Abstract
Several thieno[2,3-d]pyrimidinediones have been synthesized and examined for antibacterial activity against a range of Gram-positive and Gram-negative pathogens. Two compounds displayed potent activity (2–16 mg/L) against multi-drug resistant Gram-positive organisms, including methicillin, vancomycin-intermediate, and vancomycin-resistant Staphylococcus aureus (MRSA, VISA, VRSA) and vancomycin-resistant enterococci (VRE). Only one of these agents possessed moderate activity (16–32 mg/L) against Gram-negative strains. An examination of the cytotoxicity of these agents revealed that they displayed low toxicity (40–50 mg/L) against mammalian cell and very low hemolytic activity (2–7%). Taken together, these studies suggest that thieno[2,3-d]pyrimidinediones are interesting scaffolds for the development of novel Gram-positive antibacterial agents.
1. Introduction
The increasing prevalence of pathogenic bacteria that are resistant to currently available antibiotics represents an alarming threat to public health. The most commonly encountered antibiotic-resistant bacteria, methicillin-resistant Staphylococcus aureus (MRSA), has had a major impact on infections in both the hospital and community setting.[1, 2] While vancomycin continues to be the standard treatment option for antibiotic-resistant infections, the isolation of vancomycin-resistant Staphylococcus (VRSA) and Enterococci (VRE) foreshadows a day in which the utilization of vancomycin may become limited.[3] Unfortunately, as antibiotic-resistant organisms have become more commonplace, the pipeline for the discovery of new antimicrobial agents has decreased.[4] Thus, there is a pressing need for new antimicrobial agents that are capable of treating resistant bacterial strains.
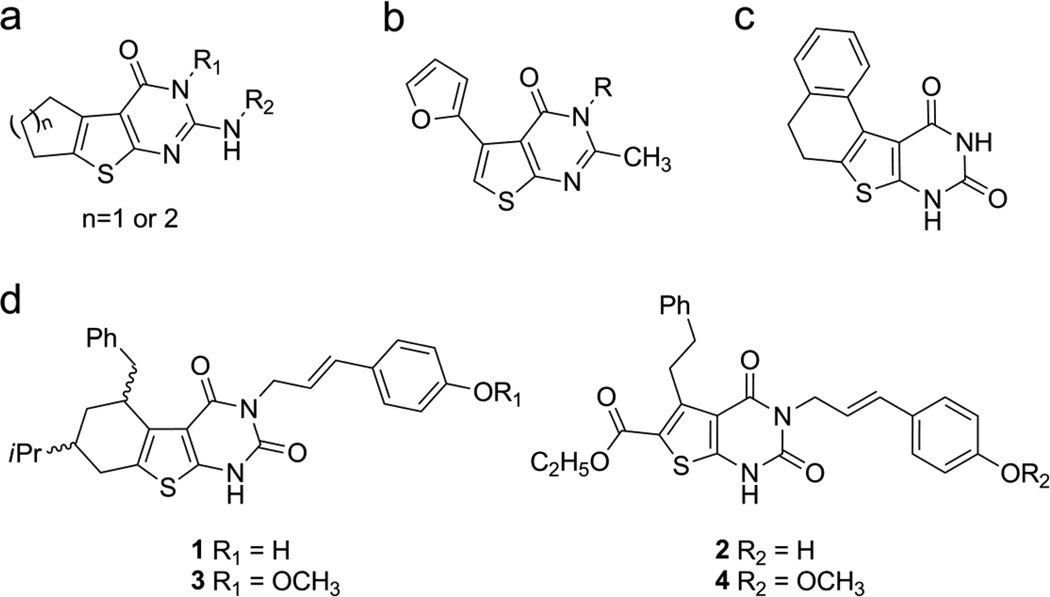
Thienopyrimidines are interesting heterocyclic compounds and a number of derivatives of these compounds display therapeutic activity as antimicrobial [5–7], antiviral [8, 9], anti inflammatory [10] antidiabetic [11] and anti cancer [12, 13] agents.[14–16] Despite the breadth of biological activities displayed by these agents, the antibacterial activity of this class of compounds has been underexplored. El-Sherbeny and colleagues examined the antimicrobial and antiviral activity of cyclopenteno and cyclohexeno [b]thieno[2,3-d]-3,4-dihydropyrimidine-4-one derivatives (Figure 1a).[8] These agents displayed -reasonable activity (MIC values 6.25–25 mg/L) against both Gram-positive and Gram-negative bacteria; however, these agents were significantly more potent against herpes simplex virus.[8] Furanyl-thieno[2,3-d]pyrimidin-4-ones (Figure 1b) were examined by Bahekar et. al. for their antibacterial activity. [7] These agents displayed MIC values in the range of 4–100 mg/L against a collection of Gram-positive and Gram-negative microbes. Interestingly, these compounds also displayed antimycobaterial activity.[7] The antibacterial activity of thieno[2,3-d]pyrimidindiones (Figure 1c) has not been reported in the literature; however, these compounds have been examined for antiviral activity.[15]
Figure 1.
