Abstract
Kynurenic acid (KYNA), an antagonist of the α7 nicotinic acetylcholine receptor and the N-methyl-D-aspartate receptor, and 3-hydroxykynurenine (3-HK), a generator of reactive oxygen species, are neuroactive metabolites of the kynurenine pathway of tryptophan degradation. In the mammalian brain as elsewhere, both compounds derive from a common bioprecursor, L-kynurenine (L-KYN). Recent studies in rats demonstrated that D-kynurenine (D-KYN), a metabolite of the bacterial amino acid D-tryptophan, can also function as a bioprecursor of brain KYNA. We now investigated the conversion of systemically administered D-KYN to KYNA in mice and also explored the possible production of 3-HK in the same animals. Thirty min after an injection of D-KYN or L-KYN (30 mg/kg, i.p.), newly produced KYNA and 3-HK were recovered from plasma, liver, forebrain and cerebellum in all cases. Using a new chiral separation method, 3-HK produced from D-KYN was positively identified as D-3-HK. L-KYN was the more effective precursor of KYNA in all tissues and also exceeded D-KYN as a precursor of brain 3-HK. In contrast, D-KYN was more potent as a precursor of 3-HK in the liver. The production of both KYNA and 3-HK from D-KYN was rapid in all tissues, peaking at 15–30 min following a systemic injection of D-KYN. These results show that biosynthetic routes other than those classically ascribed to L-KYN can account for the synthesis of both KYNA and 3-HK in vivo. This new insight may be of significant physiological or pathological relevance.
Keywords: Chiral separation, D-Aminoacid oxidase, D-Amino acids, Kynurenine pathway, Reactive oxygen species, Schizophrenia
1. Introduction
Kynurenic acid (KYNA), a metabolite produced in a side arm of the kynurenine pathway of tryptophan degradation, inhibits the α7 nicotinic acetylcholine receptor and the glycine co-agonist site of the N-methyl-D-aspartate receptor at nanomolar and low micromolar concentrations (Hilmas et al., 2001; Parsons et al., 1997). Alone or jointly, these effects likely account for the neuromodulatory and neuroprotective effects of endogenous KYNA in the mammalian brain in vivo. Thus, fluctuations in brain KYNA bi-directionally influence extracellular dopamine, glutamate and acetylcholine levels (Amori et al., 2009; Wu et al., 2010; Zmarowski et al., 2009), affect cognitive performance (Chess and Bucci, 2006; Chess et al., 2009; Erhardt et al., 2004; Pocivavsek et al., 2011; Potter et al., 2010; Shepard et al., 2003), and determine neuronal viability (Nozaki and Beal, 1992; Santamaria et al., 1996; Sapko et al., 2006). These features, as well as the fact that cerebral KYNA levels are abnormal in a number of neurological and psychiatric diseases (for reviews, see Chen and Guillemin, 2009; Erhardt et al., 2007; Müller et al., 2011; Sás et al., 2007), have raised interest in the mechanism(s) that have evolved to control KYNA biosynthesis in the brain.
3-Hydroxykynurenine (3-HK), too, is a metabolite of the kynurenine pathway, albeit in the competing, major branch of the metabolic cascade. In contrast to KYNA, 3-HK is neurotoxic (Okuda et al., 1996) and, by generating highly reactive oxygen species (Giles et al., 2003; Vazquez et al., 2000), may play a causative role in neurodegenerative diseases. Indeed, abnormal 3-HK disposition has been demonstrated in the brain of individuals dying with Huntington’s and Alzheimer’s disease (Bonda et al., 2010; Pearson and Reynolds, 1992). The in vivo effects of 3-HK are complex, however, since it can also act as an antioxidant (Leipnitz et al., 2007), and its role in brain physiology and pathology is closely linked to the function of downstream kynurenine pathway metabolites, including the neuroactive compounds 3-hydroxyanthranilic acid and quinolinic acid (Chiarugi et al., 1996). Elucidation of mechanisms that control 3-HK biosynthesis in the brain might provide insight into these complexities and is therefore of considerable relevance.
Until recently, the pivotal kynurenine pathway metabolite L-kynurenine (L-KYN) was considered the only bioprecursor of both KYNA and 3-HK in the mammalian brain, serving as a substrate of kynurenine aminotransferases (KATs; Guidetti et al., 2007; Han et al., 2010) and kynurenine 3-monooxygenase (KMO; Erickson et al., 1992; Saito et al., 1993), respectively. However, as in the periphery (Fukushima et al., 2009; Loh and Berg, 1971; Mason and Berg, 1952), brain KYNA can also be synthesized from D-kynurenine (D-KYN). This was demonstrated both in vitro, using rat and human brain tissue homogenate (Pérez-De la Cruz et al., 2012), and in vivo by intracerebral infusion of D-KYN (Ogaya et al., 2010; Pérez-De la Cruz et al., 2012) or systemic injection of D-tryptophan in rats (Ishii et al., 2011). In contrast, the in vivo formation of 3-HK from D-KYN has so far not been demonstrated either in the brain or in peripheral organs, though small amounts of 3-HK could be recovered from rabbit urine after feeding or systemic injection of D-KYN (Loh and Berg, 1971).
As D-KYN, possibly originating from bacterial D-tryptophan (Lam et al., 2009), might occur naturally in mammals, Fukushima and colleagues recently began to re-investigate the fate of peripherally administered D-KYN in rats (Fukushima et al., 2009). So far, however, these studies did not examine the effect of systemic D-KYN on brain KYNA and failed to consider the possible formation of 3-HK. The present study, performed in mice, was designed to fill this void by investigating the time- and dose-dependent degradation of systemically applied D-KYN, simultaneously assessing the production of both KYNA and 3-HK in plasma, liver and brain. In some experiments, the metabolism of D-KYN was examined side by side with its L-enantiomer for comparative purposes. A preliminary account of this work has appeared in abstract form (Wang et al., 2011).
