Abstract
Background
Abnormalities have been identified in the Cognitive Control Network (CCN) and the default mode network (DMN) during episodes of late-life depression. This study examined whether functional connectivity at rest (FC) within these networks characterize late-life depression and predict antidepressant response.
Methods
26 non-demented, non-MCI older adults were studied. Of these, 16 had major depression and 10 had no psychopathology. Depressed patients were treated with escitalopram (target dose 20 mg) for 12 weeks after a 2-week placebo phase. Resting state timeseries was determined prior to treatment. FC within the CCN was determined by placing seeds in the dACC and the DLPFC bilaterally. FC within the DMN was assessed from a seed placed in the posterior cingulate.
Results
Low resting state FC within the CCN and high FC within the DMN distinguished depressed from normal elderly subjects. Beyond this “double dissociation”, low resting state FC within the CCN predicted low remission rate and persistence of depressive symptoms and signs, apathy, and dysexecutive behavior after treatment with escitalopram. In contrast, resting state FC within the DMN was correlated with pessimism but did not predict treatment response.
Conclusions
If confirmed, these findings may serve as a signature of the brain’s functional topography characterizing late-life depression and sustaining its symptoms. By identifying the network abnormalities underlying biologically meaningful characteristics (apathy, dysexecutive behavior, pessimism) and sustaining late-life depression, these findings can provide a novel target on which new somatic and psychosocial treatments can be tested.
INTRODUCTION
Structural and functional abnormalities have been identified in depressed older adults in structures participating in cognitive and emotional regulation. In the cognitive control network (CCN), microstructural white matter abnormalities have been found in structures including the dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate cortex (ACC) (Alexopoulos et al., 2009, Bae et al., 2006). Decreased metabolic activity at rest has been observed in the dorsal ACC and the DLPFC during episodes of depression(Drevets et al., 1997, Mayberg et al., 1999, Aizenstein et al., 2009). When challenged with tasks probing the CCN both elderly (Aizenstein et al., 2009) and young (Fales et al., 2008) depressed patients exhibit decreased DLPFC activation. This hypoactivation of the DLPFC resolves after SSRI treatment (Aizenstein et al., 2009, Fales et al., 2009), but decreased task-based FC may persist(Aizenstein et al., 2009). Further, abnormalities in CCN structures during episodes of late-life depression may influence response to antidepressants(Alexopoulos et al., 2008).
A complex network of corticolimbic structures have been implicated in emotional regulation. Among these structures, ventromedial prefrontal regions play a prominent role in depression. For example, lesions in the ventromedial prefrontal cortex (VMPFC) are associated with abnormal affect-guided anticipation and planning (Damasio, 1994). Failure to anticipate and direct behavior towards positive incentives leads to “negativity bias”, a common behavioral characteristic of depressed patients. Posterior cingulate cortex pathways devoted to attentional processing, and amygdalar pathways devoted to emotional processing converge within the ventral ACC (BA24)(Davidson et al., 2002). Abnormal activation of the ventral ACC (BA24 and BA32) may be associated with blunted conscious experience of affect, hypoarousal, anhedonia, reduced coping in situations of uncertainty, conflict, and expectancy violation between the environment and the individual’s affective state(Davidson et al., 2002). Metabolic increases that occur in the ventral ACC during depressive episodes, correlate with symptom severity(Drevets et al., 1997, Mayberg et al., 1999). Further, remission of depression has been associated with metabolic changes in structures participating in emotional regulation (e.g., amygdala, ventral ACC)(Bauer et al., 2005, Drevets, 1999, Lopez-Sola et al., 2010, Mayberg et al., 2005).
Depressed elders have cortical and subcortical microstructural white matter abnormalities (Alexopoulos et al., 2009, Gunning-Dixon et al., 2008) and greater white matter hyperintensity (WMH) burden within and connecting networks critical for cognitive control and emotional regulation (e.g., the uncinate, superior and inferior longitudinal, and fronto-occipital fasciculi, and external capsule) (Sheline et al., 2008). Further, in elderly depressed subjects microstructural white matter abnormalities in emotional regulation and cognitive control systems are associated with poor antidepressant response(Alexopoulos et al., 2008), and reduced task-based in FC in the CCN persists despite treatment with an SSRI(Aizenstein et al., 2009). Further, recent data indicate that greater WMH burden is associated with hyperactivation of the subgenual cingulate in late-life depression(Aizenstein et al., 2011).
Taken together, the above findings suggest that corticolimbic connectivity, particularly in networks associated with emotional regulation and cognitive control, plays a role in geriatric depression. These observations are mainly derived from structural imaging and from studies of cerebral activation in response to specific tasks. However, late-life depression is a complex disorder with symptoms mediated by large distributed networks. Arguably, assessment of the brain’s functional connectivity (FC) at rest can offer complementary information on relationships among structures with abnormal activation patterns during depression.
FC is based on the observation that spontaneous blood oxygen level dependent (BOLD) signal fluctuations among brain regions similarly modulated by specific tasks tend to be correlated(Fox et al., 2009, Biswal et al., 1995, Cordes et al., 2001, Cordes et al., 2000, De Luca et al., 2005, Fox et al., 2006, Fox and Raichle, 2007, Lowe et al., 1998, Xiong et al., 1999). FC during rest is thought to reflect important interrelationships among structures with related functions. Most of the brain’s energy (>85%) is consumed to maintain a functionally differentiated state at rest(Fox and Raichle, 2007). Studies using differing methodology suggest that BOLD activity during a resting state is mainly driven by “intrinsic activity”, which remains consistent across different resting conditions(Fransson, 2005, Raichle and Mintun, 2006), task performance(Arfanakis et al., 2000, Arieli et al., 1996, Bartels and Zeki, 2005, Engel et al., 2001, Fair et al., 2007, Fransson, 2006, Greicius et al., 2004, Grill-Spector et al., 2004, Hampson et al., 2004, Jiang et al., 2004, Lowe et al., 2000, Marder and Weimann, 1991, Morgan and Price, 2004, Pessoa et al., 2002, Pessoa and Padmala, 2005, Ress et al., 2000, Ress and Heeger, 2003, Sapir et al., 2005, Sun et al., 2007, Tsodyks et al., 1999, Wagner et al., 1998, Waites et al., 2005), sleep(Fukunaga et al., 2006, Horovitz et al., 2008), and anesthesia (Peltier et al., 2005, Vincent et al., 2007).