Thieno[2,3-d]pyrimidineone derivatives
Recently, during a study on thieno [2,3-d]pyrimidinones, we discovered a set of compounds that possessed antibacterial activity (Figure 1d). These agents are structurally unrelated to any clinically used antibiotic and display discreet structural overlap with thieno [2,3-d]pyrimidines that have been reported in the literature. In this report, we discuss the synthesis of thieno[2,3-d]pyrimidinediones and their antibacterial and cytotoxic activities.
2. Results and Discussion
2.1. Chemistry
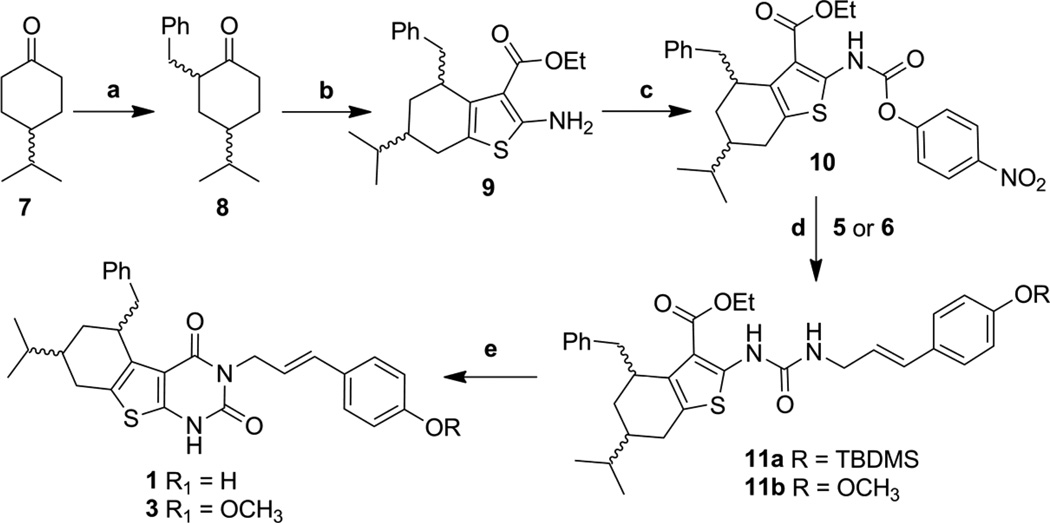
For a project on the development of antiviral therapeutics, we required the synthesis of two constrained (1 and 3) and unconstrained (2 and 4) thieno[2,3-d]pyrimidine-2,4-dione derivatives (Figure 1d), neither of which had been described in the literature. A retrosynthetic analysis of these agents suggested that an amino thiophene ester ring would be prepared first using the standard Gewald reaction. [17, 18] Once the thiophene was in hand, the pyrimidine ring could be prepared by converting the amine into a urea followed by cyclizing with the ester under basic conditions.