2. Results
2.1. KYNA and 3-HK production from D-KYN and L-KYN in vivo
The first study was designed to explore and compare the effects of D-KYN and L-KYN, administered at 30 mg/kg (i.p.), on the de novo formation of KYNA and 3-HK, respectively. Animals were killed after 30 min, and the levels of the two kynurenine pathway metabolites were measured in plasma, liver, forebrain and cerebellum.
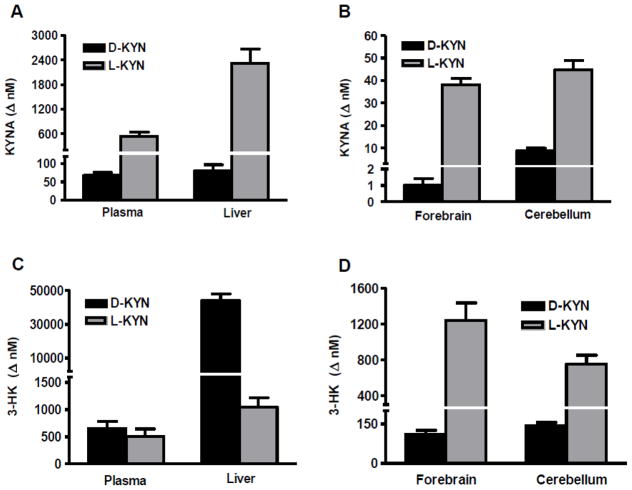
As shown in Figs. 1A and B, newly produced KYNA was recovered in all four tissues. In plasma, liver and forebrain, KYNA formation from L-KYN was 10–40-fold higher than from D-KYN. In the cerebellum, KYNA production from D-KYN was more efficient (p<0.001 vs. the forebrain) but still approximately 5 times less than from L-KYN.
Figure 1.
De novo synthesis of KYNA and 3-HK from D- and L-KYN in mouse plasma, liver, forebrain and cerebellum. D- or L-KYN (each 30 mg/kg, i.p.) were administered to adult FVB/N mice (n = 5 per group), and animals were euthanized after 30 min. Levels of newly produced (Δ nM) KYNA and 3-HK were obtained by deducting the endogenous content of the respective analyte. Data are the mean ± SEM.
In the same animals, 3-HK, too, was produced from both D-KYN and L-KYN in plasma, liver, forebrain and cerebellum (Figs. 1C and D). In contrast to the neosynthesis of KYNA, however, 3-HK formation from L-KYN exceeded synthesis from D-KYN only in the brain (by 5–10-fold). In plasma, D-KYN and L-KYN stimulated 3-HK formation approximately equally, and D-KYN was 40 times more effective than L-KYN in the liver.
Relative to respective baseline values, D-KYN (30 mg/kg, i.p.) enhanced the concentration of 3-HK in plasma, liver and forebrain more than the concentration of KYNA (Tables 1 and 2). Due to the exceptionally high conversion rate of D-KYN to KYNA in the cerebellum (Fig. 1), this discrepancy was not observed in cerebellar tissue where approximately 3-fold increases in both metabolites were found 30 min after the application of D-KYN. Effects on 3-HK and KYNA diverged especially in the liver where the administration of D-KYN caused a 700-fold increase in 3-HK within 30 min, whereas the levels KYNA were barely doubled compared to endogenous values (Tables 1 and 2).
Table 1.
KYNA production from D-KYN in vivo.
| KYNA (nM)
|
|||
|---|---|---|---|
| Ctr | D-KYN (30 mg/kg) | D-KYN (300 mg/kg) | |
| Plasma | 61.6 ± 3.9 | 129.2 ± 8.0** | 726.7 ± 171.7*** |
| Liver | 91.4 ± 7.3 | 171.2 ± 17.2* | 560.9 ± 124.6*** |
| Forebrain | 3.6 ± 0.3 | 4.6 ± 0.4 | 15.0 ± 3.0*** |
| Cerebellum | 4.1 ± 0.3 | 12.9 ± 1.0*** | 99.7 ± 14.1*** |
Mice received an i.p. injection of 30 or 300 mg/kg D-KYN (n=5 per group), and KYNA was measured in plasma, liver, forebrain and cerebellum after 30 min. Data are the mean ± SEM.
p<0.05,
p<0.01 and
p<0.001 vs. controls (Ctr; one-way ANOVA followed by Bonferroni’s post-hoc test).
Table 2.
3-HK production from D-KYN in vivo.
| 3-HK (nM)
|
|||
|---|---|---|---|
| Ctr | D-KYN (30 mg/kg) | D-KYN (300 mg/kg) | |
| Plasma | 19.6 ± 2.6 | 672.0 ± 133.4*** | 21,299.5 ± 4,920.7*** |
| Liver | 59.8 ± 18.8 | 44,074.5 ± 3,808.7*** | 403,522.1 ± 53,797.8*** |
| Forebrain | 50.9 ± 2.6 | 161.5 ± 14.5*** | 869.3 ± 122.6*** |
| Cerebellum | 76.2 ± 11.0 | 219.9 ± 11.6*** | 1,167.9 ± 170.8*** |
Mice received an i.p. injection of 30 or 300 mg/kg D-KYN (n=5 per group), and 3-HK was measured in plasma, liver, forebrain and cerebellum after 30 min. Data are the mean ± SEM.
p<0.001 vs. controls (Ctr; one-way ANOVA followed by Bonferroni’s post-hoc test).
Application of a 10-times higher dose of D-KYN (300 mg/kg, i.p.) under otherwise identical conditions revealed the same qualitative picture (i.e. 3-HK synthesis > KYNA synthesis). In quantitative terms, after deduction of basal levels, both KYNA and 3-HK production from D-KYN was essentially linear in all 4 tissues (Tables 1 and 2). Notably, the 3-HK concentration in the liver reached approximately 400 μM 30 min after the i.p. administration of 300 mg/kg D-KYN.
With one exception, statistical analyses showed significant dose effects in all tissues. Thus, after the administration of 30 mg/kg D-KYN, KYNA levels were significantly elevated compared to endogenous values in plasma (p<0.01), liver (p<0.05) and cerebellum (p<0.001), but not in forebrain. These increases reached statistical significance in all tissues after the administration of 300 mg/kg D-KYN (p<0.001). D-KYN administration (30 mg/kg and 300 mg/kg) also caused a significant increase in 3-HK levels in all tissues compared to respective baseline values (each p<0.001).
