0.16 | 0.34 | |||
| 2 | Hmong | 0.18 | 0.64 | 0.13 | 0.24 | 0.01 | <0.000 1 |
0.01 | 0.03 |
|
Non-
Hmong |
0.16 | 0.11 | 0.23 | 0.30 | 0.20 | 0.42 | |||
| 3 | Hmong | 0.13 | 0.85 | 0.09 | 0.18 | 0.01 | <0.000 1 |
0.01 | 0.03 |
|
Non-
Hmong |
0.13 | 0.09 | 0.19 | 0.31 | 0.20 | 0.45 | |||
| 4 | Hmong | 0.15 | 0.67 | 0.10 | 0.22 | 0.01 | <0.000 1 |
0.00 | 0.02 |
|
Non-
Hmong |
0.13 | 0.08 | 0.19 | 0.34 | 0.22 | 0.49 | |||
Comparing Hmong vs. non-Hmong in each quarter
3.3 1-year retention in treatment
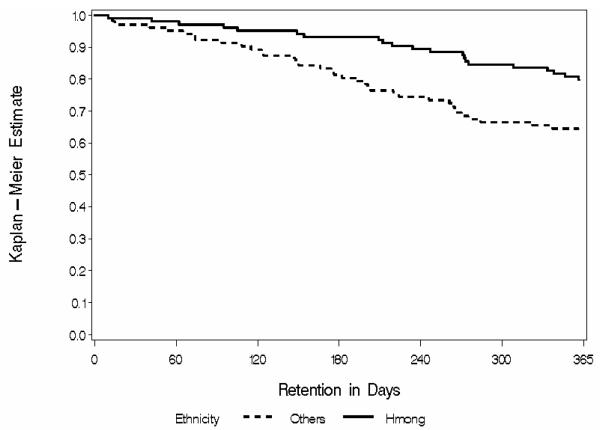
Hmong had significantly greater 1-year retention than non-Hmong, respectively 79.8% (95% CI 72.1%-87.5%) versus 63.5% (95% CI 54.1%-72.8%) (p=0.006; see Figure). Among those who did leave treatment before 1 year, reasons for leaving did not differ between Hmong and non-Hmong: loss to follow-up (38% versus 40%), discharge for behavioral reasons (24% versus 28%), transfer to another clinic (10% versus 14%), patient request for taper (14% versus 2%), incarceration (5% versus 9%), and other reasons (9% vs. 7%).
Figure.
Kaplan-Meier estimator curve for 1ear retention in treatment. Hmong retention is significantly greater than non-Hmong retention (log-rank test X2df1=7.56, p=0.006).
Considering the influence of ongoing drug use on retention, positive drug screens were significantly associated with risk of stopping treatment for opiate positive results (hazard ratio for stopping treatment 1.44, 95% CI 1.22-1.70, p<0.0001) and for non-opiate positive results (hazard ratio for stopping treatment 1.32, 95% CI 1.16-1.49, p<0.0001). After adjusting for the effect of positive drug screens, Hmong patients no longer differed significantly from non-Hmong patients (hazard ratio for stopping treatment for Hmong vs. non-Hmong 1.31, 95% CI 0.63-2.71, p=0.47).
Finally, there was a significant association between methadone dose and retention, with hazard ratio for stopping treatment 0.85 for each 10 mg increase in methadone dose (95% CI 0.76-0.95; P = 0.005). After adjusting for methadone dose, Hmong were still significantly less likely than non-Hmong to stop treatment (hazard ratio for stopping treatment 0.30, 95% CI 0.16-0.55; p<0.0001).
4. Discussion
This is the first comparison of methadone-maintained Hmong and non-Hmong populations in the literature and one of the few reports from the United States that includes an Asian methadone population. We found a significant difference in treatment retention between Hmong and non-Hmong patients enrolled in a single methadone maintenance program. Furthermore, while methadone dose was generally associated with clinical retention, the Hmong had significantly greater retention despite requiring significantly lower doses of methadone.
Retention in treatment may be one of the strongest predictors of long term outcome in methadone maintenance (Simpson & Sells, 1982). Identification of factors associated with clinical retention may help in tailoring treatment approaches to reduce the risk of leaving treatment. For example, psychiatric comorbidity has predicted poor outcome in some studies and the provision of on-site psychotherapy or treatment for depression has improved treatment outcome (Woody et al., 1982; Woody et al., 1983; Nunes et al., 1991). Using evidence-based dosing regimens rather than set ceiling doses has also resulted in improved retention (Ball & Ross, 1991; Caplehorn & Bell, 1991). Factors such as age, gender, and ethnicity which have been weakly associated with retention are not modifiable but do inform us of the importance of tailoring treatment approaches to specific populations (McLellan, 1983). Besides patient factors, other programmatic and community variables impact treatment outcome (Villafranca et al., 2006).
In this study, ongoing drug use predicted stopping treatment, whether the drug used was an opiate or a non-opiate. Several studies have found that ongoing cocaine, benzodiazepine, and alcohol use predict stopping treatment (Grella et al., 1997; Joe et al., 1999; Peles et al., 2006). We did not include marijuana use in our analysis, but a retrospective meta-analysis by Epstein and Preston did not find positive urine screens for cannabinoids to predict clinical retention (Epstein & Preston, 2003).
While we found both opiate and non-opiate drug use predicted stopping treatment, the probability of non-opiate drug use did not appear to change through the first year of treatment. It could be argued that the persistence of high non-opiate drug use in the test samples is due to selection bias, so that patients with positive tests are more likely to receive future tests. However, in this study the number of urine tests in a quarter was not associated with the fraction of tests that were positive for non-opiate drugs, either for all subjects combined (p = 0.16) or for Hmong and non-Hmong considered separately (p = 0.10 and 0.39, respectively). This indicates that it may not be the ongoing drug use itself that predicts stopping treatment but rather that ongoing drug use is a surrogate marker for unidentified destabilizing factors (e.g., medical psychiatric, legal, psychosocial) that also predict stopping treatment (Brewer et al., 1998). It is unknown whether addressing these factors alone can improve treatment retention independent of any effect on ongoing drug use. Elucidating this may help appropriately orient therapeutic priorities towards an emphasis on the contributing psychosocial factors of which ongoing drug use is but a marker, rather than on the drug use itself.
After adjusting for urine drug screen results, Hmong was no longer a predictor of treatment outcome. Their extended retention in treatment, therefore, may be a reflection of lower frequency of ongoing drug use, especially non-opiate drugs. We do not have medical or psychosocial data that could help further predict treatment outcome differences between ethnicities. In a previous report of the first forty Hmong patients enrolled in our methadone program, however, we found a high level of baseline psychiatric symptomatology: Hamilton Depression Scale (HAM-D) mean score 28.6 (range 17-44), Hamilton Anxiety Scale mean score 26.19 (range 16-41), Zung Depression Scale mean score 40.5 (range 22-65), and a Global Assessment of Functioning (GAF) mean of 56.6 (range 40-70) (Azeem et al., 2002). These levels of symptomatology are consistent with or more severe than previous reports in non-Hmong populations entering methadone maintenance (Woody et al., 1975; Weissman et al., 1976; Maremmani et al., 2007) and, therefore, it is less likely that our observed difference in treatment outcome is related to ethnic differences in psychosocial distress. Finally, the Hmong and non-Hmong patients were all cared for within a single clinical setting, thereby reducing potential differences in treatment approach that could affect outcome.
A relatively novel finding is that the Hmong required lower doses of methadone than the non-Hmong. Retention in methadone maintenance is generally dose related, as was found here; however, in several studies retention is greatest for doses above 60 mg daily (Caplehorn & Bell, 1991; Ball & Ross, 1991; Strain et al., 1999; Johnson et al., 2000). Our nearly 80% 1-year retention for the Hmong at an average of 49 mg daily is remarkable. While the Hmong do have lower body mass than non-Hmong and the milligram per kilogram methadone dose was similar in the two groups, there is no clinical or pharmacokinetic precedent to use weight-based rather than absolute milligram amount when interpreting dose-outcome data.
Variants in genes related to drug metabolism or effect may account for the superior outcome on lower doses in Hmong. This is not unprecedented in that for nicotine dependence, East Asians are more likely to have a genetic variant resulting in reduced nicotine metabolism that correlates to their lower level of cigarette consumption (Benowitz et al., 2002; Schoedel et al., 2004). Additionally, those with this variant are more likely to respond to nicotine replacement therapy (Lerman et al., 2010). Whether a similar pharmacogenetic effect exists for genes involved in methadone pharmacokinetics is unknown and worth pursuing given our results.
There are several limitations to this study. First, this is a retrospective chart review rather than a prospective study. We have attempted to control for a cohort effect by matching patients for date of admission. We were unable to control frequency of urine drug screening but we reduced its effect to some extent by aggregating tests by quarters. Second, there are likely several psychosocial and clinical process factors that contribute to treatment outcome that could not be assessed in this retrospective study. For example, we do not have information regarding levels of baseline tolerance, amount of drug use, or social stability characteristics (such as Addiction Severity Index composite scores) to help assess these influences on treatment outcome. Additionally, the lower methadone dose requirement in Hmong may be due to lower levels of tolerance related to their opium smoking versus the non-Hmong’s mostly heroin injection. While psychosocial stability, amount and frequency of drug use, and history of injection drug use are negative predictors of treatment retention (Marsch et al., 2005; Simpson et al., 1997; Kelly et al., 2011; Brewer et al., 1998), it is unclear whether route of drug administration (e.g., injection versus smoking) or type of opiate used (e.g., heroin versus opium) are predictive of methadone dose requirements. Future studies would benefit from considering other patient characteristics such as socioeconomic status, education, medical and psychiatric comorbidity, Addiction Severity Index composite scores, treatment motivation, and treatment satisfaction. Finally, there may be distinct cultural differences that influence motivation for treatment and family/cultural supports for recovery that were not measured. Prior reports of culturally oriented detoxification or abstinence-based approaches to opium addiction in Hmong found results to be as poor as those of non-Hmong (Westermeyer, 1982), thereby reducing the potential that cultural differences were the sole or major mediator of our findings.
Conclusion
We have identified a significant difference in methadone treatment retention despite lower dose requirements in Hmong versus non-Hmong patients attending a single clinical site. Identification of factors (e.g., levels of tolerance, psychosocial stability, pharmacogenetics) that appear to make methadone more effective at low doses in Hmong compared to non-Hmong may ultimately lead to more generalizable approaches to dose optimization and treatment improvement.
Acknowledgements
The authors wish to thank Dr. Ross Crosby for statistical evaluation of an earlier version of this data. The authors also thank Chomchanh Soudaly, Shoua Thao, Kong Sue Xiong, Mao Xiong, and Wesley Yang for their assistance in collecting data. This work was supported by a NIH-NIDA career development award K23 DA024663.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- The Practices and Context of Pharmacotherapy of Opioid Dependence in South-East Asia and Western Pacific Regions. World Health Organization Department of Mental Health and Substance Dependence; Geneva: 2002. [Google Scholar]
- Azeem MW, Carlson G, Soudaly C. Treatment response of opium smoking Hmong refugees to methadone maintenance. Jefferson J Psychiatry. 2002;17:3–10. [Google Scholar]
- Ball JC, Ross A. The Effectiveness of Methadone Maintenance. Springer-Verlag; New York: 1991. [Google Scholar]
- Benowitz NL, Perez-Stable EJ, Herrera B, Jacob P. Slower Metabolism and Reduced Intake of Nicotine From Cigarette Smoking in Chinese-Americans. Journal of the National Cancer Institute. 2002;94:108–115. doi: 10.1093/jnci/94.2.108. [DOI] [PubMed] [Google Scholar]
- Brewer DD, Catalano RF, Haggerty K, Gainey RR, Fleming CB. A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction. 1998;93:73–92. [PubMed] [Google Scholar]
- Caplehorn JR, Bell J. Methadone dosage and retention of patients in maintenance treatment. Med J Aust. 1991;154:195–199. [PubMed] [Google Scholar]
- Deck D, Carlson MJ. Retention in Publicly Funded Methadone Maintenance Treatment in Two Western States. Journal of Behavioral Health Services & Research. 2005;32:43–60. doi: 10.1007/BF02287327. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. Does cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? Past findings and more evidence against. Addiction. 2003;98:269–279. doi: 10.1046/j.1360-0443.2003.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella CE, Anglin MD, Wugalter SE. Patterns and predictors of cocaine and crack use by clients in standard and enhanced methadone maintenance treatment. Am J Drug Alcohol Abuse. 1997;23:15–42. doi: 10.3109/00952999709001685. [DOI] [PubMed] [Google Scholar]
- Hubbard RL, Marsden ME, Rachal JV, Harwood HJ, Cavanaugh ER, Ginzburg HM. Drug Abuse Treatment: A National Study of Effectiveness. University of North Carolina Press; Chapel Hill: 1989. [Google Scholar]
- Joe GW, Simpson DD, Greener JM, Rowan-Szal GA. Integrative modeling of client engagement and outcomes during the first 6 months of methadone treatment. Addict.Behav. 1999;24:649–659. doi: 10.1016/s0306-4603(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Kelly SM, O’Grady KE, Mitchell SG, Brown BS, Schwartz RP. Predictors of methadone treatment retention from a multi-site study: A survival analysis. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GY. The shaping of traditions: agriculture and Hmong society. Hmong Studies Journal. 2005;6:1–33. [Google Scholar]
- Lerman C, Jepson C, Wileyto EP, Patterson F, Schnoll R, Mroziewicz M, et al. Genetic Variation in Nicotine Metabolism Predicts the Efficacy of Extended-Duration Transdermal Nicotine Therapy. Clin Pharmacol Ther. 2010;87:553–557. doi: 10.1038/clpt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maremmani I, Pani PP, Pacini M, Perugi G. Substance use and quality of life over 12 months among buprenorphine maintenance-treated and methadone maintenance-treated heroin-addicted patients. Journal of Substance Abuse Treatment. 2007;33:91–98. doi: 10.1016/j.jsat.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Stephens MA, Mudric T, Strain EC, Bigelow GE, Johnson RE. Predictors of outcome in LAAM, buprenorphine, and methadone treatment for opioid dependence. Exp.Clin Psychopharmacol. 2005;13:293–302. doi: 10.1037/1064-1297.13.4.293. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane.Database.Syst.Rev. 2003 doi: 10.1002/14651858.CD002209. CD002209. [DOI] [PubMed] [Google Scholar]
- McLellan AT. Patient characteristics associated with outcome. In: Cooper J, Altman F, Brown BS, Czechowicz D, editors. Research in the Treatment of Narcotic Addiction. National Institute on Drug Abuse; Rockville, MD: 1983. pp. 500–529. [Google Scholar]
- National Institutes of Health Effective medical treatment of opiate addiction. National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. JAMA. 1998;280:1936–1943. [PubMed] [Google Scholar]
- Nunes EV, Quitkin FM, Brady R, Stewart JW. Imipramine treatment of methadone maintenance patients with affective disorder and illicit drug use. Am J Psychiatry. 1991;148:667–669. doi: 10.1176/ajp.148.5.667. [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Adelson M. Factors predicting retention in treatment: 10-year experience of a methadone maintenance treatment (MMT) clinic in Israel. Drug Alcohol Depend. 2006;82:211–217. doi: 10.1016/j.drugalcdep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics and Genomics. 2004;14 doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Broome KM, Hiller ML, Knight K, Rowan-Szal GA. Program diversity and treatment retention rates in the drug abuse treatment outcome study (DATOS) Psychology of Add Behav. 1997;11:279–293. [Google Scholar]
- Simpson DD, Sells SB. Effectiveness of treatment for drug abuse: an overview of the DARP research progrgam. Advances in Alcohol and Substance Abuse. 1982;2:7–29. [Google Scholar]
- Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate- vs high-dose methadone in the treatment of opioid dependence: a randomized trial. JAMA. 1999;281:1000–1005. doi: 10.1001/jama.281.11.1000. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime Laos opium survey 2005. 2005 http://www.unodc.org/pdf/laopdr/lao_opium_survey_2005.pdf [On-line]
- Villafranca SW, McKellar JD, Trafton JA, Humphreys K. Predictors of retention in methadone programs: a signal detection analysis. Drug Alcohol Depend. 2006;83:218–224. doi: 10.1016/j.drugalcdep.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Slobetz F, Prusoff B, Mezritz M, Howard P. Clinical depression among narcotic addicts maintained on methadone in the community. American Journal of Psychiatry. 1976;133:1434–1438. doi: 10.1176/ajp.133.12.1434. [DOI] [PubMed] [Google Scholar]
- Westermeyer J. Opium availability and the prevalence of addiction in Asia. Br J Addict. 1981;76:85–90. doi: 10.1111/j.1360-0443.1981.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Westermeyer J. Addiction among immigrants and migrants. American Journal on Addictions. 1995;5:334–350. [Google Scholar]
- Westermeyer J. Poppies, Pipes, and People: Opium and Its Use in Laos. University of California Press; Berkeley: 1982. [Google Scholar]
- Woody GE, Luborsky L, McLellan AT, O’Brien CP, Beck AT, Blaine J, et al. Psychotherapy for opiate addicts. Does it help? Arch Gen Psychiatry. 1983;40:639–645. doi: 10.1001/archpsyc.1983.04390010049006. [DOI] [PubMed] [Google Scholar]
- Woody GE, O’Brien CP, McLellan AT, Marcovici M, Evans BD. The use of antidepressants with methadone in depressed maintenance patients. Ann N.Y.Acad.Sci. 1982;398:120–127. doi: 10.1111/j.1749-6632.1982.tb39485.x. [DOI] [PubMed] [Google Scholar]
- Woody GE, O’Brien CP, Rickels K. Depression and anxiety in heroin addicts: a placebo-controlled study of doxepin in combination with methadone. American Journal of Psychiatry. 1975;132:447–450. doi: 10.1176/ajp.132.4.447. [DOI] [PubMed] [Google Scholar]
- World Health Organization . The practices and context of pharmacotherapy of opioid dependence in South-East Asia and Western Pacific regions Geneva. World Health Organization; Switzerland: 2002a. [Google Scholar]
- World Health Organization . The World Health Report 2002: Reducing Risks, Promoting Healthy Life. World Health Organization; Geneva: 2002b. [Google Scholar]