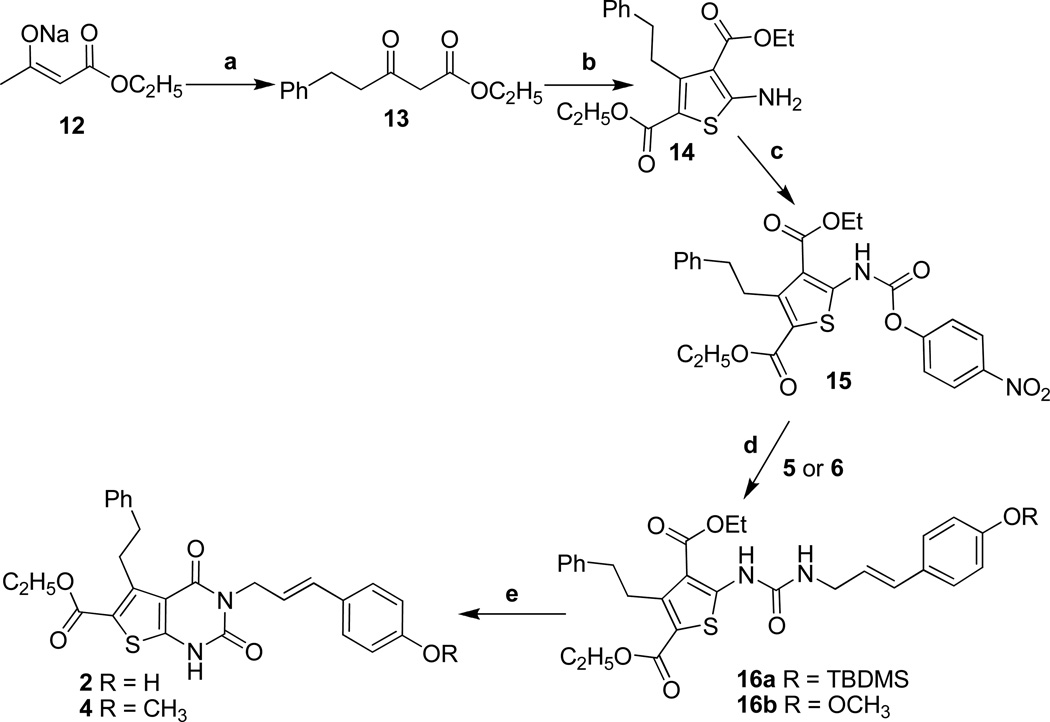
The synthesis of compounds 1–4 is shown in schemes 1–3. The synthesis of the constrained derivatives 1 and 3 starts from the commercially available ethyl-2-cyanoacetate and racemic benzylated isopropyl cyclohexanone (8, prepared from 7). These were reacted with sulfur to obtain the amino thiophene ester, 9, in decent yields. Activation of 9 with p-nitrophenyl chloroformate generated the unstable intermediate 10, which upon reaction with amines 5 or 6 produced urido compounds 11a and 11b.[19] Formation of the pyrimidinedione ring was accomplished with refluxing sodium methoxide to provide the final compounds 1 and 3 (Scheme 1).[20] The unconstrained compounds 2 and 4 were prepared using similar methodology starting from the ethyl 3-oxo-5-phenylpentanoate, 13 (Scheme 2).
Scheme 1.
