Abstract
Objective
To investigate the association between leisure-time physical activity (PA) and fecundability.
Design
Prospective cohort study.
Setting
Internet-based observational study of Danish women who were planning a pregnancy (2007–2009).
Patient(s)
3,628 women aged 18–40 years at baseline.
Intervention(s)
None.
Main outcome measure(s)
Time-to-pregnancy (TTP). Fecundability ratios (FR) and 95% confidence intervals (CI) were derived from discrete-time Cox models, with adjustment for potential confounders such as body mass index (BMI).
Result(s)
We observed an inverse monotonic association between vigorous PA and fecundability (≥5 hours/week vs. none: FR=0.68, 95% CI: 0.54, 0.85), and a weak positive association between moderate PA and fecundability (≥5 vs. <1 hours/week: FR=1.18, 95% CI: 0.98, 1.43), after mutual adjustment for both PA types. Inverse associations between high vigorous PA and fecundability were observed within subgroups of age, parity status, and cycle regularity, but not among overweight or obese women (BMI ≥ 25 kg/m2).
Conclusion(s)
There was evidence for a dose-response relation between increasing vigorous PA and delayed TTP in all subgroups of women, with the exception of overweight and obese women. Moderate PA was associated with a small increase in fecundability regardless of BMI. These findings indicate that PA of any type might improve fertility among overweight and obese women, a subgroup at higher risk of infertility. Lean women who substitute vigorous PA with moderate PA may also improve their fertility.
MeSH words: fecundability, fertility, physical activity, prospective study, cohort study
In 2006, Denmark adopted a national goal of increasing the prevalence of physical activity (PA) in the adult population to at least 75% by the year 2021, with “physically active” defined as moderate intensity activity for at least 30 minutes every day (1). Although PA has been shown to reduce the risk of several chronic health conditions such as type 2 diabetes, cardiovascular disease, depression, and some cancers (2), its relation to female fertility is less clear.
Studies of competitive female athletes have found disturbances in their menstrual cycles, including oligomenorrhea and amenhorrhea (3–7). In addition, high levels of PA have been associated with increased menstrual cycle length (6), increased follicular phase length (8), and decreased luteal phase length (9). Given that previous research shows little effect of moderate-intensity PA on menstrual characteristics, some scientists postulate that the effect of PA on fertility may be positive up to a certain level of activity and then have a deleterious effect above that threshold level of activity (10).
Epidemiologic studies of PA and infertility have been inconclusive, with some showing reduced risk among vigorous exercisers and others showing increased risk at the highest levels of frequency or intensity. Most of these studies have been based on fertility clinic populations (11, 12) or have been limited to cases of ovulatory infertility (13, 14). In one study based on a fertility clinic population, women who had engaged in PA for ≥4 hours per week for <10 years had a 40% reduced likelihood of live birth, a threefold increased risk of in vitro fertilization (IVF) cycle cancellation (i.e., oocytes not retrieved), and a twofold increased risk of implantation failure and pregnancy loss relative to women not regularly engaged in PA (11). A subsequent study of women undergoing assisted reproduction found that moderate PA was associated with higher implantation and live birth rates (12), but none of the women reported high levels of PA. Of the three investigations conducted in the general population, two have been limited to the study of ovulatory infertility (13, 14). Both reports, based on a prospective cohort study of U.S. nurses, found that higher levels of vigorous PA were associated with a decreased risk of ovulatory infertility (13, 14). The third report, based on a population-based prospective cohort study of Norwegian women, found an increased risk of infertility (all types) among women reporting the highest levels of PA intensity and frequency (10).
We examined the influence of leisure-time PA on time-to-pregnancy among Danish women enrolled in a prospective cohort study. We further stratified the results by body mass index (BMI) to assess whether the effect of PA differed according to overweight or obesity, strong predictors of fecundability in our cohort and other studies (14–17).
MATERIALS AND METHODS
Study population
The ‘Snart Gravid’ Study is an internet-based prospective cohort study of women planning a pregnancy in Denmark. The study methodology has been described in detail elsewhere (18–20). Briefly, recruitment commenced in June 2007 with placement of an advertisement on a health-related website (www.netdoktor.dk) and a coordinated media strategy involving radio, print media, online news sites, and television. Enrollment and primary data collection were conducted via a self-administered questionnaire on the study website (www.snart-gravid.dk). Before enrollment, participants read a consent form and completed an online screening questionnaire to confirm eligibility. Eligible women were aged 18–40 years, residents of Denmark, in a stable relationship with a male partner, and not receiving any type of fertility treatment. Participants provided a valid e-mail address and their Civil Personal Registration number—a unique 10-digit personal identification number assigned to each resident by the Central Office of Civil Registration (21).
The baseline questionnaire collected information on demographics; reproductive and medical history; and lifestyle and behavioral factors. During the first 6 months of the study, participants were randomized to receive either a short- or long-form baseline questionnaire, with similar completion frequencies for both versions (19). After the first 6 months, newly-enrolled participants received the long-form baseline questionnaire. Follow-up questionnaires evaluated changes in various exposures, frequency of intercourse, and clinically-recognized conception. Participants were contacted every 2 months by e-mail for 12 months or until clinically-recognized conception. Women who conceived were asked to complete one questionnaire during early pregnancy to assess changes in exposures, after which active follow-up ceased. Cohort retention after 12 months of follow-up was approximately 82% (20). The Snart Gravid study was approved by all appropriate Institutional Review Board committees, and consent was obtained from all participants via the internet.
