Abstract
Sulfur mustard (SM) is a highly toxic chemical warfare agent that remains a threat to human health. The immediate symptoms of pulmonary distress may develop into chronic lung injury characterized by progressive lung fibrosis, the major cause of morbidity among the surviving SM victims. Although SM has been intensely investigated, little is known about the mechanism(s) by which SM induces chronic lung pathology. Increasing evidence suggests that IL-17+ cells are critical in fibrosis, including lung fibrotic diseases. In this study we exposed F344 rats and cynomolgus monkeys to SM via inhalation and determined the molecular and cellular milieu in their lungs at various times after SM exposure. In rats, SM induced a burst of pro-inflammatory cytokines/chemokines within 72 h, including IL-1β, TNF-α, IL-2, IL-6, CCL2, CCL3, CCL11, and CXCL1 that was associated with neutrophilic infiltration into the lung. At 2 wk and beyond (chronic phase), lymphocytic infiltration and continued elevated expression of cytokines/chemokines were sustained. TGF-β, which was undetectable in the acute phase, was strongly upregulated in the chronic phase; these conditions persisted until the animals were sacrificed. The chronic phase was also associated with myofibroblast proliferation, collagen deposition, and presence of IL-17+ cells. At 30 days, SM inhalation promoted the accumulation of IL-17+ cells in the inflamed areas of monkey lungs. Thus, SM inhalation causes acute and chronic inflammatory responses; the latter is characterized by the presence of TGF-β, fibrosis, and IL-17+ cells in the lung. IL-17+ cells likely play an important role in the pathogenesis of SM-induced lung injury.
Keywords: Sulfur mustard, Inflammation, Apoptosis, Fibrosis, Cytokine
1. Introduction
Sulfur mustard (SM; 2-bis-chloroethyl-sulfide) is a highly toxic vesicant that has been used in war settings, causing injuries and deaths to military and civilian personnel [1, 2]. Because of the ease of production and stability, SM represents a potential terrorist threat [3]. SM exposure occurs primarily through inhalation and absorption through skin and the anterior surface of the eye, making the lungs, the skin, and the eye as major targets of SM toxicity [4]. The severity of damage depends largely on the dose and duration of exposure. Acute effects may include skin blisters, eye irritation, and breathing discomfort (chest tightness, hacking cough, rhinorrhea); however, at sublethal doses these effects are relatively temporary. During the Iran-Iraq war, immediate mortality among the Iranian soldiers exposed to SM was only 3–4%, but decades later nearly half of the victims developed chronic respiratory complications (chronic bronchitis, airway hyperreactivity, lung fibrosis, and bronchopneumonia)–the primary cause of morbidity and mortality among these soldiers [5–9].
The mechanism(s) of SM-induced respiratory toxicity are not clear. Altered immune/inflammatory responses such as decreased natural killer cell numbers [10], increased cytokine production [11], and possible changes in cytotoxic T cells [12, 13] have been reported in Iranian SM victims. Increased expression of proinflammatory cytokines has been observed in the rat skin [14] and lungs [15], and human lung cell cultures [16, 17] after SM exposure. A single lung exposure to the nitrogen mustard analogue melphalan in mice induced an acute inflammatory response with increased IL-1 and IL-6 in the bronchoalveolar lavage (BAL) as well a chronic respiratory impairment characterized by lymphocytic infiltration and lung fibrosis [18]. In a recent study exposure of mice to nitrogen mustard induced T cell-dependent long-term lung pathology [19].
The role of T cells in SM-induced lung injury is not well delineated. We have demonstrated that hairless euthymic guinea pigs dermally exposed to SM developed a SM-specific, delayed-type hypersensitivity response, suggesting that SM can activate specific T cell-mediated immune responses [20]. Th17, a subset of CD4+ T cells, has recently been implicated in a number of inflammatory, autoimmune, and chronic fibrotic lung diseases [21], and a strong relationship between the profibrotic cytokines and Th17 has been established [22]. In this communication, we show that SM exposure causes acute and delayed responses. The delayed lung responses include lung fibrosis and the presence of IL-17+ cells in the inflamed regions of the lung, suggesting that IL-17+ cells may have an important role in the development of chronic fibrosis in SM-exposed lungs.
2. Materials and Methods
2.1 Chemicals
Except where noted, all the chemicals and reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO).
2.2 Animals
Female F344 rats (Charles River Labs, Wilmington, MA, 11–13 wk, 170–190 g) and cynomolgus monkeys (Lovelace Respiratory Research Institute monkey colony) were quarantined for a minimum of 2 wk prior to use. All animal studies were approved by the Institutional Animal Care and Use Committee, conducted in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, and carried out in compliance with the Guide for the Care and Use of Laboratory Animals. Animals were provided water and food (Harlan-Teklad, Madison, WI) ad-libitum.
2.3 SM synthesis and animal exposure
Sulfur mustard, which was synthesized by proprietary methods and analyzed by gas chromatography-mass spectrometry, gas chromatography-flame ionization detector, and nuclear magnetic resonance, was determined to be greater than 99% pure [23]. Rats were exposed by tracheal intubation as described [23]. Briefly, rats were anesthetized with isoflurane (5% induction, 2% maintenance). Anesthetized rodents were intubated with a 14-gauge Teflon catheter (approximately 5 cm in length). The catheter was inserted into the trachea to a terminal location approximately half way between the bifurcation of the trachea and the larynx. The placement and seal of the catheter were verified by observing air displacement in a groundglass, gas-tight syringe during an inhalation/exhalation cycle. Intubated rodents were transferred to the exposure plenum. An airflow consisting of SM + vehicle or vehicle alone constantly ran through the exposure plenum. Rats were exposed to a single dose of 150 mg/m3 SM for 10 min and allowed to recover for observations.
Cynomolgus monkeys were restrained by the non-human primate cage-squeeze mechanism; prior to exposures, monkeys were administered ketamine as a pre-anesthetic to isoflurane and transported to the SM exposure suite. Monkeys were anesthetized with isoflurane in oxygen (5% induction) and maintained (~2.5% maintenance) during the exposure period. The monkeys were intubated and placed prone on an exposure sled; isoflurane was constantly administered to maintain anesthesia. The setup for exposure of monkeys was similar to the rodent exposure system as described [23]. Briefly, air, O2, isoflurane, and SM were delivered to the monkeys in a flow-pass manner, where the animal inhales from an exposure atmosphere “stream” maintained at a constant flow (~ 2 L/min) and concentration of SM (150 mg/m3). Oxygen was added to the system to maintain a concentration of 19% to 22% and monitored just downstream of the breathing zone. Isoflurane was kept at a maintenance level of 1 to 3%. Pulse oximetry was utilized to assist the monitoring of vital signs. Monitoring was continued after the exposures until the animals recovered from the anesthesia. The awakened monkeys were returned to their cages, which were placed in a temporary off-gassing chamber. The animals were observed continuously until the concentration of SM returned to safe levels at which time the NHPs were removed from the temporary off-gassing chamber and returned to their home rooms.