a) nBuLi, BnBr, THF, −78 °C to r.t., 18h, 80% b) ethyl 2-cyanoacetate, S8, morpholine, EtOH, Δ, 24 – 48h., 39% c) p-Nitrophenyl chloroformate, Pyridine, CH2Cl2, r.t. 6 – 12h., 77% d) Pyridine, DMAP, THF, r.t. 12–24h., 69–79% e) NaOMe, MeOH, reflux, 3h., 76–77%
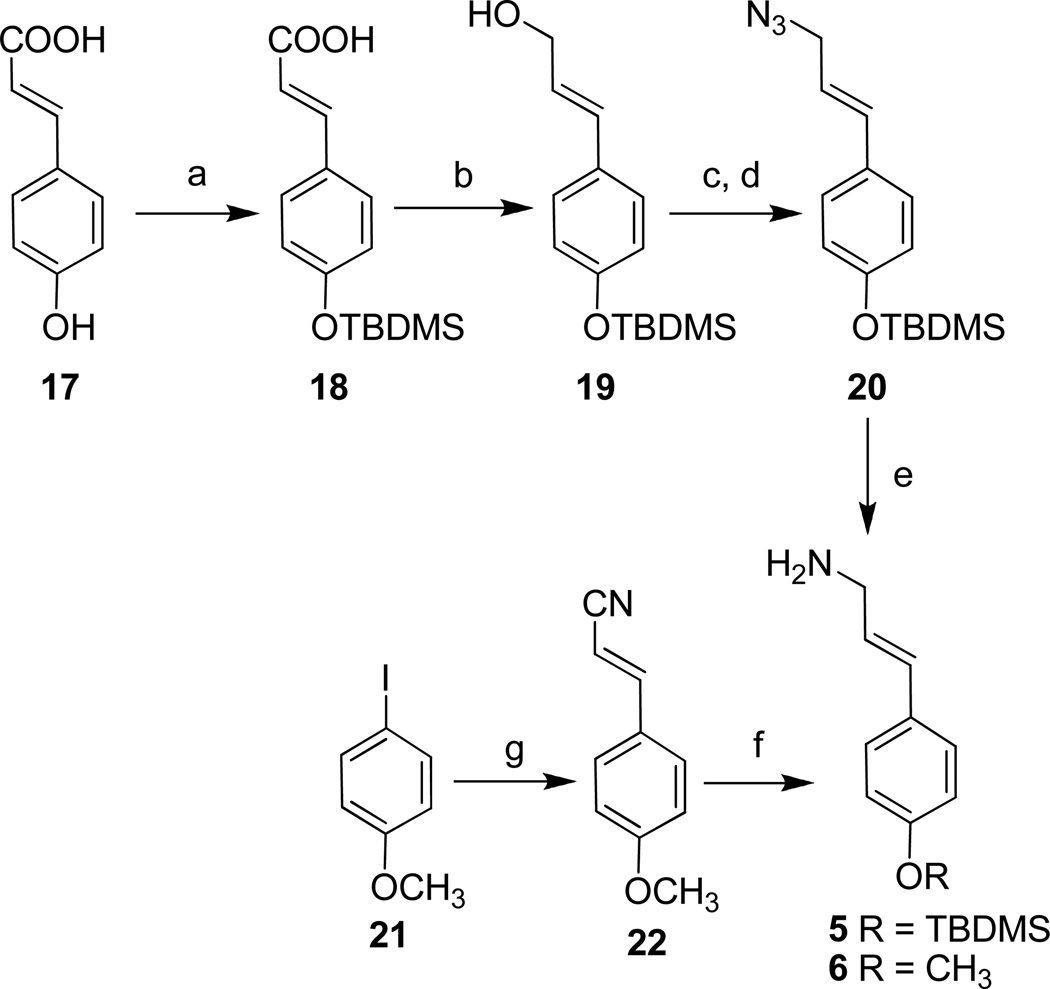
Scheme 3.
a) TBDMSCl, imidazole/DMF, r.t. 3h., 98% b) DIBAL/CH2Cl2, −78 °C, 8h., 59% c) MsCl, Et3N/THF d) NaN3/DMF, 82% e) Pd / CaCO3, H2(g)/EtOH, r.t.,93% f) LiAlH4/Et2O, r.t. 15min, 61% g) Acrylonitrile, Pd(OAc)2, Bu4NBr, NaHCO3/H2O, 48%
Scheme 2.
a) nBuLi, BnBr, THF, 0–30 °C, 18h, 66% b) ethyl 2-cyanoacetate, S8, morpholine, EtOH, Δ, 24 – 48h., 38 % c) p-Nitrophenyl chloroformate, Pyridine, CH2Cl2, r.t. 6 –12h, 77% d) Pyridine, DMAP, THF, r.t. 12–24h, 80–85% e) NaOMe, MeOH, reflux, 3h., 88–90%
The amines, 5 and 6, were prepared, as shown in the Scheme 3, from commercially available compounds (17 and 21) using procedures published for related compounds.[21–27] Amine 5 was prepared from p-hydroxycinnamic acid (17) by first protecting the phenol as the silyl ether followed by conversion of the acid into the azide (20). Selective reduction of the azide to 5 was accomplished using catalytic hydrogenation in the presence of Lindlar’s catalyst (Pd/CaCO3). Amine 6 was synthesized from p-iodoanisole (21) using a Heck reaction with acrylonitrile to generate E-cinnamonitrile (22). Reduction of the nitrile to the amine was accomplished with LiAlH4.