2.2. Time-dependency of KYNA and 3-HK production from D-KYN
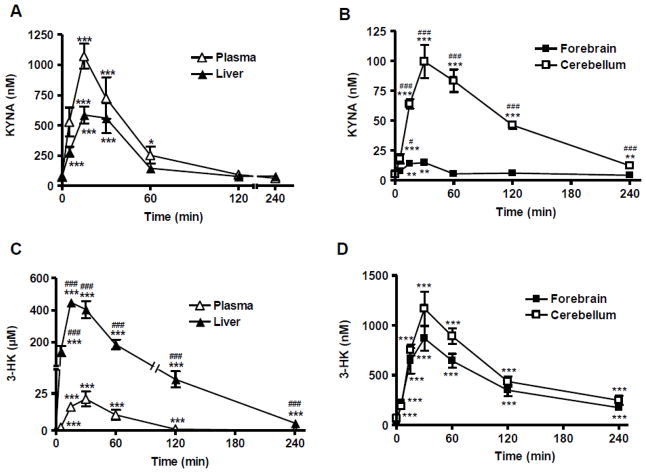
Using separate animals, we next studied the time course of the de novo production of KYNA and 3-HK from D-KYN, using 300 mg/kg (i.p.) D-KYN to drive formation of the metabolites. The study included six time points ranging from 5 min to 240 min, and results were assessed in plasma, liver, forebrain and cerebellum.
As shown in Figs. 2A and 2B, KYNA concentration increased rapidly after D-KYN administration, reaching significant difference from baseline after 15 min in all tissues. In plasma and liver, KYNA levels reached maximal concentrations (1072.2 ± 102.5 nM and 586.9 ± 70.6 nM, respectively) by 15 min and returned to endogenous values after 2 h. In the forebrain, KYNA attained a maximal concentration of 15.0 ± 3.0 nM at 30 min, and no differences from control levels were seen by 60 min. KYNA concentrations in the cerebellum reached a peak (99.7 ± 14.1nM) at 30 min, before decreasing gradually over time. Cerebellar KYNA levels remained significantly elevated until 4 h after the D-KYN injection (p<0.01). Compared to the forebrain, D-KYN-induced increases in cerebellar KYNA levels were significantly higher at all time points until 4 h (p<0.001, except p<0.05 at 5 min).
Figure 2.
Time course of KYNA and 3-HK concentrations in plasma, liver, forebrain and cerebellum of mice treated with D-KYN (300 mg/kg, i.p.). KYNA and 3-HK levels were determined at six time points from 5 to 240 min after the D-KYN injection (n = 5 per group). Data are the mean ± SEM. *p<0.05, **p<0.01 and ***p<0.001 vs. endogenous levels (one-way ANOVA followed by Bonferroni’s post-hoc test). # p<0.05 and ###p<0.001 vs. forebrain (B) or plasma (C) (two-way ANOVA followed by Bonferroni’s post-hoc test).
In the same mice, 3-HK concentrations reached maximal values 15 min after D-KYN administration in liver, and at 30 min in plasma, forebrain and cerebellum, before gradually decreasing in all tissues (Figs. 2C and 2D). In contrast to KYNA, 3-HK levels in liver, forebrain and cerebellum remained significantly higher than endogenous levels until 4 h after the administration of D-KYN (p<0.001 each). Moreover, in line with the results shown in Table 2, 3-HK levels in the liver greatly exceeded those in plasma and brain at all time points tested (p<0.001).
2.3. D-KYN is selectively converted to D-3-HK
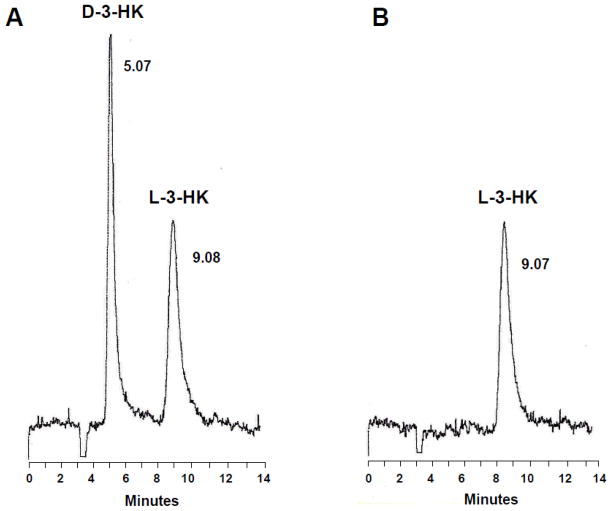
To examine the stereoselectivity of the in vivo conversion of D-KYN to 3-HK, we developed a method for the chiral separation of D-3-HK and L-3-HK. As D-3-HK is not commercially available, we used standard L-3-HK and D,L-3-HK to optimize the separation of the two enantiomers by HPLC. A series of pilot experiments with different buffer compositions and elution speeds (data not shown here) revealed optimal separation using 5 mM sodium acetate, pH 6.0, as the mobile phase and a flow rate of 0.4 ml/min (Fig. 3; see Materials and Methods for further details).
Figure 3.
Representative chromatograms of D,L-3-HK and D-3-HK standards eluted from a chiral HPLC column. Racemic D,L-3-HK (200 pmoles/20 μl; A) or L-3HK (100 pmoles/20 μl; B) were applied to the column. See 4.5. for additional experimental details.
To determine the enantioselectivity of the in vivo conversion of D-KYN to 3-HK, the precursor was administered at 300 mg/kg (i.p.), the animals were euthanized 30 min later, and the newly developed chiral separation method was used to assess the formation of D-3-HK. 3-HK levels were determined electrochemically prior to injection onto the HPLC column and in the appropriate eluate corresponding to the fraction containing D-3-HK (collection interval: ~4.5-~6.5 min; see Fig. 3 and Materials and Methods). Measurement of total 3-HK and D-3-HK showed that essentially all 3-HK produced from D-KYN in brain and peripheral tissue samples was the D-enantiomer (Table 3).
Table 3.
Production of D-3-HK from D-KYN.
| Total 3-HK (nM) | D-3-HK (nM) | D-3-HK/Total 3-HK (%) | |
|---|---|---|---|
| Plasma | 14,233 ± 775 | 14,220 ± 779 | 98.9 |
| Liver | 438,990 ± 37,413 | 435,253 ± 36,694 | 99.2 |
| Forebrain | 786 ± 97 | 766 ± 102 | 97.4 |
| Cerebellum | 970 ± 52 | 930 ± 63 | 96.0 |
Total 3-HK content and D-KYN eluted from the chiral column were quantified electrochemically in plasma, liver, forebrain and cerebellum of mice treated with D-KYN (300 mg/kg, i.p.) 30 min earlier, as detailed in the text (n = 3). % indicates the percentage of D-3-HK compared to total 3-HK in the same samples. Data are the mean ± SEM.
3. Discussion
The results of the present study provide a comprehensive view of the fate of D-KYN following its peripheral administration in the mouse. Several new findings are especially noteworthy: first, systemically applied D-KYN caused a rapid elevation in brain KYNA levels, and this effect was far more pronounced in the cerebellum than in the forebrain. Second, whereas the de novo production of KYNA from L-KYN in the brain generally exceeded neosynthesis from D-KYN, this difference in efficacy was less pronounced in the cerebellum than in the forebrain. Third, D-KYN served as an efficient precursor of 3-HK in both the brain and the periphery, and this newly formed 3-HK was identified as D-3-HK. Finally, in contrast to the brain, D-KYN greatly exceeded L-KYN as a bioprecursor of 3-HK in the liver. As both KYNA and 3-HK have distinct neuroactive properties, several of these phenomena may impact brain physiology or pathology (cf. Introduction).
Our results confirm and extend recent studies in rats, demonstrating an increase in plasma KYNA levels after a single i.p. injection of D-KYN (Fukushima et al., 2009), and KYNA formation in the brain following an intracerebral infusion of D-KYN (Ogaya et al., 2010; Pérez-De la Cruz et al., 2012). Notably, the time course of KYNA production following systemic D-KYN was similar in periphery and brain, peaking at 15 min (plasma, liver) or 30 min (brain) and gradually subsiding during the next 4 h. This suggests that D-KYN, like its enantiomer L-KYN but unlike KYNA (Fukui et al., 1991), is readily transported across the blood-brain barrier for further local sequestration. Although this has not yet been tested experimentally, D-KYN probably penetrates the blood-brain barrier via the same large neutral amino acid transporter as L-KYN, as both enantiomers use a very similar carrier for concentrative cellular uptake within the brain (system L; Speciale et al., 1989).
In line with studies in which the enantiomers of kynurenine were applied directly to forebrain structures – namely frontal cortex and striatum – by reverse dialysis (Ogaya et al., 2010; Pérez-De la Cruz et al., 2012), L-KYN was found to be the preferred bioprecursor of brain KYNA after systemic administration. Thus, compared to D-KYN, L-KYN produced >30 times more KYNA in the forebrain, possibly in part as a result of more efficient brain access. However, as L-KYN was similarly favored as a precursor of KYNA in plasma and liver (Fig. 1), the difference between the two enantiomers was more likely due to the fact that L-KYN is approximately 40 times more effective than D-KYN as a substrate of both KAT I and KAT II, as shown recently using partially purified enzyme preparations (Pérez-de la Cruz et al., manuscript in preparation). These results therefore also provide indirect support for the contention that enzymatic transamination can account for the neosynthesis of KYNA from D-KYN in mammals (Mason and Berg, 1952; Pérez-De la Cruz et al., 2012).
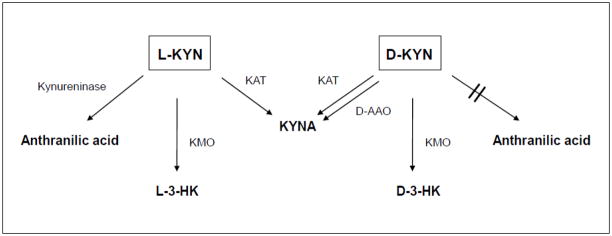
Consistent with our in vitro findings using human brain tissue homogenate (Pérez-De la Cruz et al., 2010), we noticed that the increase of KYNA following the systemic administration of D-KYN was substantially greater and longer lasting in the cerebellum than in the forebrain. This regional difference is almost certainly due to the exceptionally high concentration of D-aminoacid oxidase (D-AAO) in the cerebellum (Horiike et al., 1994; Moreno et al., 1999; Verrall et al., 2007). Like L-KYN for KATs (Han et al., 2010), D-KYN has a high Km for D-AAO (Song et al., 2010), and this enzyme is in part responsible for the in vivo generation of KYNA in the brain and elsewhere in the body (Ishii et al., 2010; Loh and Berg, 1971; Ogaya et al., 2010; Pérez-De la Cruz et al., 2010; Tashiro et al., 1961) (Fig. 4). Microdialysis studies in rats show that D-AAO and KATs are approximately equally responsible for the conversion of D-KYN to KYNA in the forebrain, whereas D-AAO accounts for more than 90% of this transformation in the cerebellum (Ogaya et al., 2010; H.-Q. Wu, unpublished data; Pérez-De la Cruz et al., 2012). By the same argument, as D-AAO activity is weak in the liver (Hamase et al., 2006), the extensive peripheral KYNA production from D-KYN seen in the present study was probably mainly due to KAT-catalyzed transamination (Mason and Berg, 1952).
Figure 4.
Schematic diagram illustrating the enzymatic processes that are involved in the degradation of L-KYN and D-KYN, respectively, in mice. D-AAO: D-Aminoacid oxidase; KAT: Kynurenine aminotransferases; KMO: Kynurenine 3-monooxygenase.
Measured in the same samples used for the determination of KYNA, we observed a time- and dose-related production of 3-HK from D-KYN in plasma, liver and brain. Using a newly developed chiral separation method, we identified essentially all newly formed 3-HK as the D-isomer. This, in accordance with the fact that D-KYN is directly converted to KYNA (see above), indicates that D-KYN did not undergo isomerization to L-KYN prior to being metabolized to 3-HK. Notably, under conditions that were optimal for the assay of KMO in vitro, 3-HK was readily formed from D-KYN in mouse liver homogenate, though about 20 times less effectively than from L-KYN (data not shown). Thus, whereas KMO was originally reported to be stereospecific with regard to its substrate (Saito et al., 1957), the neosynthesis of D-3-HK from D-KYN shown here was probably at least in part catalyzed by KMO (Fig. 4).
The liver showed disproportionately high neosynthesis of D-3-HK in the present study, surpassing the production of L-3-HK from L-KYN almost 50-fold. Although species differences may exist (Hankes and Brown, 1968; Triebwasser et al., 1976), a similar observation was made previously using tissue slices from rabbit liver (Loh and Berg, 1971). This preferential, massive accumulation of D-3-HK in the liver may be related to the fact that D-3-HK, in contrast to L-3-HK, is not recognized by the degradative enzyme, kynureninase (Tanizawa and Soda, 1979) (Fig. 4) and therefore also a poor bioprecursor of downstream metabolites such as quinolinic acid (Hankes et al., 1966). Of relevance from a neurobiological perspective, our results indicate that only a small proportion of hepatic D-3-HK is released into the bloodstream, and that the liver-derived metabolite has only little influence on brain D-3-HK (Figs. 1C and 1D). In all tissues, however, future studies should assess the likely enzymatic transamination of D-3-HK to xanthurenic acid (Hankes et al., 1972; Loh and Berg, 1971), a potential neuroactive compound (Gobaille et al., 2008), as well as possible unique biological properties of D-3-HK itself.
Although its presence in mammalian tissues has not been directly ascertained to date, D-KYN is readily formed in rabbits, dogs, rats and humans after the in vivo administration of D-tryptophan (Ishii et al., 2010; Kotake Jr. and Ito, 1937; Langner and Berg, 1955; Loh and Berg, 1971; Triebwasser et al., 1976). In natural settings, D-tryptophan may be introduced by a variety of microorganisms (Lam et al., 2009). Conversion to D-KYN is then likely catalyzed by either tryptophan-2,3-dioxygenase or indoleamine-2,3-dioxygenase, both of which recognize D-tryptophan as a substrate, albeit with different kinetic characteristics than L-tryptophan (Capece et al., 2010; Watanabe et al., 1980). As shown here, newly formed D-KYN subsequently raises both KYNA and 3-HK levels in the brain and might thereby enhance the physiological or pathophysiological roles of either metabolite. Specifically, as mentioned earlier, systemic stimulation of D-KYN synthesis can be expected to boost KYNA synthesis, leading to a reduction in α7 nicotinic acetylcholine receptor and N-methyl-D-aspartate receptor function (cerebellum>forebrain), and to affect oxidative processes by increasing cerebral 3-HK levels.
Of special interest for pathology, D-KYN formation in mammals might be enhanced during bacterial infections, both because of the infiltration of microbial pathogens and the pronounced up-regulation of indoleamine-2,3-dioxygenase, which is reliably seen under inflammatory conditions (Johnson et al., 2009). Endogenously produced D-KYN may therefore be at least in part responsible for the significant increases in brain KYNA and/or 3-HK levels seen in human brain diseases that are linked to genetic or environmental immunogenic risk factors (Brown and Derkits, 2010; Mándi and Vécsei, 2012; Raison et al., 2010; cf. Introduction). Using human tissue specimens and appropriate animal models, our current studies are designed to investigate the possible role of D-KYN in the pathophysiology of these diseases.
4. Experimental Procedure
4.1. Chemicals
D-Kynurenine (D-KYN), kynurenic acid (KYNA) and D,L-3-hydroxykynurenine (D,L-3-HK) were purchased from Sigma-Aldrich (St. Louis, MO, USA). L-Kynurenine sulfate (L-KYN) was obtained from Sai Adventium (Hyderabad, India). L-3-Hydroxykynurenine (L-3-HK) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All other biochemicals and chemicals were obtained from various commercial suppliers and were of the highest available purity.
4.2. Animals and treatment with kynurenine
Adult FVB/N wild-type mice (25–35 g; Taconic, Hudson, NY, USA) of either sex were used in all experiments. The animals were maintained on a 12:12 hour light:dark cycle in a temperature- and humidity-controlled animal care facility, and had free access to food and water. The facility was fully accredited by the American Association for the Accreditation of Laboratory Animal Care. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Maryland. The work described in the article was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving animals.
For in vivo application, both D-KYN and L-KYN were dissolved in sterile saline. The pH of the final solutions was ~6.5. D-KYN or L-KYN, or saline (control), were administered intraperitoneally (i.p.). Animals were euthanized using CO2 asphyxiation. Plasma (supernatant of blood centrifuged at 5,200 × g, 10 min), liver, forebrain and cerebellum were collected immediately, frozen on dry ice, and stored at −80°C until analysis.
4.3. KYNA measurement
Plasma was diluted (1:10, v/v), and liver (1:20, w/v), forebrain and cerebellum (each 1:5, w/v) were homogenized using deionized water. Perchloric acid (6%; 50 μl for plasma and liver, and 25 μl for forebrain and cerebellum) was then added to 100 μl of the tissue preparation, and the precipitated proteins were removed by centrifugation (16,000 × g, 15 min). Twenty μl of the resulting supernatant were injected onto a 3 μm C18 reverse phase high performance liquid chromatography (HPLC) column (80 mm × 4.6 mm; ESA, Chelmsford, Massachusetts, USA), using a mobile phase containing 250 mM zinc acetate, 50 mM sodium acetate, and 3% acetonitrile (pH adjusted to 6.2 with glacial acetic acid) at a flow rate of 1.0 ml/min. In the eluate, KYNA was quantitated fluorimetrically (excitation: 344 nm, emission: 398 nm; S200a fluorescence detector; Perkin Elmer, Waltham, Massachusetts, USA). The retention time of KYNA was approximately 7 min.
4.4. 3-HK measurement
Tissues were prepared as for KYNA measurements, except for different dilutions of plasma (1:2, v/v) and liver (1:5, w/v). Twenty μl of the supernatant obtained after the centrifugation step (see above) were then injected onto a 3 μm HPLC column (80 mm × 4.6 mm; ESA), using a mobile phase consisting of 1.5% acetonitrile, 0.9% triethylamine, 0.59% phosphoric acid, 0.27 mM EDTA, and 8.9 mM sodium heptane sulfonic acid, and a ow rate of 0.5 ml/min. In the eluate, 3-HK was detected electrochemically using a HTEC 500 detector (Eicom Corp., San Diego, CA, USA; oxidation potential: +0.5 V). The retention time of 3-HK was ~11 min.
4.5. Chiral separation of D-3-HK and L-3-HK
Where indicated, D-3-HK and L-3-HK were separated by HPLC using a chiral column. To this end, plasma was diluted (1:40, v/v), and liver (1:200; w/v), forebrain or cerebellum (each 1:5; w/v) were homogenized using deionized water. Two hundred μl of acetone were then added to 100 μl of the homogenate. After centrifugation (16,000 × g, 15 min), the resulting supernatant (~270 μl) was transferred to a new tube and evaporated to dryness in a vacuum atmosphere (~90 min). The residue was taken up in 100 μl of HPLC mobile phase (see below) and centrifuged (16,000 × g, 10 min). Twenty μl (plasma or liver) or 40 μl (forebrain or cerebellum) of the supernatant were then applied to a chiral column (150 mm × 4.0 mm, Resolvosil BSA-7, Macherey-Nagel, Bethlehem, PA, USA), using 5 mM sodium acetate (pH adjusted to 6.0 with glacial acetic acid) as the mobile phase and a flow rate of 0.4 ml/min. Under these conditions, D-3-HK and L-3-HK eluted at ~5 min and ~9 min, respectively, and were detected by UV absorbance at 365 nm (SPD-10A, Shimadzu, Columbia, MD, USA). The 2-min fraction eluting between ~4.5 min and ~6.5 min was collected, and 20 μl (plasma or liver) or 40 μl (forebrain or cerebellum) were used to determine the 3-HK content of the sample electrochemically, as described above.
4.6. Data analysis
Student’s t-test was used to compare the newly produced KYNA from D-KYN in forebrain and cerebellum. One-way ANOVA followed by Bonferroni’s post-hoc test was used to analyze the dose- and time-relationships of KYNA and 3-HK production from D-KYN. Two-way ANOVA followed by Bonferroni’s post-hoc test was used to analyze the difference in KYNA and 3-HK levels between tissues in the time curve. Prior to use of analysis of variance, a logarithmic transformation was used to normalize the data. In all cases, a p value of <0.05 was considered significant.
Highlights.
The neuroactive compounds kynurenic acid and 3-hydroxykynurenine are produced in brain and periphery following an i.p. injection of D-kynurenine.
3-Hydroxykynurenine generated from D-kynurenine is identified as the D-isomer.
In most cases, kynurenic acid and 3-hydroxykynurenine synthesis from L-kynurenine is shown to exceed formation from D-kynurenine.
Introduction of the concept that routes other than those classically ascribed to L-kynurenine can account for the synthesis of both kynurenic acid and 3-hydroxykynurenine in vivo.
Acknowledgments
This work was supported in part by NIH grant NS057715. We thank Dr. Veronica Pérez-de la Cruz for helpful discussions and Dr. Robert McMahon for his assistance with statistical analyses.
Abbreviations
- D-AAO
D-Aminoacid oxidase
- D-3-HK
D-3-Hydroxykynurenine
- D-KYN
D-Kynurenine
- D,L-3HK
D,L-3-Hydroxykynurenine
- HPLC
High performance liquid chromatography
- KAT
Kynurenine aminotransferase(s)
- KYNA
Kynurenic acid
- KMO
Kynurenine 3-monooxygenase
- L-KYN
L-Kynurenine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amori L, Wu HQ, Marinozzi M, Pellicciari R, Guidetti P, Schwarcz R. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience. 2009;159:196–203. doi: 10.1016/j.neuroscience.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda DJ, Mailankot M, Stone JG, Garrett MR, Staniszewska M, Castellani RJ, Siedlak SL, Zhu X, Lee HG, Perry G, Nagaraj RH, Smith MA. Indoleamine 2,3-dioxygenase and 3-hydroxykynurenine modifications are found in the neuropathology of Alzheimer’s disease. Redox Rep. 2010;15:161–168. doi: 10.1179/174329210X12650506623645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capece L, Arrar M, Roitberg AE, Yeh SR, Marti MA, Estrin DA. Substrate stereo-specificity in tryptophan dioxygenase and indoleamine 2,3-dioxygenase. Proteins. 2010;78:2961–2972. doi: 10.1002/prot.22819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy States. Int J Tryptophan Res. 2009;2:1–19. doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess AC, Bucci DJ. Increased concentration of cerebral kynurenic acid alters stimulus processing and conditioned responding. Behav Brain Res. 2006;170:326–332. doi: 10.1016/j.bbr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res. 2009;201:325–331. doi: 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Carpenedo R, Moroni F. Kynurenine disposition in blood and brain of mice: effects of selective inhibitors of kynurenine hydroxylase and of kynureninase. J Neurochem. 1996;67:692–698. doi: 10.1046/j.1471-4159.1996.67020692.x. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry. 2004;56:255–260. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Nilsson L, Linderholm K, Engberg G. The kynurenic acid hypothesis of schizophrenia. Physiol Behav. 2007;92:203–209. doi: 10.1016/j.physbeh.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Erickson JB, Flanagan EM, Russo S, Reinhard JF., Jr A radiometric assay for kynurenine 3-hydroxylase based on the release of 3H2O during hydroxylation of L-[3,5-3H]kynurenine. Anal Biochem. 1992;205:257–262. doi: 10.1016/0003-2697(92)90432-7. [DOI] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Sone Y, Mitsuhashi S, Tomiya M, Toyo’oka T. Alteration of kynurenic acid concentration in rat plasma following optically pure kynurenine administration: a comparative study between enantiomers. Chirality. 2009;21:468–472. doi: 10.1002/chir.20620. [DOI] [PubMed] [Google Scholar]
- Giles GI, Collins CA, Stone TW, Jacob C. Electrochemical and in vitro evaluation of the redox-properties of kynurenine species. Biochem Biophys Res Commun. 2003;300:719–724. doi: 10.1016/s0006-291x(02)02917-0. [DOI] [PubMed] [Google Scholar]
- Gobaille S, Kemmel V, Brumaru D, Dugave C, Aunis D, Maitre M. Xanthurenic acid distribution, transport, accumulation and release in the rat brain. J Neurochem. 2008;105:982–993. doi: 10.1111/j.1471-4159.2008.05219.x. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem. 2007;102:103–111. doi: 10.1111/j.1471-4159.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- Hamase K, Nagayasu R, Morikawa A, Konno R, Zaitsu K. Sensitive high-performance liquid chromatographic assay for D-amino-acid oxidase activity in mammalian tissues using a fluorescent non-natural substrate, 5-fluoro-D-tryptophan. J Chromatogr A. 2006;1106:159–164. doi: 10.1016/j.chroma.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Han Q, Cai T, Tagle DA, Li J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol Life Sci. 2010;67:353–368. doi: 10.1007/s00018-009-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankes LV, Brown RR, Schmaeler M. Metabolism of isomers of 3-hydroxykynurenine C 14 to quinolinic acid, niacin metabolites and carbon dioxide. Proc Soc Exp Biol Med. 1966;121:253–259. doi: 10.3181/00379727-121-30750. [DOI] [PubMed] [Google Scholar]
- Hankes LV, Brown RR. Metabolism of D- and L-kynurenine-keto-14C in rats and the effects of unlabeled enantiomers. Proc Soc Exp Biol Med. 1968;129:144–153. doi: 10.3181/00379727-129-33271. [DOI] [PubMed] [Google Scholar]
- Hankes LV, Brown RR, Leklem J, Schmaeler M, Jesseph J. Metabolism of C 14 labeled enantiomers of tryptophan, kynurenine and hydroxykynurenine in humans with scleroderma. J Invest Dermatol. 1972;58:85–95. doi: 10.1111/1523-1747.ep12551699. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiike K, Tojo H, Arai R, Nozaki M, Maeda T. D-amino-acid oxidase is confined to the lower brain stem and cerebellum in rat brain: regional differentiation of astrocytes. Brain Res. 1994;652:297–303. doi: 10.1016/0006-8993(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Ishii K, Ogaya T, Song Z, Iizuka H, Fukushima T. Changes in the plasma concentrations of D-kynurenine and kynurenic acid in rats after intraperitoneal administration of tryptophan enantiomers. Chirality. 2010;22:901–906. doi: 10.1002/chir.20850. [DOI] [PubMed] [Google Scholar]
- Ishii K, Iizuka H, Ogaya T, Song Z, Fukushima T. Comparative study on kynurenic acid production in the rat striatum by tryptophan enantiomers: an in vivo microdialysis study. Chirality. 2011;23(Suppl 1):E12–15. doi: 10.1002/chir.20938. [DOI] [PubMed] [Google Scholar]
- Johnson BA, 3rd, Baban B, Mellor AL. Targeting the immunoregulatory indoleamine 2,3 dioxygenase pathway in immunotherapy. Immunotherapy. 2009;1:645–661. doi: 10.2217/IMT.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake Y, Jr, Ito N. Studien über den intermediären Stoffwechsel des Tryptophans. XXV Isolierung des d-Kynurenins. J Biochem. 1937;25:71–77. [Google Scholar]
- Lam H, Oh DC, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science. 2009;325:1552–1555. doi: 10.1126/science.1178123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner RR, Berg CP. Metabolism of D-tryptophan in the normal human subject. J Biol Chem. 1955;214:699–707. [PubMed] [Google Scholar]
- Leipnitz G, Schumacher C, Dalcin KB, Scussiato K, Solano A, Funchal C, Dutra-Filho CS, Wyse AT, Wannmacher CM, Latini A, Wajner M. In vitro evidence for an antioxidant role of 3-hydroxykynurenine and 3-hydroxyanthranilic acid in the brain. Neurochem Int. 2007;50:83–94. doi: 10.1016/j.neuint.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Loh HH, Berg CP. Production of D-kynurenine and other metabolites from D-tryptophan by the intact rabbit and by rabbit tissue. J Nutr. 1971;101:465–475. doi: 10.1093/jn/101.4.465. [DOI] [PubMed] [Google Scholar]
- Mándi Y, Vécsei L. The kynurenine system and immunoregulation. J Neural Transm. 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- Mason M, Berg CP. The metabolism of d- and l-tryptophan and d- and l-kynurenine by liver and kidney preparations. J Biol Chem. 1952;195:515–524. [PubMed] [Google Scholar]
- Moreno S, Nardacci R, Cimini A, Ceru MP. Immunocytochemical localization of D-amino acid oxidase in rat brain. J Neurocytol. 1999;28:169–185. doi: 10.1023/a:1007064504007. [DOI] [PubMed] [Google Scholar]
- Müller N, Myint AM, Schwarz MJ. Kynurenine pathway in schizophrenia: pathophysiological and therapeutic aspects. Curr Pharm Des. 2011;17:130–136. doi: 10.2174/138161211795049552. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Beal MF. Neuroprotective effects of L-kynurenine on hypoxia-ischemia and NMDA lesions in neonatal rats. J Cereb Blood Flow Metab. 1992;12:400–407. doi: 10.1038/jcbfm.1992.57. [DOI] [PubMed] [Google Scholar]
- Ogaya T, Song Z, Ishii K, Fukushima T. Changes in extracellular kynurenic acid concentrations in rat prefrontal cortex after D-kynurenine infusion: an in vivo microdialysis study. Neurochem Res. 2010;35:559–563. doi: 10.1007/s11064-009-0099-1. [DOI] [PubMed] [Google Scholar]
- Okuda S, Nishiyama N, Saito H, Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc Natl Acad Sci U S A. 1996;93:12553–12558. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G, Hartmann S, Lorenz B, Wollenburg C, Baran L, Przegalinski E, Kostowski W, Krzascik P, Chizh B, Headley PM. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: electrophysiological, biochemical and behavioral characterization. J Pharmacol Exp Ther. 1997;283:1264–1275. [PubMed] [Google Scholar]
- Pearson SJ, Reynolds GP. Increased brain concentrations of a neurotoxin, 3-hydroxykynurenine, in Huntington’s disease. Neurosci Lett. 1992;144:199–201. doi: 10.1016/0304-3940(92)90749-w. [DOI] [PubMed] [Google Scholar]
- Pérez-De la Cruz V, Sathyasaikumar KV, Wang X, Schwarcz R. Distinct routes of kynurenic acid biosynthesis from D- and L-Kynurenine in the human cerebellum. Soc Neurosci Abstr. 2010;35:61. 18. [Google Scholar]
- Pérez-De la Cruz V, Amori L, Sathyasaikumar KV, Wang X, Notarangelo FM, Wu HQ, Schwarcz R. Enzymatic transamination of D-Kynurenine generates kynurenic acid in rat and human brain. J Neurochem. 2012 doi: 10.1111/j.1471-4159.2012.07653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357–2367. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu HQ, Schwarcz R. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35:1734–1742. doi: 10.1038/npp.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Quearry BJ, Saito M, Nowak TS, Jr, Markey SP, Heyes MP. Kynurenine 3-hydroxylase in brain: species activity differences and effect of gerbil cerebral ischemia. Arch Biochem Biophys. 1993;307:104–109. doi: 10.1006/abbi.1993.1567. [DOI] [PubMed] [Google Scholar]
- Saito Y, Hayaishi O, Rothberg S. Studies on oxygenases; enzymatic formation of 3-hydroxy-L-kynurenine from L-kynurenine. J Biol Chem. 1957;229:921–934. [PubMed] [Google Scholar]
- Santamaria A, Rios C, Solis-Hernandez F, Ordaz-Moreno J, Gonzalez-Reynoso L, Altagracia M, Kravzov J. Systemic DL-kynurenine and probenecid pretreatment attenuates quinolinic acid-induced neurotoxicity in rats. Neuropharmacology. 1996;35:23–28. doi: 10.1016/0028-3908(95)00145-x. [DOI] [PubMed] [Google Scholar]
- Sapko MT, Guidetti P, Yu P, Tagle DA, Pellicciari R, Schwarcz R. Endogenous kynurenate controls the vulnerability of striatal neurons to quinolinate: Implications for Huntington’s disease. Exp Neurol. 2006;197:31–40. doi: 10.1016/j.expneurol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Sás K, Robotka H, Toldi J, Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007;257:221–239. doi: 10.1016/j.jns.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Joy B, Clerkin L, Schwarcz R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology. 2003;28:1454–1462. doi: 10.1038/sj.npp.1300188. [DOI] [PubMed] [Google Scholar]
- Song Z, Ogawa T, Ishii K, Ichiba H, Iizuka H, Fukushima T. Utilization of kynurenic acid produced from D-kynurenine in an in vitro assay of D-amino acid oxidase activity. J Health Sci. 2010;56:341–346. [Google Scholar]
- Speciale C, Hares K, Schwarcz R, Brookes N. High-affinity uptake of L-kynurenine by a Na+-independent transporter of neutral amino acids in astrocytes. J Neurosci. 1989;9:2066–2072. doi: 10.1523/JNEUROSCI.09-06-02066.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizawa K, Soda K. Purification and properties of pig liver kynureninase. J Biochem. 1979;85:901–906. doi: 10.1093/oxfordjournals.jbchem.a132421. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Tsukada K, Kobayashi S, Hayaishi O. A new pathway of D-tryptophan metabolism: enzymic formation of kynurenic acid via D-kynurenine. Biochem Biophys Res Commun. 1961;6:155–160. doi: 10.1016/0006-291x(61)90120-6. [DOI] [PubMed] [Google Scholar]
- Triebwasser KC, Swan PB, Henderson LM, Budny JA. Metabolism of D- and L-tryptophan in dogs. J Nutr. 1976;106:642–652. doi: 10.1093/jn/106.5.642. [DOI] [PubMed] [Google Scholar]
- Vazquez S, Garner B, Sheil MM, Truscott RJ. Characterisation of the major autoxidation products of 3-hydroxykynurenine under physiological conditions. Free Radic Res. 2000;32:11–23. doi: 10.1080/10715760000300021. [DOI] [PubMed] [Google Scholar]
- Verrall L, Walker M, Rawlings N, Benzel I, Kew JN, Harrison PJ, Burnet PW. d-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. Eur J Neurosci. 2007;26:1657–1669. doi: 10.1111/j.1460-9568.2007.05769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-D, Notarangelo FM, Schwarcz R. Kynurenic acid and 3-hydroxykynurenine production from D-Kynurenine in mice. Soc Neurosci Abstr. 2011;36:857.812. doi: 10.1016/j.brainres.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Fujiwara M, Yoshida R, Hayaishi O. Stereospecificity of hepatic L-tryptophan 2,3-dioxygenase. Biochem J. 1980;189:393–405. doi: 10.1042/bj1890393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HQ, Pereira EF, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci. 2010;40:204–210. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmarowski A, Wu HQ, Brooks JM, Potter MC, Pellicciari R, Schwarcz R, Bruno JP. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur J Neurosci. 2009;29:529–538. doi: 10.1111/j.1460-9568.2008.06594.x. [DOI] [PubMed] [Google Scholar]