This study focuses on FC within the CCN (dorsal ACC, DLPFC, parts of the parietal lobe) and the default mode network (DMN) (posterior cingulate/precuneus, VMPFC, ventral ACC, inferior lateral parietal lobes, and parts of the temporal lobe). It targets the CCN because anatomical and functional abnormalities of its structures have been identified in late-life depression and because some of these abnormalities have been linked to poor response to antidepressants(Alexopoulos et al., 2008, Alexopoulos et al., 2009, Gunning-Dixon et al., 2008) The DMN consists of regions that consistently decrease their activity during cognitive task performance(Fox and Raichle, 2007, Raichle and Snyder, 2007). These same regions are more active at rest than during task performance. Beyond maintaining processes of the brain’s resting state (Raichle et al., 2001), structures of the DMN are central to affect regulation and have been found excessively activated during depressive episodes (Sheline et al., 2009). Many of the DMN structures participate in emotional regulation. Accordingly, this study tested the hypothesis that low resting state FC within the CCN and high FC within the DMN distinguishes depressed from normal older adults. An additional hypothesis was that lower FC of the CCN during depressive episodes predicts persistence of depressive symptoms and signs during treatment with a selective serotonin reuptake inhibitor (SSRI).
METHODS
Subjects
We studied depressed and non-depressed adults aged 60 years and older. The depressed group consisted of consecutively recruited subjects who met DSM-IV criteria for unipolar major depression without psychotic features and had a score of 18 or greater on the 24-item Hamilton Depression Rating Scale (HDRS)(Hamilton, 1960). The normal comparison subjects were recruited through advertisement and were required to have no history or presence of any psychiatric disorder. The subjects signed written informed consent approved by the IRBs of Weill-Cornell Medical College and of Nathan Kline Institute.
Individuals were excluded if they had: 1) Mini-Mental State Examination score < 24 (Folstein et al., 1975) or met the mild cognitive impairment (MCI) criteria of Petersen et al (Petersen, 2004) during the clinical interview; 2) presence of delirium, history of stroke, head trauma, multiple sclerosis, or brain degenerative diseases; 3) metastatic cancer, brain tumors, unstable cardiac, hepatic, or renal disease, myocardial infarction, or stroke within the 3 months preceding the study; 4) diseases frequently associated with depression, i.e. lymphoma, pancreatic cancer, or endocrinopathies other than diabetes; 5) treatment with drugs associated with depression, i.e. steroids, alpha-methyl-dopa, clonidine, reserpine, tamoxifen, or cimetidine; and 6) metal implants. Depressed subjects with history of other axis I psychiatric disorders prior to the onset of depression were excluded.
Assessment
DSM-IV diagnosis was based on the SCID-R, administered at entry to the study. Depressive symptoms were assessed using the Montgomery Asberg Depression Rating Scale (MADRS). Overall cognitive impairment was rated with the Mini Mental State Examination (MMSE)(Folstein et al., 1975) and the Dementia Rating Scale (Mattis, 1989). Response inhibition was assessed with the Stroop Color Word Test (Golden, 1978), visual attention and task switching with Trails A and Trails B (Reitan, 1985) and and dysexecutive behavior with the Frontal Systems Behavior Scale (FrSBe)(Grace J, 2001). Apathy was quantified with the Apathy Evaluation Scale(Marin et al., 1991). Memory was rated with the Hopkins Verbal Learning Test-Revised (Brandt, 1991).
Depressed subjects had a 2-week, single-blind, placebo, drug-wash-out phase at the end of which they had an MRI scan. Subjects who still met DMS-IV criteria for major depression and had HDRS≥18 received controlled treatment with escitalopram for 12 weeks. The starting dose was 10 mg daily for one week and increased to 20 mg daily thereafter for a total of 12 weeks. The dose was reduced to 10 mg daily in those who could not tolerate 20 mg. Subjects received their medication in one-week supply blisters. No subject received psychotherapy.
Depressed subjects had follow-up assessments at 1, 2, 3, 4, 6, 8, 10, and 12 weeks after initiation of escitalopram. Follow-up meetings consisted of a rating session with a research assistant followed by a brief session with a research psychiatrist. Research assistants administered the MADRS, obtained vital signs, questioned the subjects about medication adherence and inspected the escitalopram blister package. The AES and the FrSBe were administered at the last assessment session prior to exiting the study.
MRI
MRI Data Acquisition
MRI scans were acquired on a 1.5T Siemens Vision MR system at the Center for Advanced Brain Imaging (CABI) of the Nathan Kline Institute. Data were processed and analyzed at the Weill-Cornell Brain Imaging Analysis Laboratory. Anatomic imaging included a turbo dual echo scan and high-resolution whole brain images acquired using a 3D T1-weighted MPRAGE for coregistration with fMRI data. fMRI data were acquired using BOLD contrasts in a single-shot multi-slice echo planar image (EPI; TR = 2000 ms, a TE = 50 ms, flip-angle = 90 degrees, matrix=64×64, FOV = 224mm, 22 5mm slices, no gap), which allowed whole brain coverage.
Image Processing
Resting state (awake, closed eyes) data were processed following the procedure of Biswal et al.(Biswal et al.) To eliminate T1 relaxation effects, the first 10 images were discarded. Images were then motion corrected and time shifted using AFNI(Cox, 1996). Next, time series were smoothed using a 6-mm full width-half maximum (FWHM) Gaussian kernel, temporally filtered, and normalized to Montreal Neurological Institute (MNI)152 stereotaxic space (1 × 1 × 1 mm3 resolution using FSL; http://www.fmrib.ox.ac.uk/fsl).
MPRAGE scans were segmented using FSL’s FAST software, and these segmentations were normalized to MNI152 space using the transformations for the MPRAGE. The gray matter, cerebrospinal fluid (CSF), and total brain signal time series were then extracted using masks derived from the MPRAGE segmentation. These time series, along with the time series for the 6 motion parameters were subsequently used as covariates in a general linear model. In first level analyses, these time series were residualized from the preprocessed resting state data.
FC analysis
To calculate timeseries for each participant we used FMRIB’s Linear Image Registration Tool (FLIRT) program to transform each subject’s residualized resting-state data into MNI152 space using a 12 DOF linear affine transformation. Next, we calculated the spatial mean time series for each seed ROI. Using the locations described by Fox et al.,(Fox et al., 2005) to identify FC within the DMN, one seed was placed in the posterior cingulate cortex (PCC -5, -49, 40). Based on the literature (Sheline et al., 2010a) and regions we observed to be hypoactive in elderly depressed patients during a cognitive control task, to identify FC within the CCN, one seed was placed in each hemisphere in the dorsal ACC (−4/4, 30, 22) and the DLPFC (−36/36, 28, 34). Each seed was spherical in shape with a diameter of 8 mm.
For each seed, individual participant analyses was carried out with GLM of FSL’s FEAT toolbox using seed-based regression approaches on the residualized resting state data (Biswal et al., Hoptman et al.). The timeseries for each seed was entered as a predictor. Individual subject-level, Z-statistic images were generated for each seed.
Data Analysis
Initially, Mann-Whitney and chi-square tests were used to identify demographic and clinical variables distinguishing depressed from normal subjects and depressed subjects who achieved remission from those who remained symptomatic. Group level analyses of FC were conducted using FLAME (FMRIB’s Local Analysis of Mixed Effects), to produce thresholded z-score maps of activity associated with each seed. Images were thresholded using clusters determined by z > 2.3 and a corrected cluster significance threshold of p < 0.05 (Worsley et al., 1992). These maps revealed networks for each group (depressed patients, controls, remitters, nonremitters), as well as group difference maps for direct group comparisons (e.g., patients vs. controls, remitters vs. nonremitters). Partial correlations with age as covariate were used to study the association of FC networks with clinical characteristics at baseline. The relationship of FC within CCN and DMN at baseline to the course of depressive symptoms and signs during the 12 weeks of treatment was studied with mixed effects models. Survival analysis was used to study the relationship of resting FC within the CCN and DMN to time to remission. For clinical variables assessed at baseline and at study exit (apathy, dysexecutive behavior), regression was used to study the relationships of FC to the values of these clinical variables at the end of treatment. Two tailed significance tests were used in all comparisons.
RESULTS
Subjects
Twenty-six older adults were studied. Of these, 16 were non-demented, non-MCI subjects (mean: 69 years, SD: 5.5) with non-psychotic, unipolar major depression (baseline MADRS mean: 23.5, SD: 4.0) and 10 were normal subjects (mean age: 68.6 years, SD: 7.0). There were no differences in education (years), overall cognitive impairment (MMSE, DRS), memory (HVLT), and response inhibition (Stroop Color-Word) between depressed and normal subjects. However, depressed subjects had a higher depression (MADRS: z=4.2, p<0.0001) and disability (WHODAS: z=3.7, p<0.0001) scores than normal subjects. The depressed group was divided into remitters (MADRS ≤7 for two consecutive weeks) and non-remitters. There were no demographic or clinical differences between remitters and non-remitters at baseline with the exception of response inhibition in which non-remitters had more abnormal scores (Table 1).
Table 1.
Characteristics of Remitted and Non- Remitted Depressed Patients and Non-depressed Comparison Subjects at Baseline
| Variable | Remitted (N=16) | Non-Remitted (N=16) | Non-Depressed (N=10) |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 67.9 (4.7) | 70.1 (6.3) | 68.6 (7.0) |
| Education | 16.5 (1.4) | 17.9 (2.9) | 16.3 (3.8) |
| Ham-D1 | 24.9 (4.1) | 26.3 (3.7) | 2.4 (1.8) |
| MADRS2 | 22.3 (2.6) | 24.8 (5.0) | 2.1 (1.3) |
| MiniMental State Exam | 29.1 (1.1) | 29.1 (0.6) | 28.5 (0.97) |
| DRS3Total | 137.8 (3.0) | 135.3 (5.4) | 137.3 (3.9) |
| DRS Initiation/Perseveration | 35.8 (0.71) | 34.4 (4.3) | 35.9 (1.1) |
| Stroop Color Word | 40.3 (7.2) | 30.4 (9.4)* | 38.5 (6.8) |
| FrSBe4 | 34.6 (7.4) | 40.8 (4.7) | -- |
| Apathy Evaluation Scale | 32.8 (6.9) | 39.3 (8.6) | -- |
| Trails B | 77.7 (19.5) | 98.4 (39.4) | 75.7 (25.3) |
| Trails A | 36.8 (11.0) | 34.4 (7.2) | 30.0 (11.1) |
| HVLT-R5Immediate Recall | 26.5 (4.2) | 27.5 (5.3) | 25.5 (5.1) |
| HVLT-R Delayed Recall | 8.4 (2.4) | 9.9 (1.6) | 9.5 (2.2) |
24-item Hamilton Depression Rating Scale;
Montgomery Asberg Depression Rating Scale;
Dementia Rating Scale;
Frontal Systems Behavior Rating Scale
Hopkins Verbal Learning Test- Revised
Comparison between Remitters and Non-Remitters was significantly different Mann Whitney U=51, z=2.0, p=0.045. No other comparisons between Remitters and Non-Remitters achieved statistical significance.
Depressed subjects were scanned at the end of a 2-week single-blind placebo phase. Then, they received escitalopram at mean dose of 16.25 mg (SD: 5.0, range 10–20) daily for 12 weeks. Non-depressed subjects were scanned at study entry.
Baseline FC at Rest in Depressed Elders and Controls
Cognitive Control Network (CCN)
Both non-depressed and depressed subjects showed significant FC within a network that included the dorsal ACC, DLPFC, supramarginal. superior parietal and inferior parietal regions, and thalamus (See Figures 1a and 1b for non-depressed and depressed, respectively). Relative to depressed subjects, when a seed was placed in the left dorsal ACC, non-depressed subjects had greater FC in the left DLPFC (BA 9; MNI coordinates x =−24, y=40, z=32) (Figure 1c). When a seed was placed in the left DLPFC, relative to depressed patients, non-depressed subjects demonstrated greater FC in the bilateral inferior parietal cortices (BA 40; MNI coordinates x = 54/− 54, y=−44, z=36). No significant group differences were observed when the seed was placed either in the right DLPFC or the right ACC. Depressed subjects had no regions of greater FC than normal subjects with either the dorsal ACC or LPFC seeds.
Figure 1.
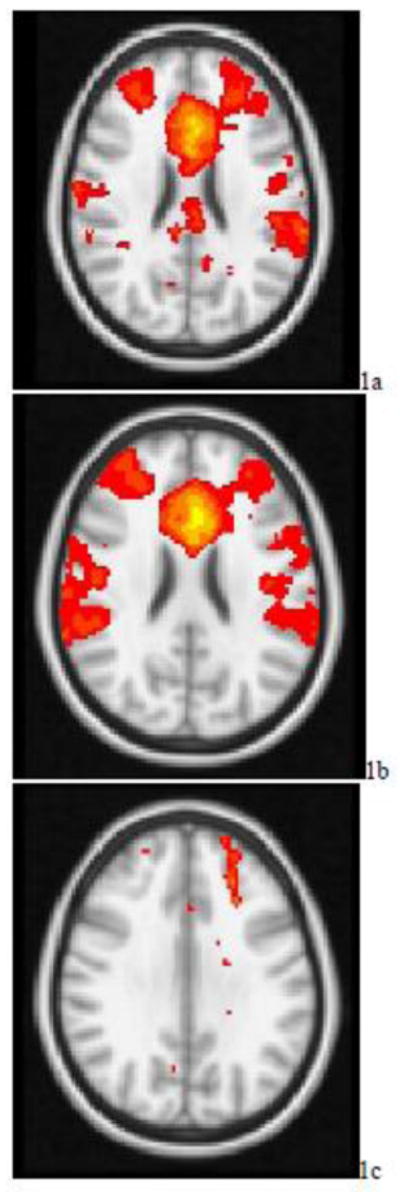
t-maps of the resting state connectivity of the cognitive control network for a) the non-depressed elderly; b) elderly depressed patients; c) non-depressed elderly > elderly depressed patients. Images were thresholded using clusters determined by z > 2.3 and a corrected cluster significance threshold of p < 0.05.
In the depressed group, partial correlation with age as covariate showed that FC within the CCN was associated with baseline Stroop Color Word scores (r=0.34, df=13, p=0.21), but this relationship did not reach significance. There was no association between FC of the CCN and Stroop Color Word in normal subjects (r=0.06), perhaps because of limited variability in Stroop values.
Default Mode Network (DMN)
Both depressed and normal subjects demonstrated significant FC within a network that included the posterior cingulate/precuneus, portions of the VMPFC, lateral parietal regions, perigenual ACC, DMPFC, and medial temporal regions (Figure 2a and 2b). Relative to non-depressed subjects, depressed subjects had greater FC in the left precuneus (BA 7; MNI coordinates x = −6, −70, 62), the subgenual ACC (BAs 25/32 MNI coordinates x = −4, 26, −10), the VMPFC (BA 11; MNI coordinates x = −4, 36, −16), and lateral parietal regions (Figure 2c).
Figure 2.
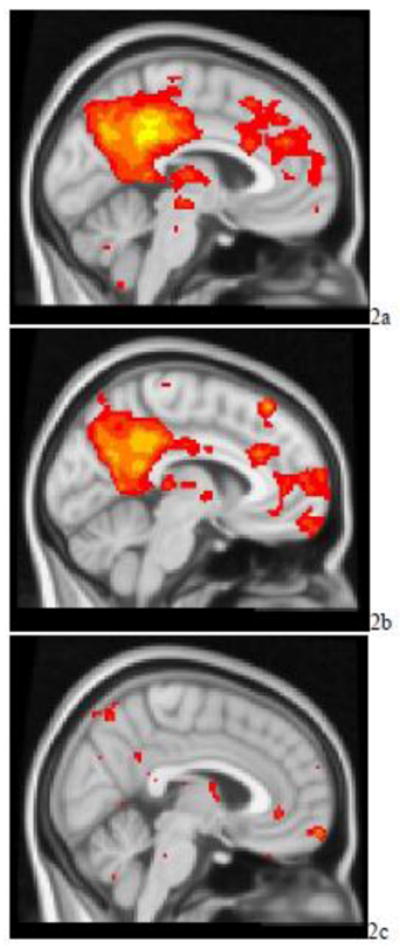
t-maps of the resting state connectivity of the default mode network for a) the non-depressed elderly subjects; b) elderly depressed patients; c) elderly depressed patients > non-depressed elderly subjects. Images were thresholded using clusters determined by z > 2.3 and a corrected cluster significance threshold of p < 0.05.
In the depressed group, partial correlation with age as the covariate showed that DMN FC (r=0.55, df=14, p=0.034) was associated with pessimism (MADRS item 9) at baseline. Age was not significantly correlated with pessimism. There was no significant association between DMN FC and overall severity of depression (MADRS).
Baseline CCN and DMN FC at Rest and the Course of Depression
Next we compared, FC in patients who remitted following treatment versus patients who remained symptomatic (Remitters, NonRemitters). Remission was defined as MADRS≤7 for two consecutive weeks and absence of DSM-IV diagnosis of depression. Seeds placed in the dorsal ACC, did not result in any significant group differences. A seed placed in the left DLPFC yielded greater FC in the bilateral dorsal ACC (BAs 24/32; MNI coordinates x= 4, y =34, z = 22, right DLPFC (BA 10; MNI coordinates x=44, y=46, z=12), and bilateral inferior parietal cortices (BA 40; MNI coordinates x= 38/−38, y =−56, z=52) in Remitters (Figure 3). When the seed was placed in the right DLPFC Remitters had greater FC than Non-Remitters in the right inferior parietal region (BA 40; MNI coordinates x= 38, y =−56, z=52). Non-Remitters did not exhibit any areas of greater FC. Analyses of the default network did not yield any significant group differences between Remitters and Non-Remitters.
Figure 3.
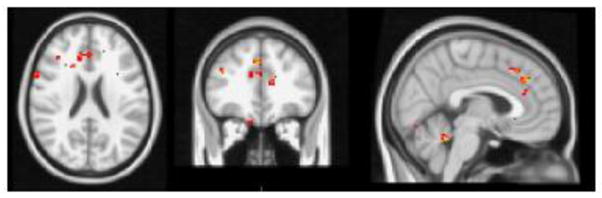
t-maps of the resting state connectivity of the cognitive control network for Remitters > NonRemitters. Images were thresholded using clusters determined by z > 2.3 and a corrected cluster significance threshold of p < 0.05.
Next, in the depressed group, we used mixed effects models to study the relationship of FC within CCN and DMN at baseline and the course of depression (MADRS) over the 12 weeks of escitalopram treatment. Age was used as a covariate. A model consisting of baseline resting FC in CCN, DMN FC, age, and time was associated with change in MADRS during the 12 week treatment phase (χ2= 61.15, p<0.0001). Baseline CCN FC predicted decline of MADRS (β= −18.19, SE= 5.3, t(89)= −3.42, p<0.001). Neither DMN FC (p=0.72) nor age (p=0.40) were significantly associated with the course of MADRS.
We used survival analysis to study the relationship of a model consisting of CCN and DMN resting FC to remission rates (MADRS≤7 for 2 consecutive weeks) during escitalopram treatment. The overall model was associated with remission (Wald χ2=13.51, df=2, p<0.001). However, only CCN FC had a significant relationship with remission (Wald χ2: 9.5, df:1, p<0.002); low FC predicted non-remission. DMN FC was not associated with remission (Wald χ2=0.48, df=1, p=0.49). Depressed subjects who achieved remission had received similar dosages of escitalopram to those who did not meet criteria for remission. Three remitters received 10 mg of escitalopram daily and five received 20 mg. The corresponding numbers for non-remitters were four and four.
Baseline Resting FC in CCN and DMN, Apathy, and Dysexecutive Behavior at 12 Weeks
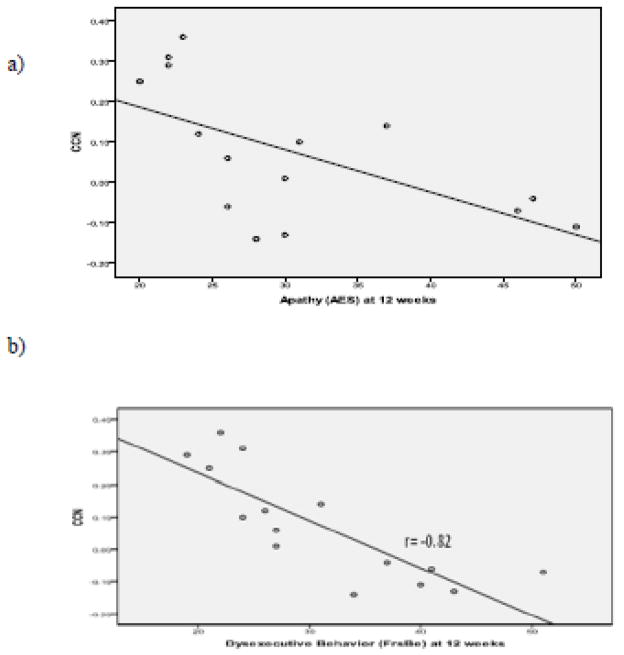
In the depressed group, apathy (AES) and dysexecutive behavior (FrSBe) were assessed at baseline and after 12 weeks of escitalopram treatment. A regression model was constructed to assess the relationship of baseline resting FC at CCN to apathy (AES) after 12 weeks of treatment with escitalopram. Baseline AES and age were used as covariates. The overall model was significant (F(3,11)=6.30, p=0.0095). Baseline CCN FC (β:-26.60, SE: 11.06, p<0.035) was associated with AES at 12 weeks (Figure 4a), while age and baseline AES had no significant association.
Figure 4.
a) Relationship between Apathy (AES) at 12 Weeks and Baseline Cognitive Control Network FC in Depressed Older Adults. b) Relationship between Dysexecutive Behavior (FrsBe) at week 12 and baseline Cognitive Control Network FC in Depressed Older Adults.
A similar analysis assessed the relationship of baseline FC within the CCN to dysexecutive behavior (FrSBe) after 12 weeks of escitalopram treatment. The overall model was significant (F(3,11)=15.1, p=0.0003). Baseline CCN FC (β=−31.28, SE=9.61, p<0.008) was associated with FrSBe at 12 weeks (Figure 4b), while age and baseline FrSBe had no significant association. Baseline FC within the DMN was not associated with either apathy or dysexecutive behavior at 12 weeks.
DISCUSSION
The principal finding of this study is that low resting FC within the CCN and high FC within the DMN characterize late-life depression. Beyond this “double dissociation” distinguishing depressed from normal older adults, resting FC at the CCN and the DMN were related to distinct clinical characteristics. Resting FC at the CCN during the depressive episode predicted poor remission rate after treatment with escitalopram and persistence of depression, apathy and dysexecutive behavior at the end of treatment. In contrast, resting FC of the DMN was correlated with pessimism during the depressive episode but was not associated with treatment response.
To our knowledge this is the first study to identify an imbalance of resting FC within the CCN and DMN in late-life depression and demonstrate a relationship between FC within CCN and poor outcomes of depression. These findings should be viewed in the context of the study’s limitations. These include the small number of subjects, the use of an 1.5 T scanner, and the absence of a placebo control group. Use of a higher field scanner may have revealed additional abnormalities. Placebo response is imbedded in escitalopram response, although the placebo effect is reduced by our placebo lead-in. A placebo control group may have allowed to distinguish escitalopram from placebo response. Nonetheless, focusing on overall response (drug + placebo) may be appropriate for the first study of its kind. A potential advantage of a non-placebo controlled study is low selection bias since placebo controlled studies tend to recruit “less sick” patients (Roose et al., 2004).
Low FC within the CCN is consistent both with the clinical presentation and with available neuroimaging findings in late-life depression. A cardinal feature of major depression is difficulty engaging in goal-directed behavior while ignoring irrelevant, mainly negatively-valenced, stimuli. These functions rely on the CCN, which enables individuals to flexibly adapt information processing to changing demands and is a central aspect of several functions, including attention allocation, working memory and cognitive inhibition (Carter and van Veen, 2007, Miller and Cohen, 2001). Moreover, the CCN influences emotional associations by biasing processing either in affective networks or in perceptual and associative memory systems (Davidson et al., 2002) and influences underdetermined responding (Barch et al., 2000), emotion regulation in anxiety (Bishop et al., 2004), and thought suppression (Anderson et al., 2004). PET and fMRI studies have shown decreased metabolic activity in dACC and DLPFC during depression (Aizenstein et al., 2009, Drevets et al., 1997, Mayberg et al., 1999). Depressed patients have decreased DLPFC activity in response to cognitive control tasks and sustained amygdala reactivity during emotional tasks (Siegle et al., 2007). Hypoactivity of the DLPFC following challenge has been documented during depression in elderly patients (Aizenstein et al., 2009). Consistent with our findings, depressed elderly patients have low FC between the DLPFC and dACC during a cognitive conflict task, which persisted after antidepressant treatment (Aizenstein et al., 2009).
The relationship of low resting FC within CCN to poor remission rate and persistence of depressive symptoms, apathy and dysexecutive behavior is consistent with earlier findings suggesting that CCN abnormalities influence response to antidepressants. Abnormal response inhibition, working memory, and processing speed, characterize depressed elderly non-remitters or predict long time to remission (Sheline et al., 2010b, Alexopoulos et al., 2005, Alexopoulos et al., 2004, Kalayam and Alexopoulos, 1999). Microstructural white matter abnormalities in the cognitive control territory have been associated with executive dysfunction (Murphy et al., 2007) and poor response to escitalopram (Alexopoulos et al., 2010, Alexopoulos et al., 2008, Alexopoulos et al., 2002), although some disagreement exists(Taylor et al., 2008). These abnormalities may disrupt the communication of the CCN with ventral limbic regions, thus interfering with normalization of their functions and inhibiting remission of depression (Alexopoulos et al., 2008). Indeed, increased activation in DLPFC, ACC and limbic regions during a task of inhibitory control was shown to predict the extent of response to escitalopram (Langenecker et al., 2007). Other studies of adults observed that remission of depression is associated with metabolic changes, mainly increases, in dACC (BA 24b), DLPFC (BA 46/9), and inferior parietal lobe (BA 40)(Drevets, 2000, Fitzgerald et al., 2006, Mayberg et al., 1997). The persistence of apathy and dysexecutive behavior is consistent with findings suggesting that low FC between the DLPFC and the dACC remains after antidepressant treatment(Aizenstein et al., 2009). Frontal lobe syndromes often include apathy and dysexecutive behaviors, including inability to plan, sequence, initiate and flexibly adjust behavior to task-related demands(Lezak et al., 2004), symptoms that often persist after improvement of depression(Royall, 1999).
Increased resting FC within the DMN is consistent with earlier findings in depressed adults (Sheline et al., 2010b) and with what is known about the function of DMN structures. The DMN is thought to mediate self-referential thinking, including the development of a personal perspective by taking into consideration the past and planning for the future and by evaluating beliefs and intentions of others(Buckner et al., 2008, Raichle et al., 2001, Raichle and Snyder, 2007, Sheline et al., 2009). Self-referential thinking is often impaired in depression and leads to a negativity bias (Herwig et al.), most pronounced during depressive episodes. Recently, it was shown that the CCN, the DMN, and other affective networks have increased resting FC with the same bilateral DMPFC region (dorsal nexus)(Sheline et al., 2010b). Further the dorsal nexus has increased FC at rest in depressed adults compared to controls. Increased FC in this region may explain the concurrent occurrence of heterogeneous symptoms in depression.
Increased FC in portions of the DMN during the depressed state in the older adults is also consistent with a recent report of increased DMN FC in the dorsomedial PFC and orbitofrontal cortex in elderly depressed patients prior to treatment (Wu et al., 2011). Some of the abnormalities in DMN FC improved with antidepressant treatment (Wu et al., 2011). Further, FC of DMN and other networks involved in emotional regulation are related to WMH burden (Wu et al., 2011) and microstructural white matter indices as measured by tractography (Steffens et al., 2011).
In conclusion, we observed that low resting FC within the CCN distinguishes depressed from normal older adults and predicts persistence of depressive symptoms and signs, low remission rate, apathy and dysexecutive behavior. In contrast, resting FC within the DMN is increased in late-life depression and correlated with pessimism but does not predict treatment response. If confirmed, these findings may serve as a signature of the brain’s functional topography characterizing late-life depression and sustaining its symptoms. By identifying the network abnormalities underlying biologically meaningful characteristics (apathy, dysexecutive behavior, pessimism) and sustaining late-life depression, these findings can provide a novel target on which new somatic and psychosocial treatments can be tested.
Acknowledgments
Role of funding source
Personnel and imaging cost of this work was supported by NIMH grants R01 MH65653, R01 MH079414, P030 MH085943, T32 MH019132 (GSA), K23 MH74818 (FGD) and the Sanchez Foundation. Escitalopram and placebo were provided free of cost by Forest Pharmaceuticals, Inc. We thank Raj Sangoi (RT)(R)(MR) for his assistance in scanning the research participants.
Footnotes
CONFLICTS OF INTEREST
Dr. Alexopoulos received grant support from Forest Pharmaceuticals and has been a member of speakers’ bureaus of Astra Zeneca, Avanir, Forest, Merck, and Lundbeck. He holds equity of Johnson and Johnson. Dr. Lim serves on the Scientific Advisory Board for Shire Corp, a biopharmaceutical company. No other authors report conflicts of interest.
Contributors
Dr. Alexopoulos conceptualized and designed and oversaw the conduct of the study, obtained funding and wrote the first draft of the manuscript. Dr. Hoptman oversaw acquisition of MRI data. Drs. Lim and Hoptman guided image analysis and provided consultation on data interpretation. Dr. Gunning performed processing and analysis of MR images and contributed to revisions of the manuscript for important technical and intellectual content. Ms. Kanellopoulos developed and managed the research database and performed statistical analyses in SPSS and SAS and contributed to the interpretation of data. Dr. Murphy aided in the design of the study and oversaw the collection of data. All authors contributed to the manuscript and have approved the final version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AIZENSTEIN H, BUTTERS M, WU M, MAZURKEWICZ L, STENGER V, GIANAROS P, BECKER J, REYNOLDS CR, CARTER C. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17:30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AIZENSTEIN HJ, ANDREESCU C, EDELMAN KL, COCHRAN JL, PRICE J, BUTTERS MA, KARP J, PATEL M, REYNOLDS CF., 3RD FMRI correlates of white matter hyperintensities in late-life depression. The American journal of psychiatry. 2011;168:1075–82. doi: 10.1176/appi.ajp.2011.10060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXOPOULOS GS, GLATT CE, HOPTMAN MJ, KANELLOPOULOS D, MURPHY CF, KELLY RE, JR, MORIMOTO SS, LIM KO, GUNNING FM. BDNF val66met polymorphism, white matter abnormalities and remission of geriatric depression. J Affect Disord. 2010;125:262–8. doi: 10.1016/j.jad.2010.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXOPOULOS GS, KIOSSES DN, CHOI SJ, MURPHY CF, LIM KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–32. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- ALEXOPOULOS GS, KIOSSES DN, HEO M, MURPHY CF, SHANMUGHAM B, GUNNING-DIXON F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–10. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- ALEXOPOULOS GS, KIOSSES DN, MURPHY C, HEO M. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29:2278–84. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- ALEXOPOULOS GS, MURPHY CF, GUNNING-DIXON FM, GLATT CE, LATOUSSAKIS V, KELLY RE, JR, KANELLOPOULOS D, KLIMSTRA S, LIM KO, YOUNG RC, HOPTMAN MJ. Serotonin transporter polymorphisms, microstructural white matter abnormalities and remission of geriatric depression. J Affect Disord. 2009;119:132–41. doi: 10.1016/j.jad.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXOPOULOS GS, MURPHY CF, GUNNING-DIXON FM, LATOUSSAKIS V, KANELLOPOULOS D, KLIMSTRA S, LIM KO, HOPTMAN MJ. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165:238–44. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- ANDERSON MC, OCHSNER KN, KUHL B, COOPER J, ROBERTSON E, GABRIELI SW, GLOVER GH, GABRIELI JD. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–5. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- ARFANAKIS K, CORDES D, HAUGHTON VM, MORITZ CH, QUIGLEY MA, MEYERAND ME. Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magn Reson Imaging. 2000;18:921–30. doi: 10.1016/s0730-725x(00)00190-9. [DOI] [PubMed] [Google Scholar]
- ARIELI A, STERKIN A, GRINVALD A, AERTSEN A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–71. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- BAE JN, MACFALL JR, KRISHNAN KR, PAYNE ME, STEFFENS DC, TAYLOR WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60:1356–63. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- BARCH DM, BRAVER TS, SABB FW, NOLL DC. Anterior cingulate and the monitoriing of response conflict: evidence from an fMRI study of overt verb generation. J Cogn Neurosci. 2000;12:298–309. doi: 10.1162/089892900562110. [DOI] [PubMed] [Google Scholar]
- BARTELS A, ZEKI S. Brain dynamics during natural viewing conditions--a new guide for mapping connectivity in vivo. Neuroimage. 2005;24:339–49. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- BAUER M, LONDON ED, RASGON N, BERMAN SM, FRYE MA, ALTSHULER LL, MANDELKERN MA, BRAMEN J, VOYTEK B, WOODS R, MAZZIOTTA JC, WHYBROW PC. Supraphysiological doses of levothyroxine alter regional cerebral metabolism and improve mood in bipolar depression. Molecular psychiatry. 2005;10:456–69. doi: 10.1038/sj.mp.4001647. [DOI] [PubMed] [Google Scholar]
- BISHOP S, DUNCAN J, BRETT M, LAWRENCE AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- BISWAL B, YETKIN FZ, HAUGHTON VM, HYDE JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- BISWAL BB, MENNES M, ZUO XN, GOHEL S, KELLY C, SMITH SM, BECKMANN CF, ADELSTEIN JS, BUCKNER RL, COLCOMBE S, DOGONOWSKI AM, ERNST M, FAIR D, HAMPSON M, HOPTMAN MJ, HYDE JS, KIVINIEMI VJ, KOTTER R, LI SJ, LIN CP, LOWE MJ, MACKAY C, MADDEN DJ, MADSEN KH, MARGULIES DS, MAYBERG HS, MCMAHON K, MONK CS, MOSTOFSKY SH, NAGEL BJ, PEKAR JJ, PELTIER SJ, PETERSEN SE, RIEDL V, ROMBOUTS SA, RYPMA B, SCHLAGGAR BL, SCHMIDT S, SEIDLER RD, SIEGLE GJ, SORG C, TENG GJ, VEIJOLA J, VILLRINGER A, WALTER M, WANG L, WENG XC, WHITFIELD-GABRIELI S, WILLIAMSON P, WINDISCHBERGER C, ZANG YF, ZHANG HY, CASTELLANOS FX, MILHAM MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 107:4734–9. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRANDT J. Clinical Neuropsychologist. Swets & Zeitlinger; 1991. The Hopkins verbal learning test: development of a new memory test with six equivalent forms. [Google Scholar]
- BUCKNER RL, ANDREWS-HANNA JR, SCHACTER DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- CARTER CS, VAN VEEN V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–79. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- CORDES D, HAUGHTON VM, ARFANAKIS K, CAREW JD, TURSKI PA, MORITZ CH, QUIGLEY MA, MEYERAND ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–33. [PMC free article] [PubMed] [Google Scholar]
- CORDES D, HAUGHTON VM, ARFANAKIS K, WENDT GJ, TURSKI PA, MORITZ CH, QUIGLEY MA, MEYERAND ME. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21:1636–44. [PMC free article] [PubMed] [Google Scholar]
- COX RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- DAMASIO AR. Descartes’ error and the future of human life. Sci Am. 1994;271:144. doi: 10.1038/scientificamerican1094-144. [DOI] [PubMed] [Google Scholar]
- DAVIDSON RJ, PIZZAGALLI D, NITSCHKE JB, PUTNAM K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–74. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- DE LUCA M, SMITH S, DE STEFANO N, FEDERICO A, MATTHEWS PM. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Exp Brain Res. 2005;167:587–94. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- DREVETS WC. Prefrontal cortical-amygdalar metabolism in major depression. Annals of the New York Academy of Sciences. 1999;877:614–37. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- DREVETS WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- DREVETS WC, PRICE JL, SIMPSON JR, JR, TODD RD, REICH T, VANNIER M, RAICHLE ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- ENGEL AK, FRIES P, SINGER W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–16. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- FAIR DA, SCHLAGGAR BL, COHEN AL, MIEZIN FM, DOSENBACH NU, WENGER KK, FOX MD, SNYDER AZ, RAICHLE ME, PETERSEN SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALES CL, BARCH DM, RUNDLE MM, MINTUN MA, MATHEWS J, SNYDER AZ, SHELINE YI. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112:206–11. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALES CL, BARCH DM, RUNDLE MM, MINTUN MA, SNYDER AZ, COHEN JD, MATHEWS J, SHELINE YI. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD PB, OXLEY TJ, LAIRD AR, KULKARNI J, EGAN GF, DASKALAKIS ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148:33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- FOLSTEIN MF, FOLSTEIN SE, MCHUGH PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- FOX MD, CORBETTA M, SNYDER AZ, VINCENT JL, RAICHLE ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–51. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX MD, RAICHLE ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- FOX MD, SNYDER AZ, VINCENT JL, CORBETTA M, VAN ESSEN DC, RAICHLE ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX MD, ZHANG D, SNYDER AZ, RAICHLE ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–83. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANSSON P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANSSON P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–45. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- FUKUNAGA M, HOROVITZ SG, VAN GELDEREN P, DE ZWART JA, JANSMA JM, IKONOMIDOU VN, CHU R, DECKERS RH, LEOPOLD DA, DUYN JH. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging. 2006;24:979–92. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- GOLDEN CJ. The Stroop Color and Word Test (Manual) Chicago: Stoetling; 1978. [Google Scholar]
- GRACE JMP. Frontal Systems Behavior Scale (FrSBe): Professional Manual. Psychological Assessment Resources 2001 [Google Scholar]
- GREICIUS MD, SRIVASTAVA G, REISS AL, MENON V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRILL-SPECTOR K, KNOUF N, KANWISHER N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7:555–62. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- GUNNING-DIXON FM, HOPTMAN MJ, LIM KO, MURPHY CF, KLIMSTRA S, LATOUSSAKIS V, MAJCHER-TASCIO M, HRABE J, ARDEKANI BA, ALEXOPOULOS GS. Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. Am J Geriatr Psychiatry. 2008;16:255–62. doi: 10.1097/JGP.0b013e3181602a66. [DOI] [PubMed] [Google Scholar]
- HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPSON M, OLSON IR, LEUNG HC, SKUDLARSKI P, GORE JC. Changes in functional connectivity of human MT/V5 with visual motion input. Neuroreport. 2004;15:1315–9. doi: 10.1097/01.wnr.0000129997.95055.15. [DOI] [PubMed] [Google Scholar]
- HERWIG U, KAFFENBERGER T, JANCKE L, BRUHL AB. Self-related awareness and emotion regulation. Neuroimage. 50:734–41. doi: 10.1016/j.neuroimage.2009.12.089. [DOI] [PubMed] [Google Scholar]
- HOPTMAN MJ, ZUO XN, BUTLER PD, JAVITT DC, D’ANGELO D, MAURO CJ, MILHAM MP. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res. 117:13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROVITZ SG, FUKUNAGA M, DE ZWART JA, VAN GELDEREN P, FULTON SC, BALKIN TJ, DUYN JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29:671–82. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG T, HE Y, ZANG Y, WENG X. Modulation of functional connectivity during the resting state and the motor task. Hum Brain Mapp. 2004;22:63–71. doi: 10.1002/hbm.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALAYAM B, ALEXOPOULOS GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56:713–8. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- LANGENECKER SA, KENNEDY SE, GUIDOTTI LM, BRICENO EM, OWN LS, HOOVEN T, YOUNG EA, AKIL H, NOLL DC, ZUBIETA JK. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol Psychiatry. 2007;62:1272–80. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEZAK M, HOWIESON D, LORING D, HANNAY H. Neuropsychological Assessment. USA: Oxford University Press; 2004. [Google Scholar]
- LOPEZ-SOLA M, PUJOL J, HERNANDEZ-RIBAS R, HARRISON BJ, CONTRERAS-RODRIGUEZ O, SORIANO-MAS C, DEUS J, ORTIZ H, MENCHON JM, VALLEJO J, CARDONER N. Effects of duloxetine treatment on brain response to painful stimulation in major depressive disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2305–17. doi: 10.1038/npp.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE MJ, DZEMIDZIC M, LURITO JT, MATHEWS VP, PHILLIPS MD. Correlations in low-frequency BOLD fluctuations reflect cortico-cortical connections. Neuroimage. 2000;12:582–7. doi: 10.1006/nimg.2000.0654. [DOI] [PubMed] [Google Scholar]
- LOWE MJ, MOCK BJ, SORENSON JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–32. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- MARDER E, WEIMANN JM. Neurobiology of Motor Program Selection: New Approaches to Mechanisms of Behavioral Choice. Manchester, UK: Manchester University Press; 1991. [Google Scholar]
- MARIN R, BIEDRZYCKI R, FIRINCIOGULLARI S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- MATTIS S. Dementia Rating Scale. Odessa: Psychological Assessment Resources; 1989. [Google Scholar]
- MAYBERG HS, BRANNAN SK, MAHURIN RK, JERABEK PA, BRICKMAN JS, TEKELL JL, SILVA JA, MCGINNIS S, GLASS TG, MARTIN CC, FOX PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- MAYBERG HS, LIOTTI M, BRANNAN SK, MCGINNIS S, MAHURIN RK, JERABEK PA, SILVA JA, TEKELL JL, MARTIN CC, LANCASTER JL, FOX PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- MAYBERG HS, LOZANO AM, VOON V, MCNEELY HE, SEMINOWICZ D, HAMANI C, SCHWALB JM, KENNEDY SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- MILLER EK, COHEN JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- MORGAN VL, PRICE RR. The effect of sensorimotor activation on functional connectivity mapping with MRI. Magn Reson Imaging. 2004;22:1069–75. doi: 10.1016/j.mri.2004.07.002. [DOI] [PubMed] [Google Scholar]
- MURPHY C, GUNNING-DIXON F, HOPTMAN MJ, LIM KO, ARDEKANI B, SHIELDS JK, HRABE J, KANELLOPOULOS D, SHANMUGHAM BR, ALEXOPOULOS GS. White-matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61:1007–10. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELTIER SJ, KERSSENS C, HAMANN SB, SEBEL PS, BYAS-SMITH M, HU X. Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport. 2005;16:285–8. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- PESSOA L, GUTIERREZ E, BANDETTINI P, UNGERLEIDER L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35:975–87. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- PESSOA L, PADMALA S. Quantitative prediction of perceptual decisions during near-threshold fear detection. Proc Natl Acad Sci U S A. 2005;102:5612–7. doi: 10.1073/pnas.0500566102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSEN RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- RAICHLE ME, MACLEOD AM, SNYDER AZ, POWERS WJ, GUSNARD DA, SHULMAN GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAICHLE ME, MINTUN MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–76. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- RAICHLE ME, SNYDER AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–90. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–9. [DOI] [PubMed] [Google Scholar]
- REITAN R, WOLFSON D. The Halstead-Reitan Neuropsychological Test Battery: Therapy and clinical interpretation 1985 [Google Scholar]
- RESS D, BACKUS BT, HEEGER DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3:940–5. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- RESS D, HEEGER DJ. Neuronal correlates of perception in early visual cortex. Nat Neurosci. 2003;6:414–20. doi: 10.1038/nn1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROOSE SP, SACKEIM HA, KRISHNAN KR, POLLOCK BG, ALEXOPOULOS G, LAVRETSKY H, KATZ IR, HAKKARAINEN H. Antidepressant pharmacotherapy in the treatment of depression in the very old: a randomized, placebo-controlled trial. Am J Psychiatry. 2004;161:2050–9. doi: 10.1176/appi.ajp.161.11.2050. [DOI] [PubMed] [Google Scholar]
- ROYALL DR. Frontal systems impairment in major depression. Semin Clin Neuropsychiatry. 1999;4:13–23. doi: 10.1053/SCNP00400013. [DOI] [PubMed] [Google Scholar]
- SAPIR A, D’AVOSSA G, MCAVOY M, SHULMAN GL, CORBETTA M. Brain signals for spatial attention predict performance in a motion discrimination task. Proc Natl Acad Sci U S A. 2005;102:17810–5. doi: 10.1073/pnas.0504678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHELINE YI, BARCH DM, PRICE JL, RUNDLE MM, VAISHNAVI SN, SNYDER AZ, MINTUN MA, WANG S, COALSON RS, RAICHLE ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHELINE YI, PIEPER CF, BARCH DM, WELSH-BOHMER K, MCKINSTRY RC, MACFALL JR, D’ANGELO G, GARCIA KS, GERSING K, WILKINS C, TAYLOR W, STEFFENS DC, KRISHNAN RR, DORAISWAMY PM. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010a;67:277–85. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHELINE YI, PRICE JL, VAISHNAVI SN, MINTUN MA, BARCH DM, EPSTEIN AA, WILKINS CH, SNYDER AZ, COUTURE L, SCHECHTMAN K, MCKINSTRY RC. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524–32. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHELINE YI, PRICE JL, YAN Z, MINTUN MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010b;107:11020–5. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGLE GJ, THOMPSON W, CARTER CS, STEINHAUER SR, THASE ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- STEFFENS DC, TAYLOR WD, DENNY KL, BERGMAN SR, WANG L. Structural integrity of the uncinate fasciculus and resting state functional connectivity of the ventral prefrontal cortex in late life depression. PloS one. 2011;6:e22697. doi: 10.1371/journal.pone.0022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN FT, MILLER LM, RAO AA, D’ESPOSITO M. Functional connectivity of cortical networks involved in bimanual motor sequence learning. Cereb Cortex. 2007;17:1227–34. doi: 10.1093/cercor/bhl033. [DOI] [PubMed] [Google Scholar]
- TAYLOR WD, KUCHIBHATLA M, PAYNE ME, MACFALL JR, SHELINE YI, KRISHNAN KR, DORAISWAMY PM. Frontal white matter anisotropy and antidepressant remission in late-life depression. PLoS One. 2008;3:e3267. doi: 10.1371/journal.pone.0003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSODYKS M, KENET T, GRINVALD A, ARIELI A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science. 1999;286:1943–6. doi: 10.1126/science.286.5446.1943. [DOI] [PubMed] [Google Scholar]
- VINCENT JL, PATEL GH, FOX MD, SNYDER AZ, BAKER JT, VAN ESSEN DC, ZEMPEL JM, SNYDER LH, CORBETTA M, RAICHLE ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–6. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- WAGNER AD, SCHACTER DL, ROTTE M, KOUTSTAAL W, MARIL A, DALE AM, ROSEN BR, BUCKNER RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–91. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- WAITES AB, STANISLAVSKY A, ABBOTT DF, JACKSON GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24:59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORSLEY KJ, EVANS AC, MARRETT S, NEELIN P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- WU M, ANDREESCU C, BUTTERS MA, TAMBURO R, REYNOLDS CF, 3RD, AIZENSTEIN H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry research. 2011;194:39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIONG J, PARSONS LM, GAO JH, FOX PT. Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum Brain Mapp. 1999;8:151–6. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<151::AID-HBM13>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]