2.2. In vitro Antibacterial activity
The initial examination of compounds 1–4 failed to detect antiviral activity. As part of a further investigation of the biological activities of these compounds, we examined the antibacterial activity of compounds 1–4 and intermediates 11a, 11b, 16a and 16b against a panel of five Gram-positive and four Gram-negative bacteria (Table 1). Compounds 1 and 2 demonstrated significant antibacterial activity with MIC values in the 2–16 mg/L range against Gram-positive bacteria such as MRSA, VRSA, VISA, VRE and S. pneumoniae. The Gram-negative activity of 1 and 2 was weak with MIC values in the range of 16 to over 32 mg/L. Compounds 3 and 4 displayed almost no antibacterial activity with the exceptions being the moderate activity (8 mg/L) of 3 displayed against E. aerogenes. Compounds 11a, 16a and 16b showed no activity against any bacterial strain; compound 11b was insoluble in media, water and DMSO and thus could not be tested. Taken together, the data support the conclusion that 1 and 2 possess Gram-positive antibacterial activity, while compounds 3, 4, 11a, 16a and 16b are essentially inactive.
Table 1.
MIC and LD50 values for compounds 1–4, 11a, 11b, 16a, and 16b
| MIC (mg/L) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
S. aureus (VISA Mu50) |
S. aureus (MRSA 494) |
S. aureus (VRSA MI) |
S. pneumoniae (ATCC 49619) |
E. faecium (VRE 7303) |
E. coli (ATCC 25922) |
P. aeruginosa (ATCC 27853) |
K. pneumoniae (CEF 2324) |
E. aerogenes (CEF 3978) |
LD50 (mg/L) |
Therapeutic Indexa |
|
| 1 | 16 | 16 | 8 | 32 | 8 | 32 | 16 | 32 | 32 | 42 | 5 |
| 2 | 2 | 2 | 2 | 8 | 4 | >32 | 32 | 32 | 16 | 52 | 26 |
| 3 | >32 | >32 | >32 | 32 | >32 | >32 | >32 | >32 | 32 | 120 | 4 |
| 4 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | 8 | 98 | 3 |
| 11a | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | nd | nd |
| 11bb | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 16a | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | nd | nd |
| 16b | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | nd | nd |
| Vancomycin | nd | 0.5 | nd | 0.5 | >32 | nd | >32 | nd | nd | nd | nd |
Therapeutic index was calculated as the ratio of LD50/MIC for VRSA.
not determined due to insolubility of compound in media.
2.3. In vitro cytotoxicity
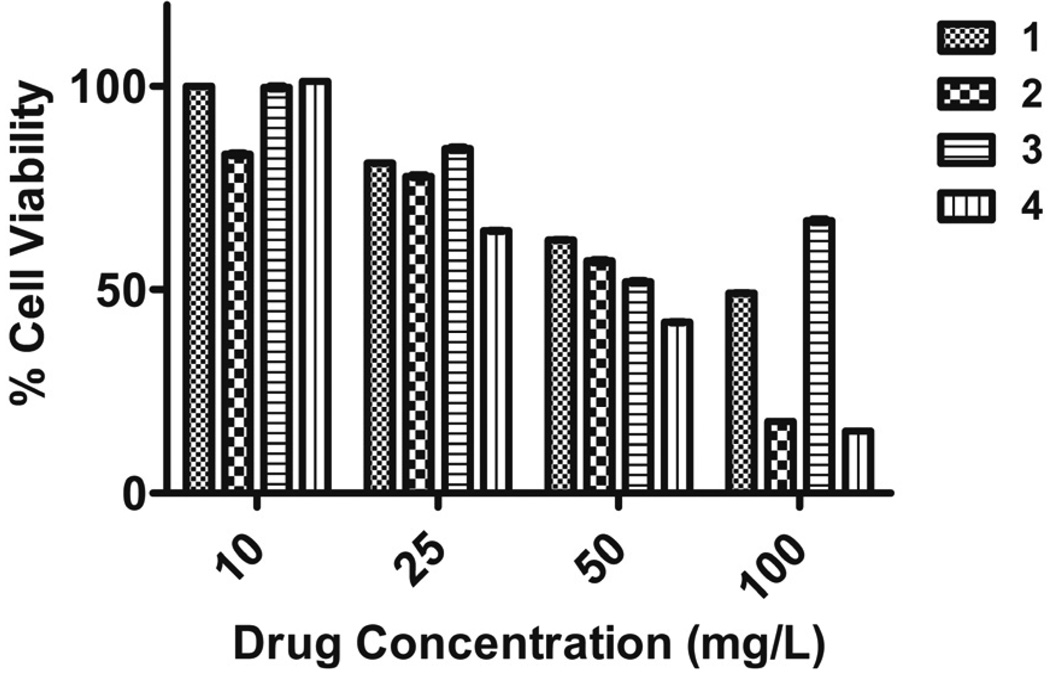
We examined the cytotoxic activity of compounds 1–4 against mammalian cells (NIH-3T3) using the MTS assay. As shown in Figure 2, all compounds displayed moderate levels of toxicity at 100 mg/L (< 50% of cell viability). The LD50 of each compound was determined from a dose-response curve of cytotoxicity versus concentration (Table 1). As shown in the table, the LD50 (~52 mg/L) of the most potent compound, 2, is 25 times higher than its MIC value indicating that 2 is selectively toxic to bacteria. In contrast, 1 displayed a therapeutic index of only 5 suggesting that the toxicity of the compound may have also played a role in its antibacterial activity.
Figure 2.
Cytotoxicity of compounds 1–4 obtained my MTS assay against mammalian cells (NIH-3T3), 5% DMSO is used as control.
2.4. In vitro Hemolytic activity
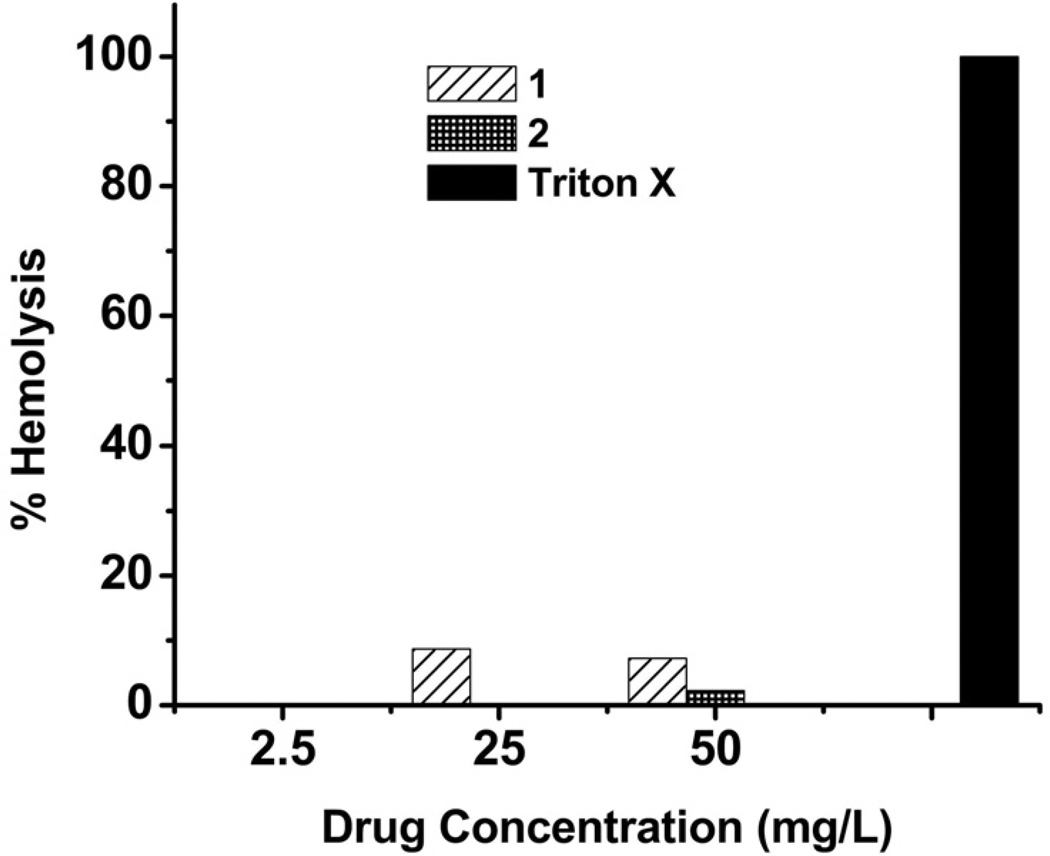
Another common measure of the toxicity of an agent is lysis of red blood cells. We examined the ability of compounds 1–2 to lyse Sheep erythrocytes. As shown in Figure 3, compound 1 displayed approximately 7% hemolysis at a concentration 25 times its MIC value. In contrast, 2, which is the most selective antibacterial agent described here, showed only 2% hemolysis at 50 times its MIC value. These results indicate that the antibacterial activity observed for compounds 1 and 2 is not due to non-specific membrane damage. This would suggest that there are specific antibacterial targets for these agents in bacteria.
Figure 3.
Hemolytic activity of compounds 1 and 2 against Sheep erythrocytes. Hemolysis was calculated using 1% Triton-X as positive control which set to 100% lysis.
2.5. Discussion
In this report we have explored thieno[2,3-d]pyrimidinedione derivatives as antibacterial agents. While thieno[2,3-d]pyrimidines have been extensively explored for their varied biological activity, the compounds reported here are unique and have not been prepared in the literature. Among the compounds synthesized, compound 2 showed selective antibacterial activity against Gram-positive antibiotic-resistant bacterial strains such as MRSA, VRSA, VISA and VRE. Unfortunately, these compounds were inactive against Gram-negative pathogens.
A complete structure-activity relationship cannot be determined from the limited set of compounds prepared here. However, inactivity of urido compounds (11a, 16a and 16b) clearly demonstrates the importance of the pyrimidine ring for the activity and our data also suggests that the presence of a phenol is critical for activity (compare compound 2 to compound 4). Furthermore, the presence of a methoxy group increases the cytotoxicity by 2-fold when compared to the phenol. A well-known method for enhancing potency of compounds is to lock flexible portions of the molecule into a fixed conformation. Tethering the side chains located on the thiophene results in the racemic compound 1. While 1 displayed antibacterial activity against Gram-positive pathogens, the MIC values were 2–8-fold higher than for the constrained analog 2. Thus, the flexibility seen in compound 2 appear to be necessary for the compound to adopt a biologically activity conformation.
The serendipitous discovery of antibacterial activity for these agents unfortunately means that the mechanism of action is unknown. While there have been reports of antibacterial activity for structurally unrelated thieno-pyrimidinones (see Introduction), the mechanism of action of those agents has never reported. Thieno-pyrimidines and thieno-pyrimidinediones have been reported as inhibitors of proteases[28], kinases[29–31] and folate utilizing enzymes[8, 9, 32, 33] suggesting that the mechanism of action of our agent could be due to inhibition of a specific protein. The inhibition of folate utilizing enzymes (i.e. dihydrofolate reductase, etc.) is especially interesting given the fact that antibacterial agents specifically targeting bacterial folate enzymes are well known. Of course, numerous other pathways could be responsible for the antibacterial activity of the compounds, including non-specific membrane effects. We are currently investigating the mechanism of action of these thieno[2,3-d]pyrimidinediones and hope to report on their mechanism in due course.
3. Conclusion
We have synthesized constrained and unconstrained thieno[2,3-d]pyrimidinedione derivatives and examined their antibacterial, cytotoxicity and hemolytic activity. Only one compound, 2, displayed potent antibacterial activity against a wide range of antibiotic-resistant bacteria including MRSA, VRSA, VISA and VRE. This compound was minimally cytotoxic against mammalian cells and possessed essentially no hemolytic activity. While the mechanism of action of this compound is unknown, efforts to determine the reason for its antibacterial activity are ongoing and will be reported in due course.
4. