Abstract
The Power of Food Scale (PFS) is a new measure that assesses the drive to consume highly palatable food in an obesogenic food environment. The data reported in this investigation evaluate whether the PFS moderates state cravings, control beliefs, and brain networks of older, obese adults following either a short-term post-absorptive state, in which participants were only allowed to consume water, or a short-term energy surfeit treatment condition, in which they consumed BOOST®. We found that the short-term post-absorptive condition,in which participants consumed water only, was associated withincreases in state cravings for desired food, a reduction in participants' confidence related to the control of eating behavior, and shifts in brain networks that parallel what is observed with other addictive behaviors. Furthermore, individuals who scored high on the PFSwere at an increased risk for experiencing these effects. Future research is needed to examine the eating behavior of persons who score high on the PFS and to develop interventions that directly target food cravings.
Keywords: aging, brain networks, food, cravings, self-efficacy
With the twofold increase in obesity over the past 20 years(Flegal, 2005) and the fact that older adults have not escaped this epidemic(Villareal, Apovian, Kushner, & Klein, 2005),there is an increased urgency to better understand the etiology of eating behavior in this population. This problem is particularly timely given the Graying of America will occur over the next 15–30 years(Manton & Vaupel, 1995). In response to this need, we examine how either a short-term post-absorptive state, in which participants were only allowed to consume water, or a short-term energy surfeit treatment condition, in which they consumed BOOST®, influence older adults'(a) craving for desired foods, (b) self-regulatory beliefstowards controlling eating behavior, and (c) brain networks. A question of particular interest iswhether these responses are moderated by scores on the Power of Food Scale (PFS)(Lowe et al., 2009).
Lowe and colleagues(Lowe et al., 2009) have shown that people differ in the drive to consume highly palatable food withinobesogenic food environments; a characteristic assessed using the PFS. These authors found that the PFS was significantly related to the disinhibition (r = .61) and hunger scales (r = .63) of the three factor eating questionnaire, as well as the emotional and external eating subscales of the Dutch Eating Behavior Questionnaire, r = .54 and r = .66, respectively. Within the appetite literature, Clark and colleagues (Clark, Abrams, Niaura, Eaton, & Rossi, 1991) have proposed that participants' confidence in their ability to resist eating due to internal states and external circumstances represents an important cognitive dimension of self-regulation.Despite favorable findings withthis construct (Linde, Rothman, Baldwin, & Jeffery, 2006; Rejeski, Mihalko, Ambrosius, Bearon, & McClelland, 2011; Richman, Loughnan, Droulers, Steinbeck, & Caterson, 2001), recent work by Nordgren and colleagues has argued that health cognitions are unstable and profoundly influenced by visceral states such as hunger and appetite (Nordgren, van der Pligt, & van, 2008; Nordgren, van, & van der Pligt, 2009). Because our interest was studying responses to two different short-term post-absorptive states, one with water and a surfeit treatment condition with BOOST®, we employed a brief state-based measure of confidence for controlling eating behavior (CCEBstate) that was developed in line with work by Bandura (1986) and a measure of state craving developed by Cepeda-Benito and colleagues(Cepeda-Benito, Gleaves, Williams, & Erath, 2000).
Finally, Alonso-Alonso and Pascual-Leone(Alonso-Alonso & Pascual-Leone, 2007) have proposed that dysfunction in the right prefrontal cortex (PFC) is a root cause of obesity. That is, rather than the appetitive drive per se being the cause of overconsumption, they argue that it is the inability of the right PFC to effectively self-regulate eating behavior. Central to the current investigation is the well documented effect that the craving to consume food increases with exposure to(Kelley, Schiltz, & Landry, 2005) and the active imaging of food cues(Pelchat, Johnson, Chan, Valdez, & Ragland, 2004), increases in cravingthat werefound to be related to activation in the hippocampus, insula, and caudate.
Therefore, in this study,we sought to determine whether scores on the PFS would be related to the CCEBstate and state cravingsfollowing two different short-term post-absorptive states, one in which participants were allowed to consumewater only and a second surfeit treatment condition in which participants consumed BOOST®. Research has shown that liquid meal replacements are an effective strategy for curbing short term hunger (Mattes & Rothacker, 2001). The hypothesis was that individuals scoring high on the PFS would exhibit a substantial increase in food craving and a loss of control related to eating behavior as compared to those scoring low on the PFS, and that this effect would be magnified in the short-term post-absorptive treatment condition in which participants consumed water only.
Additionally, we examined brain networks after repeated exposure to food cues expecting that the short-term post-absorption state, in which participants consumed water only, would most likely yield changes in the brain regions associated with craving, particularly increased connectivity in the basal ganglia and insula (Pelchat, Johnson, Chan, Valdez, & Ragland, 2004), and that these effects would be accentuated for those scoring high on the PFS. We also predicted that alterations in basal ganglia connectivity would be associated with changes in motor system connectivity as a reflection of food-seeking motivation. Parenthetically, we focused on the sensorimotor cortex because of the conceptual nature of the research question. That is, we manipulated sensory cues (i.e., palatable food) and reasoned that individual difference in the drive to consume palatable food (i.e., the PFS) would increase activity in sensorimotor networks.
Engaging external environmental cues, as promoted by the food cue manipulation employed in the current study, can result in alterations in the activity and connectivity of the default-mode brain network (DMN), since this network represents the resting state of the brain (Gusnard & Raichle, 2001; Raichle et al., 2001). The brain areas that make up the DMN include precuneus/posterior cingulate extending into the medial temporal lobe, anterior cingulate/medial prefrontal cortex, and bilateral occipito-parietal cortices. Furthermore, it has recently been demonstrated that the posterior cingulate/precuneus portion of this network is one of the most highly connected regions in the brain and serves as a brain network hub for the DMN(Hagmann et al., 2008; Sporns, Honey & Kotter, 2007). Therefore, the posterior cingulate/precuneus region was selected as the primary region of interest for this sub-network and we anticipated that there would be greater disruption of this region in the short-term post-absorptive state with water only, particularly for those scoring high on the PFSdue to a lingering preoccupation with the food cues(Kavanagh et al., 2005).
Methods
Participants
A sample (n = 22) ofobese (BMI ≥30 kg/m2 but ≤40 kg/m2), sedentaryolder adults (50–80 years of age) was recruited from Forsyth County, NC.All were Caucasians and were excluded if they were either actively dieting orinvolved in more than 60 min of structured exercise each week. Active dieting was defined as currently involved in a research study of weight loss, participating in a commercial weight loss program, or engaging in a self-directed program to lose weight. Structured exercise was any structured type of aerobic or resistance training performed in bouts lasting ≥10min. Both active dieting and structured exercise habits were assessed via interview. Other exclusion criteria included: (1) the presence of a systemic uncontrolled disease or psychiatric illness determined via self-report, (2) a binge eating disorder, (3) the inability to safely undergo magnetic resonance imaging, (4) currently undergoing active treatment for cancer, or (5) unable to read or speak English. Of the 22 that were randomized to treatment, 3 were unable to complete the study leaving a final n of 19. One individual was lost due to complications from preexisting back-pain, another became claustrophobic during the first day of scanning, and the third had a large artifact in the prefrontal region of the fMRI. Participantsreceived $225 to compensate for their time commitment.
Measures
Power of Food Scale (PFS)
The PFS assesses the drive to consume highly palatable food in an obesogenic food environment (Lowe et al., 2009); higher scores are associated with a higher drive. The total score has been shown to have good test-retest reliability (r = 0.77), is internal consistent (α = 0.91), and support exists for its construct validity(Lowe et al., 2009). Three subscale scores can be calculated: food available, food presence, and food tasted. Because the total score has such high internal consistency, we felt justified in restricting our attention to this single score of the PFS. Furthermore, examination of the separate subscales for the PFS fell beyond the scope of the current study.
Food Craving Questionnaire (theFCQstate)
The FCQstateassesses state craving for specific foods using a 5-point scale (1 = strongly disagree; 5 = strongly agree) with the mid-point being anchored by the label neutral. The FCQstateis based on a unifying construct and has a Cronbach alpha of 0.94. The FCQstateis distinct from the concept of Food Restraint and has been found to exhibit a statistically significantly reductioncompleted prior to and then following breakfast(Cepeda-Benito et al., 2000).
Confidence for Controlling Eating Behavior (CCEBstate)
We developed a 4-item measure of self-efficacy for eating behavior for the consumption of favorite foods that is state-based (Bandura, 1986). Participants rate their confidence in being able to resist or control eating their favorite food right now, at this moment. The items are rated on a 10 point scale ranging from 0 “not at all confident” to 10 “very confident”, with the anchor “moderately confident” spanning the values from 4–6 and centered at 5. The four items include the following: (1) if available, I could resist eating my favorite foods; (2) at the current time, I feel like I have good control over my appetite; (3) at the moment, I feel as if I could restrain myself from eating foods that I enjoy; and (4) currently I feel that I could avoid snacking between meals.In this sample, a principal component analysis yielded a single dimension that captured 78.4% of the item variance. All factor loadings were in excess of 0.80 with a Cronbach alpha reliability of 0.90.We have also examined the dimensionality of this measure in a larger sample of college students and found nearly identical results. Specifically, in a sample of 111 college undergraduates, 48 men and 63 women, we found that a single factor accounted for 72% of the variance in the 4 items that all items had loadings in excess of 0.70; the 4-item scale had a Cronbach alpha internal consistency reliability of 0.87.
The Interview for the Diagnosis of Eating Disorders (IDED-IV)
The semi-structured interview described by Kutlesic and colleagues(Kutlesic, Williamson, Gleaves, Barbin, & Murphy-Eberenz, 1998) was employed to exclude participants with a binge-eating disorder.
In-Person Screening & Assessments
An in-person screening visit was completed to obtain an informed consent, to gather biometric data, to assess whether participants were currently dieting, to assess volume of structured physical activity, and to screen for binge eating disorders. At this time participants were asked to both identify and rate the pleasantness of their two favorite foods. Eligible participants completed the PFS and were scheduled for two imaging visits (7–10 days apart).
Experimental Protocol for the Two Scanning Visits
Participants completed two, early morning 4-hour visits beginning around 8:00 am. During each visit, participants consumed a prepared breakfast: 350 calories for females; 450 calories for males. The breakfast meals were designed by a staff nutritionist to provide a heart healthy balance of macronutrients consisting of approximately 25% fat, 15% protein and 60% carbohydrate. Participants were allowed to choose macronutrients from a menu of options. Following breakfast, participants completed baseline assessments of the FCQstateand the CCEBstate. They then were not allowed to consume any food for 2.5-hours. During this period of food restriction, participants were only allowed to consume water and remained in the research center to be monitored by nursing staff. Approximately 45-min before the imaging procedure, participants completed an MRI safety form,if necessary a lense fitting procedure to correct for poor vision, and were then instructed and given practice on the task to be performed during the fMRI.
Once the fMRI forms and protocol had been described, participants either consumed a can of BOOST® (the short-term energy surfeit condition—240 calories) or consumed an equivalent volume of water (the short-term post-absorptive condition with water only)—NO BOOST®. They then completed a second round of the FCQstate and CCEBstate. The food restriction manipulation was counterbalanced. Specifically, 10 participants were randomly assigned to receive the BOOST® meal on their first visit, whereas the other 10 received the NO BOOST® manipulation; for the second visit, participants received the opposite treatment from the one that they received on the first visit.
Food Cue Scanning Task
Participants wore goggles in the scanner that were directly interfaced with a computer screen. The task that they performed involved the visualization of words that were presented on a computer screen for 30-sec each. There were two word blocks and 6 words in each block representing either 2 different neutral stimuli or 2 different favorite foods identified during the in-person screening. Within each block, the words were presented in random order with the restriction that each of the four words was presented at least once in each block. Each block lasted 5 min and 20 sec with a 20 sec visual cross fixation period preceding and following each word.
The instructions for the visualization phase of the task were as follows: “During the task, you will see words on the screen in front of you. Some of these words describe your favorite foods and others are non-food related. Each time a word appears, I want you to think about that word and what it represents. So, for example, if the words `baked potato' appeared, imagine the ingredients that you like to put on the potato, see the steam coming out of it, think about how it smells, its texture, and how it would taste. I want you to try to use as many senses as possible to come up with the best image you can. Hold on to that image for the entire time that the word is on the screen. Now, I want you to do the same thing for the non-food words. So, if the word `desk' appears, where is it? How many drawers does it have? Is the wood dark or light? Is it rough or smooth? Once again, hold onto that image for the entire time it is on the screen. Between each word, you will see a cross on the screen. During this time do not think about anything in particular, just focus on the cross. In addition, at two different times during the task, you will be asked to provide ratings of (1) your hunger, (2) craving for your favorite food, and (3) how vivid the image was. You will see your response to these scales using computer images that appear in your goggles.”
Immediately following each block, participants provided rating for their hunger, level of craving, and vividness of the images using visual analogue scales ranging from 0 (“not at all”) to 100 (“extreme”/”very well”). After completing the two-blocks of words and follow-up questions, participants were asked to simply lie quietly in the scanner and to focus on the cross for a final period which lasted 5 min and 20 sec. This post-exposure resting scanning period was used to examine brain networks.
Scanning Protocol
All scans were performed on a 1.5 T GE scanner using an 8-channel neurovascular head coil (GE Medical Systems, Milwaukee, WI, USA) and included anatomic imaging, perfusion imaging, two runs of fMRI with a food visualization task, and a post-exposure resting fMRI to evaluate differences in brain networks between the two treatment conditions and as moderated by scores on the PFS.
Functional images for the network analyses measured changes in the T2*-relaxation rate that accompany changes in blood oxygenation.The T2* signal is sensitive to changes in blood oxygen content. As brain activity changes the oxygen content of the blood in the same area also changes. Thus, the T2* signal is an indirect measure of changes in neural activity (Ogawa et al., 1990). Functional imaging was performed using multi-slice gradient-EPI (TR = 2000 ms; TE = 40 ms; field of view = 24 cm (frequency) × 15 cm (phase); matrix size = 96 × 86, 40 slices, 5 mm thickness, no skip; voxel resolution = 3.75 mm × 3.75 mm × 5 mm. The subjects performed no task but were asked to keep their eyes open looking at a fixation cross for the 5 min 20 s resting fMRI scan.
Statistical Analyses
The self-report data was analyzed using SAS Proc Mixed which allows testing of both fixed and random effects and inclusion of both subject level and individual measure level covariates and interaction terms. The random subject effect allows for covariances between the repeated measures within a given subject. The model we tested included (a) the baseline measure of the outcome variable for each subject assessed after breakfast prior to each experimental treatment(a covariate), (b) a fixed effect for treatment (BOOST® versus NOBOOST®), (c) the subject's score on the PFS, and (d) the interaction between treatment and the PFS that was entered as a vector. Order effects was tested in preliminary analyses and found to be non-significant.
Imaging Processing and Network Analyses
Prior to generating brain networks, all scanning images were realigned and normalized to standard space using FSL (Smith et al., 2004).The time courses were extracted for each voxel in gray matter based on the Automated Anatomical Labeling atlas(Tzourio-Mazoyer et al., 2002)and band-pass filtered to remove signals outside the range of 0.009–0.08 Hz (Biswal, Yetkin, Haughton, & Hyde, 1995; Fox et al., 2005). Mean white matter, CSF, and motion correction parameters were regressed from the filtered time series to account for physiological noise. A correlation matrix was then produced by computing Pearson correlations between all possible pairs of voxels (~21,000 voxels). This produced a 21,000 × 21,000 matrix with each cell (ij) representing the partial correlation coefficient between nodes i and j. A threshold was then applied to the correlation matrix and all cells that surpassed this threshold were assigned a value of 1. Remaining cells were set to zero. The threshold was defined such that the relationship between the number of nodes and average number of connections at each node was consistent across subjects to produce an adjacency matrix. Specifically, the relationship S=log(N)/log−(K) was the same across subjects as described previously (Hayasaka & Laurienti, 2010). For this paper, the threshold S = 2.5 was used. All remaining analyses were performed using the binary 21,000 × 21,000 adjacency matrix.
To assess network organization, two separate types of analyses were performed. The first type of analysis evaluated the number of connections between a region of interest (ROI) and the remaining brain voxels. The analysis used the pre- and post-central gyri as defined in the AAL as the region of interested. The first order connections (i.e. areas directed connected to the ROI) were assessed by counting the number of connections between the ROI and each voxel. Second order connections (i.e. connections one link away from the ROI) were assessed by counting the number of connections each brain voxel had with the first order connections. Direct connections to the sensorimotor cortex were very similar across conditions and groups, whereas second order connections revealed interesting findings. Given that the sensorimotor cortices are primary processing regions, it is not surprising that only minor differences were noted in first order connections. However, as one moves away from the primary cortices, differences in network connectivity become apparent. Thus, the results presented here focus on the second order connections from the sensorimotor cortex. Direct connections to the sensorimotor cortex were virtually identical and are not discussed.
The second type of analysis evaluatednetwork community structure. A network community, or neighborhood, is a group of nodes that are more highly interconnected with each other than they are with other neighborhoods in the network. Neighborhoods were identified using the modularity metric proposed by Newman(Newman & Girvan, 2004) using a spectral portioning algorithm(Ruan & Zhang, 2008).Group neighborhood consistency maps were generated using scaled inclusivity (SI) according to the method of Steen and colleagues (Steen, Hayasaka, Joyce, & Laurienti, 2011). The neighborhood consistency maps are derived such that the outcome is a spatial distribution showing the areas that belong to a particular neighborhood. This is a multivariate problem where membership in a community for any one voxel is dependent on the relationships between all voxels. Traditional statistical tests cannot be used due to the multivariate nature of the data. The most common method used to evaluate group community structure is to average the correlation matrices across subjects and generate a single neighborhood map. Unfortunately, such averaging does not maintain the network structure typical of the individual subjects (Simpson, Moussa, & Laurienti, in press). Thus, we prefer to use methods that demonstrate the consistency across subjects (Moussa et al., 2011; Burdette et al., 2010) and have recently demonstrated that SI effectively captures the community structure in known networks without observer bias (Steen, Hayasaka, Joyce, & Laurienti, 2011). While the SI maps are quantitative, the reader may want to view the images in a qualitative fashion to evaluate the spatial representation of the consistency of the particular community of interest. The values in each voxel (color-coded in the figures) represent how consistently across the group any one voxel belonged to the neighborhood in question. While not a traditional statistic, one can consider the value of each voxel as the confidence, relative to all other voxels, that it belongs to the neighborhood in question.
Results
Descriptive data for the sample can be found in Table 1. The success of the BOOST® manipulation is supported by the statistically significant treatmentdifference in the 3-item FCQstate hunger subscale score following 2.5h of food restriction; hunger was higher in the NO BOOST® [Mean (SE) = 8.30 (0.76)] than BOOST® condition [6.15 (0.61);Mean Difference = 2.12; t (19) = 2.33, p = .03). It is important to note, however, that ratings of hunger were not excessive in the NO BOOST®treatment condition given that the minimumscore for hunger subscale of the FCQ hunger is 3 and the maximum is 15. This is why it is best to conceptualize the NOBOOST ® condition as a short-term post-absorptive state, in which participants were only allowed to consume water, and the BOOST® condition as a short-term energy surfeit treatment condition, in which they consumed BOOST. Of interest is that fact that ratings of hunger and cravings on the 100 unit VAS scales during the presentation of food cues in the scanner werealso higher in the NOBOOST® condition than in the BOOST® condition: M (SE) for hunger = 64.87 (6.78) and 32.80 (6.61), respectively [t (19) = 4.47, p <.001]; for craving the M (SE) were 60.19 (7.39) and 42.75 (7.69), respectively [t (19) = 2.64, p = .016]. In addition, participants' reported a relatively high degree of vividness for the imagery of the food and non-food cues during the fMRI protocol, although the cues were reported to be somewhat more vivid in the NO BOOST® condition: M(SE) for NO BOOST® and BOOST® conditions were 87.21 (2.41) and 83.47 (1.98), respectively [t (19) = 2.19, p = .044].
Table 1.
Descriptive Characteristics of Participants
| Characteristic | Mean (±SD) or N (%) | |
|---|---|---|
| Age | 64.65 (±6.84) | |
| Sex | ||
| Men | 8 (40%) | |
| Women | 12(60%) | |
| Education | ||
| High School | 8 (40%) | |
| 4-year College | 6 (30%) | |
| Post-Graduate | 6 (30%) | |
| Income (annual) | ||
| <$35,000 | 6 (30%) | |
| $35,000–$49,999 | 4 (20%) | |
| $50,000–$74,999 | 5 (25%) | |
| >75,000+ | 5 (25%) | |
| BMI (kg/m2) | 33.97 (±2.67) | |
| Weekly Exercise (min) | 7.75 (±14.18) | |
| Smoking History | ||
| Never Smoked | 18 (90%) | |
| Past Smoker | 2 (10%) | |
| Comorbidities | ||
| Cardiovascular | 5 (25%) | |
| Hypertension | 12 (60%) | |
| Arthritis | 8 (40%) | |
| Diabetes | 4 (20%) | |
| Cancer | 2 (10%) |
The M ±SD for the PFS total score was 2.76 (1.05). It is also interesting to point out that BMI was not found to be related to scores on the PFS (r = .29, p = .21); however, the PFS had a strong relationshipwith scores on the IDED-IV, r = .65, p = .002.
Treatment Effects on Food Craving and Self-Efficacy
Separate mixed model ANCOVAs were employed to examine data for the FCQstate and the CCEBstate. For the food craving model, the baseline covariate was significant (p< .001) and there was a main effect for the PFS (F (1, 17) = 5.31, p = .03) and a marginal PFS by treatment interaction term (F (1, 17) = 3.95, p = .06). The main effect for the PFS indicated that, averaged over the BOOST® and the NO BOOST® treatments, those with higher PFS scores had higher FCQstate scores than those scoring low on the PFS.Table 2 provides estimates of treatment differences at 4 different values of thePFS—the average, 1.0, 2.0, 3.2, and 4.6. Note that participants with average values on the PFS or higher—3.2 and 4.6—had higher FCQstate scores (i.e., higher cravings) on the day that they did not consume BOOST® as compared to the day they did consume BOOST®. No such difference was observed for low scores on the PFS. This pattern in the data is consistent with associations between the PFS and FCQstate within each treatment condition: = .36 (p = .11) with BOOST® andr= .57 (p= .009) in the NO BOOST® condition. Moreover, when correlating PFS scores with ratings of hunger and craving during the fMRI protocol, the relationships were higher in magnitude and statistically significant in the NOBOOST® condition as compared to the BOOST® condition: r = .46 (p = .05) for hunger and r = .58 (p = .008) for craving in the NOBOOST® condition; r = .22 (p = .35) for hunger and r = .36 (p = .12) for craving in the BOOST® condition.
Table 2.
LS Mean Treatment Differences in State Craving by PFS Scores*
| PF Score | Estimated Treatment Difference | Standard Error | t Value | p Value |
|---|---|---|---|---|
| 2.76** | 7.12 | 2.34 | 3.05 | .007 |
| 1.00 | −0.86 | 4.64 | −0.19 | .855 |
| 2.00 | 3.68 | 2.90 | 1.27. | .223 |
| 3.20 | 9.12 | 2.54 | 3.58 | .002 |
| 4.60 | 15.47 | 3.21 | 13.21 | .005 |
The least square mean treatment differences, standard errors and associated tests are directly derived from the SAS Proc Mixed procedure which allows for both random (here subject) effect(s) and the aforementioned “fixed” effects of treatment, baseline covariate, PFS and the PFS*treatment interaction. Note that the estimated treatment difference varies with PFS because of the PFS*treatment interaction. As in regression, standard error of the treatment difference is smallest at the average value (2.76) of the regressor (i.e., the Power of Food Scale). The values chosen other than the mean were selected to represent the distribution of the scores.
The mean score for the Power of Food Scale.
For the CCEBstate measure, there was a significant effect for the baseline covariate (p < .001). More important, there was a significant PFS by treatment interaction term (F (1, 17) = 7.97, p = .01). As shown in Table 3, participants scoring at the average value of the PFS and those scoring above—3.20 and 4.60— had significantly lower CCEBstate scores (i.e., lower perceived self-control) when in the NOBOOST® as compared to the BOOST® condition, a pattern that makes sense give that low score on the CCEBstatedescribe contexts in whichpeople lack control. Once again, this effect was consistent with the lack of a relationship betweenPFS scores and the CCEBstatewhile on BOOST®,r= −.19 (p = .43), yetthe moderate relationship observed between these two variables in the NO BOOST® condition, r = −.56 (p < .01).
Table 3.
LS Mean Treatment Differences in Self-Efficacy by PFS Scores
| PF Score | Estimated Treatment Difference | Standard Error | t Value | p Value |
|---|---|---|---|---|
| 2.76* | −15.61 | 3.97 | −3.93 | .001 |
| 1.00 | 3.59 | 7.90 | 0.45 | .655 |
| 2.00 | −7.32 | 4.95 | −1.48 | .158 |
| 3.20 | −20.41 | 4.31 | .0002 | |
| 4.60 | −35.68 | 8.12 | −4.39 | .0004 |
represents the mean PFS Score; PFS = Power of Food Scale
Network Analyses
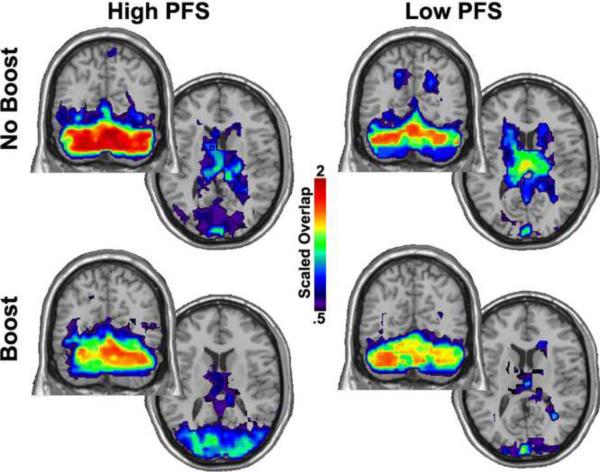
As shown in Figure 1, the neighborhood connectivity of the cerebellum included consistent interactions with the basal ganglia and thalamus in the NOBOOST® condition; this effect was particular prominent for participants that scored high on the PFS. In addition, this latter subgroup also exhibited a high connectivity between these regions and the visual cortex.
Figure 1. Neighborhood of the cerebellum by PFS category.
The figure shows a single coronal slice through the cerebellum and an axial slice through the basal ganglia and thalamus. The color bar represents the SI value (described in methods) scaled by population size. This is a relative value that indicates the consistency of the community structure across subjects. Note that the basal ganglia and thalamus are consistently in the cerebellum neighborhood in the NO BOOST condition. A larger extent of visual cortex is in the cerebellum neighborhood in the High PFS group. Also note the reduction in consistency of the cerebellum itself in the High PFS group from the NO BOOST condition to the BOOST condition. Right side of the images represents the right side of the brain in this and all subsequent images.
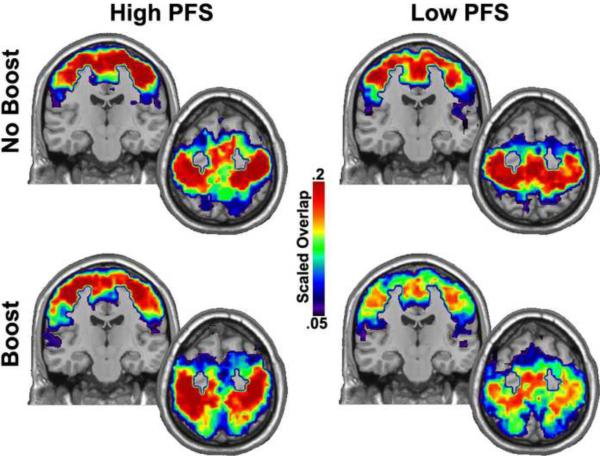
Figure 2 shows the network neighborhood for the sensorimotor cortex. This neighborhood was focused upon the pre- and post-central gyri that represent the primary sensory and motor cortices. The neighborhood also included the medial and lateral premotor areas anterior to the primary cortices. The neighborhood was highly interconnected in the NO BOOST® condition for both study populations. In the BOOST® condition the low PFS group showed a dramatic loss in consistency in this neighborhood, an effect not observed in the high PFS group. This change in neighborhood consistency suggests that the sensorimotor cortex was engaged in novel network connectivity in the BOOST® condition for the high PFS group.
Figure 2. Neighborhood of the sensorimotor cortex by PFS Category.
These Images show an axial and coronal slice through the sensorimotor area including medial regions. Also, note that the neighborhood extends anterior into premotor regions. Both groups exhibit consistent organization within this spatially focused neighborhood. There is a relative reduction in the consistency across subjects in the Low PFS group in the BOOST condition. The color bar represents the SI value (described in the methods) scaled by population size.
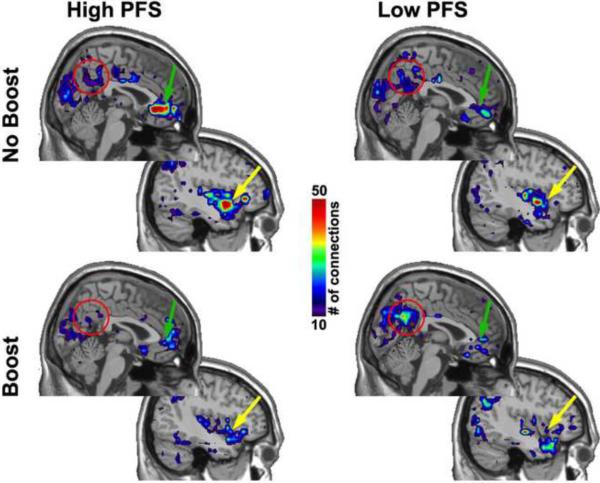
Because the precuneus is the hub of the default mode network (DMN) (Hagmann et al. 2008), a 12 mm spherical region of interest was placed in this region to calculate the average number of connections in each subject and condition. The mean connectivity in the precuneus was then compared with an independent t-test to compare groups and a dependent t-test to compare conditions within groups. The analyses revealed that connectivity was significantly increased in the Low PFS group following BOOST® compared to NO BOOST® (p = 0.04). In addition, the Low PFS group on BOOST® had higher connectivity (p=0.05) than the High PFS group on BOOST®. Other comparisons were not significant. The connectivity maps shown in Figure 3 reflect those areas that were within one step of the sensorimotor cortex. The red circle in Figure 3highlights the precuneus. Examination of the precuneus across the four panels reveals that only older adults' who consumed BOOST® and scored low on the PFS had high connectivity between the sensorimotor cortex andthis region. This suggests that the liquid meal replacement was effective in allowing the brains of these older adults to return to the default-mode after exposure to food cues. Not so for those participants who scored high on the PFS. Despite being fed a liquid meal replacement, their precuneus was not within one connection of the sensorimotor region. This pattern was consistent with connectivity between the medial prefrontal and orbital frontal cortex (green arrows) and the insula (yellow arrows), suggesting that these older adults are predisposed to process internal cues related to food and that these cues dominate conscious thought, a hypothesis that is consistent with responses to the FCQ and the CCEBstate completed just prior to entering the scanner.
Figure 3. Maps showing the connectivity to sensorimotor cortex.
These maps show two sagital slices. The upper left image is directly at midline and the other image is sliced through the insular cortex on the right side of the brain. Results on the left were virtually identical and therefore are not shown. Colored regions show the areas that are within one link of sensorimotor cortex based on functional networks. Calibration bar shows the average number of connections across subjects.
Discussion
Following ashort-term post-absorptive state in which participants were only allowed to consume water (NO BOOST®),we observed that state cravings (FCQstate) were higher and confidence for controlling eating behavior (CCEBstate) lower thanon the day that participants'consumeda BOOST® supplement—an energy surfeit state. Moreover, the effect was greater among those scoring high as opposed to low on the PFS. This finding extends the work of Lowe and colleagues(Lowe et al., 2009) in demonstrating that obese participants who score high on the PFS experience a reduction in their self-regulatory efficacy when exposed to favorite food cues 2.5h into a short-term post-absorptive state in which participants are allowed to consume water only.Indeed, it would appear that the PFS does assess the strength of the appetitive drive for palatable foods in the absence of an energy need. This reduction in self-efficacy was accompanied by increases in food craving and both effects were substantial in magnitude as supported by the shared varianceofthe PFS with both CCEBstate(r2 = 31%) and FCQstate(r2 = 32%) in the NOBOOST®treatment condition.
A unique feature of this study is the data collected on brain networks. As a number of authors have suggested, food cues are capable of triggering changes in the brain that are common to addictive agents such as cocaine and nicotine(Pelchat et al., 2004; Lowe & Butryn, 2007). Within the current study, two general points deserve comment. First, in the NO BOOST® treatment condition, the neighborhood connectivity of the cerebellum included consistent interactions with the basal ganglia and thalamus, particularly for those scoring high on the PFS; moreover, these neighborhood areas were connected with the visual cortex.Research with cats has reported that stimulation of the cerebellum leads to eating behavior(Berntson, Potolicchio, Jr., & Miller, 1973),whereas the mesocorticolimbic dopamine pathway appears to be particularly important to wanting(Robinson & Berridge, 2003)and drug addiction (Grant et al., 1996). Furthermore, in their elaborated intrusion theory of desire,Kavanagh and colleagues(Kavanagh, Andrade, & May, 2005) argue that sensory images are especially important to craving because they are networked with and stimulate the sensory and emotional qualities of the target being craved. These findings suggest that individuals who score high on the PFS may be prone to binging episodes and to consuming disproportionate amounts of palatable food even though they may not have a binge eating disorder.The relationship of the PFS to the IDED-IV certainly suggests that this pattern of behavior warrants further empirical study.
Second, we found that in the BOOST® condition the brains of participants who had scored low on the PFS returned to their default-mode network. This was not true of those who scored high on the PFS and was consistent with the connectivity observed between the medial prefrontal and orbital frontal cortex and the insula, suggesting that participantsscoring high on the PFS are predisposed to process internal cues related to food and that these cues dominate conscious thought(Kavanagh et al., 2005), a hypothesis that is consistent with responses to the FCQstateand the CCEBstate completed just prior to entering the scanner. The insula has been described as the primary gustatory cortex (Simon, de Araujo, Gutierrez, & Nicolelis, 2006), is active during the presentation of food cues (Wang et al., 2004), and has been found to be related to the anticipation of food consumption (Stice, Spoor, Ng, & Zald, 2009).
This study is not without limitations. The target sample was restricted to an older, obese population that was not currently dieting. To our knowledge, the PFS has not been validated with older adults and it is not known if responses to food cues are age-dependent. Also, it is possible that responses may have differed if participants wereinvolved in an active weight loss intervention. Second, the current methods enabled us to examine resting networks after exposure to food cues but not network activity during actual exposure to food and neutral cues. This is due to the fact that the food cues and neutral cues were presented in the same experimental run. Thus, at the current time, we do not know whether the observed effects in brain networks were due to the post-absorptive state itself or to actively imaging food cues. We are currently in the process of performing a follow-up study using a design that will allow the use of network analyses during both food cue exposure and neutral cue exposure. And third, because we did not have a normal weight control group, we cannot draw conclusions about whether the findings from this study are dependent on the obese status of our participants. Nonetheless, the brain network data are compelling, make use of cutting-edge technology, and offer strong support for the utility of the PFS in conjunction with state-based assessments in the study of eating behavior.
In summary, wedemonstrate that exposure to palatable foods even in the absence of an energy need is associated with increases in state cravings for desired food, a reduction in self-regulatory self-efficacy, and shifts in brain networks that parallel what is observed with other addictive behaviors. Clearly, individuals who score high on the PFSare at an increased risk for experiencing these effects; in fact, the consumption of food does not totally mitigate the “intrusion” that food cues have on the brain networks of this subgroup. Future research is needed to examine the eating behavior of persons who score high on the PFS and to develop interventions that directly target food cravings in this subgroup. Although one approach to treatment would be to encourage several small meals across the day to help contain their food cravings, we believe that mindfulness-based interventions warrant consideration since they have proven effective in treating obsessive-compulsive behavior(Schwartz, 1996) and analogies have been drawn between this disorder and the preoccupation and elaboration of food cues that occurs during extreme episodes of desire and craving(Kavanagh et al., 2005).
Brief restriction from foodincreases cravings and lowersself-regulatory beliefs.
Brief restriction from food alters brain networks, suggesting food is addictive.
Scores on thePower of Food Scale moderated the observed effects.
Acknowledgments
Funding Support for this study was provided by (a) National Heart, Lung, and Blood Institute grant HL076441-01A1, (b) National Institutes for Aging grant P30 AG021332, and (c) General Clinical Research Center grant, M01-RR007122.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflicting Interests The authors declare that they have no conflicts of interest with respect to their authorship or the publication of this article.
References
- Alonso-Alonso M, Pascual-Leone A. The right brain obesity. Journal of the American Medical Association. 2007;297:1819–1822. [Google Scholar]
- Bandura A. Social foundations of thought and action: A social cognitive theory. Prentice-Hall; Englewood Cliffs: 1986. [Google Scholar]
- Berntson GG, Potolicchio SJ, Jr., Miller NE. Evidence for higher functions of the cerebellum: Eating and grooming elicited by cerebellar stimulation in cats. Proceedings of the National Academy of Sciences. 1973;70:2497–2499. doi: 10.1073/pnas.70.9.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, Hayasaka S, Jennings JM, Katula JA, Kraft RA, Rejeski WJ. Using network science to evaluate exercise-associated brain changes in older adults. Frontiers in Aging Neuroscience. 2010;7:23. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Gerber RA, Leidy NK, Sexton CC, Karlsson J, et al. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: Development and measurement properties. International Journal of Obesity. 2009;33:913–922. doi: 10.1038/ijo.2009.107. [DOI] [PubMed] [Google Scholar]
- Cepeda-Benito A, Gleaves DH, Williams TL, Erath SA. The development and validation of the state and trait food-cravings questionnaires. Behavior Therapy. 2000;31:151–173. doi: 10.1016/s0005-7967(99)00141-2. [DOI] [PubMed] [Google Scholar]
- Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-Efficacy in Weight Management. Journal of Consulting and Clinical Psychology. 1991;59:739–744. doi: 10.1037//0022-006x.59.5.739. [DOI] [PubMed] [Google Scholar]
- Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiology and Behavior. 2005;86:599–602. doi: 10.1016/j.physbeh.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS One Biology. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Laurienti PJ. Comparison of characteristics between region-and voxel-based network analyses in resting-state fMRI data. Neuroimage. 2010;50:499–508. doi: 10.1016/j.neuroimage.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh DJ, Andrade J, May J. Imaginary relish and exquisite torture: The elaborated intrusion theory of desire. Psychological Review. 2005;112:446–467. doi: 10.1037/0033-295X.112.2.446. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: Studies of gene activation in corticolimbic regions. Physiology & Behavior. 2005;86:11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Kutlesic V, Williamson DA, Gleaves DH, Barbin JM, Murphy-Eberenz KP. The Interview for the Diagnosis of Eating Disorders IV: Application to DSM-IV diagnostic criteria. Psychological Assessment. 1998;10:41–48. [Google Scholar]
- Linde JA, Rothman AJ, Baldwin AS, Jeffery RW. The impact of self-efficacy on behavior change and weight change among overweight participants in a weight loss trial. Health Psychology. 2006;25:282–291. doi: 10.1037/0278-6133.25.3.282. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML. Hedonic hunger: A new dimension of appetite? Physiology & Behavior. 2007;91:432–439. doi: 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53:114–118. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Manton KG, Vaupel JW. Survival after the age of 80 in the United States, Sweden, France, England, and Japan. New England Journal of Medicine. 1995;333:1232–1235. doi: 10.1056/NEJM199511023331824. [DOI] [PubMed] [Google Scholar]
- Moussa MN, Vechlekar CD, Burdette JH, Steen MR, Hugenschmidt CE, Laurienti PJ. Changes in cognitive state alter human functional brain networks. Frontiers in Human Neuroscience. 2011;5(Article 83):1. doi: 10.3389/fnhum.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ME, Girvan M. Finding and evaluating community structure in networks. Physical Review of E Statistical Nonlinear Soft Matter Physics. 2004;69:026113. doi: 10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- Nordgren LF, van der Pligt J, van HF. The instability of health cognitions: Visceral states influence self-efficacy and related health beliefs. Health Psychology. 2008;27:722–727. doi: 10.1037/0278-6133.27.6.722. [DOI] [PubMed] [Google Scholar]
- Nordgren LF, van HF, van der Pligt J. The restraint bias: How the illusion of self-restraint promotes impulsive behavior. Psychological Science. 2009;20:1523–1528. doi: 10.1111/j.1467-9280.2009.02468.x. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: Food-craving activation during fMRI. Neuroimage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman JL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeski WJ, Mihalko SL, Ambrosius WT, Bearon LB, McClelland JW. Weight loss and self-regulatory eating efficacy in older adults: The cooperative lifestyle intervention program. Journal of Gerontology: Psychological Sciences. 2011;66:279–286. doi: 10.1093/geronb/gbq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman RM, Loughnan GT, Droulers AM, Steinbeck KS, Caterson ID. Self-efficacy in relation to eating behaviour among obese and non-obese women. International Journal of Obesity. 2001;25:907–913. doi: 10.1038/sj.ijo.0801606. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Mattes RD, Rothacker D. Beverage viscosity is inversely related to postprandial hunger in humans. Physiology & Behavior. 2001;74:551–557. doi: 10.1016/s0031-9384(01)00597-2. [DOI] [PubMed] [Google Scholar]
- Ruan J, Zhang W. Identifying network communities with a high resolution. Physical Review of E Statistical Nonlinear Soft Matter Physics. 2008;77:016104. doi: 10.1103/PhysRevE.77.016104. [DOI] [PubMed] [Google Scholar]
- Schwartz JM. Brain lock: Free yourself from obesssive-compulsive behavior. Harper-Collins Inc; New York: 1996. [Google Scholar]
- Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. The neural mechanisms of gustation: A distributed processing code. National; Review of Neuroscience. 2006;7:890–901. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- Simpson SL, Moussa MN, Laurienti PJ. An exponential random graph modeling approach to creating group-based representative whole-brain connectivity networks. NeuroImage. doi: 10.1016/j.neuroimage.2012.01.071. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kotter R. Identification and classification of hubs in brain networks. PLoS One Biology. 2007;2:e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen M, Hayasaka S, Joyce K, Laurienti P. Assessing the consistency of community strucutre in complex networks. Physical Review E: Statistical, Nonlinear, and Soft Matter Physics. 2011;84:016111. doi: 10.1103/PhysRevE.84.016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Ng J, Zald DH. Relation of and anticipatory food reward. Physiology and Behavior. 2009;97:551–560. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obesity Research. 2005;13:1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, et al. The Look AHEAD Study: A description of the lifestyle intervention and the evidence supporting it. Obesity. 2007;15:1339. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao ML, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]