Abstract
Axitinib, a tyrosine kinase inhibitor (TKI) of vascular endothelial growth factor receptors has demonstrated modest efficacy when applied as a single agent in the setting of advanced-stage melanoma. Based on the reported ability of axitinib to “normalize” the tumor vasculature, we hypothesize that combination therapy employing axitinib plus specific peptide-based vaccination would promote superior activation and recruitment of protective T cells into the melanoma microenvironment, leading to enhanced treatment benefit. Using a s.c. M05 (B16.OVA) melanoma model, we observed that a treatment regimen consisting of a 7 day course of axitinib (0.5 mg/day provided orally) combined with a s.c. vaccine (OVA peptide-pulsed syngenic dendritic cells (DC) adenovirally-engineered to produce IL-12p70) yielded superior protection against melanoma growth and extended overall survival when compared to animals receiving either single modality therapy. Treatment benefits were associated with: i.) a reduction in suppressor cell (myeloid-derived suppressor cells (MDSC) and Treg) populations in the tumor, ii.) activation of tumor vascular endothelial cells, and iii.) activation and recruitment of Type-1, vaccine-induced CD8+ T cells into tumors. These results support the therapeutic superiority of combined vaccine + axitinib immunotherapy and the translation of such approaches into the clinic for the treatment of patients with advanced-stage melanoma.
Keywords: axitinib, vaccine, dendritic cell, melanoma, myeloid derived suppressor cells, Treg
INTRODUCTION
Tumor size is physiologically constrained by a supportive vascular system that can provide a sufficient level of nutrients, while coordinately discharging waste materials [1]. Vascular endothelial growth factor receptors (VEGFRs) are critically involved in neoangiogenic pathways that support new blood vessel formation in the tumor microenvironment (TME). Production of VEGF in the TME serves to recruit, expand and differentiate (vascular) endothelial cell progenitors [2]. Clinical efforts to block the VEGF signaling cascade using bevacizumab (an antagonist anti-VEGF monoclonal antibody) in combination with standard therapies has demonstrated promising results in patients with metastatic melanoma, suggesting the utility of anti-angiogenic treatment strategies against advanced-stage disease [3–6].
An improved understanding of the molecular targets governing tumor angiogenesis has led to the development of a large number of small molecule drugs that are capable of blocking signaling pathways involved in propagating and maintaining a tumor-supportive stroma [7]. Axitinib is a potent second-generation TKI that blocks signaling through VEGFR-1, -2, and -3 (as well as the platelet-derived growth factor receptor (PDGFR) and c-kit/CD117), that has been clinically applied as a monotherapy in the setting of a broad range of cancer types [8]. FDA approval is currently being sought for the use of axitinib in patients with metastatic renal cell carcinoma (RCC), following the completion of a phase III clinical trial (AXIS trial) demonstrating an enhancement in progression-free survival in axitinib-treated patients that were refractory to first-line therapies [9].
Pre-clinical studies have also indicated that axitinib mediates anti-angiogenic and anti-tumor effects in the melanoma-bearing host [10]. In human xenograft models, axitinib monotherapy inhibited primary melanoma development and controlled distant metastasis. Axitinib also enhanced the protection associated with bevacizumab therapy when used in a combination protocol against orthotopic M24met xenografts [10].
Recently, the results of a multicenter phase II study of single-agent axitinib therapy in patients with metastatic melanoma were published [11]. Axitinib was found to be generally well-tolerated, with treatment leading to reductions in plasma levels of soluble VEGFR-2 and VEGFR-3, and increased levels of soluble VEGF ligand. Treatment with axitinib was associated with an 18.8% objective response rate (ORR), a frequency that compares favorably with standard melanoma therapies, including chemotherapy and rhIL-2 [12].
Overall, these results justify the expanded use of axitinib in the setting of melanoma, particularly when combined with non-redundant therapies (such as immunotherapies). Specific vaccination represents one valid co-applied approach since melanoma and/or melanoma associated stromal cells can be effectively targeted and destroyed by specific immune effector cells [13–15]. As vaccination also results in the expansion of memory T cell populations, such approaches have a theoretical advantage in providing sustained surveillance by a protective immune repertoire. In the current report, we observed that combined therapy of mice bearing established M05 (B16.OVA) melanoma with specific OVA peptide-based vaccination + axitinib was superior in efficacy when compared with either single component modality. Axitinib appeared to serve as an effective adjuvant to vaccination by removing endogenous regulatory cell populations and by activating the tumor vasculature, allowing for facilitated recruitment of vaccine-induced Type-1 CD8+ T cells. These results support the translational use of combined vaccine (targeting tumor cells and/or tumor-associated stromal cells [14, 15]) + axitinib-based therapies for the treatment of patients with advanced-stage melanoma.
MATERIALS AND METHODS
Mice
Female 6–8 week old C57BL/6 and OT-1 mice were purchased from the Jackson laboratory (Bar Harbor, ME). Animals were maintained in micro-isolator cages and handled under aseptic conditions following an Institutional Animal Care and Use Committee (IACUC)-approved protocol.
Cell lines and culture
The MO5 (B16.OVA) melanoma cell line was utilized for tumor challenge studies. Cells were free of Mycoplasma contamination and maintained in complete media (CM) (RPMI 1640 media supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 mM L-glutamine [all from Life Technologies, Grand Island, NY]) at 37°C with 5% CO2. Expression of transgene OVA in M05 cells was maintained under culture selection with G418 (Life Technologies).
Peptides
OVA peptides OVA257–264 (SIINFEKL; presented by H-2Kb to CD8+ T cells [16]) and OVA323–339 (ISQAVHAAHAEINEAGR; presented by H-2IAb to CD4+ T cells [16]) were synthesized to > 96% purity by 9-fluorenylmethoxycarbonyl (Fmoc) chemistry by the University of Pittsburgh Cancer Institute’s Peptide Synthesis Facility (a Shared Resource).
Vaccine generation
DCs were generated from the bone marrow of tibias/femurs from naïve mice as previously described [17, 18]. Briefly, bone-marrow-derived cells were cultured in CM over a period of 5 days with 1,000 U/ml of rmGMCSF and rmIL-4 (both from Peprotech, Rocky Hill, NJ). CD11c+ cells were subsequently purified using MACS bead positive selection (Miltenyi Biotec, Cambridge, MA) and infected at a multiplicity of infection = 50 with an adenoviral vector encoding murine IL-12p70 (Ad.mIL-12p70). The adenoviral vector was produced and provided by the University of Pittsburgh Cancer Institute’s Vector Core Facility (a Shared Resource). Transduced DCs were maintained in CM an additional 48 hours in the presence of rmGM-CSF and rmIL-4. On day 7, the IL-12 gene-modified DCs (DC.IL12) were loaded with a equimolar mixture (each at 10 μM) of the OVA257–264 and OVA323–339 peptides for 4 hours at 37°C prior to washing with PBS and injecting the antigen-loaded DC.IL12 s.c. into mice as a vaccine (VAC).
Tumor therapy model
Mice were challenged subcutaneously (s.c., right flank) with 2 × 105 MO5 melanoma cells, and tumors were allowed to progress 7 days before randomization into groups of 5 animals each with comparable mean tumor sizes/group. Animals then were left untreated, or they were vaccinated (s.c., left flank) with 1 × 106 OVA peptide-pulsed DC.IL12 cells on days 7 and 14 post-MO5 challenge. Axitinib (0.5 mg/day; Santa Cruz Biotechnology, Santa Cruz, CA) was dissolved in 50 μl Labrasol (Gattefossé Canada, Toronto, Canada) and delivered via oral gavage daily to select groups of mice for one week beginning on day 7. Tumor growth was monitored every 3–4 days and measured using Vernier calipers. MO5 tumor size was recorded in mm2 (mean +/− SD) based on product of orthogonal measurements.
Determination of OVA-specific T cell response
At time points ranging from 7–35 days post MO5 tumor inoculation, tumor infiltrating lymphocytes (TILs) and tumor draining lymph nodes (TDLNs) were harvested from euthanized mice. Single cell suspensions were generated from tumors by enzymatic digestion using DNase I, collagenase, and hyaluronidase (all from Sigma-Aldrich, St. Louis, MO), and from TDLNs by mechanical disruption, with T cells subsequently isolated using CD3-MACS beads (Miltenyi Biotec). T cells were pooled between 3 animals per group and stimulated 5 days in vitro with irradiated (100 Gy) MO5 tumor cells at an effector-to-target (E:T) ratio of 10:1. T cells were then transferred to 96-well round bottom plates (Corning, Corning, NY) and incubated with syngenic DCs pre-pulsed with the OVA257–264 (SIINFEKL) or no peptide at a 10:1 effector-to-target cell ratio for 48 hours. Cell-free supernatants were harvested and assessed for mIFN-γ content using a specific ELISA kit (BD Biosciences, San Jose, CA; with a lower detection limit of 31.3 pg/ml). Data is reported as mean IFN-γ levels (pg/ml) of triplicate determinations +/− SD.
Flow cytometry
Single cell suspensions and CD3+ TILs were stained with the following directly-conjugated anti-mouse antibodies (all from BD Biosciences): FITC-anti-CD8, FITC-anti-CD4, FITC-anti-CD11b, PE-anti-Gr1, and PE-anti-Foxp3. For Foxp3-specific staining, cells were first labeled with the FITC-anti-CD4 antibody before incubation with the PE-anti-Foxp3 antibody using a permeabilization/fixation buffer kit as recommended by the manufacturer (eBioscience, San Diego, CA). In additional experiments, a PE-conjugated H-2Kb/SIINFEKL tetramer (Beckman Coulter, Brea, CA) was utilized to assess OVA-specific CD8+ T cell frequency. Tetramer staining was carried out in accordance with the manufacturer’s suggested protocol. Flow cytometry was performed using Cell Quest software and a FACscan flow cytometer (Becton Dickinson, San Jose, CA), with FlowJo software (Tree Star, Ashland, OR) used for data analysis. Data is reported as absolute cell number ± SD, which was determined by multiplying the percentage of viable flow-gated cells by the total viable cell yield obtained from each tissue.
RT-PCR
Total RNA was extracted from tumor tissues at 7, 10, 14, 20, 28, and 35 days post MO5 inoculation using TRIzol reagent (Life Technologies). cDNA was generated using the MuLV reverse transcriptase with random hexamers (both from Applied Biosystems, Carlsbad, CA), and gene-specific PCR was carried out with AmpliTaq DNA polymerase (Applied Biosystems) and primer pairs for Tbet, IFN-γ, CXCR3, CXCL10, Foxp3 and β-actin as previously described [16]. Cycling conditions were as followed: initial denaturation at 94 °C for 2 minutes, denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and elongation at 72°C for 1 minute a total of 35–40 cycles followed by a final elongation at 72°C for 5 minutes. RT-PCR products were resolved on 2% ethidium bromide stained agarose gels (Sigma-Aldrich), and gel images were captured and analyzed using a GDS 8000 bioimaging system and Labworks software (UVP, LLC, Upland, CA). Band intensities for gene-specific products were then normalized to β-actin, which served as the endogenous housekeeping gene between samples. Normalized transcript levels are reported as mean fold change (+/− SD) compared to baseline values for each specific group at day 7 post-tumor challenge.
Immunofluorescence microscopy
Six micron tissue sections were prepared from tumors 20 days post-MO5 inoculation, as previously described [16]. Briefly, sections were first blocked in 2% BSA and washed with 0.5% BSA/PBS in subsequent steps. The tumor-derived vasculature was analyzed using rat-anti-mouse CD31 (BD Biosciences) and goat-anti-mouse VCAM-1 (R&D Systems, Minneapolis, MN) antibodies and a mixture of Alexa 488-conjugated donkey-anti-rat and CY3-conjugated donkey-anti-goat secondary antibodies (Life Technologies). After removal of unbound probes by washing, sections were treated with DAPI (Sigma-Aldrich) to stain nuclei and imaged using an Olympus BX 51 Fluorescent Microscope (Olympus America, Melville, NY). Immunofluorescence analysis was performed using Metamorph software (Molecular Devices, Sunnyvale, CA) software.
Statistical analysis
One-way ANOVA was utilized to test for overall differences between groups (StatMate III, ATMS Co., Tokyo, Japan). Differences with a p-value < 0.05 were considered significant
RESULTS
Axitinib enhances vaccine-initiated therapeutic protection against melanoma growth
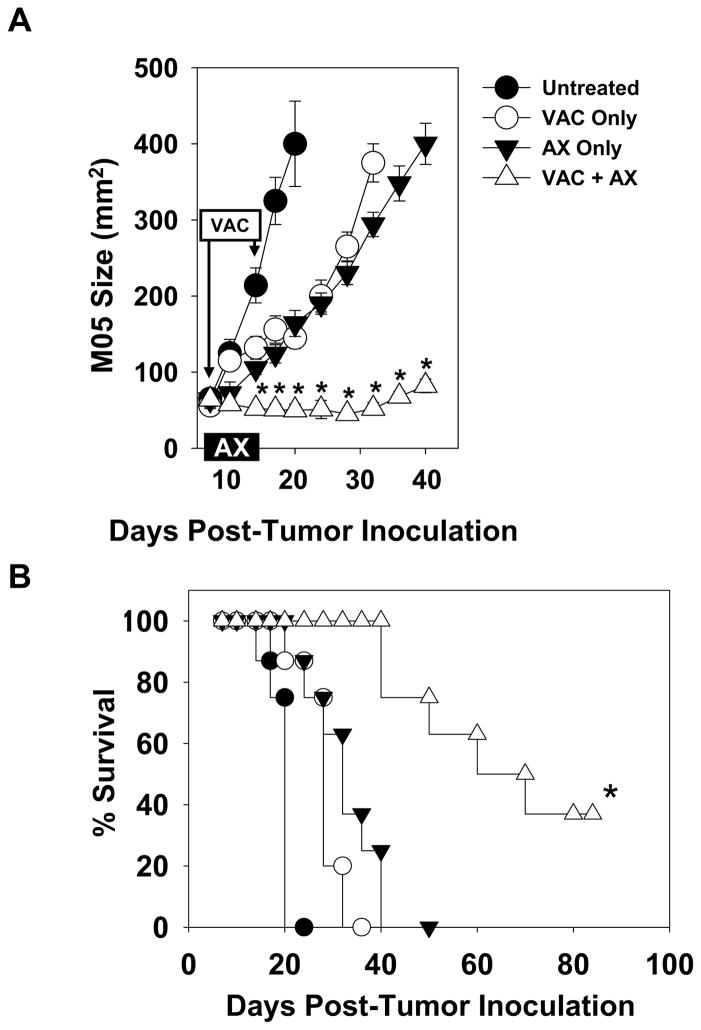
To assess the effects of axitinib in vivo, C57BL/6 mice were inoculated s.c. in the right flank with the MO5 melanoma cell line (B16.OVA). Tumors were allowed to establish and grow progressively for 7 days, at which time, treatment was initiated. Groups of animals were left untreated or they received therapies consisting of axitinib and/or specific vaccination. Axitinib (0.5 mg/daily) was administered via oral gavage for one week, while vaccines (i.e. OVA peptide-pulsed DC.IL12 that we have previously shown to promote superior anti-OVA Tc1 responses in C57BL/6 mice [15, 16]) were provided s.c. in the left flank (i.e. contralateral to the tumor) on days 7 and 14. Tumor growth was then monitored over time. Untreated animals developed rapidly progressive disease that required euthanasia by 20 days post-tumor inoculation (Fig. 1). In contrast, MO5-bearing mice that were treated with single agent vaccination or axitinib exhibited slowed tumor growth (Fig. 1A) and an extended survival period of 10–15 days (Fig. 1B). Interestingly, combination vaccine + axitinib therapy resulted in the greatest inhibition of tumor growth, which was superior to either component monotherapy (Fig. 1A, 1B; p < 0.05), with 40% of treated animals still alive 80 days post-tumor inoculation (Fig 1B).
Figure 1. Combined vaccine + axitinib therapy elicits superior anti-tumor efficacy in an established murine melanoma model.
C57BL/6 mice were injected s.c. with MO5 (B16.OVA) tumor cells. After 7 days, mice bearing established tumors were randomized into groups of 5 animals each with comparable mean tumor sizes. Animals were then left untreated, or they received a s.c. vaccine (syngenic DC.IL12 pulsed with the OVA257–264 and OVA323–339 peptides on days 7 and 14), axitinib alone (0.5 mg/day provided via oral gavage on days 7–13) or the combination of vaccine + axitinib, as outlined in Materials and Methods. Tumor growth was monitored every 2–3 days and is reported as mean +/− SD for one representative experiment of 3 performed in panel A. In B, aggregate survival data from all 3 experiments is provided in a Kaplan-Meier plot. *p < 0.05 versus all other groups.
Vaccine-induced effector T cell infiltration and activity are improved by axitinib co-therapy
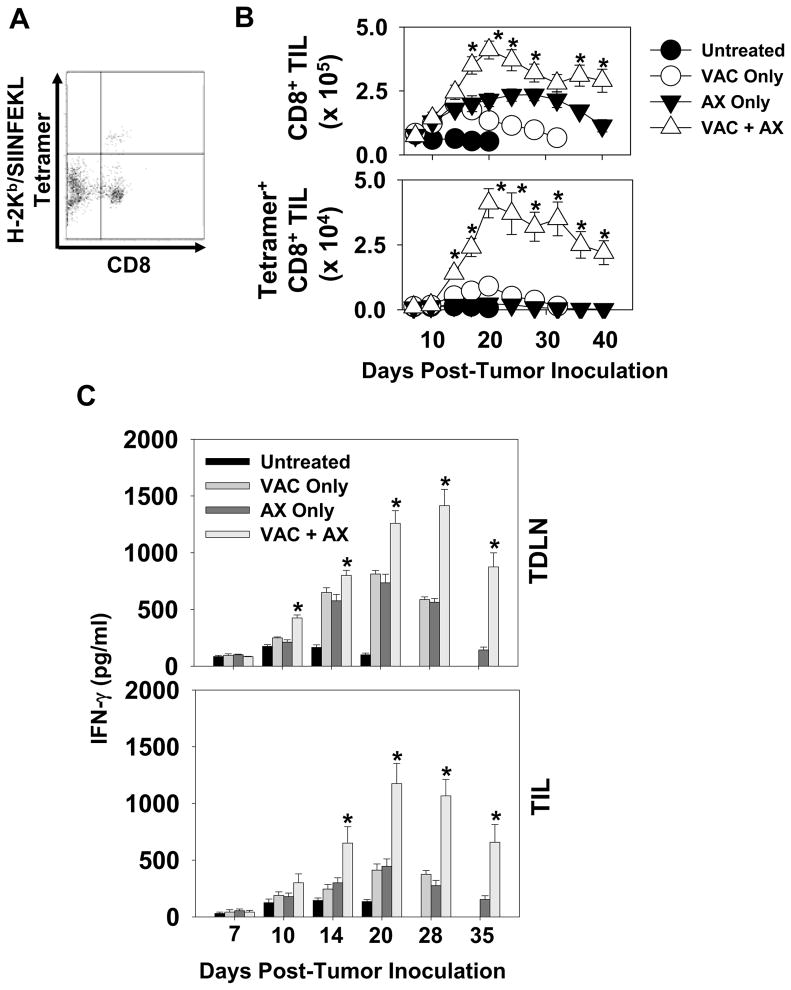
To begin to ascribe a mechanistic explanation for the superiority of combined therapy, CD8+ T cell frequency in treated tumor lesions was assessed over time. T cells were purified from enzymatically-digested tumor material by CD3-MACS, then stained and analyzed by flow cytometry for absolute CD8+ T cell numbers (Fig. 2A). As shown in Fig. 2B, levels of bulk CD8+ T cells increased in all treated groups versus untreated mice by day 14 after the initiation of treatment, with the co-therapy yielding a superior level of such TIL (versus either single modality after day 14 of the regimen; p < 0.05). Interestingly, when compared to untreated and vaccine alone cohorts, axitinib-containing cohorts displayed a prolonged period of increased CD8+ TIL infiltration/presence. Using a specific H-2Kb/OVA peptide tetramer probe, OVA-reactive CD8+ TIL frequencies were also assessed (Fig. 2A, 2B). Mice treated with either monotherapy were characterized by low, but detectable levels of tetramer+ T cells within a week of initiating treatment. A striking increase of tetramer+CD8+ TIL was observed in the vaccination + axitinib cohort of treated animals, with approximately 10% of all TIL exhibiting specificity for the model tumor antigen OVA (p < 0.05 versus all other cohorts). In concert with these therapy-associated changes in tetramer+ T cells in tumor, we also determined that Type-1 functionality (based on IFN-γ secretion) of such T cells isolated from either TIL or TDLN was enhanced and prolonged in animals treated with vaccines and axitinib, particularly those mice managed with the combination therapy (Fig. 2C).
Figure 2. Specific Type-1 CD8+ T cell infiltration is enhanced following combined vaccine + axitinib therapy.
Melanomas and TDLN were harvested on the indicated days from mice treated as outlined in Fig. 1. After enzymatic digestion/mechanical disruption, single-cell suspensions were analyzed for absolute numbers of bulk and OVA-specific (i.e. those binding the PE-conjugated H-2Kb/SIINFEKL tetramer) CD8+ tumor-infiltrating lymphocytes (TIL) by flow cytometry. An example of the flow profile data/gating obtained for the day 14 VAC + AX cohort is provided in panel A. In B, absolute cell numbers (mean ± SD) are reported. These were determined by multiplying the percentage of either gated CD8+ or tetramer-positive CD8+ T cells (based on flow cytometry analysis) by the total number of viable cells obtained from each tissue. *p < 0.05 compared to all other cohorts. In C, MACS-isolated CD8+ T cells from TIL and TDLN were longitudinally analyzed for their ability to produce IFN-γ in response to syngenic DC pulsed with OVA254–262 (SIINFEKL) peptide for 48 hours at a 10:1 E:T ratio. Cell-free supernatants were harvested and IFN-γ levels (pg/ml) were assessed using cytokine-specific ELISA, with mean +/− SD values reported from triplicate determinations. *p < 0.05 versus all groups. T cell reactivity against DC not pulsed with OVA peptide was < 50 pg/ml for all groups.
Combination vaccine + axitinib therapy effectively reduces immunoregulatory cell populations in the treated melanoma microenvironment
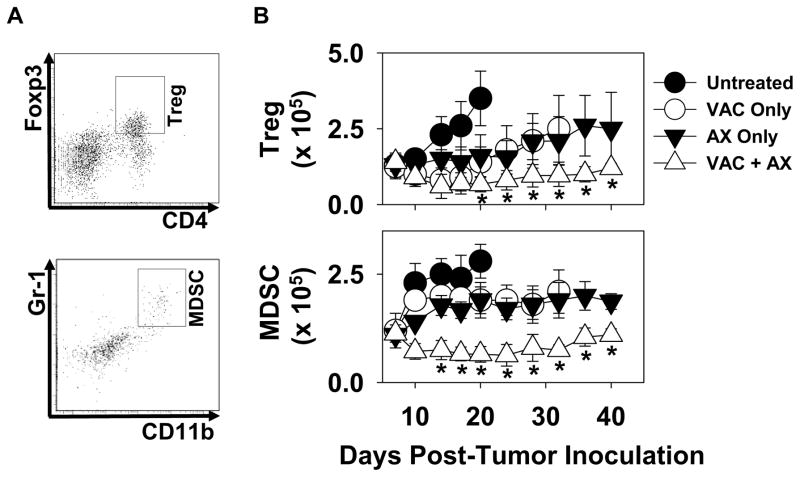
Since protective Type-1 T cells would be anticipated to mediate more robust anti-tumor function under conditions that are not negatively-influenced by regulatory cell populations [16], we next examined the impact of single and combined modality treatment on myeloid-derived suppressor cells (MDSC) and Treg cells in melanoma-bearing mice. As shown in Fig. 3, progressive growing, untreated tumors gradually accrued cells bearing MDSC (Gr1+CD11b+) and Treg (CD4+Foxp3+) phenotypes in the TME over time. Each of the single modality therapies was able to reduce the rate at which regulatory cells accumulated within B16 lesions, and the use of combined vaccination + axitinib therapy led to sustained reductions in both MDSC and Treg populations within tumors in vivo.
Figure 3. Combination vaccine + axitinib therapy reduces and/or prevents accumulation of MDSC and Treg suppressor cells in the melanoma microenvironment to a greater extent than the component single modality therapies.
Isolated melanomas harvested and processed as described in Fig. 2 were analyzed by flow cytometry for the presence of regulatory cells expressing either a Treg (CD4+Foxp3+) or an MDSC (CD11b+Gr1+) phenotype as described in Materials and Methods. Panel A provides an example of data obtained from day 14 untreated tumors, demonstrating the gating region for Treg and MDSC quantitation. In B, absolute numbers of cells bearing Treg and MDSC (mean +/−SD) phenotypes were determined by multiplying the (flow-based) percentage of double-positive cells by the overall number of viable cells obtained from tumor tissue. *p < 0.05 compared to all other groups.
Combination vaccine + axitinib therapy enforces a melanoma microenvironment conducive to Type-1 T cell recruitment/function in vivo
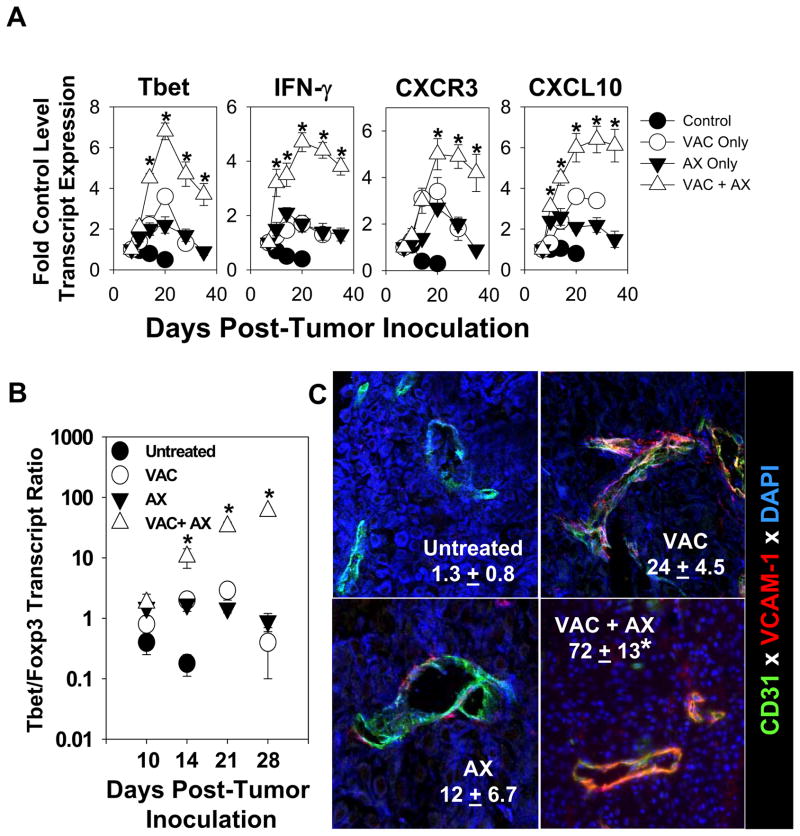
We have previously reported that protective Type-1 anti-tumor CD8+ T cells express a CXCR3+VLA-4+ phenotype which allows for recruitment into the TME based on locoregional production of CXCR3 ligand chemokines (including CXCL9/Mig, CXCL10/IP10 and CXCL11/ITAC) and expression of the VLA-4 “ligand” VCAM-1 on tumor-associated vascular endothelial cells [16, 20]. To determine how single/combined therapies altered expression of these mechanism-associated markers, we performed RT-PCR and fluorescence microscopy analyses of B16 lesions harvested 20 days after the initiation of treatment protocols. We observed that all treatments, but particularly the combined vaccination + axitinib therapy led to an increased preponderance of Type-1 transcripts (i.e. Tbet, CXCR3 and IFN-γ) and the Type-1-recruiting chemokine CXCL10 (we also observed similar tendencies for CXCL9 and CXCL11 transcript expression; data not shown) in the melanoma microenvironment (Fig 4A). Since Tbet is considered a master regulator for Type-1 function in T cells [19], these data suggest that the co-treatment most favorably reconditions the TME to allow for the effective deliver of, and therapeutic activity mediated by, Type-1 T cells. Such Tc1 activity may be further bolstered based on the coordinately reduced presence of Treg, which is suggested by a prolonged elevation in the Tbet/Foxp3 transcript ratio in tumors treated with the vaccination + axitinib regimen (Fig. 4B). Consistent with improved levels of Type-1 TIL, we observed that CD31+ vascular endothelial cells in treated melanomas appeared activated (particularly in the vaccination + axitinib combination cohort) based on their enhanced expression of VCAM-1 [21, 22], as determined in immunofluorescence microscopy analyses (Fig. 4C).
Figure 4. Combined vaccine + axitinib therapy is more effective than single modality treatment in promoting a “Type-1” melanoma microenvironment.
A, Total RNA was purified from single cell suspensions of MO5 tumors harvested on the indicated days as described in Fig. 2. RT-PCR was then performed using primer pairs specific to the Type-1 associated markers Tbet, IFN-γ, CXCR3, and CXCL10. PCR products were resolved through electrophoresis, imaged, and band intensities were normalized to the intensity of β-actin-specific amplification from paired samples. Data is presented as the fold change in transcript levels (mean ± SD) compared to baseline values from the same cohort on the day of treatment initiation (i.e. day 7 post-tumor challenge). *p < 0.05 versus all groups. In B, RT-PCR analysis was performed using Tbet- and Foxp3-specific primer pairs as outlined in panel A and Materials and Methods, with the mean ratio of Tbet/Foxp3 transcript levels reported (+/− SD) on the indicated days after treatment initiation. In C, day 20 melanomas were harvested from all groups and tissue sections analyzed by immunofluorescence microscopy for expression of CD31 and VCAM-1 as outlined in Materials and Methods. Cell nuclei were imaged by staining with DAPI. Mean (+/− SD) absolute numbers of CD31+VCAM-1+ cells per high power field (HPF) over over 10 HPFs/slide are reported. *p < 0.05 versus all other groups.
DISCUSSION
The VEGF/VEGFR signaling pathway is integral to endothelial migration and proliferation in melanoma-associated angiogenesis [2]. Pre-clinical studies support a variety of intervention strategies that may successfully disrupt VEGF/VEGFR signaling within the setting of melanoma [23–25]. This appears particularly salient since high levels of systemic VEGF correlate with poor overall prognosis in melanoma patients [26, 27], and the antagonist anti-VEGF antibody bevacizumab has demonstrated a degree of anti-melanoma efficacy when applied clinically [3–6].
An alternate method to mitigate the pro-tumor impact of VEGF involves the application of small molecule inhibitors (such as axitinib) that disrupt VEGFR-mediated signals after interaction with VEGF. Axitinib has been reported to primarily bind with high affinity to the kinase domain of VEGFR-1, -2, and -3 expressed by selected human melanoma cells, where it inhibits ligand-dependent signaling [10]. In a multi-center phase II clinical trial including 32 patients with advanced-stage melanoma, axitinib monotherapy was associated with an 18.8% objective response rate (ORR; with 1 complete response and 5 partial responses), which persisted for a median duration of 5.9 months [11]. Although ORRs in patients were similar to those obtained using more conventional treatment options such as chemotherapy and IL-2, axitinib appeared to exhibit efficacy for a larger number of patients based on secondary endpoints. A significant portion of axitinib-treated individuals experienced reductions in plasma concentrations of soluble VEGFR-2 and -3 after 2 cycles of axitinib-based therapy [11]. At least 40% of patients also experienced hypertension during exposure to axitinib, which has emerged as a biomarker for therapeutic anti-angiogenic effectiveness [28–30].
At present, it remains unclear why axitinib therapy does not mediate a higher rate of durable ORRs in advanced-stage melanoma patients. Although VEGF/VEGFR binding events comprise an important pathway involved in tumor vasculature formation [31], a potential explanation underlying the clinical limitations of axitinib might be taken from the general failure of single-agent bevacizumab as a cancer treatment [1, 32, 33]. In cancer patients, bevacizumab impacts predominantly immature blood vessels, and upon vascular “normalization” and stabilization of a mature vascular system supported by compensatory non-VEGFR-signaling pathways in the melanoma microenvironment, patient relapse can occur [33–36]. In the case of bevacizumab, this has sparked extensive research for effective second-line or co-therapeutic agents that mediate anti-tumor effects via non-overlapping mechanisms. Notably, the normalization of the tumor vasculature by many anti-angiogenic drugs leads to a transient state that is permissive for the improved delivery of chemotherapeutic agents and immune cells into the TME, which may enhance the likelihood for ORRs [3–6, 33, 37, 38]. It will, therefore, be interesting to assess whether future clinical trials incorporating combinational axitinib strategies support this “vascular normalization” hypothesis and lead to improved clinical responsiveness over treatment with single-agent axitinib. Currently, a phase II trial is recruiting metastatic melanoma patients for axitinib/chemotherapy combined therapy (Clinicaltrials.gov identifier: NCT01174238).
Active vaccination should also be considered for inclusion in combination treatment with anti-VEGF targeted strategies, since immunization may elicit protective Tc1 responses that may be consequently recruited into the TME based on the locoregional influence of drugs such as bevacizumab and axitinib, among others. The current study reports the novel result that axitinib and specific vaccination against a (model) tumor-associated antigen activates and coordinates therapeutic immunity against established melanomas. Although the individual peptide-based vaccine and axitinib monotherapies slowed tumor growth to some degree, co-administration of vaccine + axitinib led to a dramatic increase in Type-1 immune cell infiltration into B16 melanomas in association with the extended overall survival or treated animals. Improved levels of vaccine-induced CD8+ TIL observed in animals treated with the combination therapy were associated with the local activation of tumor vascular endothelial cells (based on VCAM-1 expression that serves as a ligand for Type-1 T cells expressing the VLA-4 integrin [21, 22]) and production of CXCR3 ligand chemokines (such as CXCL10/IP-10 that recruits CXCR3+ Type-1 T cells [16]). Additionally, vaccine/axitinib therapy caused acute decreases in levels of cells bearing immunosuppressive Treg and MDSC phenotypes in the TME, which were sustained at low levels for the duration of the study. The removal of MDSC and Treg allows for effector Type-1 TIL to mediate anti-tumor activity in a largely unopposed fashion [39, 40], as suggested by our observation for a dramatically increased Tbet/Foxp3 transcript ratio in the TME after treatment with the combined regimen.
Overall, axitinib appears to represent a potent “adjuvant” for use in melanoma therapy, in the sense that it reconditions the TME towards a state that is receptive to the therapeutic action of protective Type-1 immunity. Our results support the use of combined vaccine + axitinib protocols in prospective clinical trials for the treatment of patients with advanced-stage melanoma.
Acknowledgments
Sources of funding: This work was supported by NIH grants R01 CA140375 and P50 CA121973 (to W.J.S.) and the University of Pittsburgh Cancer Center Support Grant (CCSG; P30 CA047904). D.B.L. was supported by a Postdoctoral Fellowship (PF-11-151-01-LIB) from the American Cancer Society.
The authors wish to thank Dr. Jennifer L. Taylor for careful review and helpful comments provided during the preparation of this manuscript.
Footnotes
Conflicts of interest: None declared
References
- 1.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Streit M, Detmar M. Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene. 2003;22:3172–9. doi: 10.1038/sj.onc.1206457. [DOI] [PubMed] [Google Scholar]
- 3.Del Vecchio M, Mortarini R, Canova S, Di Guardo L, Pimpinelli N, Sertoli MR, et al. Bevacizumab plus fotemustine as first-line treatment in metastatic melanoma patients: clinical activity and modulation of angiogenesis and lymphangiogenesis factors. Clin Cancer Res. 2010;16:5862–72. doi: 10.1158/1078-0432.CCR-10-2363. [DOI] [PubMed] [Google Scholar]
- 4.Hainsworth JD, Infante JR, Spigel DR, Peyton JD, Thompson DS, Lane CM, et al. Bevacizumab and everolimus in the treatment of patients with metastatic melanoma: a phase 2 trial of the Sarah Cannon Oncology Research Consortium. Cancer. 2010;116:4122–9. doi: 10.1002/cncr.25320. [DOI] [PubMed] [Google Scholar]
- 5.Perez DG, Suman VJ, Fitch TR, Amatruda T, 3rd, Morton RF, Jilani SZ, et al. Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group study, N047A. Cancer. 2009;115:119–27. doi: 10.1002/cncr.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vihinen PP, Hernberg M, Vuoristo MS, Tyynela K, Laukka M, Lundin J, et al. A phase II trial of bevacizumab with dacarbazine and daily low-dose interferon-α2a as first line treatment in metastatic melanoma. Melanoma Res. 2010;20:318–25. doi: 10.1097/CMR.0b013e3283390365. [DOI] [PubMed] [Google Scholar]
- 7.Ghoreschi K, Laurence A, O’Shea JJ. Selectivity and therapeutic inhibition of kinases: to be or not to be? Nat Immunol. 2009;10:356–60. doi: 10.1038/ni.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma S, Abhyankar V, Burgess RE, Infante J, Trowbridge RC, Tarazi J, et al. A phase I study of axitinib (AG-013736) in combination with bevacizumab plus chemotherapy or chemotherapy alone in patients with metastatic colorectal cancer and other solid tumors. Ann Oncol. 2010;21:297–304. doi: 10.1093/annonc/mdp489. [DOI] [PubMed] [Google Scholar]
- 9.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 10.Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272–83. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 11.Fruehauf J, Lutzky J, McDermott D, Brown CK, Meric JB, Rosbrook B, et al. Multicenter, Phase II Study of Axitinib, a Selective Second-Generation Inhibitor of Vascular Endothelial Growth Factor Receptors 1, 2, and 3, in Patients with Metastatic Melanoma. Clin Cancer Res. 2011;17:7462–9. doi: 10.1158/1078-0432.CCR-11-0534. [DOI] [PubMed] [Google Scholar]
- 12.Emmett MS, Dewing D, Pritchard-Jones RO. Angiogenesis and melanoma - from basic science to clinical trials. Am J Cancer Res. 2011;1:852–68. [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao X, Bose A, Komita H, Taylor JL, Kawabe M, Chi N, et al. Intratumoral IL-12 gene therapy results in the crosspriming of Tc1 cells reactive against tumor-associated stromal antigens. Mol Ther. 2011;19:805–14. doi: 10.1038/mt.2010.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao X, Bose A, Komita H, Taylor JL, Chi N, Lowe DB, et al. Vaccines targeting tumor blood vessel antigens promote CD8+ T cell-dependent tumor eradication or dormancy in HLA-A2 transgenic mice. J Immunol. 2012 Jan 13; doi: 10.4049/jimmunol.1101644. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bose A, Taylor JL, Alber S, Watkins SC, Garcia JA, Rini BI, et al. Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int J Cancer. 2011;129:2158–70. doi: 10.1002/ijc.25863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komita H, Zhao X, Taylor JL, Sparvero LJ, Amoscato AA, Alber S, et al. CD8+ T-cell responses against hemoglobin-β prevent solid tumor growth. Cancer Res. 2008;68:8076–84. doi: 10.1158/0008-5472.CAN-08-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son YI, Egawa S, Tatsumi T, Redlinger RE, Jr, Kalinski P, Kanto T. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J Immunol Methods. 2002;262:145–57. doi: 10.1016/s0022-1759(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 19.Qu Y, Chen L, Pardee AD, Taylor JL, Wesa AK, Storkus WJ. Intralesional delivery of dendritic cells engineered to express T-bet promotes protective type 1 immunity and the normalization of the tumor microenvironment. J Immunol. 2010;185:2895–902. doi: 10.4049/jimmunol.1001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki K, Zhao X, Pardee AD, Ueda R, Fujita M, Sehra S, et al. Stat6 signaling suppresses VLA-4 expression by CD8+ T cells and limits their ability to infiltrate tumor lesions in vivo. J Immunol. 2008;181:104–8. doi: 10.4049/jimmunol.181.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffioen AW, Vyth-Dreese FA. Angiostasis as a way to improve immunotherapy. Thromb Haemost. 2009;101:1025–31. doi: 10.1160/th08-08-0552. [DOI] [PubMed] [Google Scholar]
- 22.Dedrick RL, Bodary S, Garovoy MR. Adhesion molecules as therapeutic targets for autoimmune diseases and transplant rejection. Expert Opin Biol Ther. 2003;3:85–95. doi: 10.1517/14712598.3.1.85. [DOI] [PubMed] [Google Scholar]
- 23.Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest. 2010;120:3953–68. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sini P, Samarzija I, Baffert F, Littlewood-Evans A, Schnell C, Theuer A, et al. Inhibition of multiple vascular endothelial growth factor receptors (VEGFR) blocks lymph node metastases but inhibition of VEGFR-2 is sufficient to sensitize tumor cells to platinum-based chemotherapeutics. Cancer Res. 2008;68:1581–92. doi: 10.1158/0008-5472.CAN-06-4685. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Cao Z, Tian H, Shen G, Ma Y, Xie H, et al. SKLB1002, a novel potent inhibitor of VEGF receptor 2 signaling, inhibits angiogenesis and tumor growth in vivo. Clin Cancer Res. 2011;17:4439–50. doi: 10.1158/1078-0432.CCR-10-3109. [DOI] [PubMed] [Google Scholar]
- 26.Pelletier F, Bermont L, Puzenat E, Blanc D, Cairey-Remonnay S, Mougin C, et al. Circulating vascular endothelial growth factor in cutaneous malignant melanoma. Br J Dermatol. 2005;152:685–9. doi: 10.1111/j.1365-2133.2005.06507.x. [DOI] [PubMed] [Google Scholar]
- 27.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–83. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 28.Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–73. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rini BI, Schiller JH, Fruehauf JP, Cohen EE, Tarazi JC, Rosbrook B, et al. Diastolic blood pressure as a biomarker of axitinib efficacy in solid tumors. Clin Cancer Res. 2011;17:3841–9. doi: 10.1158/1078-0432.CCR-10-2806. [DOI] [PubMed] [Google Scholar]
- 30.Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–30. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 31.Mahabeleshwar GH, Byzova TV. Angiogenesis in melanoma. Semin Oncol. 2007;34:555–65. doi: 10.1053/j.seminoncol.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varker KA, Biber JE, Kefauver C, Jensen R, Lehman A, Young D, et al. A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon-α2b in metastatic malignant melanoma. Ann Surg Oncol. 2007;14:2367–76. doi: 10.1245/s10434-007-9389-5. [DOI] [PubMed] [Google Scholar]
- 33.Helfrich I, Scheffrahn I, Bartling S, Weis J, von Felbert V, Middleton M, et al. Resistance to anti-angiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J Exp Med. 2010;207:491–503. doi: 10.1084/jem.20091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 35.Li JL, Sainson RC, Oon CE, Turley H, Leek R, Sheldon H, et al. DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res. 2011;71:6073–83. doi: 10.1158/0008-5472.CAN-11-1704. [DOI] [PubMed] [Google Scholar]
- 36.Fan F, Samuel S, Gaur P, Lu J, Dallas NA, Xia L, et al. Chronic exposure of colorectal cancer cells to bevacizumab promotes compensatory pathways that mediate tumour cell migration. Br J Cancer. 2011;104:1270–7. doi: 10.1038/bjc.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 38.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–80. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260–8. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–75. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]