Abstract
Athletes, body builders, and military personnel use dietary creatine as an ergogenic aid to boost physical performance in sports involving short bursts of high-intensity muscle activity. Lesser known is the essential role creatine, a natural regulator of energy homeostasis, plays in brain function and development. Creatine supplementation has shown promise as a safe, effective, and tolerable adjunct to medication for the treatment of brain-related disorders linked with dysfunctional energy metabolism, such as Huntington’s Disease and Parkinson’s Disease. Impairments in creatine metabolism have also been implicated in the pathogenesis of psychiatric disorders, leaving clinicians, researchers and patients alike wondering if dietary creatine has therapeutic value for treating mental illness. The present review summarizes the neurobiology of the creatine-phosphocreatine circuit and its relation to psychological stress, schizophrenia, mood and anxiety disorders. While present knowledge of the role of creatine in cognitive and emotional processing is in its infancy, further research on this endogenous metabolite has the potential to advance our understanding of the biological bases of psychopathology and improve current therapeutic strategies.
Keywords: creatine, creatine kinase, phosphocreatine, ATP, energy metabolism, psychiatry, mental illness, CAM therapy, antidepressant, neuroleptics, sex differences
1. Introduction
The prevalence and severity of psychiatric disorders are well substantiated by epidemiologic data, where an estimated 26–30% of the U.S. population is affected by at least one mental illness annually (Kessler et al., 1994; Kessler et al., 2005). Compounding this issue, psychotropic medications have had limited treatment success owing to delayed onset of therapeutic activity, partial or no response, individual variability, poor tolerability, high cost, and stigma associated with use (Masand, 2003; Usala et al., 2008; Young et al., 2009). An array of possible side effects contribute to low adherence rates (28–44% discontinue use within 3 months), particularly impairments in memory, attention and executive processes, extrapyramidal effects, sexual dysfunction, weight gain, and sleep disturbances (Kennedy, 2006; Masand, 2003). With these concerns in mind, researchers, clinicians and patients alike are increasingly seeking out complementary and alternative medicines (CAM), or natural forms of therapy, to improve the speed and efficacy of relief, to reduce the occurrence of adverse events, and possibly to uncover innovative mechanisms of drug action. According to the 2007 National Health Interview Survey (Nahin et al., 2009), 38.3% of Americans reported using CAM annually and consumers spent more than $15 billion on non-vitamin/non-mineral products, which contain nutritional ingredients intended to supplement the diet.
Creatine monohydrate is one of the most popular of these naturally occurring compounds, with an estimated annual market value of $400 million (Metzl et al., 2001). The primary physiological function of creatine is to buffer energy concentrations in tissues with significant and fluctuating energy demands, especially in muscles and the brain (Wyss and Schultze, 2002). Interest in creatine has centered primarily on its use as an ergogenic aid to enhance sports performance (Benzi, 2000). Nevertheless, it is becoming increasingly evident that endogenous creatine plays a critical role in a range of cognitive functions, including learning, memory, attention, speech and language, and possibly emotion.
Impaired brain energy metabolism and alterations in neuronal plasticity are among the leading hypotheses in the pathogenesis of psychiatric disorders (Kondo et al., 2011a; Martin et al., 2009; Stork and Renshaw, 2005; Wood et al., 2009; Yildiz-Yesiloglu and Ankerst, 2006; Young et al., 2009). Logic would suggest that interventions like creatine that modulate energetic, oxidative and neurotrophic parameters would improve therapeutic efficacy in psychiatric patients. With this end in mind, it is important to know whether psychiatric disorders show reliable alterations in creatine metabolism, and if so, what the location and directionality of these changes are with respect to each disorder. Much of the recent evidence on changes in brain creatine metabolism in humans has been provided by studies using magnetic resonance spectroscopy (MRS), a neuroimaging tool that enables scientists to noninvasively measure major metabolites like creatine and phosphocreatine in various brain regions in vivo (for review, see Kondo et al., 2011a; Maddock and Buonocore, 2012).
Understanding the relationships between the creatine-phosphocreatine circuit, stress, and psychiatric disorders may inspire novel hypotheses for the biological bases of these disorders or provide insight for mechanisms of drug action for more rapid, effective treatment. Accordingly, the purpose of this article is to review studies linking endogenous creatine with psychopathology, to weigh available evidence for the use of dietary creatine to treat psychiatric symptoms, and to discuss plausible mechanisms of action relevant to these disorders. Specifically, this paper will (1) provide a comprehensive description of the neurobiology of creatine, (2) summarize reports on the bioavailability, safety, and tolerability of creatine supplementation in humans and animals, and (3) compare and contrast changes in creatine metabolism observed in schizophrenia, mood and anxiety disorders. The discussion will focus on whether there is value in dietary creatine for treating psychiatric disorders and clinical implications will be addressed, including sex differences in creatine metabolism and response to supplementation and the effects of psychotropic medications on creatine metabolism.
2. Neurobiology of creatine
2.1. Creatine synthesis, transport, and loss
Creatine is a constituent of a normal diet of protein-based foods, such as milk, meat, and nuts. It is not considered an essential nutrient because the kidneys, liver, pancreas, and possibly brain cells are able to synthesize this compound endogenously from the amino acids arginine, glycine, and methionine (Andres et al., 2008; Béard and Braissant 2010; Wyss and Kaddurah-Daouk, 2000). It is estimated that approximately half of an individual’s daily requirement comes from alimentary creatine, while the remainder is replenished by the body (Brosnan and Brosnan, 2007).
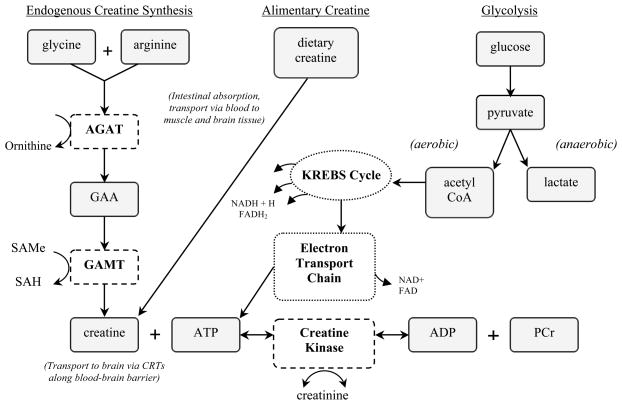
The synthesis of creatine is a simple, albeit metabolically demanding, two-step process that follows an inter-organ pathway (Figure 1). Production of creatine begins in the kidney with L-arginine:glycine amidinotransferase (AGAT), an enzyme which catalyzes the conversion of arginine and glycine to form guanidinoacetate and ornithine. Evidence indicates that guanidinoacetate is then released from the kidney and taken up by the liver. In the liver, the second enzyme in this process, glycine N-methyltransferase (GAMT), recruits S-adenosylmethionine (SAMe) to methylate guanidinoacetate to form creatine and S-adenylhomocysteine (SAH). Creatine synthesis is estimated to consume between 40% to 70% of available methyl groups provided by SAMe, a considerable demand upon amino acid metabolism (Brosnan et al., 2007; Mudd and Poole, 1975; Stead et al., 2001; Wyss and Kaddurah-Daouk, 2000; Wyss and Walliman, 1994).
Figure 1.
De novo synthesis of creatine and relation to ATP. The machinery needed to produce endogenous creatine (dashed boxes) and ATP (dotted boxes) is expressed within neurons, oligodendrocytes, and astrocytes, but it is unknown to what extent this arrangement contributes to total brain creatine content. ATP synthesis from carbohydrate (glucose) occurs via three series of metabolic pathways: glycolysis, citric acid cycle (Krebs), and the electron transport chain. When ATP is rapidly depleted, creatine kinase catalyzes the donation of a phosphate group from phosphocreatine (PCr) to ADP, producing more ATP to buffer energy needs. Conversely, when energy is released, an individual phosphate group is cleaved from ATP and bound to creatine to rejoin the PCr pool. This reversible reaction causes a spontaneous byproduct (creatinine) that is excreted from the body, which is why creatine must be replenished daily (AGAT = arginine:glycine amidino transferase; CRT, creatine transporter; GAMT, guanidinoacetate methyltransferace; GAA, guanidinoacetate; SAMe, s-adenylmethionine; SAH, s-adenosylhomocysteine).
Endogenous synthesis of creatine possibly occurs in the brain as AGAT and GAMT are expressed in most cell types, particularly neurons, oligodendrocytes, and astrocytes (Braissant et al., 2005). However, contrary findings raise uncertainty about whether central synthesis of creatine is possible, and if it is, whether it contributes significantly to total brain creatine content (Mak et al., 2009). For instance, it was found that only ~12% of cortical cells co-expressed AGAT and GAMT, while more than 30% of cells did not express either enzyme in rat brain (Braissant and Henry, 2008). In addition, individuals born with a mutation in the SLC6A8 creatine transporter gene, which inhibits the uptake of creatine into brain cells, display significantly reduced creatine levels and severe metabolic and cognitive impairments despite normal functioning of AGAT and GAMT. One explanation is that brain synthesis of creatine follows an inter-cell pathway. It may be that AGAT-containing cells synthesize and release guanidinoacetate, which then would be taken up by GAMT-containing cells to be converted to creatine, but more research is required to clarify this hypothesis. It is generally agreed that the majority of creatine synthesis occurs peripherally in the kidney (via AGAT), liver and pancreas (via GAMT).
Following biosynthesis or ingestion, creatine is transferred from blood plasma by specific sodium and chloride-dependent creatine transporters (CRT) located in skeletal muscle, kidney, heart, brain and liver (Snow and Murphy, 2001). Importantly, creatine enters the brain via these specialized CRTs at the blood-brain barrier, which underscores the potential benefits of oral creatine supplementation to treat brain-related disorders (Nash et al., 1994; Ohtsuki et al., 2002). Daily supplementation with creatine monohydrate reliably produces measurable increases of creatine and phosphocreatine in the brain in humans and animals after several weeks (Dechent et al., 1999; Ferrante et al., 2000; Ipsiroglu et al., 2001; Lyoo et al., 2003; Persky et al., 2003a, b; Stöckler et al., 1996). However, entry of creatine into the brain does not occur as rapidly as uptake into muscle tissue because astrocytic feet, which lack CRTs, limit the surface area of microcapillaries and partially obstruct central nervous system (CNS) access (Andres et al., 2008; Ohtsuki et al., 2002).
Once creatine passes the blood brain barrier, it is taken up from extracellular fluid by neurons and oligodendrocytes by CRTs, where it serves its primary role as an energy shuttle and regulator of energy homeostasis. Recent work mapping the regional and cellular locations of CRTs found the highest level of expression in neurons within the olfactory bulb, hippocampus (granulate cells of dentate gyrus), cerebral cortex (pyramidal neurons), cerebellum (Purkinje cells), brain stem (motor and sensory cranial nerves), and the spinal cord (dorsal and ventral horns), whereas the lowest levels of CRT expression was found in the basal ganglia and white matter (Mak et al., 2009). It is important to note that many of the brain regions that express CRTs are compromised in conditions like Alzheimer’s Disease (Bürklen et al., 2006), Huntington’s Disease (Ryu et al., 2005), and psychiatric disorders (Andres et al., 2008), to name a few. Loss of CRT-containing tissue may further contribute to cognitive or emotional deficits observed in numerous brain-related disorders.
Constant replenishment of creatine from either the diet or biosynthesis is necessary. Once inside a cell, creatine is phosphorylated for energy storage, a process to be described in more detail below. This reversible conversion results in a spontaneous non-enzymatic byproduct called creatinine, which is excreted from the body by the kidneys. High energy demands required by numerous cellular processes also deplete stores of phosphorylated creatine. The rate of depletion of creatine resources is estimated at approximately 1.7% of the total pool per day (Benzi, 2000; Walliman et al., 1992). Since the majority of creatine is found in skeletal muscle, the rate of reduction varies across gender and age (Brosnan and Brosnan, 2007).
2.2. Function of the creatine/creatine kinase/phosphocreatine network
Creatine is most commonly recognized as an ergogenic aid used by athletes, body builders, and military personnel to enhance muscle mass and physical performance during brief intervals of extremely intense activities, which rapidly deplete muscle energy stores, such as bench press lifting, sprinting, or swimming (Benzi, 2000). As an example, imagine the burst of energy required by a sprinter taking off from a starting block at the beginning of a track meet. The runner’s muscles launch the body forward by hydrolyzing significant amounts of adenosine triphosphate (ATP) molecules, the energy currency within cells. The actual concentrations of ATP within muscle cells normally do not fluctuate markedly because pools of stored creatine (phosphocreatine) immediately and constantly replenish energy as it is being used. Of significance, this reaction also occurs continually within brain cells to buffer ATP for many types of energy-requiring brain functions, especially Na+ transport, Ca2+ transport, the processing of neurotransmitters (e.g., synthesis, uptake, release), intracellular signaling, and axonal and dendritic transport (for review, see Ames, 2000).
The majority of ATP synthesis occurs during aerobic cellular respiration, beginning with glycolysis (i.e., catabolism of carbohydrates) and ending with the coupling of adenosine diphosphate (ADP) + phosphate group via oxidative phosphorylation (Figure 1). However, these complex, multi-step metabolic pathways require time and energy. Bearing this in mind, the creatine-creatine kinase-phosphocreatine circuit may be thought of as a bioenergetic thermostat that quickly replenishes ATP in tissue to maintain stable levels when there are sudden and significant energy demands (Wyss and Schulze, 2002). Creatine kinase isoenzymes catalyze the phosphorylation of creatine to form pools of phosphocreatine, which serve as stored energy reserves (Figure 1). When energy is needed, creatine kinase catalyzes the transfer of the phosphate group from phosphocreatine to ADP to form ATP. Thus, phosphocreatine is the rate-limiting step in the rapid resynthesis of ATP when energy demand increases. Mitochondrial dysfunction may arise when phosphocreatine is repeatedly depleted because it is no longer available to replenish ATP. Instead, the cell must shift back to the less efficient glycolysis pathway to meet energy needs, which may set the stage for a cascade of events that lead to brain-related pathology (Stork and Renshaw, 2005).
So-called “hot spots” of creatine kinase expression in the brain are found in the granule layer of the cerebellum, granule and pyramidal cells of the hippocampus, and epithelial cells of the choroid plexus (Kaldis et al., 1996). The majority of tissues express a combination of two types of creatine kinase within a single cell out of a total of four that exist: muscle-type, sarcomeric mitochondrial-type, brain-type, and ubiquitous mitochondrial-type (Kaldis et al., 1996, Walliman et al., 1992). In the brain, the ubiquitious mitochondrial-type, which dephosphorylates mitochondrial generated ATP, is typically expressed together with the brain-type, located in the cytosol and dephosphorylates glycolytically generated ATP, to produce large pools of phosphocreatine (Andres et al., 2008). The phosphorylation of creatine (phosphocreatine), by way of the dephosphorylation of ATP, is a reversible reaction so that the creatine kinases can replenish ATP when cellular energy needs increase.
The creatine-phosphocreatine system is described as a spatial energy buffer because it acts as an energy transport system that carries high-energy phosphates from mitochondrial production sites to energy utilization sites. In addition, it is a temporal energy buffer because it maintains energy homeostasis by buffering ADP and ATP ratios (Brosnan and Brosnan, 2007). The following example summarizes the buffering activities of the creatine circuit in neuronal tissue. After ATP is produced by ATP synthase on the mitochondrial inner membrane, it is released to the intermembrane space in exchange for ADP through the adenine nucleotide transporter (reviewed in Andres et al., 2008; Brosnan et al., 2007). If energy is not needed, the ubiquitous mitochondrial-type creatine kinase, found within the intermembrane space of the mitochondria, transfers the phosphoryl group from ATP to creatine, which then leaves the mitochondria to join the pool of phosphocreatine in the cytosol. When energy demand increases, such as during signal transduction, brain-type creatine kinase transfers a phosphoryl group from phosphocreatine to ADP to replenish ATP concentrations to avoid energy imbalance.
3. Creatine supplementation
3.1. Bioavailability, dosage, and administration
While the physiologic importance of creatine has been studied extensively, much less is understood about the pharmacokinetics of dietary creatine. The absolute bioavailability of supraphysiological doses of creatine is unknown. McCall and Persky (2007) outline four reasons why bioavailability may be less than 100%. First, creatine can be degraded in an acidic environment like the stomach. However, creatine spends little time in the stomach so it is assumed little creatine is lost this way. Second, creatine and its metabolite, creatinine, may be degraded by intestinal bacteria and lost in feces. Third, creatine absorption is a time-consuming process, and it is possible that excess creatine is pushed by intestinal flow beyond areas of the intestinal tract where creatine can be transported across the intestinal wall by CRTs. Fourth, decreased bioavailability may occur because creatine will not be absorbed unless if it is fully dissolved.
It is hypothesized that lower doses of creatine, defined as less than 5 g in humans, will have larger bioavailability than higher doses, defined as more than 10 g in humans, because saturation of target sites with creatine may result in the downregulation of CRT function or number (McCall and Persky, 2007; Persky, 2003; Perskey et al., 2003a, b). In addition, larger doses slow the absorption of creatine, which otherwise occurs very rapidly, usually less than 2 hours (McCall and Persky, 2007). Furthermore, tissues with lower presupplementation concentrations of creatine will show greater accumulation of creatine after supplementation (Ipsiroglu et al., 2001). In humans and in animals, research has consistently shown that administration of creatine over a prolonged period of time results in measurable increases of creatine concentrations in brain and muscle tissue, which is most pronounced after 4 weeks (Dechent et al., 1999; Ferrante et al., 2000; Ipsiroglu et al., 2001; Persky et al., 2003a, b; Stöckler et al., 1996). For instance, in humans, Dechent et al. (1999) reported significant increases in total brain creatine, particularly in the thalamus, cerebellum, white matter, and gray matter, in healthy young adults following administration of creatine monohydrate (4 × 5 g per day) for four weeks. Persky et al. (2003b) showed that repeated dosing with creatine (e.g., 5 g creatine four times/day over six days) resulted in less clearance than volunteers given a single 5 g dose of creatine, likely owing to saturation of creatine pools in muscle tissue. In animals, Ipsiroglu et al. (2001) found that 4 weeks of oral creatine supplementation produced the most pronounced increases in creatine concentrations in the muscle, brain, kidney and lungs of guinea pigs, mice and rats (and there were no systematic differences in organ distribution of creatine across species). In contrast, single or repeated administration of creatine (160 mg/kg, i.p.) to rats within a period of one to two days produced negligible increases in brain creatine (1% from baseline), underscoring the necessity of daily supplementation in the diet over several days to achieve optimal blood-brain barrier penetration and significant accumulation in the brain (Perasso et al., 2003). In summary, creatine supplementation increases brain concentrations of creatine and phosphocreatine in humans given approximately 0.13 to 0.80 g/kg/day for 14 days (Bianchi et al., 2000, Dechent et al., 1999, Lyoo et al., 2003) and animals fed 2.2 to 2.6 g/kg/day for 10 days (Allen et al., unpublished results; Michaelis et al., 1999, Wick et al., 1999).
In patient populations, the schedule of creatine administration varies from clinical study to clinical study because guidelines have not been definitively established. However, to increase brain creatine levels in human populations, it has been recommended that investigators implement a loading phase (ranging from 15 to 20 g/d creatine for approximately 3–7 days) to ensure tissue saturation, followed by a maintenance phase of ~5–10 grams for at least 2–3 months (McCall and Persky, 2007). Nevertheless, few studies have evaluated where or how much brain creatine concentrations increase on the basis of dose or schedule of administration, though estimates are around 10% (Lyoo et al., 2003; McCall and Persky, 2007).
In addition to the dosage amount, another important point when considering creatine as a therapeutic intervention is the method in which it is administered. While creatine is rapidly absorbed, different dosage forms (e.g. solutions, powders, capsules) can influence the bioavailability of creatine. Harris et al. (2002) reported that 2 g of creatine resulted in different peak concentration-time profiles depending on the form it was administered, whereby the solution form of creatine reached maximal concentration before the suspension and lozenge forms, and all of these forms peaked before meat (e.g., solution > suspension = lozenge > meat). Therefore, solid forms like meat increase plasma creatine but require thorough digestion and dissolution, which reduces the rate at which this source of creatine is absorbed and made available to the body.
3.2. Reported side effects
Extensive monographs that describe side effects associated with creatine supplementation are available, such as Natural Standard (2012) and Health Canada (2011). Creatine supplementation is generally considered safe and well-tolerated by humans and animals (Rodriguez et al., 2009). For instance, no significant adverse effects have been reported in studies of patients with inborn error of creatine synthesis given up to 0.8 g/kg daily for two years (reviewed in Braissant and Henry, 2008). Likewise, no major health issues were reported in a study of 9 healthy athletes administered 1–20 g/day from 1–4 times/day for up to five years (Poortsman and Francaux, 1999). Nonetheless, empirical and anecdotal accounts do exist that describe mild to moderate side effects of daily creatine supplementation. The majority of these reported side effects are of weight gain, gastrointestinal distress, altered insulin production, inhibition of endogenous creatine synthesis, renal dysfunction, or dehydration in study participants. Fewer have reported disturbances in mood and anxiety. Experts generally agree that there is sufficient evidence to be confident that 5 g/day of creatine is generally harmless to healthy adults, but there is not enough evidence to make an informed recommendation in favor or against doses higher than 5 g/day (Shao et al., 2006).
3.2.1. Gastrointestinal Distress
Sufficient evidence has been documented and reviewed to support gastrointestinal distress as a consequence of daily creatine supplementation. Mild to moderate gastrointestinal issues have been reported in a number of experimental and clinical trials, including diarrhea, nausea, and/or vomiting (Astorino et al., 2005; Chrusch et al., 2001; Cox et al., 2002; Groeneveld et al., 2003; Juhn et al., 1999; Tarnopolsky, 2000). In addition, abdominal discomfort, increased or decreased appetite, weight gain, stomach distress, and loose stool have been reported (Juhn et al., 1999; Kendall et al., 2005; Tarnopolsky, 2000; Volek et al., 2000).
3.2.2. Renal Dysfunction
While some case studies have suggested that creatine causes renal dysfunction, most empirical studies in humans and animals indicate that it is more probable that creatine worsens pre-existing renal disease (Kuehl et al., 1998; Koshy et al., 1999; Edmunds et al., 2001; Pritchard and Kalra, 1998; Sheth et al., 2006; Thornsteindottir et al., 2006). In other words, it is unlikely that healthy adults without a history of renal disease will develop kidney problems as a consequence of creatine supplementation unless there are other exacerbating factors involved, such as use of illicit anabolic-androgenic steroids, non-steroidal anti-inflammatory agents, nephrotoxic or renally-cleared medications, or diuretics (Natural Standard, 2012). It has also been argued that creatine supplementation can confound renal analyses because serum creatinine is the most widely used marker of renal function (Gualano et al., 2008). Creatine supplementation increases levels of creatinine, which can be falsely interpreted as an indication of renal dysfunction because most laboratories factor in serum creatinine levels when estimating glomerular filtration rate. The frequency with which physicians who are not kidney specialists misdiagnose patients that use creatine and protein supplements with renal disease is high enough that the British Medical Journal published a “Lesson of the Week” to highlight this issue (Willis et al., 2010). While research is ongoing, the majority of studies conclude that creatine supplementation is generally not harmful to the kidneys when used as directed (Dalbo et al., 2008).
3.2.3. Dehydration
The media often perpetuates anecdotal reports of creatine-induced dehydration, however few empirical studies using control groups and blinding have documented dehydration as a side effect of creatine supplementation (Juhn et al., 1999, Poortsman and Francaux, 1999). To err on the side of caution, physicians typically advise drinking extra water and avoiding caffeine when taking creatine supplements (Vahedi et al., 2000).
3.2.4. Body weight and water retention
Increased body weight has been reported in humans following creatine supplementation. This is most probably due to the fact that creatine is an osmotically active substance that increases water retention inside cells (for review, see Bemben and Lamont, 2005; Sobolewski et al., 2011). This water weight is lost following discontinuation of creatine supplements.
3.2.5. Mood and anxiety
Negative changes in mood or anxiety following supplementation with creatine have been documented in two human trials (Roitman et al., 2007; Volek et al., 2000) and one animal experiment (Allen et al., 2010). Specifically, in an open-label clinical trial of creatine, Roitman et al. (2007) reported that two patients diagnosed with bipolar disorder exhibited hypomania or mania following daily supplementation with 3–5 g creatine. In a clinical trial examining the effectiveness of creatine to enhance heavy resistance training, Volek et al. (2000) noted that two subjects reported feeling more aggressive and nervous after 1 week of creatine supplementation (25 g/day). In rodents, Allen et al. (2010) observed increased depression-like behavior in male rats supplemented with 4% creatine for five weeks, although this effect was not replicated in male rats in a follow-up study (Allen et al., in press). Taken together, there remains the possibility that creatine can increase risk of mania or depression in susceptible individuals. It is also possible that long-term high dosing of creatine alters creatine transporter function or creatine kinase activity in a manner that adversely affects emotional regulation. Further research is required before definitive conclusions are drawn, but caution is warranted in at-risk individuals.
4. Linking creatine with cognition and emotion
4.1. Non-psychiatric populations
4.1.1. Healthy adults
The majority of research on creatine has focused on the ability of creatine monohydrate to improve muscle strength and physical endurance in healthy adult athletes. Systematic reviews of studies assessing the ergogenic effects of creatine have found consistent beneficial effects in sporting activities that involve repeated, short bouts of intense exercise, including football and soccer (for review, see Bemben and Lamont, 2005). The favorable effects of creatine supplementation on muscle energy metabolism and function increase the plausibility of creatine having a positive effect on brain energy metabolism, cognitive processes and/or mood states. This is particularly interesting because metabolic dysfunction is hypothesized to be a contributing factor for thought and mood disturbances. Accordingly, evidence for the association of creatine with cognition and mood in healthy and creatine deficient populations are considered in this section to facilitate understanding of how creatine metabolism differs in psychiatric populations.
Virtually no human or animal studies have directly examined the association of brain creatine metabolism to mood or cognition in healthy adults. However, converging evidence is bringing to light the importance of endogenous creatine for normal brain development and cognitive function. The expression of the majority of creatine kinase iosenzymes in the hippocampus and frontal cortex provides one clue that creatine metabolism participates in higher mental functioning (Kaldis et al., 1996). Another clue is the unanticipated finding that, in healthy volunteers, low phosphocreatine levels were associated with better performance on a specific frontal lobe task (Wisconsin Card Sorting Task)(Volz et al., 1998b). This evidence suggests that individuals that more efficiently replenish ATP, which relies upon the creatine-phosphocreatine circuit, have a cognitive advantage in tasks that heavily tax the frontal cortex (Volz et al., 1998b).
On the other hand, there has been significant research interest in the potential for creatine supplementation to enhance cognitive performance and brain function in healthy adults. In human intervention studies, most placebo-controlled, double-blind studies reported positive findings. In healthy volunteers, creatine supplementation reduced mental fatigue following a stressful time-pressured serial calculation test (Watanabe et al., 2002). Additionally, creatine improved working memory and intelligence scores in vegetarians and vegans, who are more likely to have diminished phosphocreatine reserves due to limited meat consumption (Rae et al., 2003). In non-vegetarians undergoing significant sleep deprivation paired with mild exercise (> 24 hours), creatine improved mood and reduced fatigue and performance decline on a choice reaction task (McMorris et al., 2006). In a follow-up study, creatine supplementation enhanced performance on central executive and working memory tasks after 36-hours of sleep deprivation (McMorris et al., 2007a). In older adults (~76 years of age), creatine buffered age-related cognitive decline, with improvement in verbal and spatial short-term memory and long-term memory after one week of daily supplementation (McMorris et al., 2007b). Most recently, adults supplemented with creatine exhibited better short-term memory and trended towards better abstract reasoning than placebo controls (Hammett et al., 2010). Post-supplementation fMRI Blood oxygen dependent responses were reduced compared to baseline, which indicates reduced metabolic demand, changes in vascular response, increased oxygen uptake, or direct neuromodulatory effects (e.g., modulating glutamatergic or GABAergic receptors).
The positive effects of creatine on cognitive behavior may be mediated in situations of stress, which significantly reduce available phosphocreatine reserves. For instance, creatine did not improve the performance of rested non-vegetarians on a battery of neurocognitive tests (Rawson et al., 2008). As mentioned, studies of vegetarian and vegan subjects may have shown greater benefit in cognitive processing post-supplementation because they presumably have lower brain creatine levels due to dietary restrictions (e.g., Rae et al., 2003). Indeed, tissues with lower pre-supplementation concentrations of creatine will show greater accumulation of creatine after supplementation (Ipsiroglu et al., 2001; Pan and Takahashi, 2006). Moreover, many other studies that have shown creatine-induced differences in cognitive function involved conditions that reduced brain creatine, such as significant sleep deprivation, mild exercise, and stressful mathematical tasks (McMorris et al., 2006; Watanabe et al., 2002).
In rodent models of cognition, female mice supplemented with 1% creatine performed better on the object recognition test, lived 9% longer, exhibited fewer markers of ageing (lipofuscin) and less oxidative stress in the hippocampus than unsupplemented mice, suggesting improved memory capabilities in these mice (Bender et al., 2008). In male rats, it was observed that intra-hippocampal administration of creatine (2.5 and 7.5 nmol) improved spatial learning, specifically by reducing escape time and the mean number of errors on subsequent trials of the Barnes Maze test (Oliveira et al., 2008). Interestingly, these researchers found that blocking the polyamine-binding site at the NMDA receptor by co-administration of arcaine (0.02 nmol/hippocampus) prevented this creatine-induced spatial learning enhancement, while co-administration of the polyamine binding site agonist spermidine (0.02 nmol/hippocampus) intensified this effect. Complementing these findings, creatine applied to hippocampal brain slices in vitro enhanced synaptic plasticity, but this effect was not observed in the presence of AP5, an NMDA antagonist (Royes et al., 2008). Taken together, creatine may exert positive cognitive effects by modifying the activity of the NMDA receptor (Brosnan and Brosnan, 2007).
4.1.2. Creatine deficiency syndromes
Severe cognitive deficits are observed in patients diagnosed with an inborn error of creatine synthesis or an X-linked creatine transporter defect (reviewed in Braissant and Henry, 2008). Briefly, defects in the AGAT or GAMT biosynthesis genes render the body unable to synthesize creatine endogenously, whereas the presence of a mutation in the SLC6A8 gene causes creatine transporter dysfunction that prevents the uptake of creatine into muscle and brain cells. Individuals with these types of inborn errors exhibit significant brain atrophy, mitochondrial abnormalities, severe developmental delays, speech and language impairments, autism, and epilepsy (Andres et al., 2008; Braissant and Henry, 2008; Schulze et al., 2003).
Cognitive and behavioral improvement is observed in creatine deficiency patients with AGAT and GAMT deficiencies after long-term daily oral treatment with high doses of creatine monohydrate (with 0.30 – 0.80 g/kg ), however supplementation is ineffective in treating SLC6A8 transporter defects (Battini et al., 2002, Bianchi et al., 2007; Bizzi et al., 2002; Mercimek-Mahmutoglu et al., 2006; Stöckler et al., 1996; Salomons et al., 2001). In addition, creatine supplementation was safe and effective in treating a 4-month-old infant diagnosed with AGAT deficiency (Battini et al., 2006). Early intervention with creatine monohydrate may reduce or prevent deficits that occur early in life. This is important because de novo synthesis and uptake of creatine is critical to normal brain development in humans. Expression of creatine kinases have been found in the hindbrain, midbrain, and fore brain of fetuses at 8-weeks after conception (Andres et al., 2008; Braissant et al., 2005).
In animal models of creatine deficiency, transgenic mice lacking mitochondrial- and brain-type creatine kinases exhibit smaller hippocampi, larger pyramidal mossy-fiber fields, decreased creatine content, and undetectable levels of phosphocreatine relative to values observed in wild-type animals (in ‘t Zandt et al., 2004; Streijger et al., 2005). Behaviorally, these animals show severe impairments in spatial learning, less nest-building activity, less pre-pulse inhibition, and poor acoustic and startle reflex compared to wild-type controls (Jost et al., 2002; Streijger et al., 2004; Streijger et al., 2005). Mice deficient of only one creatine kinase isoenzyme (single knock-out) exhibit less severe physical and behavioral phenotypes, with intact sensory and motor systems, most likely because the intact isoenzyme compensates for the absence of the other (Streijger et al., 2005).
4.2. Psychiatric populations
4.2.1. Psychological Stress
Psychiatric illness is characterized by diverse neural abnormalities, running the gamut from neuroendocrine and neurotransmission systems to neuroanatomical and neurotrophic factors, and these processes have significant energy requirements. Psychological stress is associated with impairments in energy metabolism, which increase the susceptibility of neurons to the negative effects of reactive oxygen species (oxidative stress), including lipid peroxidation, protein carboxylation, DNA damage and apoptosis (Seifried et al., 2007). In view of this evidence, chronic psychological stress is considered a precipitating factor in the onset of psychiatric disorders (Caspi et al., 2003; Duman and Monteggia, 2006; Pittenger and Duman; 2008, Sapolsky, 2000). For instance, in mice, chronic mild stress damaged mitochondrial structure and function in the hippocampus and prefrontal cortex and increased depression-like behavior (Gong et al., 2011). Given that creatine metabolism depends on mitochondrial function, it is hypothesized that stress causes changes in creatine, phosphocreatine, or creatine kinase in brain areas linked with mental illness. If this is the case, stress-related psychiatric disorders may benefit from creatine supplementation or other agents that stimulate creatine kinase to reverse the deleterious effects of stress on mitochondrial dysfunction. Moreover, creatine supplementation may be beneficial in safeguarding the brain because it prevents oxidative damage from the formation of reactive oxygen species through direct antioxidant activity (Sestili et al., 2006; Young et al., 2010).
The effects of psychological stress on brain creatine metabolism have not been directly studied in humans, but stress-induced impairments in brain metabolite concentrations have been investigated in animal models of stress. Using MRS neuroimaging and histological techniques, researchers have found that subordinate animals exposed repeatedly to experiences of psychosocial defeat by dominant animals exhibit significantly less total creatine (the sum of creatine + phosphocreatine), reduced hippocampal volume, and impaired neurogenesis (Czéh et al., 2001; Fuchs et al., 2002; van der Hart et al., 2003). Moreover, these studies showed that the effects of stress on total creatine concentrations and neuroplasticity were reversed after treatment with tianeptine (Stablon) and clomipramine (Anafranil), and a novel substance P antagonist (L-760,735). Another study using MRS imaging ex vivo found that rats subjected to single prolonged stress showed reduced creatine concentrations in the medial prefrontal cortex compared to non-stress controls (Knox et al., 2010). On the basis of these findings, it is difficult to know whether the effects of stress on creatine occur upstream or downstream of mitochondria, but the creatine-phosphocreatine circuit may be an important mediator or target of drug action.
One known study in animals has directly assessed the effects of creatine supplementation on stress-induced impairments. Young chickens that had creatine (2 μg) administered directly into their brains prior to social separation displayed significantly fewer stress responses, including fewer vocalizations, less spontaneous activity, and reduced plasma corticosterone concentrations (Koga et al., 2005). These effects were blocked by concomitant administration of picrotoxin, a GABAA antagonist, suggesting that creatine exerts protective effects against stress, at least in part, by modifying GABAA receptor activity.
4.2.2. Schizophrenia
Schizophrenia is a behaviorally and biochemically heterogeneous disorder characterized by psychotic episodes, which are periods of time in which the individual experiences significant disturbances in thought and/or loses contact with reality. Symptoms manifestations include delusions, hallucinations, disorganized thought or speech, catatonic behavior, and/or negative symptoms (e.g., flattened affect, alogia, or avolition). The lifetime prevalence of schizophrenia is approximately 0.5–1% of the population (Bhugra, 2005; McGrath et al., 2008).
Impairments in metabolic function, neuronal density and cellular integrity in the frontal and temporal lobes, basal ganglia, and hippocampus have been observed in schizophrenic patients (Bertolino et al., 1998; Fukuzako et al., 1995; Fujimoto et al., 1996; Yurgelun-Todd et al., 1996). Decreased metabolic activity diminishes the brain’s ability to efficiently generate or process neural signals, which may account for the positive or negative symptoms of schizophrenia described above. Consistent with this evidence of impaired metabolic processes, abnormalities in the creatine-phosphocreatine pathways have also been observed within emotional and executive brain regions of schizophrenic patients. Additionally, some studies have found an association between creatine metabolism and cognitive function in schizophrenia, generating much interest in the role creatine plays in the pathogenesis of the disorder.
One of the earliest and most robust findings in clinical reports of schizophrenia is the incidence of psychosis-associated creatine kinase-emia (PACK), which refers to a marked spike in serum creatine kinase levels at the onset of acute psychotic episodes (Coffey et al., 1970; Faulstich et al., 1984; Gosling et al., 1972; Hermesh et al., 2002; Meltzer 1968, 1973, 1976; Meltzer et al., 1969, 1970; Martin et al., 1972; Schweid et al., 1972). Studies have reported that approximately half of acutely psychotic schizophrenic patients exhibit muscle-type PACK, though interestingly normal creatine kinase activity is observed in patients experiencing chronic episodes of psychosis (Hermesh et al., 2002; Melkersson et al., 2006). Moreover, sex differences have been observed in PACK, with men showing significantly greater increases in muscle-type creatine kinase activity than women, and this pattern is more likely to reoccur in men than women (Hermesh et al., 2002; Manor et al., 1998).
In recent years, advancements in neuroimaging technology have better enabled scientists to research creatine metabolism in the brain of schizophrenic patients (Table 1a,e). Reports have described alterations specific to schizophrenia, but the evidence is inconsistent and sometimes contradictory. In studies that specifically measured phosphocreatine, elevated levels have been observed in the dorsolateral prefrontal cortex (DLPFC)(Volz et al.,1998b). Additionally, levels of phosphocreatine were also found to be asymmetrical in the temporal lobes in schizophrenia, with the left side displaying lower levels than the right, but there were no overall differences compared with controls (Deicken et al., 1995). In contrast, less phosphocreatine has been found in the frontal region of schizophrenics and first-degree relatives of schizophrenics (Deicken et al., 1994; Klemm et al., 2001; Volz et al., 2000). Other studies did not observe differences in phosphocreatine in the DLPFC (Volz et al., 1998a) or the temporal lobes (Calabrese et al., 1992; Fujimoto et al., 1992). Reduced total creatine has been reported in the DLPFC (Ohrmann et al., 2007), anterior cingulate cortex (ACC)(Öngür et al., 2009; Tayoshi et al., 2009), the left thalamus (Yoo et al., 2009), parietal-occipital cortex (Öngür et al., 2009), and white matter (Auer et al., 2001). Conversely, increased total creatine concentrations have been reported in the left frontal-parietal region (Fujimoto et al., 1992), ACC (Jensen et al., 2004; O’Neill et al., 2003), hippocampus (Lutkenhoff et al., 2010), left temporal lobe (Fukuzako et al.,1999), medial temporal lobe (Wood et al., 2008), and parietal cortex (Bluml et al., 1999). Numerous other neuroimaging studies have not detected any differences in creatine metabolism in these areas when comparing schizophrenic patients to healthy controls (Table 1a,e).
Table 1.
Magnetic resonance spectroscopy studies of brain creatine metabolism in psychiatric disorders
| Reference | Subject comparison | ROI | Alteration in total creatine | Medication status and clarifying comments |
|---|---|---|---|---|
| (a) Schizophrenia | ||||
| Lutkenhoff et al. (2010) | 14 SCZ twin pairs (2 MZ, 12 DZ), 13 HC twin pairs (4 MZ, 9 DZ) | medial PFC GM, left PFC WM, left HPC | (1) HPC: SCZ > HC; (2) HPC: SCZ twin > unaffected co-twin | After exclusions, 12 co-twins, 9 probands, 21 HC analyzed. Psychotropic use unclear, but medication use by SCZ mentioned in discussion |
| Keshavan et al. (2009) | 40 HR-SCZ, 48 HC (adolescents) | WM, ACC, caudate, thalamus, POC | Caudate: HR-SCZ < HC | All subjects psychotropic medication naïve |
| Tayoshi et al. (2009) | 30 SCZ, 25 HC | ACC, left basal ganglia | (1) ACC: male SCZ < male HC; (2) Both regions: female SCZ = female HC; (3) left basal ganglia: males < females | Eleven patients received benzodiazepines and three patients received paroxetine |
| Yoo et al. (2009) | 22 HR-SCZ, 22 HC | ACC, DLPFC, thalamus | Left thalamus: HR-SCZ < HC | All participants were free of psychotropic drugs |
| Wood et al. (2008) | 34 SCZ (15 med- naïve), 19 HC | medial temporal lobes | medicated SCZ > HC = medication-naïve SCZ | Fifteen of the patients were antipsychotic-naïve |
| Ohrmann et al. (2007) | 15 first-episode SCZ, 20 chronic SCZ, 20 HC | DLPFC | chronic SCZ < first-episode SCZ < HC | First-episode SCZ patients were treatment naïve. All chronic SCZ patients were taking psychotropic medications |
| Jensen et al. (2004) | 15 SCZ, 15 HC | thalamus, cerebellum, HPC, ACC, PCC, PFC, POC | ACC: SCZ > HC | All but 5 were free of psychotropic medication |
| O’Neill et al. (2003) | 11 SCZ, 20 HC (children/adolescents) | ACC, frontal cortex, striatum, thalamus, parietal cortex, WM | ACC: SCZ > HC | Ongoing medication use in all but two treatment naïve patients |
| Sigmundsson et al. (2002) | 25 SCZ, 26 HC | DLPFC | SCZ = HC | All patients were receiving psychotropic medications at the time of the study |
| Klemm et al. (2001) | 14 HR-SCZ, 14 HC (children/adolescents) | frontal region | [PCr only] HR-SCZ < HC (trend, p = .05) | All subjects psychotropic medication naïve |
| Auer et al. (2001) | 32 SCZ, 17 HC | GM, WM, thalamus | (1) WM: SCZ < HC; (2) WM tCr correlated positively with BPRS scores | Ongoing psychotropic medications in all patients |
| Ende et al. (2000) | 13 SCZ, 15 HC | thalamus, basal ganglia, HPC | All regions: SCZ = HC | Ongoing psychotropic medications in all patients |
| Volz et al. (2000) | 11 SCZ, 11 HC | frontal lobes | [PCr only] SCZ < HC | All patients were medication free at time of study |
| Bluml et al. (1999) | 13 SCZ, 15 HC | parietal cortex | SCZ > HC | Ongoing medication in all but two patients |
| Fukuzako et al. (1999) | 17 SCZ, 17 HC | temporal lobes | SCZ > HC on left side | All patients medication naïve |
| Volz et al. (1998a) | 26 SCZ, 23 HC | frontal lobes | [PCr only] (1) SCZ = HC; (2) PCr correlates with frontal lobe task in controls only | Ongoing medication in all patients |
| Volz et al. (1998b) | 50 SCZ, 36 HC | DLPFC | (PCr) SCZ > HC | Ongoing medication in all patients |
| Volz et al. (1997) | 26 medicated SCZ, 10 drug-free SCZ, 36 HC | frontal lobes | [PCr only] medicated SCZ > drug-free = HC | Ongoing medication in all but ten patients |
| Kato et al. (1995) | 27 SCZ (14 high SANS, 13 low SANS), 26 HC | frontal lobes | [PCr only] left frontal: SCZ with high negative symptoms > SCZ low negative symptoms = HC | 10 patients were medication free (3 drug naïve), and ongoing medication in remainder |
| Deicken et al. (1995) | 18 SCZ, 14 HC | temporal lobes | [PCr only] temporal: SCZ asymmetry, right > left | Ongoing medication in all but 5 patients |
| Deicken et al. (1994) | 20 SCZ, 16 HC | frontal lobes, parietal lobes | [PCr only] frontal: SCZ < HC | Ongoing medications in all but 6 patients |
| Calabrese et al. (1992) | 11 SCZ, 9 HC | temporal lobes | [PCr only] (1) SCZ = HC; (2) PCr/ATP asymmetry: right lobe > left lobe | Two patients were medication free for 1 week prior to the study, ongoing treatment in 9 remaining patients |
| Fujimoto et al. (1992) | 16 SCZ, 20 HC | temporal lobes, frontoparietal region | [PCr only] left frontoparietal: SCZ < HC | Ongoing medication in all patients |
| Pettegrew et al. (1991) | 11 SCZ, 10 HC | DLPFC | SCZ = HC | All patients drug-naïve |
| (b) Major Depressive Disorder | ||||
| Merkl et al. (2011) | 25 MDD, 27 HC | DLPFC, ACC | All regions: MDD = HC | Ongoing psychotropic medication in all patients |
| Nery et al. (2009) | 37 MDD, 40 HC | DLPFC | Male MDD < Male HC; Female MDD > Female HC | All medication free for at least 2 weeks prior to scan |
| Ventkatramar et al. (2009) | 14 MDD, 12 HC (elderly) | medial PFC | MDD < HC | All but 1 patient on psychotropic meds at time of scan |
| Ende et al. (2007) | 11 MDD, 10 HC | HPC, putamen | All regions: MDD = HC | Ongoing psychotropic medication in all patients |
| Gabbay et al. (2007) | 14 MDD, 10 HC (adolescents) | Left/right caudate, putamen, thalamus | Left caudate: MDD > HC | 6 medication free (4 treatment naïve), 8 on psychotropic medications at time of scan |
| Caetano et al. (2005) | 14 MDD, 22 HC (all children/adolescents) | left DLPFC | MDD = HC | 8 medication free (6 treatment naïve), 6 on psychotropic meds |
| Hasler et al. (2005) | 16 remitted MDD, 15 HC | PFC (dorsal- medial/dorsal- anterolateral) | MDD = HC | All medication free for at least 3 months prior to study |
| Brambilla et al. (2004) | 19 MDD, 19 HC | left DLPFC | MDD = HC | All patients were free of psychotropic medications. |
| Mirza et al. (2004) | 14 MDD, 13 HC (children/adolescents) | ACC, occipital cortex | ACC: MDD < HC | All patients were psychotropic medication- naïve. |
| Sanacora et al. (2004) | 33 MDD, 38 HC | occipital cortex | MDD = HC | All medication free for at least 2 weeks prior |
| Gruber et al. (2003) | 17 MDD, 17 HC | frontal lobe | MDD > HC | All medication free for at least 4 weeks prior |
| Michael et al. (2003a) | 12 MDD, 12 HC | left DLPFC | (1) MDD = HC; (2) more severe depression negatively correlated with lower tCr | All medication free for at least 3–8 d |
| Michael et al. (2003b) | 28 MDD, 28 HC | left amygdalar region | MDD = HC | All medication free for at least 3–8 d |
| Pfleiderer et al. (2003) | 17 MDD, 17 HC | left ACC | MDD = HC | All but 1 patient off psychotropic medications for at least ~5 d |
| Kumar et al. (2002) | 20 MDD, 18 HC (elderly) | left DLPFC, ACC | All regions: MDD = HC | All medication free for at least 2 weeks, except Lorazepam |
| Farchione et al. (2002) | 11 MDD, 11 HC (all children/adolescents) | DLPFC | MDD = HC | All patients treatment-naïve |
| Auer et al. (2000) | 19 MDD, 18 HC | ACC, parietal WM | All regions: MDD = HC | 7 patients medication free, remainder on antidepressant therapy |
| Ende et al. (2000) | 17 MDD, 24 HC, 6 remitted MDD | hippocampus | MDD = remitted MDD = HC | Washout period unclear, but medications free at least 8 d prior to ECT treatment |
| Rosenberg et al. (2000) | 13 MDD, 13 HC (children/adolescents) | left caudate, occipital cortex | All regions: MDD = HC | All patients medication-naïve |
| Volz et al. (1998) | 14 MDD, 8 HC | frontal lobe | MDD = HC | All but 3 patients on psychotropic medications |
| (c) Bipolar Disorder | ||||
| Caetano et al. (2011) | 43 BP, 38 HC (children/adolescents) | mPFC, DLPFC, ACC, occipital lobes | Left mPFC, DLPFC: BP < HC | 12 patients medication free, remainder receiving treatment |
| Patel et al. (2008) | 28 BP, 10 HC (children/adolescents) | ACC, VLPFC | (1) VLPFC: BP > HC; (2) ACC: BP > HC (trend, p = .07) | All patients were medication free for ~ 18 d |
| Frey et al. (2007a) | 32 BP, 32 HC | DLPFC | Left side: BP < HC | All patients medication free for at least 2 weeks (6 weeks for fluoxetine) |
| Frey et al. (2007b) | 35 BP (24 val/val, 11 val/met), 40 HC | DLPFC (left) | (1) BP = HC; (2) val/met BP < val/val BP; (3) val/val BP > HC | Twenty-two patients received ongoing treatment, and 13 were unmedicated for at least 2 weeks |
| Frye et al. (2007) | 23 BP, 12 HC | ACC/mPFC | BP > HC | All subjects free of psychotropic medication except 5 patients using lithium |
| Frey et al. (2005) | 10 BP, 10 HC | DLPFC | BP = HC | Ongoing psychotropic medication in all patients |
| Sassi et al. (2005) | 14 BP, 18 HC | DLPFC (left) | BP < HC (trend, p = .08) | Ongoing psychotropic medication in all but two patients |
| Dager et al. (2004) | 32 BP, 26 HC | cingulate gyrus | (1) BP = HC; (2) inverse correlation with white matter tCr and HAMD scores | All patients medication free |
| Hamakawa et al. (1999) | 23 BP, 20 HC | frontal lobes | Depressive state BP < HC | Ongoing psychotropic medication in all patients. All patients scanned in euthymic stage, and 8 scanned again during depressive stage |
| Murashita et al. (2000) | 19 BP (9 responders, 10 resistant), 25 HC | occipital region | [PCr only] BP resistant < BP responder = HC (post-photic stimulation) | Ongoing psychotropic medications in all but 1 patient |
| Deicken et al. (1995) | 12 BP, 14 HC | temporal lobes | [PCr only] BP = HC | All patients medication free for at least 1 week |
| Kato et al. (1994) | 29 BP (15 BP-II, 14 BP-I), 59 HC | frontal lobes | [PCr only] BP II < BP I = HC | Ongoing medication in all patients, which changed on basis of psychiatric states (hypomanic, depressed, euthymic) |
| (d) Anxiety Disorders | ||||
| Coplan et al. (2006) | 15 GAD, 15 HC | centrum semiovale (cerebral WM) | GAD < HC | Six patients were medication-naïve, and all were off medication for at least 4 weeks |
| Yucel et al. (2008) | 20 OCD, 26 HC | ACC | OCD = HC | 12 patients on psychotropic medications |
| Massana et al. (2002) | 11 PD, 11 HC | medial temporal lobe, medial PFC | Medial temporal: PD < HC | All patients free of medications for at least 2 weeks |
| Shiori et al. (1996) | 18 PD, 18 HC | frontal lobes | [PCr only] asymmetry in PD, left lobe > right lobe | All patients on psychotropic medications |
| (e) Comparisons between disorders | ||||
| Öngür et al. (2010) | 15 BP, 15 SCZ, 20 HC | ACC, POC | ACC: BP < SCZ = HC | Differences reflect shorter metabolite T2 relation times. Ongoing medication in all but 1 BP patents and 1 SCZ patient |
| Öngür et al. (2009) | 15 BP, 15 SCZ, 22 HC | ACC, POC | All regions: BP = HC > SCZ | Ongoing medication in all patients |
| Mirza et al. (2006) | 18 MDD, 27 OCD, 18 HC (children/adolescents) | medial thalamus | OCD > MDD = HC | All patients medication free |
| Cecil et al. (2003) | 7 BP, 2 MDD, 10 HC (children/adolescents) | PFC, cerebellum | All regions: MDD/BP < HC (trend, p = .07) | All patients medication free except 1 |
| Hamakawa et al. (1998) | 22 MDD, 18 BP (11 depressive, 16 euthymic), 20 HC | basal ganglia | (1) MDD = BP = HC; (2) medicated BP (n = 7) > unmedicated BP (n = 11) | Ongoing psychotropic medication in majority of patients |
| Kato et al. (1992) | 12 MDD, 10 BP, 22 HC | frontal lobe | [PCr only] (1) BP = HC; (2) severe MDD < mild MDD | Ongoing psychotropic medication in all patients |
All comparisons refer to baseline spectroscopic analyses (pre-treatment, if applicable) of total creatine (creatine + phosphocreatine) except where noted. Greater-than (>) and less-than (<) signs represent statistically significant differences in creatine metabolism (p < .05) excluding if a trend is described. Correlations between total creatine, phosphocreatine and behavior were included only if these associations reached significance (p < .05). Studies reporting ratio calculations, as opposed to absolute metabolite values, were excluded due to limitations in interpreting the directionality of metabolite changes.
Abbreviations: [Metabolites] tCr, total creatine; PCr, phosphocreatine; [Diagnoses] HC, healthy controls; HR, high risk; MDD, major depression; BP, bipolar disorder; GAD, generalized anxiety disorder; OCD, obsessive-compulsive disorder; PTSD, post-traumatic stress disorder; PD, panic disorder; SCZ, schizophrenia; MZ, monozygotic; DZ, dizygotic; [Scales] BPRS, Brief Psychotic Rating Scale; [Brain regions] ACC, anterior cingulate cortex; DLPFC, dorsal-lateral prefrontal cortex; HPC, hippocampus; PCC, posterior cingulate cortex; POC, parietal-occipital cortex; VLPFC, ventro-lateral prefrontal cortex
Behavioral measures correlated with creatine metabolism in certain studies. For instance, total thalamic creatine and frontal phosphocreatine were associated with psychiatric symptoms, measured using the Brief Psychotic Rating Scale (Auer et al., 2000; Deicken et al., 1994). In particular, low frontal phosphocreatine was observed in schizophrenics with high hostility-suspiciousness scores and high anxiety-depression scores (Deicken et al., 1994). Moreover, schizophrenics with high negative symptom scores exhibited greater phosphocreatine levels in the frontal lobes than schizophrenics with few negative symptoms and healthy controls (Kato et al.,1995). Additionally, asymmetry of phosphocreatine/ATP in the temporal lobes was also associated with severity of psychiatric symptoms (Calabrese et al., 1992), and reduced left temporal phosphocreatine and greater phosphocreatine asymmetry were associated with more severe thinking disturbances (Deicken et al., 1995). As a whole, reduced brain phosphocreatine levels correspond with earlier findings of hyper creatine kinase activity (PACK).
One intervention study in humans has been carried out using a randomized, double-blind cross-over design to examine the potential for creatine to buffer abnormal energy metabolism in schizophrenia (Kapstan et al., 2007). Creatine supplementation was not superior to placebo in treating symptoms. The heterogeneity of schizophrenia, both in neuropsychological symptoms and treatment effects, may contribute to variability in this small study (Stroup, 2007; Sautter et al., 1995). Additionally, the severity or duration of illness or difficulties in measuring symptom improvement may generate a false negative result. On the other hand, perhaps creatine supplementation would show more benefit in first-episode, treatment-naïve patients. Moreover, as significant neurobiological evidence supports the plausibility that schizophrenia is a disorder of neurodevelopment (Weinberger, 1996), creatine may prove more essential during a critical point in cortical development. As mentioned previously, creatine plays a significant role in embryonic development, as well as in the survival and differentiation of GABA-ergic and dopaminergic neurons (Andres et al., 2005; Ducray et al., 2007a, b).
4.2.3. Mood disorders
4.2.3.1. Major depressive disorders
Major depressive disorders are characterized by at least one major depressive episode, or a period of at least two weeks during which an individual experiences a depressed mood or loss of pleasure in activities once enjoyed. To receive a clinical diagnosis of major depression, this episode must be accompanied by four or more additional depressive symptoms, such as difficulty concentrating, changes in sleep, appetite or weight, decreased energy, feelings of worthlessness or guilt, or suicidal ideation, plans, or attempts. Currently, an estimated 5–6% of the U.S. population suffers from major depression annually, whereas 13–16% of the population is at risk of experiencing depression over the course of a lifetime (Hasin et al., 2005; Kessler et al., 2003).
The role of dysfunctional creatine metabolism in the neurochemical underpinnings of depression was first considered over three decades ago (Agren and Niklasson, 1984, 1988). In the first of these studies, a significantly negative correlation between levels of creatinine in cerebral spinal fluid and self-reported suicidal ideation was identified in depressed patients (Agren and Niklasson, 1984). Moreover, a positive relationship between CSF dopamine and serotonin metabolites (HVA and 5-HIAA) and levels of creatine and creatinine was subsequently discovered in depressed patients (Agren and Niklasson, 1988). Altogether, these data hinted at the fact that functional neurotransmission depends on intracellular energy metabolism supported by the creatine-phosphocreatine system.
Imaging studies have found conflicting alterations in absolute concentrations of phosphocreatine or total creatine for patients diagnosed with depression (Table 1b,e). One study reported increased total creatine in the left caudate of depressed patients (Gabbay et al., 2007). Two studies found decreased total creatine in the ACC (Mirza et al., 2004) and medial PFC (Venkatraman et al., 2009), though the latter study was exclusively an elderly population. One other finding reported an intriguing sex by diagnosis interaction for total creatine in the DLPFC, where depressed men exhibited less total creatine than healthy men and depressed women exhibited more total creatine than healthy women (Nery et al., 2009). This sex-dependent outcome will be considered in the clinical implications section below.
Assessments of symptom severity in serum and neuroimaging studies support a link between brain creatine metabolism and depression. Serum creatine kinase activity (Segal et al., 2007), levels of white matter creatine (Dager et al., 2004), left DLPFC total creatine (Michael et al., 2003), and frontal lobe phosphocreatine (Kato et al., 1992) are correlated with the severity of depression. Reduced creatine metabolism is associated with a less favorable course and outcome of depressive illness.
Increasing evidence supports the use of creatine monohydrate for preventing or treating depression. Data from preclinical animal studies indicate that daily supplementation with 4% creatine for five weeks produces antidepressant-like effects in female rats tested in the forced swim test, a behavioral assay that is sensitive to pharmacological agents known to alter depressive symptoms in humans (Allen et al., 2010). Moreover, in a follow up study, Allen et al. (in press) observed that sub-acute treatment with low-dose fluoxetine (5 mg/kg, or a total of 15 mg/kg) modestly augmented the antidepressant-like behavioral effects of dietary creatine in female rats. In humans, two open-label, add-on intervention studies have reported improved mood in adult patients with treatment-resistant depression following daily supplementation with 3–5 g creatine for four weeks (Amital et al., 2006a, Roitman et al., 2007). In adolescents, another preliminary open-label, add-on study reported that 4 g of creatine administered daily for 8 weeks improved depressive symptoms and increased brain phosphocreatine concentrations in treatment-resistant females concurrently prescribed the SSRI fluoxetine (Kondo et al., 2011b). Complementing these reports are MRS imaging data showing that high baseline levels of phosphocreatine predicted treatment response in depressed adults after four weeks of daily medication with a selective serotonin reuptake inhibitor (SSRI) and triiodothyronine, a thyroid hormone known to increase brain energy metabolism (Iosifescu et al., 2008). The promising results of these initial studies, in tandem with plausible neurobiological hypotheses to be discussed in later sections, have motivated four clinical trials that are currently underway to evaluate the potential for creatine supplementation to improve clinical relief in patients who have previously failed to respond to adequate trials of antidepressant drugs (NCT00729755, NCT01175616, NCT00313417, NCT00851006).
4.2.3.2. Bipolar disorder
Bipolar disorder is a severe and disabling mental illness characterized by at least one manic episode, or a period of time in which the individual experiences extreme euphoria, confidence, or recklessness. Frequently individuals also experience at least one major depressive episode, which results in recurring shifts in mood states from extreme highs (mania, hypomania) to extreme lows (dysthymia, depression). Lifetime prevalence of bipolar disorder is estimated at about 4% of the U.S. population (Kessler et al., 2005).
There has been significant interest in the role of the creatine-phosphocreatine circuit in the pathogenesis of bipolar disorder, especially as evidence of mitochondrial dysfunction in bipolar patients continues to grow (Kato, 2008; Young et al., 2007). Postmortem analyses have revealed significant downregulation of brain-type creatine kinase and ubiquitous mitochondrial creatine kinase mRNA levels in the DLPFC and hippocampus of bipolar patients compared with schizophrenic and healthy controls (MacDonald et al., 2006). Moreover, important insight into the relationship between mood state and creatine metabolism has been gained through the evaluation of alternating periods of depression and mania. A number of reports have described elevated serum creatine kinase activity levels during the manic state compared to the euthymic and depressive states, which may indicate that changes in creatine kinase activity correspond with mood states or cognitive speed in bipolar patients (Chung et al., 2009; Danivas et al., 2010; Feier et al., 2010; Segal et al., 2007).
Neuroimaging studies frequently observe reduced levels of PCr or total creatine in areas within the frontal lobe in bipolar patients (Table 1c,e)(Kato et al., 1994; Hamakawa et al., 1999), particularly total creatine within the PFC (Cecil et al., 2003), left medial PFC (Caetano et al., 2011), and DLPFC (Caetano et al., 2011; Frey et al., 2007a; Sassi et al., 2005). One exception is a study reporting greater total creatine concentrations in the VLPFC (Patel et al., 2008). Findings in the ACC are mixed, with reports of increased total creatine in ACC/mPFC (Frye et al., 2007), a trend towards greater total creatine in the ACC (Patel et al., 2008), and reductions in total creatine in the ACC (Öngür et al., 2010). Reduced total creatine has also been reported in the cerebellum (Cecil et al., 2003). Lastly, treatment resistant bipolar patients exhibited lower total creatine in the occipital cortex after photic stimulation compared to treatment responders and healthy controls (Murashita et al., 2000). It is important to note that, for each of these regions of interest, there also exists neuroimaging research on creatine metabolism that did not find differences between bipolar patients and healthy controls (see Table 1c,e).
There were no associations reported between cognitive or symptom assessments and creatine in bipolar disorder. Moreover, essentially no studies have directly examined creatine supplementation in bipolar patients, with the exception of Roitman et al. (2007). It was reported that creatine supplementation might have precipitated manic episodes in two bipolar subjects approximately three weeks post-creatine treatment. Although there are no double-blind, placebo-controlled clinical trials evaluating creatine supplementation for the treatment of bipolar disorder, other purported metabolic enhancers are increasingly being studied in this patient population, including acetyl-L-carnitine, alpha-lipoic acid, Coenzyme Q10, N-acetyl cysteine, and uridine (for review, see Sanches et al., 2010).
4.3. Anxiety disorders
Anxiety disorders are the most common class of mental illness, affecting approximately 18% of the U.S population over the course of a lifetime (Kessler et al., 2005). This category broadly refers to a constellation of chronic and distressing conditions that share the primary feature of excessive and uncontrollable anxiousness. For instance, generalized anxiety disorder, which has a lifetime prevalence of 3% in the U.S. (Kessler et al., 2005), is characterized by uncontrollable worrying nearly every day for at least six months, and causes the individual to feel restless, fatigued, distracted, or irritable.
Identifying differences in creatine metabolism may help distinguish among the different anxiety disorders or facilitate treatment (Table 1d,e). In the case of general anxiety disorder, investigators detected reduced levels of total creatine in cerebral white matter of patients (Coplan et al., 2006). This effect was only observed in patients without history of early trauma. Additionally, there was no relationship between total creatine and self-reported worry or intelligence. In a study of panic disorder, patients that were actively experiencing panic attacks exhibited reduced levels of total creatine in the right amygdalohippocampal region (Massana et al., 2002). Another study of panic disorder detected asymmetry of phosphocreatine levels in patients only, with the right frontal lobe having higher concentrations than the left (Shiori et al., 1996). In patients diagnosed with post-traumatic stress disorder (PTSD), research has shown reductions in total creatine in right and left hippocampal regions compared to control subjects (Schuff et al., 2001; Villareal et al., 2002).
Preliminary intervention studies provide support for the therapeutic value of creatine for treating PTSD, but its use in other anxiety disorders has not yet been evaluated. An initial case study reported creatine had value for treating a 52 year-old woman suffering from PTSD, depression, and fibromyalgia (Amital et al., 2006b). This patient had abnormally low muscle levels of phosphocreatine and ATP and was unresponsive to psychotropic medications prior to creatine supplementation. Following daily supplementation this patient reported improvements on measures of depression and fibromyalgia and showed a 30% improvement in her overall quality of life. An open-label, add-on trial found that creatine improved symptoms in men and women with treatment resistant PTSD, with the greatest benefit in patients diagnosed with comorbid depression (Amital et al., 2006a).
5. Clinical implications
There is considerable evidence to support creatine as a biological correlate of psychopathology. Examinations of creatine metabolites in vivo reliably detected differences in schizophrenia and bipolar disorder, particularly in the frontal and limbic regions, but the direction of these differences were less consistent. The majority of spectroscopy data for depression did not detect differences in creatine metabolism. Nonetheless, significant reductions in frontal and white matter creatine and decreased serum creatine kinase activity were reported for severe cases of depression, suggesting altered creatine metabolism is related to the course of the disorder. Too few studies are available to draw conclusions for anxiety disorders. Summarized below are important clinical considerations for future preclinical and clinical research designs, including a discussion on how creatine fits into existing pharmacological mechanisms and sex differences in creatine metabolism and treatment response. Finally, methodological challenges inherent in current research will be discussed.
5.1. Potential therapeutic mechanisms of creatine action
Alterations in the creatine circuit observed in psychiatric populations are presumed to be a compensatory mechanism initiated by neurons to combat general metabolic deficits. Mitochondrial dysfunction in mental illness has been hypothesized to reflect a shift from the rapid creatine circuit towards the less efficient, time-consuming glycolysis pathway to keep up with energy requirements (Stork and Renshaw, 2005). In the simplest terms, significant energy demands reduce ATP availability (hypometabolism), thereby stimulating creatine kinase activity to use remaining phosphocreatine stores to maintain energy homeostasis (Figure 1). Constant exhaustion of phosphocreatine may contribute to impairments in cognitive function or altered mood because the brain has to rely on the catabolism of glucose, which is slower and less effective at replenishing ATP than phosphocreatine. Mentally ill individuals may benefit from creatine monohydrate because daily long-term supplementation increases brain levels of phosphocreatine (see Section 2.1.).
Some of the human trials reviewed above are supportive of this premise. Chronic administration of creatine monohydrate improves symptoms of depression and PTSD in humans and female rats (Allen et al., 2010; Amital et al., 2008; Roitman et al., 2007). However, there was no benefit of creatine for treating schizophrenia, and some evidence suggested that creatine is contraindicated for bipolar disorder, specifically by triggering manic switch in two patients post-supplementation (Roitman et al., 2007). Lastly, three studies have found increased symptoms of depression or anxiety in men and male rats (Section 3.2.5). These negative reports are unsettling because there also exist two MRS studies that demonstrated creatine monohydrate, administered daily to healthy young adults (between 20–30 years old), reduced metabolite levels associated with neuronal integrity, function, and energy homeostasis, namely N-acetylcholine (NAA), choline and ATP (Dechent et al., 1999, Lyoo et al., 2003; for review of metabolites, see Kondo et al., 2011a). In particular, Dechent et al. (1999) found reduced brain levels of NAA in the thalamus and cerebellum and decreased choline in the thalamus only following administration of creatine monohydrate (4 × 5 g per day) for four weeks. Lyoo et al. (2003) observed reduced β-NTP, a measure that mainly reflects ATP signaling, following administration of creatine monohydrate (0.03 g/kg – 0.3g/kg) for two weeks. Lyoo et al. hypothesized that the brain needs to make compensatory changes, expressly by reducing ATP synthesis, to maintain energy balance in response to significant increases in creatine and phosphocreatine. Taken together, it is plausible that creatine supplementation benefits metabolically impaired brains (e.g. psychiatric patients) with exhausted phosphocreatine stores but can be harmful when over-saturating otherwise healthy brains by shifting metabolite concentrations to preserve equilibrium. Nevertheless, these contradictory biochemical and behavioral outcomes in the creatine literature highlight the need to advance current knowledge of the neurobiological mechanisms of creatine’s actions to determine its level of efficacy and safety.
Our current understanding of the potential therapeutic mechanisms of creatine action has largely been facilitated by promising research on the use of dietary creatine to treat numerous neurodegenerative disorders linked with mitochondrial dysfunction and brain atrophy, including Huntington’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis (for review, see Allen et al., 2011; Andres et al., 2008; Gualano et al., 2010; Wyss and Schulze, 2002). Dietary creatine confers neuroprotection against a range of toxic substances and can minimize physical damage from traumatic insults in rodent models of disease (Brustovetsky et al., 2001; Matthews et al., 1998; 1999; Sullivan et al., 2000; Roy et al., 2002). The proposed mechanisms underlying these effects include the ability of creatine to increase energy availability, to promote neuronal proliferation and survival, and to reduce oxidative stress, apoptosis and necrosis of neurons (Andres et al., 2005; Brdiczka et al., 1998; Dolder et al., 2003; Ducray et al., 2007a, b; Lawler et al., 2001; Sestilli et al., 2006, 2011).
The antioxidant effects of creatine are one plausible neurobiological mechanism for the treatment of psychiatric disease. Metabolic disturbances in the frontal and limbic regions have been described for schizophrenia, mood and anxiety disorders (see Section 4.2.). Briefly, a decline in ATP concentrations, which is typically observed in psychiatric illness, leads to an accumulation of intracellular calcium (Ca2+), the formation of radical and reactive oxygen species, and ultimately mitochondrial damage from oxidative stress. Creatine supplementation prevents oxidative damage through direct antioxidant activity in mammalian cell cultures (Lawler et al., 2002; Sestili et al., 2006; Young et al., 2010).
Creatine also has a neuromodulatory function in the CNS, and may influence neurotransmitter systems or neuroplasticity factors associated with mental illness. Evidence indicates that creatine can be released in an excitotic, action-potential dependent manner in response to membrane depolarization (Almeida et al., 2006). Specifically, researchers have demonstrated that depolarization of rat brain tissue produced an influx of Ca2+ and then subsequent release of creatine. When Ca2+ was not present or when Na+ channels were blocked by tetrodotoxin, creatine was not released. This is usually typical of neurotransmitter release. This and other work indicates that creatine acts as a partial agonist of central GABAA receptors (Almeida et al., 2006; Koga et al., 2005), and creatine has also been shown to modify NMDA receptor activity (Oliveira et al., 2008; Royes et al., 2008). Other evidence has suggested creatine is related to serotonin and dopamine (Agren and Niklasson, 1988; Allen et al., 2010; Andres et al., 2005). Nevertheless, it is clear that significantly more research is needed to understand how creatine interacts with neurotransmitter systems and where in the brain these changes occur.
Another possible mechanism of therapeutic action for treating psychiatric disorders involves the relationship between creatine and one-carbon metabolism. As mentioned previously (Figure 1), de novo creatine synthesis is metabolically demanding because it requires up to 70% of available SAMe, the universal methyl donor (Brosnan et al., 2007; Mudd and Poole, 1975; Stead et al., 2001; Wyss and Walliman, 1994). Consequently, less SAMe is available for other essential reactions, like the methylation of neurotransmitters that are important for mood regulation and cognition, including dopamine and serotonin. Creatine supplementation may exert antidepressant effects because increased dietary intake reduces the need for de novo synthesis of creatine and increases available SAMe (Stead et al., 2001). Further, creatine supplementation lowers homocysteine concentrations (Deminice et al., 2008; Korzun, 2004; Lobley et al., 1996; Stead et al., 2001), and elevated homocysteine levels are increasingly being associated with psychopathology (Almeida et al., 2008; Bjelland et al., 2003; Muntjewerff et al., 2006; Osher et al., 2004).
5.2. Use of creatine markers in psychiatric disorders
An important question is whether alterations in creatine metabolism are a function of the course of psychiatric illness or due to predisposing genetic factors. In the research described, abnormal creatine metabolism in the frontal and limbic regions in schizophrenia, mood and anxiety disorders is correlated with a less favorable course and outcome of illness. However, this evidence is insufficient to parse out the contributions of mood states, treatment, and neurodevelopment to changes in the creatine circuit. Does creatine metabolism influence mood states, or do mood states influence creatine metabolism? Perhaps there is a third variable that mediates this relationship. Another important question, if these changes in creatine are reliable, is whether creatine values can predict the severity of disease or treatment success.
Research is now beginning to clarify whether altered creatine metabolism represents trait or state markers of mental illness. For instance, healthy adolescents at high risk of developing schizophrenia, defined as having at least one first-degree schizophrenic relative, exhibited significantly less total creatine in the caudate than healthy low-risk controls (Keshavan et al., 2009) and trended towards less phosphocreatine in the frontal region (Klemm et al., 2001), which supports trait-dependency. On the other hand, in a study of twin pairs discordant for schizophrenia, affected twins exhibited greater total hippocampal creatine than unaffected co-twins, which suggests creatine is a disease-state marker for schizophrenia (Lutkenhoff et al., 2010).
Other intriguing genetic findings have associated alterations in brain creatine metabolism with a functional polymorphism of the brain-derived neurotrophic factor (BDNF) gene, which substitutes a valine for a methionine at the codon 66 (val66met)(for review, see Dincheva et al., 2012). Briefly, BDNF is an essential mediator of synaptic plasticity that is linked with energy metabolism. Met-carriers (val/met) exhibit cognitive and neuronal abnormalities, have increased risk of psychopathology, and tend to show poorer clinical outcome (Forlenza et al., 2010, Hashimoto et al., 2010; Vinberg et al., 2009; Spalletta et al., 2010). With regard to creatine metabolism, it was found that bipolar met-carriers exhibited less total creatine in the left DLPFC than val/val bipolar patients, and val/val patients had greater total creatine than val/val healthy controls (Frey et al., 2007b). This BDNF polymorphism has also been associated with reduced hippocampal creatine metabolism in healthy met-carriers (Gallinat et al., 2010).
Though studies are beginning to indicate that alterations in creatine metabolism are genetically driven, it is debatable whether components of this system can be used as meaningful diagnostic markers. As discussed above, multiple disorders exhibit similar deficits in creatine metabolism in the frontal and limbic regions, meaning this marker likely represents a broader metabolic dilemma. In addition, altered serum creatine kinase activity is not diagnosis-specific, but rather it appears that marked changes are symptom-specific (e.g., mood state, excitation, psychosis, or agitation). For instance, significant elevations in creatine kinase activity have been observed during acute psychosis in both schizophrenia and bipolar disorder, when patients reported experiencing grandiose ideas, auditory hallucinations, disorientation, or aggressive behavior (see Sections 4.2.2. Schizophrenia and 4.2.3.2 Bipolar Disorder; Danivas et al., 2010). It is probable that increased psychomotor activity that is associated with acute psychosis, and perhaps antipsychotic drug treatment, influence creatine metabolism. Further complicating matters, marked elevations in creatine kinase have not been consistently observed in cases of acute psychosis (Tuason et al., 1974), and have also been described in nonpsychotic depressed patients compared to psychotic depressed patients (Segal et al., 2007) and in non-psychotic chronic alcoholics (Ikeda et al., 1977).
Nevertheless, creatine, phosphocreatine, and creatine kinase abnormalities do commonly occur in psychiatric disorders, and a less favorable outcome is associated with greater impairments in creatine metabolism (state marker). These changes likely represent general metabolic deficits that are similar across psychiatric disorders but are not sensitive or specific enough for diagnostic purposes (Segal et al., 2007). An important avenue for consideration is the potential for creatine metabolism to serve as a treatment marker. Few studies have investigated this possibility, but creatine metabolism may be an important therapeutic target for pharmacological agents known to improve psychiatric symptoms (Iosifescu et al., 2003).
5.3. Creatine metabolism and psychoactive drugs
Antidepressants may exert their effects through modulation of energy metabolism. Recent investigations have found that different classes of psychoactive drugs affect different components of the creatine-phosphocreatine system in various ways. A novel hypothesis currently under investigation is whether dietary creatine supplementation can enhance the effects of psychoactive drugs or reduce associated side effects (see Section 4.2.3.1. Major Depressive Disorders; Allen et al., in press; Kondo et al., 2011b).
In the case of SSRIs, it has been shown in rats that four weeks of paroxetine (Paxil) increased creatine transport to the hippocampus and prefrontal cortex (Lugenbiel et al., 2010), and fluoxetine (Prozac) and escitalopram (Lexapro) both altered creatine kinase activity in hippocampus, striatum, and prefrontal cortex, albeit in different directions (Agostinho et al., 2009; Santos et al., 2009). In the case of mixed or dual uptake inhibitors, four weeks of either clomipramine (Anafranil) or tianeptine (Stablon) have been shown to normalize total creatine in the forebrain of stressed tree shrews (Czéh et al., 2001; Fuchs et al., 2002; van der Hart et al., 2002), four weeks of desipramine (Norpramine) increased total creatine in the DLPFC of male C57BL/6 mice (Kim et al., 2010), and acute imipramine (Tofranil) increased creatine kinase activity in the striatum, cerebellum, and PFC of rats (Assis et al., 2009; Réus et al., 2011, 2012). For atypical antipsychotics, acute treatment with aripiprazole (Abilify) or olanzapine (Zyprexa) increased creatine kinase activity in the striatum and cerebellum (Assis et al., 2009). Moreover, combined antipsychotic and SSRI treatment using Prozac and Zyprexa has been shown to decrease creatine kinase activity in the cerebellum, striatum, and PFC after four weeks of treatment in rats (Agostinho et al., 2009). The mood stabilizers lithium (Eskalith) and valproate (Depakote) decreased total creatine in the whole brain of rats after two weeks of treatment (O’Donnell et al., 2000), and the anesthetic ketamine (Ketalar) increased creatine kinase the striatum, cerebellum, and PFC (Assis et al., 2009). Varying protocols of electroconvulsive shock have been shown to increase creatine transport (Lugenbiel et al., 2010), increase total creatine in the hippocampus (Sartorious et al., 2003), and alter creatine kinase in the prefrontal cortex, hippocampus, cerebellum, and pons/medulla (Búrigo et al., 2006; Erakovic et al., 2001). In sum, psychoactive drugs alter creatine, phosphocreatine, or creatine kinase activity, but it is also evident that the direction and brain regions affected vary on the basis of drug class, dosage, and administration schedule.
5.4. Sex differences in creatine metabolism and treatment response
Another clinically important development comes from a growing number of reports finding that the relationship between creatine and mood is sex-dependent (Allen et al., 2010, Hamakawa et al., 1999; Kato et al., 1992, 1994; Nery et al., 2009; Moore et al., 1997; Volz et al., 1998). Generally, sex differences have been observed in creatine transport into the brain, total creatine concentrations, and patterns of creatine kinase expression and activity levels (Gledhill et al., 1988; Hamakawa et al., 1999; Ramirez and Jimenez, 2002; Riehemann et al., 1999; Wong et al., 1983). For instance, it has been reported that healthy women have less phosphocreatine in the frontal lobe compared to healthy men (Riehemann et al., 1999). Moreover, female bipolar and depressed patients have lower creatine levels in the right frontal lobe compared with male patients (Hamakawa et al., 1999). Given this evidence, gender differences in creatine metabolism may confer differences in therapeutic efficacy of creatine supplementation.
Preclinical studies are increasingly substantiating this premise. Long-term creatine supplementation showed significant benefit in female rats, but not male rats, in an animal model of antidepressant efficacy (Allen et al., 2010), and this effect is most robust when levels of ovarian hormones are highest (Allen et al., in press). Further, studies of electroconvulsive shock therapy (ECS) in rats show sex-dependent differences in creatine metabolism in brain regions associated with depression, expressly increasing creatine kinase activity in females (Erakovic et al., 2001) but decreased creatine kinase activity in males (Búrigo et al., 2006).
Equally fascinating are results of a recent spectroscopy study, which revealed a gender by diagnosis interaction of total creatine within the DLPFC. Specifically, total creatine levels in depressed men were lower than those in healthy men whereas creatine in depressed women were higher than those in healthy women (Nery et al., 2009). These observations are especially important because depression is twice as common in women than men (Bebbington et al., 2003; Kessler et al., 2003), and studies have reported that women experience longer depressive episodes, report greater distress, are more severely impaired, are more likely to attempt suicide, and relapse more frequently than men (Kornstein et al., 2000; Marcus et al., 2005; Nolen-Hoeksema, 1993; Young et al., 2009).
The neural mechanisms underlying sex-specific creatine alterations are unknown, however it is likely that organizational and/or activational effects of sex hormones are implicated (Allen et al., 2010). All together, these studies provide the impetus for more basic and translational research on sex differences in creatine metabolism. Advancements in this area could possibly lead to sex-specific therapeutic strategies in the treatment of brain-related disorders.
5.5. Methodological challenges
Several unavoidable difficulties inherent in psychiatric research have limited the interpretation of brain creatine metabolism and treatment effects in humans. Generally speaking, the most significant issues observed in the research evaluated in this review include the recruitment of small sample sizes, use of a single creatine dosage, diagnostic validity, diagnostic comorbidity, concomitant medication use, lack of placebo control, and add-on intervention designs. Additionally, because of the novelty of this topic, there are a limited number of published reports and many have not yet been replicated.
One important issue relates to the recruitment of adequate sample sizes within specific patient populations. Psychiatric patients are burdened by significant emotional, social, or occupational impairments that make it difficult for these individuals to complete studies or adhere to medications. This is particularly true of neuroimaging work, given the anxiogenic nature of brain scanning procedures. For instance, it has been difficult for investigators to compare differences in creatine metabolism in patients suffering from anxiety disorders. As a result, few imaging studies on creatine and anxiety are available for evaluation in the present review. Of the available evidence for schizophrenia and mood disorders, many studies have included patient populations with multiple diagnoses of psychopathology (e.g., Roitman et al., 2007).
Another issue is the ethical dilemma associated with discontinuing psychotropic medications in at-risk psychiatric patients. However, inconsistencies in the directionality of creatine metabolite changes may be a function of medication status. Concomitant medication use was most often reported in studies of schizophrenia. Medicated schizophrenics with neuroleptics have displayed greater phosphocreatine levels than unmedicated patients and healthy controls in the frontal lobes (Jayakumar et al., 2010; Volz et al., 1997; Volz et al., 2000). Another report found that benzodiazepine use positively correlated with total creatine levels in the left basal ganglia of schizophrenic patients and healthy controls (Tayoshi et al., 2009). Moreover, low baseline levels of phosphocreatine in medication-free schizophrenics normalized after one year of antipsychotic treatment, and this change in metabolic activity was associated with symptom improvement (Jayakumar et al., 2010). However, the possibility that neuroleptic medications confound creatine metabolism is not clear-cut because other studies exist that have not observed differences in phosphocreatine as a function of neuroleptic medications (Deicken et al., 1995; Ohrmann et al., 2007; Volz et al., 1998b). Similarly, in the case of mood disorders, use of mood stabilizers or antidepressants may obscure differences in creatine metabolism, especially in mild cases, as animal studies have shown that these drugs alter total creatine and creatine kinase (see Section 5.3.).
A more general issue that has limited available neuroimaging evidence, and consequently our understanding of creatine alterations in psychiatric populations, is the use of ratio calculations in studies using MRS. In the present review, only studies that reported absolute concentrations of phosphocreatine or total creatine (creatine + phosphocreatine) were considered. It is common practice for MRS studies to report ratio calculations (e.g., choline/creatine or N-acetyl-aspartate/creatine) because creatine is typically used as an internal standard (Malhi et al., 2002). However, because creatine is not stable under stressful conditions and differences in brain creatine metabolism are observed in most psychiatric populations, it is impossible to separate out the direction of creatine changes from ratio measures. When metabolite values are combined, it is impossible to know how the signals for creatine, phosphocreatine, and creatine kinase change individually with respect to the equation for the creatine-phosphocreatine equilibrium reaction (Figure 1).
The described methodological challenges obscure the relationship between brain creatine and the pathogenesis of psychiatric disorders. In summary, these issues highlight the need for caution when interpreting the positive findings for creatine in treating depression and anxiety-related disorders. The few extant clinical studies recruited small comorbid samples and did not include a placebo comparison group. In addition, because these studies used add-on designs, it is unknown whether treatment with creatine alone is effective or if the benefits of creatine occur in combination with psychotropic medications.
6. Conclusions and recommendations
The ability of dietary creatine to alter brain energetics, promote neurogenesis, and improve brain function safely and effectively is opening up the exciting possibility for creatine monohydrate to provide a novel, natural strategy for the treatment of psychiatric disorders. To overcome methodological hurdles described in previous studies, it is advised that greater attention be paid to the dose and duration of creatine supplementation, as well as individual patient characteristics, such as age, sex, medical condition, genetic origin, or severity of psychiatric symptoms, which may alter the effects of creatine in the brain. The ideal clinical trial would take these factors into consideration, implement selective screening practices (e.g., exclude comorbidity), recruit large samples of men and women, evaluate multiple endpoints for response and remission, and assess changes in brain creatine and phosphocreatine levels in vivo using neuroimaging techniques concomitantly with neurocognitive testing. In addition, cause-and-effect evidence from animal models and cellular studies are needed to advance mechanistic understanding, particularly in terms of identifying the location, function, and severity of creatine alterations in the brain that are associated with cognitive and emotional dysfunction.
Too few data are available to advise on when or how much creatine is required to produce therapeutic effects in psychiatric populations. The difficulty in characterizing the value of creatine supplementation is in part due to differences in study design (add-on versus monotherapy, comorbid diagnoses, differing doses, dietary influences). Some investigators have proposed that therapeutic intervention should be defined as more than 6 g daily in humans, which is three-times greater than the normal ~2 g daily clearance rate (Benzi, 2000). However, many clinical trials (other than studies of individuals with inborn errors of creatine metabolism) have treated patients with 5 g or less because possible side effects of larger doses have not been carefully investigated. Nonetheless, given the severity of metabolic impairments observed, psychiatric patients may benefit from larger daily doses in the 20–40 g range (administered in smaller 5 g doses, 4x day for optimal bioavailability), as in the cases for other brain-related disorders with significant metabolic impairments, like creatine deficiency syndromes. As such, careful documentation of side effects of doses greater than 5 g daily is strongly encouraged. This is especially important in consideration of reports finding that creatine supplementation can adversely affect mood (Allen et al., 2010; Roitman et al., 2007; Volek et al., 2000). Ultimately, further work is required to titrate the optimal dose of creatine given the population being studied because metabolic need will likely vary as a function of age, gender, physical activity, and brain-related pathology. Assessment of multiple doses and schedules of creatine administration is needed to clarify whether creatine kinetics are sex- or diagnosis-dependent.
The classical and nontraditional mechanisms of creatine action remain important avenues of research that could pave the way for the development of complementary or alternative treatments strategies. For instance, few studies have directly evaluated the potential for creatine monohydrate to oppose the negative consequences of stress on the brain, but future research elucidating the neural mechanisms could aid in the understanding of stress-related psychiatric disorders. More work using animal models of psychopathology are encouraged to enhance our mechanistic understanding of how endogenous creatine influences neurochemistry and behavior, how creatine supplementation might mitigate the effects of stress on these parameters, and how other agents with therapeutic efficacy affect the creatine-phosphocreatine circuit. More rigorous clinical trials are needed to determine the true safety and efficacy of creatine to treat or prevent mental illness.
More generally speaking, basic and translational research on CAM therapy should be made a priority to help clinicians and patients make informed decisions, as trends indicate the use of these novel strategies will continue to increase. Cognitive and emotional health is strongly associated with the quality of the diet because of the important role micronutrients and macronutrients have in brain structure, function, and development (Bodnar and Wisner, 2005; Christensen, 2001; Lieberman, 2003; McGowan et al., 2008). Food constituents provide the building blocks for cell growth and proliferation, as well as participate in neurotransmitter synthesis, synaptic transmission, neural plasticity, gene expression, and energy metabolism. Of significance, poor dietary patterns are linked with increased risk of mental illness (Akbaraly et al., 2009; Jacka et al., 2010, 2011; Kuczmarski et al., 2010; Li et al., 2010; Sanchez-Villegas et al., 2009), which may be prevented by informed use of dietary supplements like creatine, either as monotherapy or adjunct therapy with conventional psychotropic medications (Bodnar and Wisner, 2005). Taken together, understanding the complex relationships between dietary factors and the pathogenesis of psychopathology may uncover innovative targets for drug action to improve treatment options.
An increasingly burgeoning area of research, the role of creatine in developing and maintaining brain health cannot be understated. This accumulation of positive reports should encourage scientific inquiry among psychology, neuroscience and nutrition communities to further clarify the importance of creatine in cognition and emotion. Greater understanding of relationships between the creatine-phosphocreatine circuit and psychiatric disorders may inspire innovative strategies to treat or prevent these disorders. Creatine supplementation has the potential to serve as an adjunct for treatment, but because this compound is still the early stages of investigation, an affirmative answer would be premature. More rigorous clinical designs need to be carried out, and the pharmacokinetics and dose-response effects in psychiatric populations need to be more thoroughly described.
Highlights.
Creatine is an antioxidant, neuromodulator, and key regulator of energy metabolism.
Alterations in the brain creatine pathway are linked with psychiatric disorders.
Creatine supplements, commonly consumed by athletes, are bioavailable to the brain.
Intervention studies report benefit of creatine treatment for depression and PTSD.
Possible therapeutic mechanisms and methodological challenges are considered.
Acknowledgments
I am grateful to the Tufts Psychology Department for encouraging fourth year doctoral students to write and publish reviews in their area of expertise, as well as to Robin Kanarek and Gina Kuperberg for feedback that greatly improved the manuscript. Financial support was provided by Award Number F31AT006292 from the National Center of Complementary and Alternative Medicine (NCCAM). The content is solely the responsibility of the author and does not necessarily represent the official views of NCCAM or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostinho FR, Scaini G, Ferreira GK, Jeremias IC, Réus GZ, Rezin GT, Castro AA, Zugno AI, Quevedo J, Streck EL. Effects of olanzapine, fluoxetine and olanzapine/fluoxetine on creatine kinase activity in rat brain. Brain Res Bull. 2009;80:337–340. doi: 10.1016/j.brainresbull.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Agren H, Niklasson F. Creatinine and creatine in CSF: indices of brain energy metabolism in depression. Short note J Neural Transm. 1988;74:55–59. doi: 10.1007/BF01243575. [DOI] [PubMed] [Google Scholar]
- Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry. 2009;195:408–413. doi: 10.1192/bjp.bp.108.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PJ, D’Anci KE, Kanarek RB. Creatine, brain functioning, and behavior. In: Kanarek RB, Lieberman HR, editors. Diet, Brain and Behavior: Practical Implications. Taylor and Francis; New York: 2011. pp. 215–236. [Google Scholar]
- Allen PJ, D’Anci KE, Kanarek RB, Renshaw PF. Chronic creatine supplementation alters depression-like behavior in rodents in a sex-dependent manner. Neuropsychopharmacology. 2010;35:534–546. doi: 10.1038/npp.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PJ, D’Anci KE, Kanarek RB, Renshaw PF. Sex-specific antidepressant effects of dietary creatine with and without sub-acute fluoxetine in rats. Pharmacol Biochem Behav. doi: 10.1016/j.pbb.2012.03.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida LS, Salomons GS, Hogenboom F, Jakobs C, Schoffelmeer AN. Exocytotic release of creatine in rat brain. Synapse. 2006;60:118–123. doi: 10.1002/syn.20280. [DOI] [PubMed] [Google Scholar]
- Almeida OP, McCaul K, Hankey GJ, Norman P, Jamrozik K, Flicker L. Homocysteine and depression in later life. Arch Gen Psychiatry. 2008;65:1286–1294. doi: 10.1001/archpsyc.65.11.1286. [DOI] [PubMed] [Google Scholar]
- Ames A., 3rd CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Amital D, Vishne T, Roitman S, Kotler M, Levine J. Open study of creatine monohydrate in treatment-resistant posttraumatic stress disorder. J Clin Psychiatry. 2006;67:836–837. doi: 10.4088/jcp.v67n0521c. [DOI] [PubMed] [Google Scholar]
- Amital D, Vishne T, Rubinow A, Levine J. Observed effects of creatine monohydrate in a patient with depression and fibromyalgia. Am J Psychiatry. 2006;163:1840–1841. doi: 10.1176/ajp.2006.163.10.1840b. [DOI] [PubMed] [Google Scholar]
- Andres R, Ducray A, Schlattner U, Wallimann T, Widmer H. Functions and effects of creatine in the central nervous system. Brain Res Bull. 2008;76:329–343. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Andres R, Huber A, Schlattner U, Perezbouza A, Krebs S, Seiler R, Wallimann T, Widmer H. Effects of creatine treatment on the survival of dopaminergic neurons in cultured fetal ventral mesencephalic tissue. Neuroscience. 2005;133:701–713. doi: 10.1016/j.neuroscience.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Assis LC, Scaini G, Di-Pietro PB, Castro AA, Comim CM, Streck EL, Quevedo J. Effect of antipsychotics on creatine kinase activity in rat brain. Basic Clin Pharmacol Toxicol. 2007;101:315–319. doi: 10.1111/j.1742-7835.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- Astorino TA, Marrocco AC, Gross SM, Johnson DL, Brazil CM, Icenhower ME, Kneessi RJ. Is running performance enhanced with creatine serum ingestion? J Strength Cond Res. 2005;19:730–734. doi: 10.1519/R-16744.1. [DOI] [PubMed] [Google Scholar]
- Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Auer DP, Wilke M, Grabner A, Heidenreich JO, Bronisch T, Wetter TC. Reduced NAA in the thalamus and altered membrane and glial metabolism in schizophrenic patients detected by 1H-MRS and tissue segmentation. Schizophr Res. 2001;52:87–99. doi: 10.1016/s0920-9964(01)00155-4. [DOI] [PubMed] [Google Scholar]
- Battini R, Alessandri MG, Leuzzi V, Moro F, Tosetti M, Bianchi MC, Cioni G. Arginine:glycine amidinotransferase (AGAT) deficiency in a newborn: early treatment can prevent phenotypic expression of the disease. J Pediatr. 2006;148:828–830. doi: 10.1016/j.jpeds.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Battini R, Leuzzi V, Carducci C, Tosetti M, Bianchi MC, Item CB, Stöckler-Ipsiroglu S, Cioni G. Creatine depletion in a new case with AGAT deficiency: clinical and genetic study in a large pedigree. Mol Genet Metab. 2002;77:326–331. doi: 10.1016/s1096-7192(02)00175-0. [DOI] [PubMed] [Google Scholar]
- Béard E, Braissant O. Synthesis and transport of creatine in the CNS: importance for cerebral functions. J Neurochem. 2010;115:297–313. doi: 10.1111/j.1471-4159.2010.06935.x. [DOI] [PubMed] [Google Scholar]
- Bebbington P, Dunn G, Jenkins R, Lewis G, Brugha T, Farrell M, Meltzer H. The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric Morbidity. Int Rev Psychiatry. 2003;15:74–83. doi: 10.1080/0954026021000045976. [DOI] [PubMed] [Google Scholar]
- Bender A, Beckers J, Schneider I, Hölter SM, Haack T, Ruthsatz T, Vogt-Weisenhorn DM, Becker L, Genius J, Rujescu D, Irmler M, Mijalski T, Mader M, Quintanilla-Martinez L, Fuchs H, Gailus-Durner V, de Angelis MH, Wurst W, Schmidt J, Klopstock T. Creatine improves health and survival of mice. Neurobiol Aging. 2008;29:1404–1411. doi: 10.1016/j.neurobiolaging.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Benzi G. Is There a Rationale for the Use of Creatine Either as Nutritional Supplementation or Drug Administration in Humans Participating in a Sport? Pharmacol Res. 2000;41:255–264. doi: 10.1006/phrs.1999.0618. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Callicott JH, Elman I, Mattay VS, Tedeschi G, Frank JA, Breier A, Weinberger DR. Regionally specific neuronal pathology in untreated patients with schizophrenia: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 1998;43:641–648. doi: 10.1016/s0006-3223(97)00555-6. [DOI] [PubMed] [Google Scholar]
- Bhugra D. The global prevalence of schizophrenia. PLoS Med. 2005;2:372–373. doi: 10.1371/journal.pmed.0020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MC, Tosetti M, Battini R, Leuzzi V, Alessandri MG, Carducci C, Antonozzi I, Cioni G. Treatment monitoring of brain creatine deficiency syndromes: a 1H- and 31P-MR spectroscopy study. AJNR Am J Neuroradiol. 2007;28:548–554. [PMC free article] [PubMed] [Google Scholar]
- Bianchi MC, Tosetti M, Fornai F, Alessandri MG, Cipriani P, De Vito G, Canapicchi R. Reversible brain creatine deficiency in two sisters with normal blood creatine level. Ann Neurol. 2000;47:511–513. [PubMed] [Google Scholar]
- Bizzi A, Bugiani M, Salomons GS, Hunneman DH, Moroni I, Estienne M, Danesi U, Jakobs C, Uziel G. X-linked creatine deficiency syndrome: a novel mutation in creatine transporter gene SLC6A8. Ann Neurol. 2002;52:227–231. doi: 10.1002/ana.10246. [DOI] [PubMed] [Google Scholar]
- Bjelland I, Tell GS, Vollset SE, Refsum H, Ueland PM. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60:618–626. doi: 10.1001/archpsyc.60.6.618. [DOI] [PubMed] [Google Scholar]
- Bluml S, Tan J, Harris K, Adatia N, Karme A, Sproull T, Ross B. Quantitative proton-decoupled 31P MRS of the schizophrenic brain in vivo. J Comput Assist Tomogr. 1999;23:272–275. doi: 10.1097/00004728-199903000-00017. [DOI] [PubMed] [Google Scholar]
- Bodnar L, Wisner K. Nutrition and Depression: Implications for Improving Mental Health Among Childbearing-Aged Women. Biol Psychiatry. 2005;58:679–685. doi: 10.1016/j.biopsych.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braissant O, Henry H. AGAT, GAMT and SLC6A8 distribution in the central nervous system, in relation to creatine deficiency syndromes: A review. J Inherit Metab Dis. 2008 doi: 10.1007/s10545-008-0826-9. [DOI] [PubMed] [Google Scholar]
- Braissant O, Henry H, Villard AM, Speer O, Wallimann T, Bachmann C. Creatine synthesis and transport during rat embryogenesis: spatiotemporal expression of AGAT, GAMT and CT1. BMC Dev Biol. 2005;5:9. doi: 10.1186/1471-213X-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brdiczka D, Beutner G, Ruck A, Dolder M, Wallimann T. The molecular structure of mitochondrial contact sites. Their role in regulation of energy metabolism and permeability transition. Biofactors. 1998;8:235–242. doi: 10.1002/biof.5520080311. [DOI] [PubMed] [Google Scholar]
- Brosnan JT, Brosnan ME. Creatine: Endogenous Metabolite, Dietary, and Therapeutic Supplement. Annu Rev Nutr. 2007;27:241–261. doi: 10.1146/annurev.nutr.27.061406.093621. [DOI] [PubMed] [Google Scholar]
- Brosnan ME, Edison EE, da Silva R, Brosnan JT. New insights into creatine function and synthesis. Adv Enzyme Regul. 2007;47:252–260. doi: 10.1016/j.advenzreg.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Brustovetsky T, Dubinsky JM. On the mechanisms of neuroprotection by creatine and phosphocreatine. J Neurochem. 2001;76:425–434. doi: 10.1046/j.1471-4159.2001.00052.x. [DOI] [PubMed] [Google Scholar]
- Búrigo M, Roza CA, Bassani C, Feier G, Dal-Pizzol F, Quevedo J, Streck EL. Decreased Creatine Kinase Activity Caused by Electroconvulsive Shock. Neurochem Res. 2006;31:877–881. doi: 10.1007/s11064-006-9091-1. [DOI] [PubMed] [Google Scholar]
- Bürklen TS, Schlattner U, Homayouni R, Gough K, Rak M, Szeghalmi A, Wallimann T. The Creatine Kinase/Creatine Connection to Alzheimer’s Disease: CK Inactivation, APP-CK Complexes and Focal Creatine Deposits. J Biomed Biotech. 2006;2006:1–12. doi: 10.1155/JBB/2006/35936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano SC, Olvera RL, Hatch JP, Sanches M, Chen HH, Nicoletti M, Stanley JA, Fonseca M, Hunter K, Lafer B, Pliszka SR, Soares JC. Lower N-acetyl-aspartate levels in prefrontal cortices in pediatric bipolar disorder: a (1)H magnetic resonance spectroscopy study. J Am Acad Child Adolesc Psychiatry. 2011;50:85–94. doi: 10.1016/j.jaac.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Calabrese G, Deicken RF, Fein G, Merrin EL, Schoenfeld F, Weiner MW. 31Phosphorus magnetic resonance spectroscopy of the temporal lobes in schizophrenia. Biol Psychiatry. 1992;32:26–32. doi: 10.1016/0006-3223(92)90139-q. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cecil KM, DelBello MP, Sellars MC, Strakowski SM. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J Child Adolesc Psychopharmacol. 2003;13:545–555. doi: 10.1089/104454603322724931. [DOI] [PubMed] [Google Scholar]
- Christensen L. The effect of food intake on mood. Clin Nutr. 2001;20:161–166. [Google Scholar]
- Chrusch MJ, Chilibeck PD, Chad KE, Davison KS, Burke DG. Creatine supplementation combined with resistance training in older men. Med Sci Sports Exerc. 2001;33:2111–2117. doi: 10.1097/00005768-200112000-00021. [DOI] [PubMed] [Google Scholar]
- Chung S, Choi MS, Ha TS, Yang JC, Park TW, Chung YC, Jung AJ. Creatine kinase level may not be associated with motor activity but with simple mood state in Korean unmedicated bipolar patients. Eur Neuropsychopharmacol. 2009;19:S465–S466. [Google Scholar]
- Coffey JW, Heath RG, Guschwan AF. Serum creatine kinase, aldolase, and copper in acute and chronic schizophrenics. Biol Psychiatry. 1970;2:331–339. [PubMed] [Google Scholar]
- Coplan J, Mathew S, Mao X, Smith E, Hof P, Coplan P, Rosenblum L, Gorman J, Shungu D. Decreased choline and creatine concentrations in centrum semiovale in patients with generalized anxiety disorder: Relationship to IQ and early trauma. Psychiatry Res Neuroimaging. 2006;147:27–39. doi: 10.1016/j.pscychresns.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Cox G, Mujika I, Tumilty D, Burke L. Acute creatine supplementation and performance during a field test simulating match play in elite female soccer players. Int J Sport Nutr Exerc Metab. 2002;12:33–46. doi: 10.1123/ijsnem.12.1.33. [DOI] [PubMed] [Google Scholar]
- Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, Dunner DL, Renshaw PF. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–458. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- Dalbo VJ, Roberts MD, Stout JR, Kerksick CM. Putting to rest the myth of creatine supplementation leading to muscle cramps and dehydration. Br J Sports Med. 2008;42:567–573. doi: 10.1136/bjsm.2007.042473. [DOI] [PubMed] [Google Scholar]
- Danivas V, Behere RV, Varambally S, Rao NP, Venkatasubramanian G, Gangadhar BN. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26:278–279. doi: 10.1097/yct.0b013e3181da848f. [DOI] [PubMed] [Google Scholar]
- Dechent P, Pouwels PJ, Wilken B, Hanefeld F, Frahm J. Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. Am J Physiol. 1999;277:R698–704. doi: 10.1152/ajpregu.1999.277.3.R698. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Calabrese G, Merrin EL, Meyerhoff DJ, Dillon WP, Weiner MW, Fein G. 31phosphorus magnetic resonance spectroscopy of the frontal and parietal lobes in chronic schizophrenia. Biol Psychiatry. 1994;36:503–510. doi: 10.1016/0006-3223(94)90613-0. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Calabrese G, Merrin EL, Vinogradov S, Fein G, Weiner MW. Asymmetry of temporal lobe phosphorous metabolism in schizophrenia: a 31phosphorous magnetic resonance spectroscopic imaging study. Biol Psychiatry. 1995;38:279–286. doi: 10.1016/0006-3223(94)00372-A. [DOI] [PubMed] [Google Scholar]
- Deminice R, Portari GV, Vannucchi H, Jordao AA. Effects of creatine supplementation on homocysteine levels and lipid peroxidation in rats. Br J Nutr. 2008;102:110. doi: 10.1017/S0007114508162985. [DOI] [PubMed] [Google Scholar]
- Dincheva I, Glatt CE, Lee FS. Impact of the BDNF Val66Met polymorphism on cognition: Implications for behavioral genetics. Neuroscientist. 2012 doi: 10.1177/1073858411431646. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder M, Walzel B, Speer O, Schlattner U, Wallimann T. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem. 2003;278:17760–17766. doi: 10.1074/jbc.M208705200. [DOI] [PubMed] [Google Scholar]
- Ducray A, Qualls R, Schlattner U, Andres R, Dreher E, Seiler R, Wallimann T, Widmer H. Creatine promotes the GABAergic phenotype in human fetal spinal cord cultures. Brain Res. 2007;1137:50–57. doi: 10.1016/j.brainres.2006.12.038. [DOI] [PubMed] [Google Scholar]
- Ducray AD, Schläppi JA, Qualls R, Andres RH, Seiler RW, Schlattner U, Wallimann T, Widmer HR. Creatine treatment promotes differentiation of GABA-ergic neuronal precursors in cultured fetal rat spinal cord. J Neurosci Res. 2007;85:1863–1875. doi: 10.1002/jnr.21337. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Edmunds JW, Jayapalan S, DiMarco NM, Saboorian MH, Aukema HM. Creatine supplementation increases renal disease progression in Han:SPRD-cy rats. Am J Kidney Dis. 2001;37:73–78. doi: 10.1053/ajkd.2001.20590. [DOI] [PubMed] [Google Scholar]
- Erakovic V, Zupan G, Varljen J, Laginja J, Simonic A. Altered activities of rat brain metabolic enzymes in electroconvulsive shock-induced seizures. Epilepsia. 2001;42:181–189. doi: 10.1046/j.1528-1157.2001.30499.x. [DOI] [PubMed] [Google Scholar]
- Faulstich ME, Brantley PJ, Barkemeyer CA. Creatine phosphokinase, the MMPI, and psychosis. Am J Psychiatry. 1984;141:584–586. doi: 10.1176/ajp.141.4.584. [DOI] [PubMed] [Google Scholar]
- Feier G, Valvassori SS, Rezin GT, Burigo M, Streck EL, Kapczinski F, Quevedo J. Creatine kinase levels in patients with bipolar disorder: depressive, manic, and euthymic phases. Rev Bras Psiquiatr. 2011 doi: 10.1590/s1516-44462011005000005. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlenza OV, Diniz BS, Teixeira AL, Ojopi EB, Talib LL, Mendonça VA, Izzo G, Gattaz WF. Effect of brain- derived neurotrophic factor Val66Met polymorphism and serum levels on the progression of mild cognitive impairment. World J Biol Psychiatry. 2010;11:774–780. doi: 10.3109/15622971003797241. [DOI] [PubMed] [Google Scholar]
- Freitas TP, Scaini G, Corrêa C, Santos PM, Ferreira GK, Rezin GT, Moretti M, Valvassori SS, Quevedo J, Streck EL. Evaluation of brain creatine kinase activity in an animal model of mania induced by ouabain. J Neural Transm. 2010;117:149–153. doi: 10.1007/s00702-009-0337-3. [DOI] [PubMed] [Google Scholar]
- Frey BN, Stanley JA, Nery FG, Monkul ES, Nicoletti MA, Chen HH, Hatch JP, Caetano SC, Ortiz O, Kapczinski F, Soares JC. Abnormal cellular energy and phospholipid metabolism in the left dorsolateral prefrontal cortex of medication-free individuals with bipolar disorder: an in vivo 1H MRS study. Bipolar Disord. 2007a;9(Suppl 1):119–127. doi: 10.1111/j.1399-5618.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- Frey BN, Walss-Bass C, Stanley JA, Nery FG, Matsuo K, Nicoletti MA, Hatch JP, Bowden CL, Escamilla MA, Soares JC. Brain-derived neurotrophic factor val66met polymorphism affects prefrontal energy metabolism in bipolar disorder. Neuroreport. 2007b;18:1567–1570. doi: 10.1097/WNR.0b013e3282ef7082. [DOI] [PubMed] [Google Scholar]
- Frye MA, Watzl J, Banakar S, O’Neill J, Mintz J, Davanzo P, Fischer J, Chirichigno JW, Ventura J, Elman S, Tsuang J, Walot I, Thomas MA. Increased Anterior Cingulate/Medial Prefrontal Cortical Glutamate and Creatine in Bipolar Depression. Neuropsychopharmacology. 2007;32:2490–2499. doi: 10.1038/sj.npp.1301387. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Czéh B, Kole M, Michaelis T, Lucassen P. Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol. 2004;14:S481–S490. doi: 10.1016/j.euroneuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Czéh B, Michaelis T, de Biurrun G, Watanabe T, Frahm J. Synaptic plasticity and tianeptine: structural regulation. Eur Psychiatry. 2002;17(Suppl 3):311–317. doi: 10.1016/s0924-9338(02)00652-1. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Nakano T, Takano T, Hokazono Y, Asakura T, Tsuji T. Study of chronic schizophrenics using 31P magnetic resonance chemical shift imaging. Acta Psychiatr Scand. 1992;86:455–462. doi: 10.1111/j.1600-0447.1992.tb03297.x. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Nakano T, Takano T, Takeuchi K, Yamada K, Fukuzako T, Akimoto H. Proton magnetic resonance spectroscopy of basal ganglia in chronic schizophrenia. Biol Psychiatry. 1996;40:14–18. doi: 10.1016/0006-3223(95)00316-9. [DOI] [PubMed] [Google Scholar]
- Fukuzako H, Fukuzako T, Hashiguchi T, Kodama S, Takigawa M, Fujimoto T. Changes in levels of phosphorus metabolites in temporal lobes of drug-naive schizophrenic patients. Am J Psychiatry. 1999;156:1205–1208. doi: 10.1176/ajp.156.8.1205. [DOI] [PubMed] [Google Scholar]
- Fukuzako H, Takeuchi K, Hokazono Y, Fukuzako T, Yamada K, Hashiguchi T, Obo Y, Ueyama K, Takigawa M, Fujimoto T. Proton magnetic resonance spectroscopy of the left medial temporal and frontal lobes in chronic schizophrenia: preliminary report. Psychiatry Res. 1995;61:193–200. doi: 10.1016/0925-4927(95)02622-5. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Hess DA, Liu S, Babb JS, Klein RG, Gonen O. Lateralized caudate metabolic abnormalities in adolescent major depressive disorder: a proton MR spectroscopy study. Am J Psychiatry. 2007;164:1881–1889. doi: 10.1176/appi.ajp.2007.06122032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Schubert F, Brühl R, Hellweg R, Klär AA, Kehrer C, Wirth C, Sander T, Lang UE. Met carriers of BDNF Val66Met genotype show increased N-acetylaspartate concentration in the anterior cingulate cortex. Neuroimage. 2010;49:767–771. doi: 10.1016/j.neuroimage.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Gledhill RF, Van der Merwe CA, Greyling M, Van Niekerk MM. Race-gender differences in serum creatine kinase activity: a study among South Africans. J Neurol Neurosurg Psychiatry. 1988;51:301–304. doi: 10.1136/jnnp.51.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Chai Y, Ding JH, Sun XL, Hu G. Chronic mild stress damages mitochondrial ultrastructure and function in mouse brain. Neurosci Lett. 2011;488:76–80. doi: 10.1016/j.neulet.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Gosling R, Kerry RJ, Orme JE, Owen G. Creatine phosphokinase activity in newly admitted psychiatric patients. Br J Psychiatry. 1972;121:351–355. doi: 10.1192/bjp.121.4.351. [DOI] [PubMed] [Google Scholar]
- Groeneveld GJ, Veldink JH, van der Tweel I, Kalmijn S, Beijer C, de Visser M, Wokke JH, Franssen H, van den Berg LH. A randomized sequential trial of creatine in amyotrophic lateral sclerosis. Ann Neurol. 2003;53:437–445. doi: 10.1002/ana.10554. [DOI] [PubMed] [Google Scholar]
- Gualano B, Artioli GG, Poortmans JR, Lancha Junior AH. Exploring the therapeutic role of creatine supplementation. Amino Acids. 2010;38:31–44. doi: 10.1007/s00726-009-0263-6. [DOI] [PubMed] [Google Scholar]
- Gualano B, Ugrinowitsch C, Novaes RB, Artioli GG, Shimizu MH, Seguro AC, Harris RC, Lancha AH., Jr Effects of creatine supplementation on renal function: a randomized, double-blind, placebo-controlled clinical trial. Eur J Appl Physiol. 2008;103:33–40. doi: 10.1007/s00421-007-0669-3. [DOI] [PubMed] [Google Scholar]
- Hamakawa H, Kato T, Shioiri T, Inubushi T, Kato N. Quantitative proton magnetic resonance spectroscopy of the bilateral frontal lobes in patients with bipolar disorder. Psychol Med. 1999;29:639–644. doi: 10.1017/s0033291799008442. [DOI] [PubMed] [Google Scholar]
- Hammett ST, Wall MB, Edwards TC, Smith AT. Dietary supplementation of creatine monohydrate reduces the human fMRI BOLD signal. Neurosci Lett. 2010;479:201–205. doi: 10.1016/j.neulet.2010.05.054. [DOI] [PubMed] [Google Scholar]
- Harris RC, Nevill M, Harris DB, Fallowfield JL, Bogdanis GC, Wise JA. Absorption of creatine supplied as a drink, in meat or in solid form. J Sports Sci. 2002;20:147–151. doi: 10.1080/026404102317200855. [DOI] [PubMed] [Google Scholar]
- Health Canada. Creatine monohydrate. [Last accessed March 1, 2012];Health Canada Natural Health Products Ingredients Database. 2011 from http://webprod.hc-sc.gc.ca/nhpid-bdipsn/atReq.do?atid=creatine.mono&lang=eng.
- Hermesh H, Stein D, Manor I, Shechtmann T, Blumensohn R, Meged S, Shiloh R, Benjamini Y, Weizman A. Serum creatine kinase levels in untreated hospitalized adolescents during acute psychosis. J Am Acad Child Adolesc Psychiatry. 2002;41:1045–1053. doi: 10.1097/00004583-200209000-00004. [DOI] [PubMed] [Google Scholar]
- Ikeda H. Serum creatine phosphokinase activity in newly admitted chronic alcoholics. Folia Psychiatr Neurol Jpn. 1977;31:9–16. doi: 10.1111/j.1440-1819.1977.tb02678.x. [DOI] [PubMed] [Google Scholar]
- in ‘t Zandt HJ, Renema WK, Streijger F, Jost C, Klomp DW, Oerlemans F, Van der Zee CE, Wieringa B, Heerschap A. Cerebral creatine kinase deficiency influences metabolite levels and morphology in the mouse brain: a quantitative in vivo 1H and 31P magnetic resonance study. J Neurochem. 2004;90:1321–1330. doi: 10.1111/j.1471-4159.2004.02599.x. [DOI] [PubMed] [Google Scholar]
- Iosifescu DV, Bolo NR, Nierenberg AA, Jensen JE, Fava M, Renshaw PF. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry. 2008;63:1127–1134. doi: 10.1016/j.biopsych.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Ipsiroglu OS, Stromberger C, Ilas J, Hoger H, Muhl A, Stöckler-Ipsiroglu S. Changes of tissue creatine concentrations upon oral supplementation of creatine-monohydrate in various animal species. Life Sci. 2001;69:1805–1815. doi: 10.1016/s0024-3205(01)01268-1. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O’Reilly SL, Nicholson GC, Kotowicz MA, Berk M. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167:305–311. doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Pasco JA, Mykletun A, Williams LJ, Nicholson GC, Kotowicz MA, Berk M. Diet quality in bipolar disorder in a population-based sample of women. J Affect Disord. 2011;129:332–337. doi: 10.1016/j.jad.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Jayakumar PN, Gangadhar BN, Venkatasubramanian G, Desai S, Velayudhan L, Subbakrishna D, Keshavan MS. High energy phosphate abnormalities normalize after antipsychotic treatment in schizophrenia: A longitudinal 31P MRS study of basal ganglia. Psychiatry Research: Neuroimaging. 2010;181:237–240. doi: 10.1016/j.pscychresns.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Jensen JE. Focal changes in brain energy and phospholipid metabolism in first-episode schizophrenia: 31P-MRS chemical shift imaging study at 4 Tesla. The British Journal of Psychiatry. 2004;184:409–415. doi: 10.1192/bjp.184.5.409. [DOI] [PubMed] [Google Scholar]
- Jost CR, Van Der Zee CE, In ‘t Zandt HJ, Oerlemans F, Verheij M, Streijger F, Fransen J, Heerschap A, Cools AR, Wieringa B. Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur J Neurosci. 2002;15:1692–1706. doi: 10.1046/j.1460-9568.2002.02001.x. [DOI] [PubMed] [Google Scholar]
- Juhn MS, O’Kane JW, Vinci DM. Oral creatine supplementation in male collegiate athletes: a survey of dosing habits and side effects. J Am Diet Assoc. 1999;99:593–595. doi: 10.1016/s0002-8223(99)00145-5. [DOI] [PubMed] [Google Scholar]
- Kaldis P, Hemmer W, Zanolla E, Holtzman D, Wallimann T. ‘Hot spots’ of creatine kinase localization in brain: cerebellum, hippocampus and choroid plexus. Dev Neurosci. 1996;18:542–554. doi: 10.1159/000111452. [DOI] [PubMed] [Google Scholar]
- Kaptsan A, Odessky A, Osher Y, Levine J. Lack of efficacy of 5 grams daily of creatine in schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:881–884. doi: 10.4088/jcp.v68n0609. [DOI] [PubMed] [Google Scholar]
- Kato T. Molecular neurobiology of bipolar disorder: a disease of ‘mood-stabilizing neurons’? Trends Neurosci. 2008;31:495–503. doi: 10.1016/j.tins.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Kato T, Shioiri T, Murashita J, Hamakawa H, Inubushi T, Takahashi S. Lateralized abnormality of high-energy phosphate and bilateral reduction of phosphomonoester measured by phosphorus-31 magnetic resonance spectroscopy of the frontal lobes in schizophrenia. Psychiatry Res. 1995;61:151–160. doi: 10.1016/0925-4927(95)02752-j. [DOI] [PubMed] [Google Scholar]
- Kato T, Takahashi S, Shioiri T, Inubushi T. Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord. 1992;26:223–230. doi: 10.1016/0165-0327(92)90099-r. [DOI] [PubMed] [Google Scholar]
- Kato T, Takahashi S, Shioiri T, Murashita J, Hamakawa H, Inubushi T. Reduction of brain phosphocreatine in bipolar II disorder detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord. 1994;31:125–133. doi: 10.1016/0165-0327(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Kendall RW, Jacquemin G, Frost R, Burns SP. Creatine supplementation for weak muscles in persons with chronic tetraplegia: a randomized double-blind placebo-controlled crossover trial. J Spinal Cord Med. 2005;28:208–213. doi: 10.1080/10790268.2005.11753814. [DOI] [PubMed] [Google Scholar]
- Kennedy S. A review of antidepressant treatments today. Eur Neuropsychopharmacol. 2006;16:S619–S623. [Google Scholar]
- Keshavan MS, Dick RM, Diwadkar VA, Montrose DM, Prasad KM, Stanley JA. Striatal metabolic alterations in non-psychotic adolescent offspring at risk for schizophrenia: A 1H spectroscopy study. Schizophr Res. 2009;115:88–93. doi: 10.1016/j.schres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The Epidemiology of Major Depressive Disorder: Results From the National Comorbidity Survey Replication (NCS-R) JAMA: The Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kim S-Y, Lee Y-J, Kim H, Lee D-W, Woo D-C, Choi C-B, Chae J-H, Choe B-Y. Desipramine attenuates forced swim test-induced behavioral and neurochemical alterations in mice: An in vivo 1H-MRS study at 9.4T. Brain Res. 2010;1348:105–113. doi: 10.1016/j.brainres.2010.05.097. [DOI] [PubMed] [Google Scholar]
- Klemm S, Rzanny R, Riehemann S, Volz HP, Schmidt B, Gerhard UJ, Filz C, Schonberg A, Mentzel HJ, Kaiser WA, Blanz B. Cerebral phosphate metabolism in first-degree relatives of patients with schizophrenia. Am J Psychiatry. 2001;158:958–960. doi: 10.1176/appi.ajp.158.6.958. [DOI] [PubMed] [Google Scholar]
- Knox D, Perrine SA, George SA, Galloway MP, Liberzon I. Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neurosci Lett. 2010;480:16–20. doi: 10.1016/j.neulet.2010.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga Y, Takahashi H, Oikawa D, Tachibana T, Denbow D, Furuse M. Brain creatine functions to attenuate acute stress responses through GABAnergic system in chicks. Neuroscience. 2005;132:65–71. doi: 10.1016/j.neuroscience.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kondo DG, Hellem TL, Sung YH, Kim N, Jeong EK, DelMastro KK, Shi X, Renshaw PF. Review: Magnetic Resonance Spectroscopy Studies of Pediatric Major Depressive Disorder. Depression Research and Treatment. 2011a;2011:1–13. doi: 10.1155/2011/650450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo DG, Sung YH, Hellem TL, Fiedler KK, Shi X, Jeong EK, Renshaw PF. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31-phosphorus magnetic resonance spectroscopy study. J Affect Disord. 2011b;135:354–361. doi: 10.1016/j.jad.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Ryan CE, Hess AL, Harrison W, Davis SM, Keller MB. Gender differences in chronic major and double depression. J Affect Disord. 2000;60:1–11. doi: 10.1016/s0165-0327(99)00158-5. [DOI] [PubMed] [Google Scholar]
- Korzun WJ. Oral creatine supplements lower plasma homocysteine concentrations in humans. Clin Lab Sci. 2004;17:102–106. [PubMed] [Google Scholar]
- Koshy KM, Griswold E, Schneeberger EE. Interstitial nephritis in a patient taking creatine. N Engl J Med. 1999;340:814–815. doi: 10.1056/NEJM199903113401017. [DOI] [PubMed] [Google Scholar]
- Kuczmarski MF, Cremer Sees A, Hotchkiss L, Cotugna N, Evans MK, Zonderman AB. Higher Healthy Eating Index- 2005 scores associated with reduced symptoms of depression in an urban population: findings from the Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS) study. J Am Diet Assoc. 2010;110:383–389. doi: 10.1016/j.jada.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl K, Goldberg L, Elliot D. Re: Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med Sci Sports Exerc. 2000;32:248–249. doi: 10.1097/00005768-200001000-00037. [DOI] [PubMed] [Google Scholar]
- Lawler J. Direct Antioxidant Properties of Creatine. Biochem Biophys Res Commun. 2002;290:47–52. doi: 10.1006/bbrc.2001.6164. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang J, McKeown R. Cross-sectional assessment of diet quality in individuals with a lifetime history of attempted suicide. Psychiatry Res. 2009;165:111–119. doi: 10.1016/j.psychres.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Lieberman H. Nutrition, brain function and cognitive performance★. Appetite. 2003;40:245–254. doi: 10.1016/s0195-6663(03)00010-2. [DOI] [PubMed] [Google Scholar]
- Lobley GE, Connell A, Revell D. The importance of transmethylation reactions to methionine metabolism in sheep: effects of supplementation with creatine and choline. Br J Nutr. 1996;75:47–56. doi: 10.1079/bjn19960109. [DOI] [PubMed] [Google Scholar]
- Lugenbiel P, Sartorius A, Vollmayr B, Schloss P. Creatine transporter expression after antidepressant therapy in rats bred for learned helplessness. World Journal of Biological Psychiatry. 2010;11:329–333. doi: 10.1080/15622970903131597. [DOI] [PubMed] [Google Scholar]
- Lutkenhoff ES, van Erp TG, Thomas MA, Therman S, Manninen M, Huttunen MO, Kaprio J, Lönnqvist J, O’Neill J, Cannon TD. Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 2010;15:308–318. doi: 10.1038/mp.2008.87. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kong SW, Sung SM, Hirashima F, Parow A, Hennen J, Cohen BM, Renshaw PF. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine- monohydrate. Psychiatry Res. 2003;123:87–100. doi: 10.1016/s0925-4927(03)00046-5. [DOI] [PubMed] [Google Scholar]
- MacDonald ML, Naydenov A, Chu M, Matzilevich D, Konradi C. Decrease in creatine kinase messenger RNA expression in the hippocampus and dorsolateral prefrontal cortex in bipolar disorder. Bipolar Disord. 2006;8:255–264. doi: 10.1111/j.1399-5618.2006.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak CSW, Waldvogel HJ, Dodd JR, Gilbert RT, Lowe MTJ, Birch NP, Faull RLM, Christie DL. Immunohistochemical localisation of the creatine transporter in the rat brain. Neuroscience. 2009;163:571–585. doi: 10.1016/j.neuroscience.2009.06.065. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Valenzuela M, Wen W, Sachdev P. Magnetic resonance spectroscopy and its applications in psychiatry. Aust N Z J Psychiatry. 2002;36:31–43. doi: 10.1046/j.1440-1614.2002.00992.x. [DOI] [PubMed] [Google Scholar]
- Manor I, Hermesh H, Weizman A, Munitz H. Elevated serum levels of creatine kinase in acute psychotic patients. Harefuah. 1999;136:639–641. [PubMed] [Google Scholar]
- Marcus S, Young E, Kerber K, Kornstein S, Farabaugh A, Mitchell J, Wisniewski S, Balasubramani G, Trivedi M, Rush A. Gender differences in depression: Findings from the STAR*D study. J Affect Disord. 2005;87:141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, Nemeroff CB. The Neurobiology of Anxiety Disorders: Brain Imaging, Genetics, and Psychoneuroendocrinology. Psychiatr Clin North Am. 2009;32:549–575. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WA, Garey RE, Heath RG. Cerebrospinal fluid creatine kinase in acutely psychotic patients. J Neurol Neurosurg Psychiatry. 1972;35:726–729. doi: 10.1136/jnnp.35.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25:2289–2304. doi: 10.1016/s0149-2918(03)80220-5. [DOI] [PubMed] [Google Scholar]
- Massana G, Gastó C, Junqué C, Mercader J-Ma, Gómez B, Massana J, Torres X, Salamero M. Reduced Levels of Creatine in the Right Medial Temporal Lobe Region of Panic Disorder Patients Detected with 1H Magnetic Resonance Spectroscopy. Neuroimage. 2002;16:836–842. doi: 10.1006/nimg.2002.1083. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, Kaddurah-Daouk R, Beal MF. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington’s disease. J Neurosci. 1998;18:156–163. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall W, Persky AM. Pharmacokinetics of creatine. Subcell Biochem. 2007;46:261–273. [PubMed] [Google Scholar]
- McGowan P, Meaney M, Szyf M. Diet and the epigenetic (re)programming of phenotypic differences in behavior. Brain Res. 2008;1237:12–24. doi: 10.1016/j.brainres.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: A Concise Overview of Incidence, Prevalence, and Mortality. Epidemiol Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- McMorris T, Harris R, Howard A, Langridge G, Hall B, Corbett J, Dicks M, Hodgson C. Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol Behav. 2007a;90:21–28. doi: 10.1016/j.physbeh.2006.08.024. [DOI] [PubMed] [Google Scholar]
- McMorris T, Harris RC, Swain J, Corbett J, Collard K, Dyson RJ, Dye L, Hodgson C, Draper N. Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology (Berl) 2006;185:93–103. doi: 10.1007/s00213-005-0269-z. [DOI] [PubMed] [Google Scholar]
- McMorris T, Mielcarz G, Harris RC, Swain JP, Howard A. Creatine supplementation and cognitive performance in elderly individuals. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007b;14:517–528. doi: 10.1080/13825580600788100. [DOI] [PubMed] [Google Scholar]
- Melkersson K. Serum creatine kinase levels in chronic psychosis patients – a comparison between atypical and conventional antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1277–1282. doi: 10.1016/j.pnpbp.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Meltzer H. Creatine kinase and aldolase in serum: abnormality common to acute psychoses. Science. 1968;159:1368–1370. doi: 10.1126/science.159.3821.1368. [DOI] [PubMed] [Google Scholar]
- Meltzer H. Increased activity of creatine phosphokinase and aldolase activity in the acute psychoses: case reports. J Psychiatr Res. 1970;7:249–262. doi: 10.1016/0022-3956(70)90019-1. [DOI] [PubMed] [Google Scholar]
- Meltzer H, Elkun L, Moline RA. Serum-enzyme changes in newly admitted psychiatric patients. I Arch Gen Psychiatry. 1969;21:731–738. doi: 10.1001/archpsyc.1969.01740240091011. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Creatine phosphokinase activity and clinical symptomatology. A study in acute schizophrenic patients. Arch Gen Psychiatry. 1973;29:589–593. doi: 10.1001/archpsyc.1973.04200050005001. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Serum creatine phosphokinase in schizophrenia. Am J Psychiatry. 1976;133:192–197. doi: 10.1176/ajp.133.2.192. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15:395–405. doi: 10.1016/0893-133X(95)00276-J. [DOI] [PubMed] [Google Scholar]
- Mercimek-Mahmutoglu S, Stöckler-Ipsiroglu S, Adami A, Appleton R, Araujo HC, Duran M, Ensenauer R, Fernandez- Alvarez E, Garcia P, Grolik C, Item CB, Leuzzi V, Marquardt I, Muhl A, Saelke-Kellermann RA, Salomons GS, Schulze A, Surtees R, van der Knaap MS, Vasconcelos R, Verhoeven NM, Vilarinho L, Wilichowski E, Jakobs C. GAMT deficiency: features, treatment, and outcome in an inborn error of creatine synthesis. Neurology. 2006;67:480–484. doi: 10.1212/01.wnl.0000234852.43688.bf. [DOI] [PubMed] [Google Scholar]
- Metzl JD, Small E, Levine SR, Gershel JC. Creatine use among young athletes. Pediatrics. 2001;108:421–425. doi: 10.1542/peds.108.2.421. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol Med. 2003;33:1277–1284. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- Michaelis T, Wick M, Fujimori H, Matsumura A, Frahm J. Proton MRS of oral creatine supplementation in rats. Cerebral metabolite concentrations and ischemic challenge. NMR Biomed. 1999;12:309–314. doi: 10.1002/(sici)1099-1492(199908)12:5<309::aid-nbm572>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Mirza Y, O’Neill J, Smith EA, Russell A, Smith JM, Banerjee SP, Bhandari R, Boyd C, Rose M, Ivey J, Renshaw PF, Rosenberg DR. Increased medial thalamic creatine-phosphocreatine found by proton magnetic resonance spectroscopy in children with obsessive-compulsive disorder versus major depression and healthy controls. J Child Neurol. 2006;21:106–111. doi: 10.1177/08830738060210020201. [DOI] [PubMed] [Google Scholar]
- Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am J Psychiatry. 1997;154:116–118. doi: 10.1176/ajp.154.1.116. [DOI] [PubMed] [Google Scholar]
- Mudd SH, Poole JR. Labile methyl balances for normal humans on various dietary regimens. Metabolism. 1975;24:721–735. doi: 10.1016/0026-0495(75)90040-2. [DOI] [PubMed] [Google Scholar]
- Muntjewerff JW, Kahn RS, Blom HJ, den Heijer M. Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: a meta-analysis. Mol Psychiatry. 2006;11:143–149. doi: 10.1038/sj.mp.4001746. [DOI] [PubMed] [Google Scholar]
- Murashita J, Kato T, Shioiri T, Inubushi T, Kato N. Altered brain energy metabolism in lithium-resistant bipolar disorder detected by photic stimulated 31P-MR spectroscopy. Psychol Med. 2000;30:107–115. doi: 10.1017/s0033291799001439. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009:1–14. [PubMed] [Google Scholar]
- Nash SR, Giros B, Kingsmore SF, Rochelle JM, Suter ST, Gregor P, Seldin MF, Caron MG. Cloning, pharmacological characterization, and genomic localization of the human creatine transporter. Receptors Channels. 1994;2:165–174. [PubMed] [Google Scholar]
- Natural Standard. Creatine. [Last accessed March 1, 2012];Natural Standard Professional Monographs. 2012 from http://www.naturalstandard.com/index-abstract.asp?create-abstract=creatine.asp&title=Creatine.
- Nery FG, Stanley JA, Chen HH, Hatch JP, Nicoletti MA, Monkul ES, Matsuo K, Caetano SC, Peluso MA, Najt P. Normal metabolite levels in the left dorsolateral prefrontal cortex of unmedicated major depressive disorder patients: A single voxel 1H spectroscopy study. Psychiatry Research: Neuroimaging. 2009;174:177–183. doi: 10.1016/j.pscychresns.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Niklasson F, Agren H. Brain energy metabolism and blood-brain barrier permeability in depressive patients: analyses of creatine, creatinine, urate, and albumin in CSF and blood. Biol Psychiatry. 1984;19:1183–1206. [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. J Abnorm Psychol. 1993;102:20–28. doi: 10.1037//0021-843x.102.1.20. [DOI] [PubMed] [Google Scholar]
- O’Donnell T, Rotzinger S, Nakashima TT, Hanstock CC, Ulrich M, Silverstone PH. Chronic lithium and sodium valproate both decrease the concentration of myo-inositol and increase the concentration of inositol monophosphates in rat brain. Brain Res. 2000;880:84–91. doi: 10.1016/s0006-8993(00)02797-9. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Levitt J, Caplan R, Asarnow R, McCracken J, Toga A, Alger J. H MRSI evidence of metabolic abnormalities in childhood-onset schizophrenia. Neuroimage. 2004;21:1781–1789. doi: 10.1016/j.neuroimage.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Ohrmann P, Siegmund A, Suslow T, Pedersen A, Spitzberg K, Kersting A, Rothermundt M, Arolt V, Heindel W, Pfleiderer B. Cognitive impairment and in vivo metabolites in first-episode neuroleptic-naive and chronic medicated schizophrenic patients: A proton magnetic resonance spectroscopy study. J Psychiatr Res. 2007;41:625–634. doi: 10.1016/j.jpsychires.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Tachikawa M, Takanaga H, Shimizu H, Watanabe M, Hosoya K, Terasaki T. The blood-brain barrier creatine transporter is a major pathway for supplying creatine to the brain. J Cereb Blood Flow Metab. 2002;22:1327–1335. doi: 10.1097/01.WCB.0000033966.83623.7D. [DOI] [PubMed] [Google Scholar]
- Oliveira M, Furian A, Fighera M, Fiorenza N, Ferreira J, Rubin M, Mello C, Royes L. The involvement of the polyamines binding sites at the NMDA receptor in creatine-induced spatial learning enhancement. Behav Brain Res. 2008;187:200–204. doi: 10.1016/j.bbr.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Öngür D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Research: Neuroimaging. 2009;172:44–48. doi: 10.1016/j.pscychresns.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JW, Takahashi K. Cerebral energetic effects of creatine supplementation in humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1745–1750. doi: 10.1152/ajpregu.00717.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NC, Cecil KM, Strakowski SM, Adler CM, DelBello MP. Neurochemical Alterations in Adolescent Bipolar Depression: A Proton Magnetic Resonance Spectroscopy Pilot Study of the Prefrontal Cortex. J Child Adolesc Psychopharmacol. 2008;18:623–627. doi: 10.1089/cap.2007.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perasso L. Kinetics of creatine in blood and brain after intraperitoneal injection in the rat. Brain Res. 2003;974:37–42. doi: 10.1016/s0006-8993(03)02547-2. [DOI] [PubMed] [Google Scholar]
- Persky AM, Brazeau GA, Hochhaus G. Pharmacokinetics of the dietary supplement creatine. Clin Pharmacokinet. 2003;42:557–574. doi: 10.2165/00003088-200342060-00005. [DOI] [PubMed] [Google Scholar]
- Persky AM, Müller M, Derendorf H, Grant M, Brazeau GA, Hochhaus G. Single- and multiple-dose pharmacokinetics of oral creatine. J Clin Pharmacol. 2003;43:29–37. doi: 10.1177/0091270002239703. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Poortmans JR, Francaux M. Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med Sci Sports Exerc. 1999;31:1108–1110. doi: 10.1097/00005768-199908000-00005. [DOI] [PubMed] [Google Scholar]
- Pritchard NR, Kalra PA. Renal dysfunction accompanying oral creatine supplements. Lancet. 1998;351:1252–1253. doi: 10.1016/s0140-6736(05)79319-3. [DOI] [PubMed] [Google Scholar]
- Rae C, Digney AL, McEwan SR, Bates TC. Oral creatine monohydrate supplementation improves brain performance: a double-blind, placebo-controlled, cross-over trial. Proceedings of the Royal Society B: Biological Sciences. 2003;270:2147–2150. doi: 10.1098/rspb.2003.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez O, Jimenez E. Sexual dimorphism in rat cerebrum and cerebellum: different patterns of catalytically active creatine kinase isoenzymes during postnatal development and aging. Int J Dev Neurosci. 2002;20:627–639. doi: 10.1016/s0736-5748(02)00102-8. [DOI] [PubMed] [Google Scholar]
- Rawson E, Lieberman H, Walsh T, Zuber S, Harhart J, Matthews T. Creatine supplementation does not improve cognitive function in young adults. Physiol Behav. 2008;95:130–134. doi: 10.1016/j.physbeh.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Stringari RB, Gonçalves CL, Scaini G, Carvalho-Silva M, Jeremias GC, Jeremias IC, Ferreira GK, Streck EL, Hallak JE, Zuardi AW, Crippa JA, Quevedo J. Administration of harmine and imipramine alters creatine kinase and mitochondrial respiratory chain activities in the rat brain. Depression Research and Treatment. 2012;2012:1–7. doi: 10.1155/2012/987397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réus GZ, Stringari RB, Rezin GT, Fraga DB, Daufenbach JF, Scaini G, Benedet J, Rochi N, Streck EL, Quevedo J. Administration of memantine and imipramine alters mitochondrial respiratory chain and creatine kinase activities in rat brain. J Neural Transm. 2011 doi: 10.1007/s00702-011-0718-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Riehemann S, Volz HP, Wenda B, Hubner G, Rossger G, Rzanny R, Sauer H. Frontal lobe in vivo (31)P-MRS reveals gender differences in healthy controls, not in schizophrenics. NMR Biomed. 1999;12:483–489. doi: 10.1002/(sici)1099-1492(199912)12:8<483::aid-nbm589>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Rodriguez NR, DiMarco NM, Langley S. American College of Sports Medicine position stand. Nutrition and Athletic Performance Med Sci Sports Exerc. 2009;41(3):709–31. doi: 10.1249/MSS.0b013e31890eb86. [DOI] [PubMed] [Google Scholar]
- Roitman S, Green T, Osher Y, Karni N, Levine J. Creatine monohydrate in resistant depression: a preliminary study. Bipolar Disord. 2007;9:754–758. doi: 10.1111/j.1399-5618.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- Roy BD, Bourgeois JM, Mahoney DJ, Tarnopolsky MA. Dietary supplementation with creatine monohydrate prevents corticosteroid-induced attenuation of growth in young rats. Can J Physiol Pharmacol. 2002;80:1008–1014. doi: 10.1139/y02-129. [DOI] [PubMed] [Google Scholar]
- Royes L, Fighera M, Furian A, Oliveira M, Fiorenza N, Ferreira J, Dasilva A, Priel M, Ueda E, Calixto J. Neuromodulatory effect of creatine on extracellular action potentials in rat hippocampus: Role of NMDA receptors. Neurochem Int. 2008;53:33–37. doi: 10.1016/j.neuint.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Ryu H, Rosas HD, Hersch SM, Ferrante RJ. The therapeutic role of creatine in Huntington’s disease. Pharmacol Ther. 2005;108:193–207. doi: 10.1016/j.pharmthera.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Salomons GS, van Dooren SJ, Verhoeven NM, Cecil KM, Ball WS, Degrauw TJ, Jakobs C. X-linked creatine- transporter gene (SLC6A8) defect: a new creatine-deficiency syndrome. Am J Hum Genet. 2001;68:1497–1500. doi: 10.1086/320595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches M, Newberg AR, Soares JC. Emerging drugs for bipolar disorder. Expert Opin Emerg Drugs. 2010;15:453–466. doi: 10.1517/14728214.2010.492393. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martinez-Gonzalez MA. Association between folate, vitamin B(6) and vitamin B(12) intake and depression in the SUN cohort study. J Hum Nutr Diet. 2009;22:122–133. doi: 10.1111/j.1365-277X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- Santos PM, Scaini G, Rezin GT, Benedet J, Rochi N, Jeremias GC, Carvalho-Silva M, Quevedo J, Streck EL. Brain creatine kinase activity is increased by chronic administration of paroxetine. Brain Res Bull. 2009;80:327–330. doi: 10.1016/j.brainresbull.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Vollmayr B, Neumann-Haefelin C, Ende G, Hoehn M, Henn FA. Specific creatine rise in learned helplessness induced by electroconvulsive shock treatment. Neuroreport. 2003;14:2199–2201. doi: 10.1097/00001756-200312020-00013. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Stanley JA, Axelson D, Brambilla P, Nicoletti MA, Keshavan MS, Ramos RT, Ryan N, Birmaher B, Soares JC. Reduced NAA levels in the dorsolateral prefrontal cortex of young bipolar patients. Am J Psychiatry. 2005;162:2109–2115. doi: 10.1176/appi.ajp.162.11.2109. [DOI] [PubMed] [Google Scholar]
- Sautter FJ, McDermott BE, Cornwell J, Johnson J, Borges A, Wilson AF, Vasterling JJ, Foundas AL. A preliminary study of the neuropsychological heterogeneity of familial schizophrenia. Schizophr Res. 1995;18:1–7. doi: 10.1016/0920-9964(95)00015-1. [DOI] [PubMed] [Google Scholar]
- Schulze A, Bachert P, Schlemmer H, Harting I, Polster T, Salomons GS, Verhoeven NM, Jakobs C, Fowler B, Hoffmann GF, Mayatepek E. Lack of creatine in muscle and brain in an adult with GAMT deficiency. Ann Neurol. 2003;53:248–251. doi: 10.1002/ana.10455. [DOI] [PubMed] [Google Scholar]
- Schuff N, Neylan T, Foxbosetti S, Lenoci M, Samuelson K, Studholme C, Kornak J, Marmar C, Weiner M. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Research: Neuroimaging. 2008;162:147–157. doi: 10.1016/j.pscychresns.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweid DE, Steinberg JS, Sudak HS. Creatine phosphokinase and psychosis. Arch Gen Psychiatry. 1972;26:263–265. doi: 10.1001/archpsyc.1972.01750210071013. [DOI] [PubMed] [Google Scholar]
- Segal M, Avital A, Drobot M, Lukanin A, Derevenski A, Sandbank S, Weizman A. Serum creatine kinase level in unmedicated nonpsychotic, psychotic, bipolar and schizoaffective depressed patients. Eur Neuropsychopharmacol. 2007;17:194–198. doi: 10.1016/j.euroneuro.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Seifried HE. Oxidative stress and antioxidants: a link to disease and prevention? J Nutr Biochem. 2007;18:168–171. doi: 10.1016/j.jnutbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Sestili P, Martinelli C, Bravi G, Piccoli G, Curci R, Battistelli M, Falcieri E, Agostini D, Gioacchini AM, Stocchi V. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med. 2006;40:837–849. doi: 10.1016/j.freeradbiomed.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Sestili P, Martinelli C, Colombo E, Barbieri E, Potenza L, Sartini S, Fimognari C. Creatine as an antioxidant. Amino Acids. 2011;40:1385–1396. doi: 10.1007/s00726-011-0875-5. [DOI] [PubMed] [Google Scholar]
- Shao A, Hathcock JN. Risk assessment for creatine monohydrate. Regul Toxicol Pharmacol. 2006;45:242–251. doi: 10.1016/j.yrtph.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Sheth NP, Sennett B, Berns JS. Rhabdomyolysis and acute renal failure following arthroscopic knee surgery in a college football player taking creatine supplements. Clin Nephrol. 2006;65:134–137. doi: 10.5414/cnp65134. [DOI] [PubMed] [Google Scholar]
- Shioiri T, Kato T, Murashita J, Hamakawa H, Inubushi T, Takahashi S. High-energy phosphate metabolism in the frontal lobes of patients with panic disorder detected by phase-encoded 31P-MRS. Biol Psychiatry. 1996;40:785–793. doi: 10.1016/0006-3223(95)00487-4. [DOI] [PubMed] [Google Scholar]
- Snow RJ, Murphy RM. Creatine and the creatine transporter: a review. Mol Cell Biochem. 2001;224:169–181. doi: 10.1023/a:1011908606819. [DOI] [PubMed] [Google Scholar]
- Sobolewski EJ, Thompson BJ, Smith AE, Ryan ED. The physiological effects of creatine supplementation on hydration: A review. Amer J Lifestyle Med. 2011 doi: 10.1177/1559827611406071. Epub ahead of print. [DOI] [Google Scholar]
- Soni SD. Serum creatine phosphokinase in acute psychosis. Br J Psychiatry. 1976;128:181–183. doi: 10.1192/bjp.128.2.181. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Morris DW, Angelucci F, Rubino IA, Spoletini I, Bria P, Martinotti G, Siracusano A, Bonaviri G, Bernardini S. BDNF Val66Met polymorphism is associated with aggressive behavior in schizophrenia. Eur Psychiatry. 2010;25:311–313. doi: 10.1016/j.eurpsy.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Stead LM, Au KP, Jacobs RL, Brosnan ME, Brosnan JT. Methylation demand and homocysteine metabolism: effects of dietary provision of creatine and guanidinoacetate. Am J Physiol Endocrinol Metab. 2001;281:E1095–1100. doi: 10.1152/ajpendo.2001.281.5.E1095. [DOI] [PubMed] [Google Scholar]
- Stöckler S, Hanefeld F, Frahm J. Creatine replacement therapy in guanidinoacetate methyltransferase deficiency, a novel inborn error of metabolism. Lancet. 1996;348:789–790. doi: 10.1016/s0140-6736(96)04116-5. [DOI] [PubMed] [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Streck E, Amboni G, Scaini G, Dipietro P, Rezin G, Valvassori S, Luz G, Kapczinski F, Quevedo J. Brain creatine kinase activity in an animal model of mania. Life Sci. 2008;82:424–429. doi: 10.1016/j.lfs.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Streijger F, Jost CR, Oerlemans F, Ellenbroek BA, Cools AR, Wieringa B, Van der Zee CE. Mice lacking the UbCKmit isoform of creatine kinase reveal slower spatial learning acquisition, diminished exploration and habituation, and reduced acoustic startle reflex responses. Mol Cell Biochem. 2004:256–257. 305–318. doi: 10.1023/b:mcbi.0000009877.90129.e3. [DOI] [PubMed] [Google Scholar]
- Streijger F, Oerlemans F, Ellenbroek B, Jost C, Wieringa B, Vanderzee C. Structural and behavioural consequences of double deficiency for creatine kinases BCK and UbCKmit. Behav Brain Res. 2005;157:219–234. doi: 10.1016/j.bbr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Stroup T. Heterogeneity of Treatment Effects in Schizophrenia. Am J Med. 2007;120:S26–S31. doi: 10.1016/j.amjmed.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Ann Neurol. 2000;48:723–729. [PubMed] [Google Scholar]
- Tarnopolsky MA. Potential benefits of creatine monohydrate supplementation in the elderly. Curr Opin Clin Nutr Metab Care. 2000;3:497–502. doi: 10.1097/00075197-200011000-00013. [DOI] [PubMed] [Google Scholar]
- Tayoshi SY, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, Iga J-i, Nakataki M, Ueno S-i, Harada M, Ohmori T. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS) Schizophr Res. 2009;108:69–77. doi: 10.1016/j.schres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Thorsteinsdottir B, Grande JP, Garovic VD. Acute renal failure in a young weight lifter taking multiple food supplements, including creatine monohydrate. J Ren Nutr. 2006;16:341–345. doi: 10.1053/j.jrn.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Tuason VB, Oleshansky MA, Jaranson JM. Creatine phosphokinase in functional psychosis. Compr Psychiatry. 1974;15:435–438. doi: 10.1016/0010-440x(74)90042-x. [DOI] [PubMed] [Google Scholar]
- Usala T, Clavenna A, Zuddas A, Bonati M. Randomised controlled trials of selective serotonin reuptake inhibitors in treating depression in children and adolescents: A systematic review and meta-analysis. Eur Neuropsychopharmacol. 2008;18:62–73. doi: 10.1016/j.euroneuro.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Vahedi K, Domigo V, Amarenco P, Bousser MG. Ischaemic stroke in a sportsman who consumed MaHuang extract and creatine monohydrate for body building. J Neurol Neurosurg Psychiatry. 2000;68:112–113. doi: 10.1136/jnnp.68.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hart MGC, Czéh B, de Biurrun G, Michaelis T, Watanabe T, Natt O, Frahm J, Fuchs E. Substance P receptor antagonist and clomipramine prevent stress-induced alterations in cerebral metabolites, cytogenesis in the dentate gyrus and hippocampal volume. Mol Psychiatry. 2002;7:933–941. doi: 10.1038/sj.mp.4001130. [DOI] [PubMed] [Google Scholar]
- Venkatraman TN, Krishnan RR, Steffens DC, Song AW, Taylor WD. Biochemical abnormalities of the medial temporal lobe and medial prefrontal cortex in late-life depression. Psychiatry Res Neuroimaging. 2009;172:49–54. doi: 10.1016/j.pscychresns.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal G, Petropoulous H, Hamilton DA, Rowland LM, Horan WP, Griego JA, Moreshead M, Hart BL, Brooks WM. Proton magnetic resonance spectroscopy of the hippocampus and occipital white matter in PTSD. Can J Psychiatry. 2002;47:666–670. doi: 10.1177/070674370204700709. [DOI] [PubMed] [Google Scholar]
- Vinberg M, Trajkovska V, Bennike B, Knorr U, Knudsen GM, Kessing LV. The BDNF Val66Met polymorphism: Relation to familiar risk of affective disorder, BDNF levels and salivary cortisol. Psychoneuroendocrinology. 2009;34:1380–1389. doi: 10.1016/j.psyneuen.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Volek JS, Duncan ND, Mazzetti SA, Putukian M, Gomez AL, Kraemer WJ. No effect of heavy resistance training sand creatine supplementation on blood lipids. Int J Sport Nutr Exerc Metab. 2000;10:144–156. doi: 10.1123/ijsnem.10.2.144. [DOI] [PubMed] [Google Scholar]
- Volz HP, Hubner G, Rzanny R, Rossger G, Preussler B, Eichhorn M, Kreitschmann-Andermahr I, Kaiser WA, Sauer H. High-energy phosphates in the frontal lobe correlate with Wisconsin Card Sort Test performance in controls, not in schizophrenics: a 31phosphorus magnetic resonance spectroscopic and neuropsychological investigation. Schizophr Res. 1998;31:37–47. doi: 10.1016/s0920-9964(97)00157-6. [DOI] [PubMed] [Google Scholar]
- Volz HP, Rzanny R, Riehemann S, May S, Hegewald H, Preussler B, Hubner G, Kaiser WA, Sauer H. 31P magnetic resonance spectroscopy in the frontal lobe of major depressed patients. Eur Arch Psychiatry Clin Neurosci. 1998;248:289–295. doi: 10.1007/s004060050052. [DOI] [PubMed] [Google Scholar]
- Volz HP, Rzanny R, Rossger G, Hubner G, Kreitschmann-Andermahr I, Kaiser WA, Sauer H. Decreased energy demanding processes in the frontal lobes of schizophrenics due to neuroleptics? A 31P-magneto-resonance spectroscopic study. Psychiatry Res. 1997;76:123–129. doi: 10.1016/s0925-4927(97)00047-4. [DOI] [PubMed] [Google Scholar]
- Volz HR, Riehemann S, Maurer I, Smesny S, Sommer M, Rzanny R, Holstein W, Czekalla J, Sauer H. Reduced phosphodiesters and high-energy phosphates in the frontal lobe of schizophrenic patients: a (31)P chemical shift spectroscopic- imaging study. Biol Psychiatry. 2000;47:954–961. doi: 10.1016/s0006-3223(00)00235-3. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281 ( Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Kato N, Kato T. Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neurosci Res. 2002;42:279–285. doi: 10.1016/s0168-0102(02)00007-x. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology. 1996;14:1S–11S. doi: 10.1016/0893-133X(95)00199-N. [DOI] [PubMed] [Google Scholar]
- Wick M, Fujimori H, Michaelis T, Frahm J. Brain water diffusion in normal and creatine-supplemented rats during transient global ischemia. Magn Reson Med. 1999;42:798–802. doi: 10.1002/(sici)1522-2594(199910)42:4<798::aid-mrm23>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Willis J, Jones R, Nwokolo N, Levy J. Protein and creatine supplements and misdiagnosis of kidney disease. BMJ. 2010;340:b5027. doi: 10.1136/bmj.b5027. [DOI] [PubMed] [Google Scholar]
- Wong ET, Cobb C, Umehara MK, Wolff GA, Haywood LJ, Greenberg T, Shaw ST., Jr Heterogeneity of serum creatine kinase activity among racial and gender groups of the population. Am J Clin Pathol. 1983;79:582–586. doi: 10.1093/ajcp/79.5.582. [DOI] [PubMed] [Google Scholar]
- Wood S, Berger G, Wellard R, Proffitt T, McConchie M, Velakoulis D, McGorry P, Pantelis C. A 1H-MRS investigation of the medial temporal lobe in antipsychotic-naïve and early-treated first episode psychosis. Schizophr Res. 2008;102:163–170. doi: 10.1016/j.schres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Yucel M, Pantelis C, Berk M. Neurobiology of schizophrenia spectrum disorders: the role of oxidative stress. Ann Acad Med Singapore. 2009;38:396–396. [PubMed] [Google Scholar]
- Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- Wyss M, Schulze A. Health implications of creatine: can oral creatine supplementation protect against neurological and atherosclerotic disease? Neuroscience. 2002;112:243–260. doi: 10.1016/s0306-4522(02)00088-x. [DOI] [PubMed] [Google Scholar]
- Wyss M, Wallimann T. Creatine metabolism and the consequences of creatine depletion in muscle. Mol Cell Biochem. 1994:133–134. 51–66. doi: 10.1007/BF01267947. [DOI] [PubMed] [Google Scholar]
- Yildiz-Yesiloglu A, Ankerst D. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: A meta-analysis. Psychiatry Research: Neuroimaging. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Yoo SY, Yeon S, Choi CH, Kang DH, Lee JM, Shin NY, Jung WH, Choi JS, Jang DP, Kwon JS. Proton magnetic resonance spectroscopy in subjects with high genetic risk of schizophrenia: Investigation of anterior cingulate, dorsolateral prefrontal cortex and thalamus. Schizophr Res. 2009;111:86–93. doi: 10.1016/j.schres.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Young EA, Kornstein SG, Marcus SM, Harvey AT, Warden D, Wisniewski SR, Balasubramani GK, Fava M, Trivedi MH, John Rush A. Sex differences in response to citalopram: A STAR*D report. J Psychiatr Res. 2009;43:503–511. doi: 10.1016/j.jpsychires.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JF, Larsen LB, Malmendal A, Nielsen NC, Straadt IK, Oksbjerg N, Bertram HC. Creatine-induced activation of antioxidative defence in myotube cultures revealed by explorative NMR-based metabonomics and proteomics. J Int Soc Sports Nutr. 2010;7:9. doi: 10.1186/1550-2783-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LT. Is bipolar disorder a mitochondrial disease? J Psychiatry Neurosci. 2007;32:160–161. [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Renshaw PF, Gruber SA, Ed M, Waternaux C, Cohen BM. Proton magnetic resonance spectroscopy of the temporal lobes in schizophrenics and normal controls. Schizophr Res. 1996;19:55–59. doi: 10.1016/0920-9964(95)00071-2. [DOI] [PubMed] [Google Scholar]