Assessment of physical activity
On the baseline questionnaire, women reported the average number of hours per week that they engaged in PA during the past year. They were asked to report moderate and vigorous types of activity separately. Categories of response were: none, <1, 1, 2, 3–4, 5–6, 7–9, ≥10 hours per week. Examples were provided for vigorous (“running, fast cycling, aerobics, gymnastics, or swimming”) and moderate (“brisk walking, leisurely cycling, golfing, or gardening”) types of activity. Based on the Compendium of Physical Activities (22), we estimated total metabolic equivalents (METs) of PA per week by summing the metabolic equivalents from vigorous exercise (hours/week multiplied by 7.0) and moderate exercise (hours/week multiplied by 3.5).
Assessment of covariates
Data on age, weight, height, parity, smoking history, current alcohol consumption, last method of contraception, and frequency of intercourse were self-reported on the baseline questionnaire and were updated every 2 months by follow-up questionnaire. At baseline, women reported whether their cycles were currently regular and, if so, their usual cycle length when not using hormonal contraception (“number of days from the first day of one menstrual period to the first day of the next menstrual period”). We calculated BMI as [weight in kilograms]/[height in meters]2. Self-reported height and weight among women who delivered infants conceived during our study showed excellent agreement with measures provided by the Danish Medical Birth Registry (15).
Assessment of pregnancy and cycles at risk
On each follow-up questionnaire, women reported the date of their last menstrual period, whether they were currently pregnant, and whether they had experienced any other pregnancies since the date of their last questionnaire, including miscarriage, induced abortion, or ectopic pregnancy. Total cycles at risk (rounded to the nearest whole number) were calculated as follows: (days of attempt time at study entry/usual cycle length) + (((last menstrual period date (LMP) from most recent follow-up questionnaire - date of baseline questionnaire completion)/usual cycle length) + 1), with observed cycles at risk defined as those contributed after study entry. For women with irregular cycles, we estimated usual cycle length based on the baseline LMP date, expected date of their next menstrual period, and LMP dates recorded over follow-up. Because we anticipated that the results would be less reliable among women with irregular cycles, we evaluated results separately among women with and without regular cycles.
Exclusions
After 30 months of recruitment, 5,460 women registered at the study website. Of these, we excluded 1,063 (19%) women who had been trying to conceive for >6 cycles at study entry, 274 (5%) women with insufficient or implausible information about their LMP date or date of first pregnancy attempt, and 495 (9%) women who did not complete a follow-up survey. After these exclusions, 3,628 women remained in this study. The 601 (16.6%) women who were subsequently lost to follow-up at some point during the year after enrollment (mean follow-up of 5.3 months) had lower parity (28.6% vs. 34.4%), higher BMI (mean: 24.7 vs. 24.0 kg/m2), and heavier smoking histories (mean: 2.8 vs. 2.0 pack-years) than the 3,027 women who were followed to a study endpoint. The two groups were similar with respect to total PA (mean: 24.1 vs. 24.9 MET-hours/week) and other baseline characteristics (e.g., mean age: 28.2 vs. 28.5 years; mean alcoholic drinks per week: 3.0 vs. 2.9; >4 years of higher education: 20.1% vs. 25.0%; gravidity: 43.6% vs. 45.8%; and use of oral contraceptives as last method of contraception: 61.4% vs. 61.6%).
Data analysis
We analyzed vigorous PA in categories of none (reference), <1, 1, 2, 3–4, and ≥5 hours per week, and moderate PA in categories of <1 (reference), 1, 2, 3–4, and ≥5 hours per week. Continuous variables for moderate and vigorous PA were coded as the midpoint of each category (assigning 11 hours/week to the top category). We categorized total MET-hours per week in 10-unit increments, with 20–29 as the reference category (because it was associated with the highest fecundability in our cohort) and ≥60 as the maximum exposure category. We allowed for the possibility of a non-linear relation or threshold effect of each PA variable on fecundability by fitting a restricted cubic spline model (23, 24).
The fecundability ratio (FR) represents the cycle-specific probability of conception among exposed women divided by that among unexposed women. We used a discrete-time analogue of the Cox proportional hazards model to estimate FRs and 95% confidence intervals (CI) for moderate, vigorous, and total METs of physical activity in association with time-topregnancy, in cycles (25). We evaluated time to any pregnancy, regardless of pregnancy outcome. Women were censored if they did not conceive after 12 cycles, the typical amount of time after which couples seek medical assistance for infertility (25, 26). Women contributed cycles at risk until they reached a study endpoint—pregnancy, use of fertility treatments, loss to follow-up, or the end of observation (12 cycles), whichever occurred first. The Cox model allowed for “delayed entry” into the risk set—which occurs when women enter the study after having tried to conceive for 1 or more cycles. Therefore, risk sets were based only on cycles at risk observed after study entry (15).
We selected potential confounders from a list of variables associated with PA at baseline that met criteria for confounding based on a review of the literature and assessment of a causal graph (27). We then controlled for potential confounders that changed the adjusted FR by more than 5 percent relative to the unadjusted FR (27). Based on these criteria, we controlled for female age (<25, 25–29, 30–34, ≥35 years), partner’s age (<25, 25–29, 30–34, ≥35 years), BMI (<20, 20–24, 25–29, ≥30 kg/m2), alcohol consumption (drinks per day), pack-years of smoking (never smoked, <5, 5–9, ≥10 pack-years), frequency of intercourse (<1, 1, 2–3, ≥4 times/week), and last method of contraception (barrier methods, oral contraceptives, other hormonal contraceptives, natural family planning). Alcohol consumption and frequency of intercourse were modeled as time-varying variables. Further control for “doing something to time intercourse” made very little difference in the effect estimates for vigorous or moderate PA (<1% in FRs). To assess the independent effects of vigorous and moderate PA, we further controlled for each type of PA simultaneously in the final multivariable model. The proportion of missing data at baseline ranged from as low as 0.19% (age at menarche) to as high as 4% (packyears of smoking); proportions were 0.25% and 0.27% for vigorous and moderate PA, respectively. We used multiple imputation methods to impute missing covariate values (28). In SAS, we used PROC MI to create five imputed datasets and PROC MIANALYZE to combine results across the five datasets (29). All potential confounders were included in the imputation procedure.
In secondary analyses, we evaluated the extent to which the associations changed when pregnancy losses were excluded from the outcome definition. In these analyses, women who reported an abortion or ectopic pregnancy were censored at their estimated time-to-pregnancy (30). We stratified by age, parity status, BMI, cycle regularity, and number of cycle attempts before study entry. We assessed departure from the proportional hazards assumption by plotting the log-log survivor functions for each exposure variable in categorical form, where parallel loglog survivor curves indicated proportional hazards. We used SAS statistical software (version 9.2) for all analyses (29).
RESULTS
Baseline characteristics of the study population according to hours of vigorous PA per week are presented in Table 1. Vigorous PA was positively associated with education and higher frequency of intercourse, and was inversely associated with BMI, waist circumference, caffeine, current smoking, parity, and the report of “doing something to time intercourse.” Women in the highest category of total MET-hours/week of PA tended to have longer and irregular cycles (data not shown).
Table 1.
Baseline characteristics of 3,628 Danish women participating in a prospective cohort study of pregnancy planners, according to level of vigorous physical activity
| Vigorous physical activity, hours per week | ||||||
|---|---|---|---|---|---|---|
| Characteristic a | None | <1 | 1 | 2 | 3–4 | ≥5 |
| Number of women | 720 | 821 | 631 | 749 | 513 | 194 |
| Age, years (mean) | 28.9 | 28.4 | 28.5 | 28.3 | 28.2 | 27.8 |
| Partner’s age, years (mean) | 31.0 | 30.7 | 30.9 | 30.7 | 30.7 | 31.1 |
| Age at menarche, years (mean) | 13.0 | 13.0 | 12.9 | 12.9 | 12.9 | 13.2 |
| Regular cycles (%) | 74.3 | 75.6 | 75.8 | 77.6 | 78.3 | 74.5 |
| Cycle length <27 days (%) | 10.9 | 11.9 | 11.0 | 13.8 | 13.1 | 11.1 |
| Cycle length ≥32 days (%) | 24.0 | 24.4 | 24.0 | 23.8 | 22.8 | 25.6 |
| BMI, kg/m2 (mean) | 24.9 | 24.6 | 23.8 | 23.6 | 23.6 | 22.9 |
| Waist circumference, inches (mean) | 84.5 | 82.9 | 80.9 | 79.8 | 79.7 | 78.0 |
| Moderate physical activity, hrs/wk (mean) | 3.4 | 3.6 | 4.1 | 4.2 | 4.4 | 5.8 |
| Total physical activity, MET-hrs/wk (mean) | 11.8 | 16.1 | 21.2 | 28.8 | 39.9 | 65.1 |
| Higher education >4 years (%) | 16.3 | 21.2 | 27.4 | 27.4 | 28.9 | 30.7 |
| Parous (%) | 47.6 | 40.6 | 31.1 | 25.2 | 22.3 | 17.4 |
| Current regular smoker, yes (%) | 18.8 | 13.7 | 10.1 | 9.2 | 9.0 | 7.7 |
| Pack-years of ever smoking (mean) | 6.3 | 5.5 | 5.0 | 5.0 | 4.5 | 5.3 |
| Alcohol intake, drinks/wk (mean) | 2.6 | 2.7 | 2.9 | 3.0 | 3.3 | 3.0 |
| Caffeine intake, mg/day (mean) | 148 | 140 | 130 | 136 | 133 | 120 |
| Intercourse frequency, ≥4 times/wk (%) | 17.2 | 15.8 | 16.5 | 20.0 | 25.0 | 33.0 |
| Doing something to time intercourse (%) | 48.5 | 49.5 | 44.8 | 44.6 | 41.0 | 41.2 |
| Last method of contraception (%) | ||||||
| Barrier methods | 30.4 | 30.5 | 27.9 | 25.4 | 26.6 | 20.6 |
| Hormonal contraceptives | 59.7 | 59.1 | 60.8 | 65.5 | 65.8 | 67.8 |
| Withdrawal, charting, or other | 9.7 | 10.1 | 11.3 | 9.0 | 7.7 | 11.0 |
All characteristics, with exception of participant’s age, are age-standardized to cohort at baseline.
In multivariable models that mutually controlled for both types of PA, we observed a monotonic inverse association between vigorous PA and fecundability, and a weak positive association between moderate PA and fecundability (Table 2). When we considered the contribution of both types of PA to total activity levels, we found that higher levels of PA were associated with reduced fecundability (≥60 vs. 20–29 MET-hours of total activity/week: FR=0.74, 95% CI=0.56, 0.97).
Table 2.
Physical activity at baseline and time to pregnancy
| Pregnancies | Cycles | Unadjusted model | Adjusted modela | |||
|---|---|---|---|---|---|---|
| FR | 95% CI | FR | 95% CI | |||
| Vigorous physical activity, hrs/wk | ||||||
| None | 500 | 2,856 | 1.00 | (ref.) | 1.00 | (ref.) |
| <1 | 566 | 3,492 | 0.91 | 0.80, 1.04 | 0.88 | 0.77, 1.01 |
| 1 | 440 | 2,607 | 0.94 | 0.81, 1.08 | 0.87 | 0.76, 1.01 |
| 2 | 520 | 3,156 | 0.93 | 0.82, 1.07 | 0.84 | 0.73, 0.97 |
| 3–4 | 342 | 2,295 | 0.82 | 0.70, 0.95 | 0.73 | 0.63, 0.86 |
| ≥5 | 116 | 819 | 0.77 | 0.61, 0.96 | 0.68 | 0.54, 0.85 |
| Moderate physical activity, hrs/wk | ||||||
| <1 | 161 | 1,150 | 1.00 | (ref.) | 1.00 | (ref.) |
| 1 | 227 | 1,543 | 1.02 | 0.82, 1.27 | 1.00 | 0.80, 1.25 |
| 2 | 538 | 3,273 | 1.17 | 0.96, 1.41 | 1.15 | 0.95, 1.40 |
| 3–4 | 720 | 4,283 | 1.19 | 0.99, 1.44 | 1.16 | 0.95, 1.40 |
| ≥5 | 838 | 4,976 | 1.20 | 0.99, 1.45 | 1.18 | 0.98, 1.43 |
| Total metabolic equivalents, hrs/wk | ||||||
| <10 | 374 | 2,445 | 0.87 | 0.75, 1.09 | 0.95 | 0.82, 1.11 |
| 10–19 | 784 | 4,747 | 0.93 | 0.82, 1.05 | 0.96 | 0.85, 1.09 |
| 20–29 | 533 | 3,095 | 1.00 | (ref.) | 1.00 | (ref.) |
| 30–39 | 404 | 2,345 | 0.98 | 0.85, 1.13 | 0.97 | 0.84, 1.12 |
| 40–49 | 201 | 1,392 | 0.82 | 0.69, 0.98 | 0.81 | 0.68, 0.97 |
| 50–59 | 118 | 688 | 0.97 | 0.77, 1.20 | 0.93 | 0.75, 1.17 |
| ≥60 | 70 | 513 | 0.75 | 0.58, 0.99 | 0.74 | 0.56, 0.97 |
Note: FR = fecundability ratio, CI = confidence interval. Unadjusted model controls for cycle number.
Adjusted for cycle number, age, partner’s age, BMI, alcohol consumption, pack-years of smoking, intercourse frequency, and last method of contraception. Vigorous activity is adjusted for moderate activity, and vice versa.
The effect of vigorous PA was relatively uniform across levels of age, parity, and attempt time at study entry (Table 3). While we also observed an inverse association between vigorous PA and fecundability among women with BMI <25 (vigorous PA ≥5 vs. 0 hours/week: FR=0.58, 95% CI=0.45, 0.75), there was no evidence of an inverse association among overweight and obese women (BMI ≥25) (vigorous PA ≥5 vs. 0 hours/week: FR= 1.22, 95% CI = 0.74, 2.02). In fact, for most categories of vigorous PA above “none” among overweight and obese women, there were weak positive associations between vigorous PA and fecundability (FRs ranged from 1.12–1.22, with the exception of FR=0.76 for 3–4 hours/week). The inverse association between vigorous PA and fecundability among lean women (BMI <25) was still apparent after the exclusion of underweight women, defined as BMI <18.5 kg/m2 (data not shown). Within subgroups of selected covariates, increasing levels of moderate PA were either weakly positively associated with fecundability or not associated with fecundability (Supplemental Table 1). Women who engaged in 20–39 MET-hours per week of PA (from all activity sources) had the highest fecundability in our cohort, regardless of BMI (Supplemental Table 2).
Table 3.
Vigorous PA and time to pregnancy, stratified by selected factors
| Vigorous physical activity, hours per week | ||||||
|---|---|---|---|---|---|---|
| Characteristic | None | <1 | 1 | 2 | 3–4 | ≥5 |
| Age at baseline, years: | ||||||
| <30 | ||||||
| Pregnancies | 287 | 353 | 263 | 340 | 232 | 85 |
| Cycles | 1,659 | 2,173 | 1,636 | 2,023 | 1,542 | 571 |
| FR (95% CI) a | 1.00 (ref.) | 0.90 (0.76, 1.07) | 0.84 (0.69, 1.01) | 0.88 (0.73, 1.05) | 0.75 (0.62, 0.91) | 0.70 (0.53, 0.91) |
| ≥30 | ||||||
| Pregnancies | 213 | 213 | 177 | 180 | 110 | 31 |
| Cycles | 1,197 | 1,319 | 971 | 1,133 | 753 | 248 |
| FR (95% CI) a | 1.00 (ref.) | 0.86 (0.69, 1.06) | 0.93 (0.74, 1.17) | 0.80 (0.64, 1.01) | 0.72 (0.55, 0.93) | 0.61 (0.40, 0.93) |
| Parity: | ||||||
| Parous | ||||||
| Pregnancies | 275 | 261 | 153 | 135 | 81 | 19 |
| Cycles | 1,257 | 1,211 | 709 | 705 | 473 | 107 |
| FR (95% CI) a | 1.00 (ref.) | 0.92 (0.75, 1.12) | 0.86 (0.68, 1.09) | 0.78 (0.61, 0.99) | 0.66 (0.50, 0.89) | 0.68 (0.39, 1.16) |
| Nulliparous | ||||||
| Pregnancies | 225 | 305 | 287 | 385 | 261 | 97 |
| Cycles | 1,599 | 2,281 | 1,898 | 2,451 | 1,822 | 712 |
| FR (95% CI) a | 1.00 (ref.) | 0.89 (0.74, 1.08) | 0.96 (0.79, 1.17) | 0.99 (0.82, 1.19) | 0.86 (0.70, 1.05) | 0.80 (0.61, 1.04) |
| Body mass index (kg/m2): | ||||||
| <25 | ||||||
| Pregnancies | 337 | 365 | 311 | 392 | 267 | 93 |
| Cycles | 1,700 | 2,148 | 1,798 | 2,355 | 1,627 | 688 |
| FR (95% CI) a | 1.00 (ref.) | 0.79 (0.66, 0.93) | 0.79 (0.66, 0.94) | 0.76 (0.64, 0.89) | 0.72 (0.60, 0.87) | 0.58 (0.45, 0.75) |
| ≥25 | ||||||
| Pregnancies | 163 | 201 | 129 | 128 | 75 | 23 |
| Cycles | 1,156 | 1,344 | 809 | 801 | 668 | 131 |
| FR (95% CI) a | 1.00 (ref.) | 1.12 (0.89, 1.41) | 1.15 (0.88, 1.48) | 1.16 (0.89, 1.51) | 1.76 (0.56, 1.03) | 1.22 (0.74, 2.02) |
| Cycle attempts before study entry: | ||||||
| ≤2 | ||||||
| Pregnancies | 353 | 420 | 334 | 391 | 272 | 91 |
| Cycles | 1,948 | 2,409 | 1,808 | 2,189 | 1,687 | 583 |
| FR (95% CI) a | 1.00 (ref.) | 0.91 (0.78, 1.07) | 0.92 (0.77, 1.08) | 0.88 (0.75, 1.04) | 0.77 (0.64, 0.92) | 0.72 (0.55, 0.94) |
| 3–6 | ||||||
| Pregnancies | 147 | 146 | 106 | 129 | 70 | 25 |
| Cycles | 908 | 1,083 | 799 | 967 | 608 | 236 |
| FR (95% CI) a | 1.00 (ref.) | 0.80 (0.62, 1.03) | 0.75 (0.56, 0.99) | 0.73 (0.56, 0.96) | 0.64 (0.46, 0.88) | 0.55 (0.35, 0.88) |
| Moderate PA, hrs/wk: | ||||||
| <5 | ||||||
| Pregnancies | 360 | 392 | 297 | 340 | 208 | 49 |
| Cycles | 2,184 | 2,591 | 1,744 | 2,072 | 1,351 | 307 |
| FR (95% CI) a | 1.00 (ref.) | 0.89 (0.76, 1.04) | 0.96 (0.81, 1.14) | 0.91 (0.77, 1.08) | 0.83 (0.69, 1.01) | 0.87 (0.62, 1.21) |
| ≥5 | ||||||
| Pregnancies | 140 | 174 | 143 | 180 | 134 | 67 |
| Cycles | 672 | 901 | 863 | 1,084 | 944 | 512 |
| FR (95% CI) a | 1.29 (1.03, 1.61) | 1.09 (0.89, 1.34) | 0.92 (0.74, 1.12) | 0.91 (0.75, 1.12) | 0.75 (0.60, 0.94) | 0.69 (0.52, 0.92) |
Note: FR = fecundability ratio, CI = confidence interval.
Adjusted for cycle number, age, partner’s age, BMI, alcohol consumption, pack-years of smoking, intercourse frequency, last method of contraception, and moderate PA (when applicable).
Results for vigorous PA within levels of moderate PA (<5 vs. ≥5 hours/week) are shown at the bottom of Table 3, using a single reference category of women engaged in <5 hours of moderate PA and no vigorous PA. Fecundability was lowest for the women engaged in ≥5 hours/week of both moderate and vigorous PA (Table 3). Within each of the two levels of moderate PA, increasing levels of vigorous PA were associated with decreasing fecundability. Notably, women who engaged in ≥5 hours of moderate PA but reported no vigorous PA had increased fecundability relative to the least active women (<5 hours of moderate PA and no vigorous PA).
The overall results were virtually unchanged when we further controlled for cycle length and cycle irregularity, which are potential mediators of the PA-TTP association (vigorous PA ≥5 vs. 0 hours/week: FR= 0.68, 95% CI = 0.54, 0.85; total PA ≥60 vs. 20–29 MET-hrs/week: FR=0.75, 95% CI: 0.57, 0.99). Among women with regular cycles, point estimates were less precise but consistent with the results based on all women (vigorous PA ≥5 vs. 0 hours/week: FR= 0.66, 95% CI = 0.51, 0.86; total PA ≥60 vs. 20–29 MET-hrs/week: FR=0.74, 95% CI: 0.53, 1.02). Similar associations were observed when pregnancy losses were excluded from the outcome definition (data not shown).
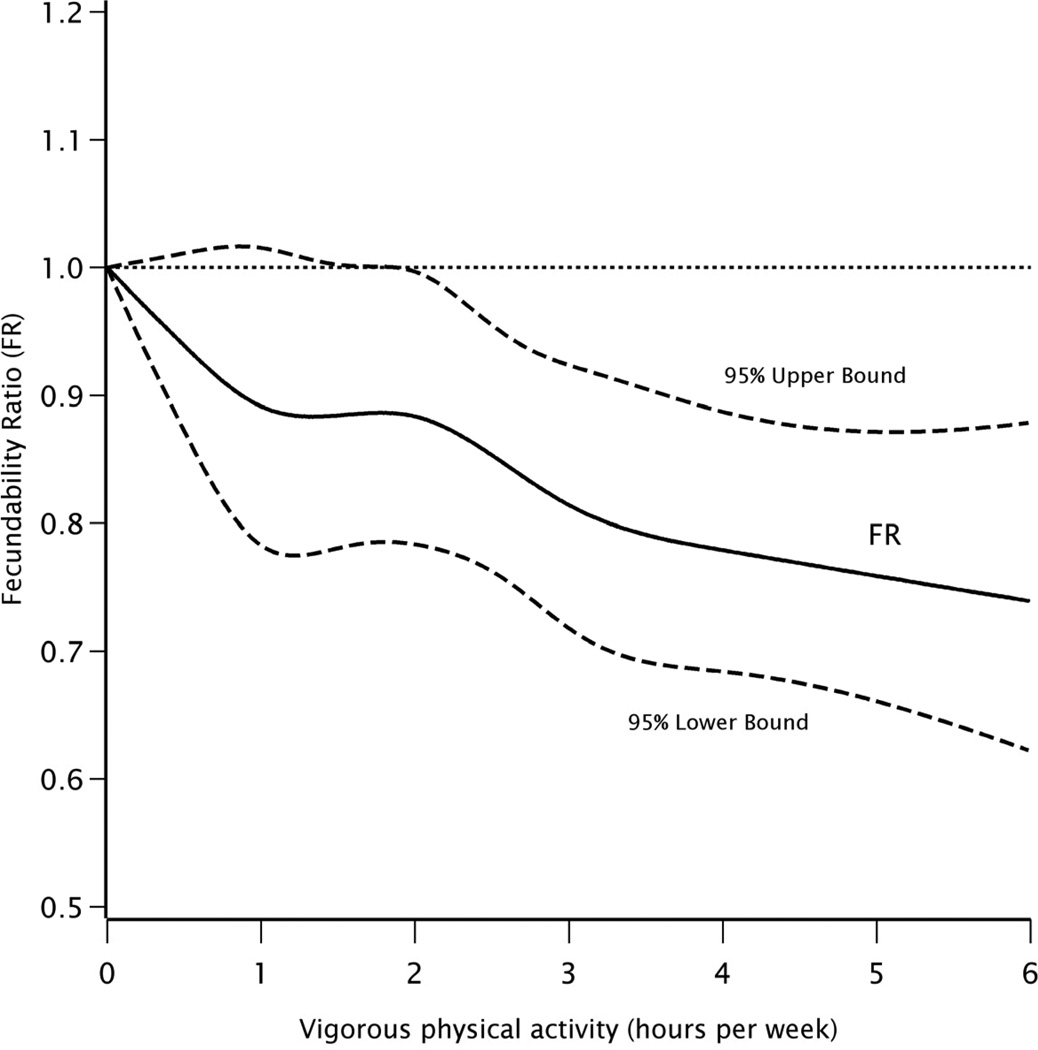
Figure 1 shows the overall association between vigorous PA (hours per week) and fecundability using restricted cubic splines. Results are also presented according to categories of BMI at baseline (Supplemental Figures 1 and 2). The observed patterns were consistent with the categorical results presented above: fecundability decreased with increasing hours of vigorous PA per week, in a dose-response fashion, overall and among lean women. In contrast, low levels of vigorous PA were associated with increased fecundability among overweight and obese women up until about 2 hours per week, after which vigorous PA had little effect on fecundability.
Figure 1.
Association between hours of vigorous physical activity per week and fecundability, fitted by restricted cubic splines. The reference level for the fecundability ratio is 0 hours/week. The curves are adjusted for cycle number, age, partner’s age, BMI, alcohol consumption, pack-years of smoking, intercourse frequency, last method of contraception, and hours of moderate physical activity per week. There were 5 knots located at 0.5, 1, 2, 3, and 4 hours/week.
DISCUSSION
In this prospective cohort study of Danish women aged 18–40 years, vigorous PA was associated with reduced fecundability in all subgroups of women examined, with the exception of overweight and obese women (BMI ≥25), among whom PA of any type either modestly increased or had little effect on fecundability. In contrast, moderate PA was associated with a modest increase in fecundability overall and did not appear to have any deleterious effect on fertility among lean or overweight/obese women.
The finding of reduced fecundability among the highest-intensity exercisers agrees with some (10, 11) but not all previous epidemiologic investigations (13, 14). Furthermore, the finding that any PA, regardless of type, may be associated with a modest increase in fecundability among overweight/obese women is supported by an intervention study showing that moderate PA coupled with weight loss can enhance fertility in obese women (31). The observation that women engaged in moderate PA or intermediate levels of total PA (20–39 MET-hours/week) had higher fecundability agrees with another study based on a fertility clinic population (12).
The conflicting results across studies regarding the effect of high intensity PA on female infertility may be attributable to the type of infertility studied. Two previous studies focused only on ovulatory infertility (13, 14). The mechanisms by which high intensity PA has a deleterious effect on female fertility might involve factors other than ovulation, such as impaired implantation. In support of this hypothesis, the Morris et al. study of the success of IVF treatment reported a higher rate of implantation failures among women with high levels of PA (11). Morris et al. also found that associations were stronger among the women who engaged in cardiovascular activities (e.g., running, aerobics, or bicycling) as their primary exercise relative to those who engaged in walking (11), which agrees with our results for vigorous vs. moderate types of PA.
Not all women entered our study when they were first attempting to conceive, introducing potential for both differential and non-differential misclassification of PA. However, the observation of reduced fecundability among the high-intensity exercisers with ≤2 cycle attempts before study entry suggests that bias due to left truncation did not have a large influence on our results. More than 96% of women in our cohort with a viable pregnancy reported using home pregnancy tests to confirm their pregnancy, suggesting that bias due to differential recognition of early pregnancy loss (which may be as high as 25% (32)) is unlikely to explain our results. Although rates of unintended pregnancy are considerably lower in Denmark than in other developed countries (33), the study’s restriction to pregnancy planners entailed the omission of a non-negligible fraction of total pregnancies. If pregnancy intention was related both to PA and fertility potential, our results would not apply to women with unplanned pregnancies.
Another limitation is that we did not validate our measures of physical activity. However, vigorous PA was correlated with other lifestyle and behavioral variables (e.g., education, BMI, and parity) in the expected direction. In addition, we did not ask about specific types of PA, but rather ascertained the number of hours of activity per week for all types of vigorous or moderate PA combined. Given that specific sports with varying intensities may have different effects on fecundability—for instance, an Iranian study showed that endurance and weight category sports may confer a higher risk of amenorrhea or oligomenorrhea than other sports (5)—our results may have been influenced by non-differential misclassification, which most likely would have biased the effect of high PA towards the null. Use of a baseline measure of physical activity, as opposed to one that was updated over follow-up, may have resulted in misclassification. For example, women who took longer to conceive could have modified their exercise patterns, thereby introducing differential exposure misclassification. Nevertheless, after stratifying the data by attempt time at entry into the study, we did not find strong evidence of bias in our findings. We were also unable to examine the different causes of subfertility in this study. The TTP measure represents a combination of different causes contributing to couples’ subfertility, and thus the associations reported in this study likely reflect the overall effect of PA on fertility.
Cohort retention in the present study was similar to that reported in other large volunteer cohort studies (34, 35). PA levels were similar for the small proportion of women lost to follow-up and women followed to a study endpoint, implying that bias due to selective losses is unlikely. Another consideration is that this study enrolled a self-selected sample of pregnancy planners recruited via the internet, but there is little reason to believe that such women would differ from the general population of women seeking a pregnancy in ways that would lead to biased effect estimates. Two Scandinavian birth cohort studies, in which population registry data were used to compare differences between study participants and all women giving birth in the general population, showed that nonparticipation at study outset had a small impact on effect estimates (36, 37).
In summary, while the present study found evidence for a dose-response relation between increasing vigorous PA and delayed TTP, results were equivocal among overweight and obese women. Moderate PA was associated with a small increase in fecundability regardless of BMI. These findings indicate that PA of any type might improve fertility among overweight and obese women, a subgroup at higher risk of infertility. Lean women who substitute vigorous PA with moderate PA may also improve their fertility. Future research investigating individual types of physical activity in relation to fertility, and whether overweight or obese women might benefit from increased physical activity when planning a pregnancy, is warranted.
Supplementary Material
ACKNOWLEDGMENTS
We thank the study staff and all the women who participated in the Snart Gravid study. The authors also thank Ms. Tina Christensen for her support with data collection and media contact, Dr. Donna Baird for her feedback on questionnaire development, and Mr. Thomas Jensen for his assistance with website design. We also thank Ms. Kristen Hahn, Ms. Rose Radin, and Ms. Kristen Banholzer for their general assistance with the manuscript.
FUNDING: This work was supported by the National Institute of Child Health and Human Development (R21-050264) and the Danish Medical Research Council (271-07-0338). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We have no conflicts of interest to declare.
REFERENCES
- 1.Copenhagen, Nordic Council of Ministers; 2006. [accessed 22 December 2010]. Nordic Plan of Action on better health and quality of life through diet and physical activity. http://www.norden.org/en/publications/publications/2006-746/at_download/publicationfile. [Google Scholar]
- 2.Blair SN, Morris JN. Healthy Hearts and the Universal Benefits of Being Physically Active: Physical Activity and Health. Annals of epidemiology. 2009;19:253–256. doi: 10.1016/j.annepidem.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Frisch RE, Gotz-Welbergen AV, McArthur JW, Albright T, Witschi J, Bullen B, et al. Delayed menarche and amenorrhea of college athletes in relation to age of onset of training. Jama. 1981;246:1559–1563. [PubMed] [Google Scholar]
- 4.Loucks AB. Effects of exercise training on the menstrual cycle: existence and mechanisms. Med Sci Sports Exerc. 1990;22:275–280. [PubMed] [Google Scholar]
- 5.Dadgostar H, Razi M, Aleyasin A, Alenabi T, Dahaghin S. The relation between athletic sports and prevalence of amenorrhea and oligomenorrhea in Iranian female athletes. Sports Med Arthrosc Rehabil Ther Technol. 2009;1:16. doi: 10.1186/1758-2555-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper GS, Sandler DP, Whelan EA, Smith KR. Association of physical and behavioral characteristics with menstrual cycle patterns in women age 29–31 years. Epidemiology. 1996;7:624–628. doi: 10.1097/00001648-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 7.De Souza MJ, Toombs RJ, Scheid JL, O'Donnell E, West SL, Williams NI. High prevalence of subtle and severe menstrual disturbances in exercising women: confirmation using daily hormone measures. Hum Reprod. 2010;25:491–503. doi: 10.1093/humrep/dep411. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Gold EB, Lasley BL, Johnson WO. Factors affecting menstrual cycle characteristics. Am J Epidemiol. 2004;160:131–140. doi: 10.1093/aje/kwh188. [DOI] [PubMed] [Google Scholar]
- 9.De Souza MJ, Miller BE, Loucks AB, Luciano AA, Pescatello LS, Campbell CG, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220–4232. doi: 10.1210/jcem.83.12.5334. [DOI] [PubMed] [Google Scholar]
- 10.Gudmundsdottir SL, Flanders WD, Augestad LB. Physical activity and fertility in women: the North-Trøndelag Health Study. Human Reproduction. 2009;24:3196–3204. doi: 10.1093/humrep/dep337. [DOI] [PubMed] [Google Scholar]
- 11.Morris SN, Missmer SA, Cramer DW, Powers RD, McShane PM, Hornstein MD. Effects of lifetime exercise on the outcome of in vitro fertilization. Obstet Gynecol. 2006;108:938–945. doi: 10.1097/01.AOG.0000235704.45652.0b. [DOI] [PubMed] [Google Scholar]
- 12.Kucuk M, Doymaz F, Urman B. Effect of energy expenditure and physical activity on the outcomes of assisted reproduction treatment. Reprod Biomed Online. 2010;20:274–279. doi: 10.1016/j.rbmo.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol. 2007;110:1050–1058. doi: 10.1097/01.AOG.0000287293.25465.e1. [DOI] [PubMed] [Google Scholar]
- 14.Rich-Edwards JW, Spiegelman D, Garland M, Hertzmark E, Hunter DJ, Colditz GA, et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002;13:184–190. doi: 10.1097/00001648-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25:253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5:247–250. doi: 10.1097/00001648-199403000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Cramer DW, Barbieri RL, Xu H, Reichardt JKV. Determinants of basal follicle stimulating hormone levels in premenopausal women. J Clin Endocrinol Metab. 1994;79:1105–1109. doi: 10.1210/jcem.79.4.7962282. [DOI] [PubMed] [Google Scholar]
- 18.Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sorensen HT. Cohort Profile: The Danish Web-based Pregnancy Planning Study--'Snart-Gravid'. Int J Epidemiol. 2009;38:938–943. doi: 10.1093/ije/dyn191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothman KJ, Mikkelsen EM, Riis A, Sørensen HT, Wise LA, Hatch EE. Randomized Trial of Questionnaire Length. Epidemiology. 2009;20:154. doi: 10.1097/EDE.0b013e31818f2e96. [DOI] [PubMed] [Google Scholar]
- 20.Huybrechts KF, Mikkelsen EM, Christensen T, Riis AH, Hatch EE, Wise LA, et al. A successful implementation of e-epidemiology: the Danish pregnancy planning study 'Snart-Gravid'. Eur J Epidemiol. 2010;25:297–304. doi: 10.1007/s10654-010-9431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank L. When an entire country is a cohort. Science. 2000;287:2398–2399. doi: 10.1126/science.287.5462.2398. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. The SAS LGTPHCURV8 Macro. Boston, MA: Channing Laboratory; 2004. [Google Scholar]
- 24.Durrleman S, Simon R. Flexible regression models with cubic splines. Statist Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 25.Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol. 1986;124:470–480. doi: 10.1093/oxfordjournals.aje.a114417. [DOI] [PubMed] [Google Scholar]
- 26.Bonde JP, Joffe M, Sallmén M, Kristensen P, Olsen J, Roeleveld N, et al. Validity issues relating to time-to-pregnancy studies of fertility. Epidemiology. 2006;17:347–349. doi: 10.1097/01.ede.0000210239.80406.46. [DOI] [PubMed] [Google Scholar]
- 27.Greenland S, Rothman KJ. Introduction to stratified analysis: selecting confounders for control. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. New York: Lippincott Williams & Wilkins; 2008. pp. 261–263. [Google Scholar]
- 28.Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20:1541–1549. doi: 10.1002/sim.689. [DOI] [PubMed] [Google Scholar]
- 29.SAS Institute Inc. 2008. SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute Inc.; 2008. Cary, NC: SAS Institute. [Google Scholar]
- 30.Joffe M, Key J, Best N, Keiding N, Scheike T, Jensen TK. Studying time to pregnancy by use of a retrospective design. Am J Epidemiol. 2005;162:115–124. doi: 10.1093/aje/kwi172. [DOI] [PubMed] [Google Scholar]
- 31.Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod. 1995;10:2705–2712. doi: 10.1093/oxfordjournals.humrep.a135772. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 33.Jones EF, Forrest JD, Henshaw SK, Silverman J, Torres A. Unintended Pregnancy, Contraceptive Practice and Family Planning Services in Developed Countries. Family Planning Perspectives. 1988;20:53–67. [Google Scholar]
- 34.Russell C, Palmer JR, Adams-Campbell LL, Rosenberg L. Follow-up of a large cohort of Black women. Am J Epidemiol. 2001;154:845–853. doi: 10.1093/aje/154.9.845. [DOI] [PubMed] [Google Scholar]
- 35.Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, et al. The Danish National Birth Cohort--its background, structure and aim. Scand J Public Health. 2001;29:300–307. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 36.Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 37.Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias? Epidemiology. 2006;17:413–418. doi: 10.1097/01.ede.0000220549.14177.60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.