2.4 Bronchoalveolar lavage and tissue collection
Rats were deeply anesthetized and exsanguinated by clipping the vena cava. A 16-gauge needle was introduced into the trachea and secured using 3/0 suture. The thoracic cavity was then opened to expose the trachea and lung. The left lung was lavaged three times with 3 ml aliquots of sterile saline. The lavage fluid was pooled, centrifuged at 300×g for 10 min and the supernatant collected and stored at −80°C until analyses. The left lobe along with the right cranial, medial, caudal, and accessory lobes of the lung were removed, snap frozen in liquid nitrogen, and stored at −80°C. A similar procedure was used to collect BAL and lung tissues from the monkeys.
2.5 Real-time PCR
Total RNA was isolated from lung tissues using TRI-Reagent (Molecular Research Center, Cincinnati, OH) as described [24]. Briefly, lung tissues were homogenized in 1 ml Tri-Reagent containing 100 μl BCP (Molecular Research Center), and the homogenates were centrifuged at 13,000× g for 10 min at 4°C. The aqueous layer was collected and mixed with 600 μl of isopropanol. After 15 min at room temperature, samples were centrifuged (13,000× g; 10 min) and the pellets resuspended in 75% ethanol, centrifuged, and air dried. The air-dried samples were resuspended in diethyl-pyrocarbonate-treated water (55°C for 10 min to dissolve RNA) and quantitated spectrophotometrically. The real-time polymerase chain reaction (qPCR) analysis was performed on the ABI PRISM 7900HT Real-Time PCR System using the One-Step RT-PCR Master Mix (Applied Biosystems, Foster City, CA). The relative expression of each mRNA was calculated by the method described earlier [24]. mRNAs were normalized with 18S rRNA or GAPDH; both gave similar results. All primer/probe sets for cytokines/chemokines and GAPDH were purchased from Applied Biosystems.
2.6 Immunohistochemistry
For immunohistochemistry (IHC), lung sections were made as described [24]. Briefly, the lung was inflated for 2 h at a constant hydrostatic pressure of 25 cm with 10% neutral buffered formalin and immersed in the same solution for 48 h. The fixed lung was trimmed, embedded in paraffin, and cut into 5 μm sections. The endogenous peroxidase was quenched by incubating the slides containing tissue sections in 2% hydrogen peroxide diluted in methanol for 1 min. The slides were washed with deionized water followed by washes with Dulbecco’s polybuffered saline (pH 7.4) containing 0.05% Brij. Proteins were unmasked by incubating the tissue sections with trypsin solution (Zymed Laboratory, San Francisco, CA) at 37°C for 10 min. After blocking nonspecific binding by 1% horse serum containing 2% BSA and 0.1% Triton X-100, the slides were incubated overnight at 4°C in a humidified chamber with primary antibody diluted in blocking buffer, followed by incubation with biotinylated secondary antibody (VECTASTAIN® Elite ABC kit, Vector Laboratories, Burlingame, CA). Binding was visualized using an avidin-biotinylated enzyme complex (VECTASTAIN® Elite ABC kit) with 3, 3′-diaminobenzidine (DAB) as substrate.
2.7 TUNEL assay
Terminal deoxynucleotidyl transferase (TUNEL) staining and making the positive and negative controls were performed on paraffin-embedded lung sections by the TACS 2 TdT-DAB In Situ Apoptosis Detection Kit (Trevigen, Gaithersburg, MD) as per manufacturer’s instructions.
2.8 Statistical analysis
All statistical calculations were performed using GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA). Results are expressed as mean values ± standard deviations. Statistical comparisons were determined by the unpaired Student’s t-test. The differences with P values of ≤ 0.05 were regarded as significant.
3. RESULTS
3.1 SM induces early expression of proinflammatory cytokines/chemokines and promotes leukocytic infiltration in the lung
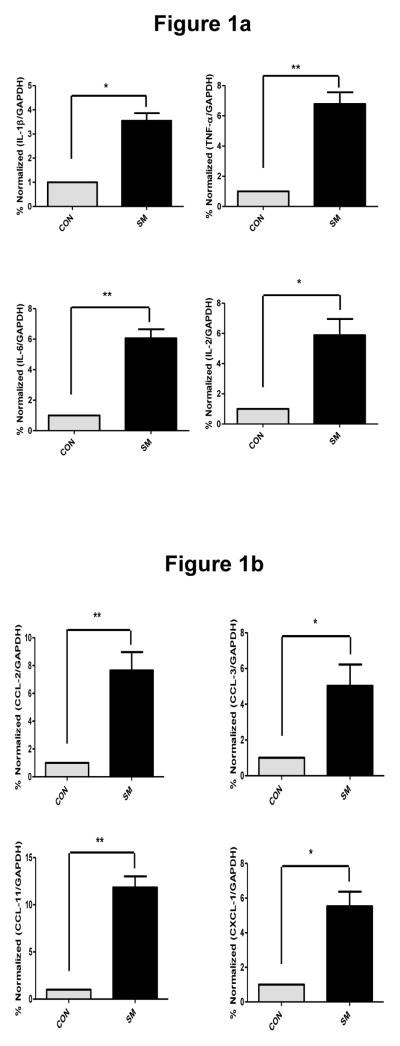
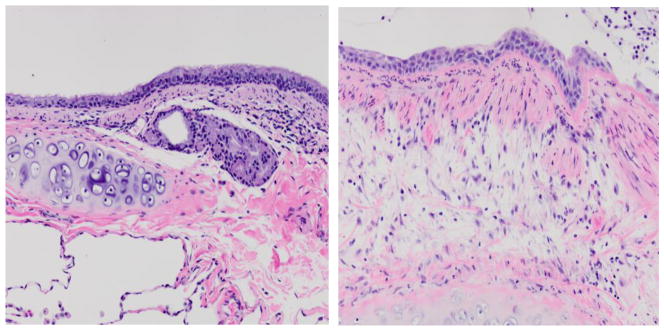
To investigate whether inhalation of SM induces an acute proinflammatory response in the lung, we determined the expression of pro-inflammatory cytokines and chemokines in the lung tissues and BAL fluid of control and SM-exposed rats by qPCR and ELISA. At 3 days after SM exposure, qPCR analysis indicated that compared to vehicle control, SM induced significant increases in the lung expression of proinflammatory cytokines (IL-1β, TNF-α, IL-2, and IL-6) (Fig. 1a) and proinflammatory chemokines (Fig. 1b), including CCL2 (MCP-1), CCL3 (MIP-1α), CCL11 (eotaxin), and CXCL1 (Gro-α). ELISA determinations of BALF indicated that SM exposure caused a significant rise in the immunoreactive protein content of TNF-α (19.2 ± 4.4 PG/ML) compared to control (3.1 ± 2.2 PG/ML) and IL-1β (11.5 + 2.4 PG/ML) compared to control (4.3 ± 2.0 PG/ML). Interestingly, the expression (qPCR and ELISA analysis) of the profibrotic cytokines (e.g., TGF-β, IL-13) was not significantly different between the control and SM-exposed lung at this time point (not shown). These results suggest that SM inhalation exposure leads to early upregulated expression of proinflammatory cytokines and chemokines but not profibrotic cytokines in the rat lung. Histopathology of the lung sections from SM-exposed rats at 3 days post exposure also indicated a significant increase in neutrophilic infiltration in the lung (not shown). Similarly, histopathology of the lungs from monkeys at 2 wk post SM exposure showed significant increases in neutrophilic infiltration and proliferative changes in mucosal epithelium (Fig. 2). Thus, the early lung inflammatory response is the predominant feature of SM exposure.
Figure 1. SM induces early expression of proinflammatory cytokines (a) and chemokines (b) in rat lungs.
F344 rats were exposed to 150 mg/m3 of SM or vehicle for 10 min by tracheal intubation; animals were sacrificed after 3 days to collect lung tissue. Total RNA was isolated from lung tissues as described in “Materials and Methods.” The qPCR analysis was performed; relative expression of each mRNA and cytokine were calculated. Data are expressed as mean ± SD (6–8 animals in each group). *p<0.05, **p<0.01.
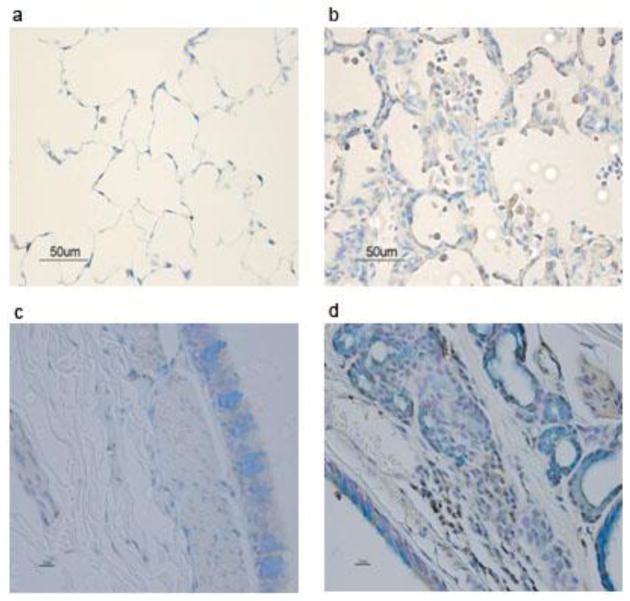
Figure 2. SM exposure causes histopathological changes in mucosal epithelium and neutrophil infiltration in cynomolgus macaque lungs.
Monkeys were exposed to SM or vehicle by tracheal intubation. Sections of lung bronchus were prepared 2 wk after exposure, stained with H&E and examined microscopically. Normal bronchial mucosal epithelium of a control monkey (left panel) is ciliated with mucus-secreting cells; mucosal epithelium is replaced by stratified squamous epithelium after SM exposure (right panel). The figure is a representative of 2 monkeys in each group.
3.2 SM induces apoptosis in lung cells
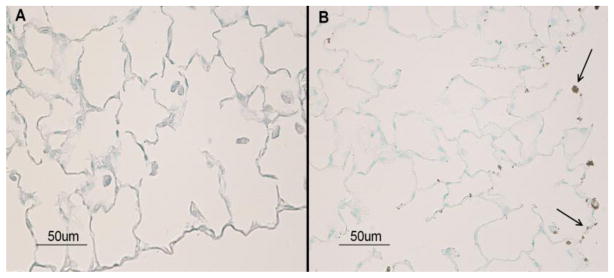
Early apoptotic cell death has been suggested as the critical event in the initiation of a number of fibrotic diseases, including lung fibrotic diseases [25]. To ascertain whether SM-induced lung injury involved apoptotic cell death, we performed a TUNEL assay on lung sections from control and SM-exposed rats at 3 days post exposure. Results showed that SM induced a significant increase in TUNEL-positive cells in the lung (Fig. 3). Thus, as with other fibrotic lung diseases, prior to a significant fibrotic response, SM exposure triggers an early apoptotic cell death.
Figure 3. SM exposure induces apoptosis in the rat lung cells.
Animals were exposed to 150 mg/m3 of SM and sacrificed 48 h post exposure to collect lungs. Tissue sections from control (left) and SM-exposed (right) rats were examined by TUNEL assay. Arrows indicate TUNEL-positive cells (nuclear condensates) present in the lung sections from SM-exposed animals. The figure is a representative of 4 animals per group.
3.3 SM promotes delayed proliferation of myofibroblast and lung fibrosis
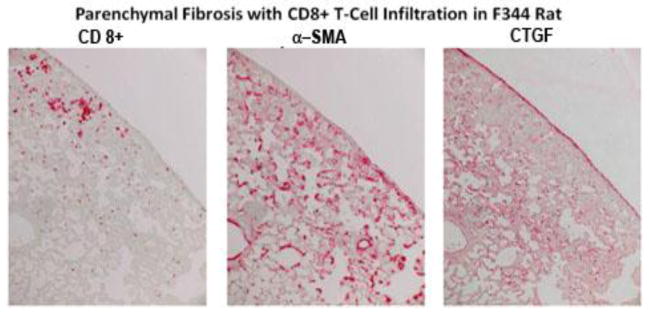
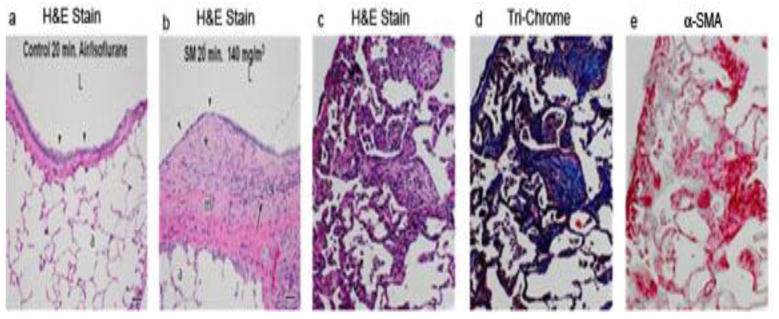
Lung fibrosis is the major cause of morbidity and mortality in SM-exposed Iranian victims [26]. To determine whether SM exposure leads to lung fibrosis, we determined whether SM inhalation causes lung fibrosis in rats at various times following SM exposure. Pro-fibrotic changes as judged by histopathology were minimal up to 14 days post SM exposure (not shown); however, significant thickening of the submucosa with fibroblastic tissue was seen at day 21 (see later, Fig. 6b) and increased progressively thereafter. At 28 days after SM exposure, IHC staining suggested that the lungs from rats exposed to SM had increased expression of the fibrosis marker CTGF [27], the myofibroblast marker α-smooth muscle actin (α-SMA), and infiltration of CD8+ cells (Fig. 4). At 39 days post exposure, hematoxylin and eosin (H&E staining) (Fig. 5a–c), trichrome staining (Fig. 5d), and IHC staining for α-SMA (Fig. 5e) of adjacent lung sections suggested that SM promotes chronic lung inflammation, collagen deposition, and myofibroblast proliferation, respectively. These results indicate that SM inhalation induces a delayed profibrotic response in the lung. In addition, SM induces emigration of CD8+ cells in the lung; the role of these cells in lung fibrosis is not clear.
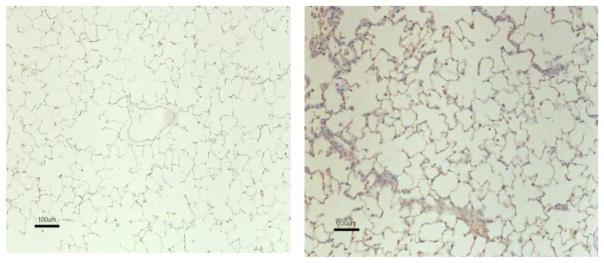
Figure 6. SM stimulates the production of TGF-β in the lung.
F344 rats were exposed to vehicle or SM (150 mg/m3) as described in “Materials and Methods.” At day 28 days post exposure, rats were sacrificed to collect lung tissues; IHC staining on lung sections was performed for TGF-β. Tissue sections treated with vehicle (left panel) and SM (right panel) were evaluated for TGF-β expression. Brown staining represents TGF-β reactivity. The figure is representative of 6 animals in each group.
Figure 4. SM increases CTGF and promotes infiltration of CD8+ T cells in the lung.
Rats were exposed to vehicle or 150 mg/m3 SM and sacrificed at day 39 post exposure. Adjacent lung sections were stained for CD8, α-SMA, and CTGF. Parenchymal fibrosis with CD8+ T cell infiltration is evident in these sections. The control section showed minimal staining under these conditions. The figure is representative of 6 animals in each group.
Figure 5. SM promotes proliferation of myofibroblasts and fibrosis in the lung.
Rats were exposed to vehicle (control) or SM (150 mg/m3 for 10 min). Animals were sacrificed at day 21 (a, b) or at day 39 (c, d, and e) after exposure. Lung sections were stained with H&E (a, b, c), trichrome (d), or α-SMA-specific antibody (e). Note the thickened fibroblastic tissues after SM exposure (b).
3.4 SM stimulates the accumulation of TGF-β+ cells in the lungs
TGF-β is the major profibrotic cytokine that is produced primarily by T cells and macrophages [28]. To determine the role of TGF-β in SM-induced chronic lung injury and development of pulmonary fibrosis, control and SM-exposed rat lungs were stained for TGF-β+ cells by IHC at 3 days and 28 days post SM exposure. While at 3 days, staining of TGF-β was not significant (not shown), whereas at 28 d post SM exposure, lungs showed a strong presence of TGF-β+ cells (Fig. 6). These results suggest that SM inhalation causes delayed expression of TGF-β in the lung.
3.5 SM inhalation promotes accumulation of IL-17+ cells in rat and monkey lungs
SM-induced lung fibrosis is progressive and appears late after SM inhalation, suggesting an “autoimmune-like” etiology. Many autoimmune and fibrotic lung diseases are associated with the presence of Th17 cells. To determine whether SM promotes the accumulation of IL-17+ cells in the lung, lung sections from vehicle control and SM-exposed rats (28 days post SM/air inhalation); and vehicle control and SM-exposed monkeys (60 days post SM/air inhalation) were evaluated for the presence of IL-17+ cells by IHC staining. As shown in Fig. 7, SM inhalation exposure induced the migration of IL-17+ cells into the rat lung (Fig. 7a and 7b) and monkeys (Fig. 7c and 7d) at 28 days and 60 days, respectively. Interestingly, in monkeys, IL17+ cells were not seen in significant numbers prior to 30 days post SM exposure, where only a few IL-17+ cells were seen in the lung (not shown); however, a strong increase in the presence of these cells was observed at day 60 post SM exposure. Moreover, the infiltration of IL-17+ cells was observed only in the areas of lung inflammation/fibrosis. These results suggest that IL-17+ may be intimately associated with SM-induced chronic lung inflammation and fibrosis.
Figure 7. SM induces accumulation of IL-17+ cells in the lung.
F344 rats and cynomolgus macaques were exposed to vehicle or SM (150 mg/m3) as described in “Materials and Methods.” Lung sections were processed for intracellular IHC staining to visualize IL-17+ cells. The sections represent rat control (a), rat SM (b), monkey control (c), and monkey SM (d). Brown staining represents IL-17+ cells (right panels); vehicle control sections show minimal staining (left panels).
4. DISCUSSION
The surviving SM victims of the Iran-Iraq war continue to suffer from chronic respiratory complication of SM exposure [7]. In humans, respiratory exposure to mustard agents (sulfur and nitrogen mustards) produces acute lung injury followed by chronic progressive lung fibrosis [6, 7, 26, 29, 30]. Moreover, the SM-exposed patients show persistent signs of immune dysfunction and accumulation of CD8+ cells in the lung [10–13]. Thus, sublethal exposures to mustard agents may induce two distinct but partially overlapping phases of lung injury: the acute phase involving production of proinflammatory factors and the chronic phase characterized by immunological alterations leading to progressive lung fibrosis.
To better define the early and late events in SM-induced lung injury in this study, we exposed rats and monkeys to sublethal doses of SM. In the rat model, within 3 days after SM exposure, mRNA expression of the prototypic inflammatory cytokines (e.g., IL-1β, IL-6, TNF-α, IL-17) and chemokines (CCL2, CCL3, CCL11 and CXCL1) was significantly higher in the lung. This was accompanied with higher protein content of proinflammatory cytokines (IL-1β and TNF-α) in the BALF from SM-exposed animals. Histopathology of lung tissues indicated increased numbers of neutrophils, eosinophils, and lymphocytes in the lungs of SM-exposed rats. It is conceivable that increased leukocytic infiltration resulted from the increased expression of proinflammatory cytokines and chemokines. Cytokine chemoattractants such as CCL2, CCL3, and CXCL1 are pivotal in the development of inflammatory responses. CCL2 (MCP-1) is a small secreted protein that has chemoattractant activity for not only monocytes, but also memory T cells, natural killer cells, and perhaps dendritic cells resulting in their recruitment to the sites of tissue injury and inflammation [31–33]. CCL2 is also implicated in chronic inflammatory/autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, glomerulonephritis [34] and pulmonary fibrosis [35]. CCL3 (MIP-1α) is produced by a variety of cells including monocyte/macrophages, mast cells, epithelial cells, and lymphocytes. It has been shown as a neutrophil chemoattractant in allergen and infection associated lung inflammations [36, 37]. Interestingly, CCL3-mediated neutrophilic immigration in the lung may be mediated by IL-1β and TNF-α [36, 38]–both the proinflammatory cytokines were upregulated in the SM lung. CXCL1 (Gro-α) acts like IL-8 and is a strong chemoattractant for neutrophils in experimental models of human inflammatory diseases [39, 40]. Eotaxin is an established chemoattractant for eosinophils in the lung [41], and it is likely that increased levels of eotaxin contributes to the eosinophilic infiltration seen in SM-exposed lungs. In addition to these cytokines/chemokines, SM caused moderate increases (2–3 fold) in the expression of IL-4 and IL-5 (not shown); interestingly; however, IL-13 expression in the SM lung did not increase significantly. While IL-5 promotes eosinophilic responses, IL4 and IL-13 are fibrogenic in several fibrotic conditions [42]. Moreover, in rats at 3 days post SM exposure, the expression of TGF-β or IL-13 had not increased significantly. These results suggest that early response in the lung is mostly inflammatory and not profibrotic.
Apoptosis is a tightly regulated mechanism for cell death that eliminates unwanted, damaged, and infected cells. Apoptosis is believed to have contributed to the lung injury in Iranian victims of SM exposure [43]. Increased LDH activity (cell death) was observed in guinea pig lungs [44] and tracheal epithelial cells of pigs exposed to SM inhalation [45]. If the cell injury/death involves an apoptotic process, the nuclear condensation of apoptotic cells can be visualized by TUNEL staining [46]. Our results indicated significant damage to the epithelial cells and the presence of TUNEL-positive cells in SM-exposed but not control lungs 3 days after SM inhalation. Malaviya et al. [15] also reported an early activation (6 h after SM inhalation) of an apoptotic response (TUNEL-positive cells and caspase 3/9 activation) in the lungs. Increasing evidence suggests that xenobiotic-induced apoptotic cell death in the lung is associated with lung fibrosis, and blocking apoptosis ameliorates fibrotic response in in vitro and in vivo models of lung injury [47–49]. On the other hand, fibroblasts obtained from established lung fibrotic disease may become resistant to apoptosis and contribute to the progressive fibrotic process [50–52]. Together these results suggest that SM inhalation induces a potent inflammatory response in the lungs that is associated with apoptotic cell death, which may initiate the fibrotic response. Early interventions might be useful in inhibiting the extensive fibrotic response seen at later stages of SM exposure.
The major cause of morbidity and mortality among human SM victims is lung fibrosis; however, the mechanism of lung fibrosis is not clearly understood. It is generally accepted that neutrophils and eosinophils are the abundant cell type during the early stages of lung injury. The cytokines and chemokines released at the site of injury stimulate angiogenesis and activate T cells that are the major producers of the profibrotic cytokines IL-4/IL-13 and TGF-β [28, 42]. These cytokines, particularly TGF-β, activate macrophages and fibroblasts, and the activated fibroblasts may transform into α-SMA-expressing myofibroblasts [53]. Apart from TGF-β, IL-13 and IL-4 have been identified as key fibrogenic cytokines in many fibrotic conditions [42, 54, 55]. However, our results show that a single sublethal dose of SM did not induce significant expression of IL-13 at any time after SM exposure. There was only a moderate transitory increase in IL-4, which, in addition to being an archetypal Th2 cytokine, promotes TGF-β production from eosinophils [56]. Moreover, increased TGF-β expression was detected only at later times (2 wk after SM inhalation), and the increased fibrotic response as seen by trichrome staining and augmented expression of CTGF and α-SMA was detected several weeks after SM exposure. Coincident with lung fibrosis, was the accumulation of CD8+ and IL-17+ cells in the lung. The presence of CD8+ cells has been reported in other fibrotic lung diseases, including COPD [57–59], idiopathic pulmonary fibrosis [60–62], and bronchiolitis obliterans [63]. Interestingly, increased numbers of CD8+ cells are present in the BAL of SM-exposed patients who show signs of bronchiolitis obliterans [13, 64]. We also observed the presence of CD8+ cells in skin sections of dermally SM-exposed guinea pigs [20]. Thus, CD8+ cells seem to be associated with a number of fibrotic diseases including SM-exposed humans and animals; however, whether CD8+ cells perpetuate the injury as cytotoxic cells or play a direct role in lung fibrosis is not clear.
Based on cytokine/transcription factor expression, CD4+ T cells have been divided into three subpopulations: Th1 (IFN-γ+/T-bet+), Th2 (IL4+/STAT3+), and recently added Th17 (IL-17+/ROR-γt) [65]. IL-17+ cells have been implicated in a number of autoimmune and experimental fibrotic lung diseases, including cystic fibrosis [66], sarcoidosis [67], systemic sclerosis-related pulmonary fibrosis [68], and bleomycin-induced lung fibrosis [69]. IL-1β, IL-6, and TGF-β are critically important for differentiation of Th17 cells, production of IL-17, and lung fibrosis [69, 70]. Our results indicate that SM significantly increases the expression of IL-17, IL-6, and TGF-β; however, the expression of TGF-β was delayed and preceded the development of frank lung fibrosis. Because, IL-17 is also produced by neutrophils, it is likely that early expression of IL-17 was associated with the infiltration of neutrophils in the lungs of SM-exposed animals. Most of these studies were carried out in rats; however, in a limited number of monkeys, SM caused delayed lung fibrosis that was associated with the presence of IL-17+ cells in the lung. Moreover, in monkeys, SM caused a patchy inflammatory response, and IL-17+ cells were abundantly present only in inflamed areas of the lung. Thus, IL-17+ cells are clearly associated with lung inflammation. We also have preliminary evidence suggesting that SM can induce similar responses in C57BL/6 mice, including the increased presence of CD4+ IL-17+ T cells and cellular and molecular imprints of lung fibrosis (Mishra et al., unpublished observation). Thus, our results indicate that IL-17 may be involved in SM-induced lung fibrosis across the mammalian species and a potential target for therapeutic interventions.
Highlights.
Sulfur mustard is a highly toxic warfare agent and remains threat to human health.
SM inhalation causes acute and chronic inflammatory responses.
SM inhalation causes proliferation of myofibroblast and deposition of collagen.
SM inhalation was associated with the presence of IL-17+ cells in the lung.
IL-17+ cells play an important role in the pathogenesis of SM-induced lung injury.
Acknowledgments
This work was supported by the CounterACT Program, the National Institutes of Health Office of the Director, and the National Institute of Neurological Diseases and Stroke (grant number 5U54NS058185). The authors would like to thank Drs. Tom March and Julie Hutt for pathological assessment of the slides
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kehe K, Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214(3):198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Ghanei M, Naderi M, Kosar AM, Harandi AA, Hopkinson NS, Poursaleh Z. Long-term pulmonary complications of chemical warfare agent exposure in Iraqi Kurdish civilians. Inhal Toxicol. 2010;22(9):719–24. doi: 10.3109/08958371003686016. [DOI] [PubMed] [Google Scholar]
- 3.Wattana M, Bey T. Mustard gas or sulfur mustard: an old chemical agent as a new terrorist threat. Prehosp Disaster Med. 2009;24(1):19–29. doi: 10.1017/s1049023x0000649x. discussion 30–1. [DOI] [PubMed] [Google Scholar]
- 4.Ghabili K, Agutter PS, Ghanei M, Ansarin K, Panahi Y, Shoja MM. Sulfur mustard toxicity: history, chemistry, pharmacokinetics, and pharmacodynamics. Crit Rev Toxicol. 2011;41(5):384–403. doi: 10.3109/10408444.2010.541224. [DOI] [PubMed] [Google Scholar]
- 5.Balali-Mood M, Hefazi M. Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic Clin Pharmacol Toxicol. 2006;99(4):273–82. doi: 10.1111/j.1742-7843.2006.pto_429.x. [DOI] [PubMed] [Google Scholar]
- 6.Ghanei M, Naderi M, Kosar AM, Harandi AA, Hopkinson NS, Poursaleh Z. Long-term pulmonary complications of chemical warfare agent exposure in Iraqi Kurdish civilians. Inhal Toxicol. 2010;22(9):719–24. doi: 10.3109/08958371003686016. [DOI] [PubMed] [Google Scholar]
- 7.Balali-Mood M, Afshari R, Zojaji R, Kahrom H, Kamrani M, Attaran D, et al. Delayed toxic effects of sulfur mustard on respiratory tract of Iranian veterans. Hum Exper Toxicol. 2010;30(9):1141–9. doi: 10.1177/0960327110389501. [DOI] [PubMed] [Google Scholar]
- 8.Adelipour M, Imani Fooladi AA, Yazdani S, Vahedi E, Ghanei M, Nourani MR. Smad molecules expression pattern in human bronchial airway induced by sulfur mustard. Iran J Allergy Asthma Immunol. 2011;10(3):147–54. [PubMed] [Google Scholar]
- 9.Taghaddosinejad F, Fayyaz AF, Behnoush B. Pulmonary complications of mustard gas exposure: a study on cadavers. Acta Med Iran. 2011;49(4):233–6. [PubMed] [Google Scholar]
- 10.Ghotbi L, Hassan Z. The immunostatus of natural killer cells in people exposed to sulfur mustard. Int Immunopharmacol. 2002;2(7):981–5. doi: 10.1016/s1567-5769(02)00053-x. [DOI] [PubMed] [Google Scholar]
- 11.Emad A, Emad Y. Levels of cytokine in bronchoalveolar lavage (BAL) fluid in patients with pulmonary fibrosis due to sulfur mustard gas inhalation. J Interferon Cytokine Res. 2007;27(1):38–43. doi: 10.1089/jir.2006.0084. [DOI] [PubMed] [Google Scholar]
- 12.Emad A, Emad Y. CD4/CD8 ratio and cytokine levels of the BAL fluid in patients with bronchiectasis caused by sulfur mustard gas inhalation. Journal of Inflammation (London, England) 2007;4:2. doi: 10.1186/1476-9255-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emad A, Emad Y. Increased in CD8 T lymphocytes in the BAL fluid of patients with sulfur mustard gas-induced pulmonary fibrosis. Respir Med. 2007;101(4):786–92. doi: 10.1016/j.rmed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Gao X, Anderson DR, Brown AW, Lin H, Amnuaysirikul J, Chua AL, et al. Pathological studies on the protective effect of a macrolide antibiotic, roxithromycin, against sulfur mustard inhalation toxicity in a rat model. Toxicol Pathol. 2011;39(7):1056–64. doi: 10.1177/0192623311422079. [DOI] [PubMed] [Google Scholar]
- 15.Malaviya R, Sunil VR, Cervelli J, Anderson DR, Holmes WW, Conti ML, et al. Inflammatory effects of inhaled sulfur mustard in rat lung. Toxcol Appl Pharmacol. 2010;248(2):89–99. doi: 10.1016/j.taap.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emmler J, Hermanns MI, Steinritz D, Kreppel H, Kirkpatrick CJ, Bloch W, et al. Assessment of alterations in barrier functionality and induction of proinflammatory and cytotoxic effects after sulfur mustard exposure of an in vitro coculture model of the human alveolo-capillary barrier. Inhal Toxicol. 2007;19(8):657–65. doi: 10.1080/08958370701353726. [DOI] [PubMed] [Google Scholar]
- 17.Seagrave J, Weber WM, Grotendorst GR. Sulfur mustard vapor effects on differentiated human lung cells. Inhal Toxicol. 2010;22(11):896–902. doi: 10.3109/08958378.2010.493901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wigenstam E, Rocksen D, Ekstrand-Hammarstrom B, Bucht A. Treatment with dexamethasone or liposome-encapsuled vitamin E provides beneficial effects after chemical-induced lung injury. Inhal Toxicol. 2009;21(11):958–64. doi: 10.1080/08958370802596298. [DOI] [PubMed] [Google Scholar]
- 19.Ekstrand-Hammarstrom B, Wigenstam E, Bucht A. Inhalation of alkylating mustard causes long-term T cell-dependent inflammation in airways and growth of connective tissue. Toxicology. 2011;280(3):88–97. doi: 10.1016/j.tox.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Mishra NC, Rir-Sima-Ah J, March T, Weber W, Benson J, Jaramillo R, et al. Sulfur mustard induces immune sensitization in hairless guinea pigs. Int Immunopharmacol. 2010;10(2):193–9. doi: 10.1016/j.intimp.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123(5):986–94. doi: 10.1016/j.jaci.2009.03.033. quiz 95–6. [DOI] [PubMed] [Google Scholar]
- 22.Han G, Li F, Singh TP, Wolf P, Wang XJ. The Pro-inflammatory Role of TGFbeta1: A Paradox? Int J Biol Sci. 2012;8(2):228–35. doi: 10.7150/ijbs.8.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber WM, Kracko DA, Lehman MR, Irvin CM, Blair LF, White RK, et al. Inhalation exposure systems for the development of rodent models of sulfur mustard-induced pulmonary injury. Toxicol Mech Meth. 2010;20(1):14–24. doi: 10.3109/15376510903483730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra NC, Rir-Sima-Ah J, Langley RJ, Singh SP, Pena-Philippides JC, Koga T, et al. Nicotine primarily suppresses lung Th2 but not goblet cell and muscle cell responses to allergens. J Immunol. 2008;180(11):7655–63. doi: 10.4049/jimmunol.180.11.7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2(2):103–21. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghanei M, Adibi I, Farhat F, Aslani J. Late respiratory effects of sulfur mustard: how is the early symptoms severity involved? Chron Respir Dis. 2008;5(2):95–100. doi: 10.1177/1479972307087191. [DOI] [PubMed] [Google Scholar]
- 27.Ponticos M, Holmes AM, Shi-wen X, Leoni P, Khan K, Rajkumar VS, et al. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum. 2009;60(7):2142–55. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- 28.Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, Peng X, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol. 2011;43(1):154–62. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Balali-Mood M, Afshari R, Zojaji R, Kahrom H, Kamrani M, Attaran D, et al. Delayed toxic effects of sulfur mustard on respiratory tract of Iranian veterans. Hum Exp Toxicol. 2011;30(9):1141–9. doi: 10.1177/0960327110389501. [DOI] [PubMed] [Google Scholar]
- 30.Ghabili K, Agutter PS, Ghanei M, Ansarin K, Panahi Y, Shoja MM. Sulfur mustard toxicity: history, chemistry, pharmacokinetics, and pharmacodynamics. Crit Rev Toxicol. 2011;41(5):384–403. doi: 10.3109/10408444.2010.541224. [DOI] [PubMed] [Google Scholar]
- 31.Balkwill F. Cancer and the chemokine network. Nat Rev. 2004;4(7):540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 32.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95(9):858–66. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 33.Ajuebor MN, Flower RJ, Hannon R, Christie M, Bowers K, Verity A, et al. Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J Leukoc Biol. 1998;63(1):108–16. doi: 10.1002/jlb.63.1.108. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Patel L, Pienta KJ. Targeting chemokine (C-C motif) ligand 2 (CCL2) as an example of translation of cancer molecular biology to the clinic. Prog Mol Biol Transl Sci. 2010;95:31–53. doi: 10.1016/B978-0-12-385071-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore BB, Paine R, 3rd, Christensen PJ, Moore TA, Sitterding S, Ngan R, et al. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol. 2001;167(8):4368–77. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- 36.Ramos CD, Canetti C, Souto JT, Silva JS, Hogaboam CM, Ferreira SH, et al. MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4. J Leukoc Biol. 2005;78(1):167–77. doi: 10.1189/jlb.0404237. [DOI] [PubMed] [Google Scholar]
- 37.Das AM, Ajuebor MN, Flower RJ, Perretti M, McColl SR. Contrasting roles for RANTES and macrophage inflammatory protein-1 alpha (MIP-1 alpha) in a murine model of allergic peritonitis. Clin Exp Immunol. 1999;117(2):223–9. doi: 10.1046/j.1365-2249.1999.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroder JM. Chemoattractants as mediators of neutrophilic tissue recruitment. Clin Dermatol. 2000;18(3):245–63. doi: 10.1016/s0738-081x(99)00117-0. [DOI] [PubMed] [Google Scholar]
- 39.Koch AE, Kunkel SL, Shah MR, Hosaka S, Halloran MM, Haines GK, et al. Growth-related gene product alpha. A chemotactic cytokine for neutrophils in rheumatoid arthritis. J Immunol. 1995;155(7):3660–6. [PubMed] [Google Scholar]
- 40.MacDermott RP. Chemokines in the inflammatory bowel diseases. J Clin Immunol. 1999;19(5):266–72. doi: 10.1023/a:1020583306627. [DOI] [PubMed] [Google Scholar]
- 41.Graziano FM, Cook EB, Stahl JL. Cytokines, chemokines, RANTES, and eotaxin. Allergy Asthma Proc. 1999;20(3):141–6. doi: 10.2500/108854199778553055. [DOI] [PubMed] [Google Scholar]
- 42.Wynn TA. IL-13 effector functions. Ann Rev Immunol. 2003;21:425–56. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 43.Ghanei M, Harandi AA. Molecular and cellular mechanism of lung injuries due to exposure to sulfur mustard: a review. Inhal Toxicol. 2011;23(7):363–71. doi: 10.3109/08958378.2011.575413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allon N, Amir A, Manisterski E, Rabinovitz I, Dachir S, Kadar T. Inhalation exposure to sulfur mustard in the guinea pig model: clinical, biochemical and histopathological characterization of respiratory injuries. Toxicol App Pharmacol. 2009;241(2):154–62. doi: 10.1016/j.taap.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Fairhall SJ, Jugg BJ, Read RW, Stubbs SJ, Rutter SJ, Smith AJ, et al. Exposure-response effects of inhaled sulfur mustard in a large porcine model: a 6-h study. Inhal Toxicol. 2010;22(14):1135–43. doi: 10.3109/08958378.2010.527398. [DOI] [PubMed] [Google Scholar]
- 46.Torchinsky MB, Garaude J, Blander JM. Infection and apoptosis as a combined inflammatory trigger. Curr Opin Immunol. 2010;22(1):55–62. doi: 10.1016/j.coi.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borges VM, Falcao H, Leite-Junior JH, Alvim L, Teixeira GP, Russo M, et al. Fas ligand triggers pulmonary silicosis. J Exp Med. 2001;194(2):155–64. doi: 10.1084/jem.194.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang WD, Wang JZ, Lu YQ, Di YM, Jiang JK, Zhang Q. Lysine acetylsalicylate ameliorates lung injury in rats acutely exposed to paraquat. Chin Med J. 2011;124(16):2496–501. [PubMed] [Google Scholar]
- 49.Imazu Y, Yanagi S, Miyoshi K, Tsubouchi H, Yamashita S, Matsumoto N, et al. Ghrelin ameliorates bleomycin-induced acute lung injury by protecting alveolar epithelial cells and suppressing lung inflammation. Eur J Pharmacol. 2011;672(1–3):153–8. doi: 10.1016/j.ejphar.2011.09.183. [DOI] [PubMed] [Google Scholar]
- 50.Lappi-Blanco E, Soini Y, Paakko P. Apoptotic activity is increased in the newly formed fibromyxoid connective tissue in bronchiolitis obliterans organizing pneumonia. Lung. 1999;177(6):367–76. doi: 10.1007/pl00007654. [DOI] [PubMed] [Google Scholar]
- 51.Cha SI, Groshong SD, Frankel SK, Edelman BL, Cosgrove GP, Terry-Powers JL, et al. Compartmentalized expression of c-FLIP in lung tissues of patients with idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;42(2):140–8. doi: 10.1165/rcmb.2008-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wynes MW, Edelman BL, Kostyk AG, Edwards MG, Coldren C, Groshong SD, et al. Increased cell surface Fas expression is necessary and sufficient to sensitize lung fibroblasts to Fas ligation-induced apoptosis: implications for fibroblast accumulation in idiopathic pulmonary fibrosis. J Immunol. 2011;187(1):527–37. doi: 10.4049/jimmunol.1100447. [DOI] [PubMed] [Google Scholar]
- 53.Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep. 2006;8(2):145–50. doi: 10.1007/s11926-006-0055-x. [DOI] [PubMed] [Google Scholar]
- 54.Emura M, Nagai S, Takeuchi M, Kitaichi M, Izumi T. In vitro production of B cell growth factor and B cell differentiation factor by peripheral blood mononuclear cells and bronchoalveolar lavage T lymphocytes from patients with idiopathic pulmonary fibrosis. Clin Exp Immunol. 1990;82(1):133–9. doi: 10.1111/j.1365-2249.1990.tb05416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallace WA, Ramage EA, Lamb D, Howie SE. A type 2 (Th2-like) pattern of immune response predominates in the pulmonary interstitium of patients with cryptogenic fibrosing alveolitis (CFA) Clin Exp Immunol. 1995;101(3):436–41. doi: 10.1111/j.1365-2249.1995.tb03131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elovic AE, Ohyama H, Sauty A, McBride J, Tsuji T, Nagai M, et al. IL-4-dependent regulation of TGF-alpha and TGF-beta1 expression in human eosinophils. J Immunol. 1998;160(12):6121–7. [PubMed] [Google Scholar]
- 57.O’Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155(3):852–7. doi: 10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- 58.Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, et al. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(3 Pt 1):822–6. doi: 10.1164/ajrccm.157.3.9709027. [DOI] [PubMed] [Google Scholar]
- 59.Nadigel J, Prefontaine D, Baglole CJ, Maltais F, Bourbeau J, Eidelman DH, et al. Cigarette smoke increases TLR4 and TLR9 expression and induces cytokine production from CD8+ T cells in chronic obstructive pulmonary disease. Respir Res. 2011;12:149. doi: 10.1186/1465-9921-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papiris SA, Kollintza A, Karatza M, Manali ED, Sotiropoulou C, Milic-Emili J, et al. CD8+ T lymphocytes in bronchoalveolar lavage in idiopathic pulmonary fibrosis. J Inflamm (London, England) 2007;4:14. doi: 10.1186/1476-9255-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagai S, Handa T, Ito Y, Takeuchi M, Izumi T. Bronchoalveolar lavage in idiopathic interstitial lung diseases. Semin Respir Crit Care Med. 2007;28(5):496–503. doi: 10.1055/s-2007-991522. [DOI] [PubMed] [Google Scholar]
- 62.Kopinski P, Przybylski G, Balicka-Slusarczyk B, Chorostowska-Wynimko J, Pinis G, Plato M, et al. Cigarette smoking results in the number of CD8+Fas Ligand+ T cytotoxic lymphocytes in bronchoalveolar lavage (BAL) fluid of patients with idiopathic pulmonary fibrosis (IPF) Przeglad Lekarski. 2007;64(10):689–94. [PubMed] [Google Scholar]
- 63.Hodge S, Hodge G, Ahern J, Liew CL, Hopkins P, Chambers DC, et al. Increased levels of T cell granzyme b in bronchiolitis obliterans syndrome are not suppressed adequately by current immunosuppressive regimens. Clin Exp Immunol. 2009;158(2):230–6. doi: 10.1111/j.1365-2249.2009.04008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowell M, Kehe K, Balszuweit F, Thiermann H. The chronic effects of sulfur mustard exposure. Toxicology. 2009;263(1):9–11. doi: 10.1016/j.tox.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 65.Awasthi A, Kuchroo VK. Th17 cells: from precursors to players in inflammation and infection. Int Immunol. 2009;21(5):489–98. doi: 10.1093/intimm/dxp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2011;184(2):252–8. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dagur PK, Biancotto A, Wei L, Sen HN, Yao M, Strober W, et al. MCAM-expressing CD4(+) T cells in peripheral blood secrete IL-17A and are significantly elevated in inflammatory autoimmune diseases. J Autoimmun. 2011;37(4):319–27. doi: 10.1016/j.jaut.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsu E, Shi H, Jordan RM, Lyons-Weiler J, Pilewski JM, Feghali-Bostwick CA. Lung tissues in systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum. 2010 Nov 19; doi: 10.1002/art.30159. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207(3):535–52. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454(7202):350–2. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]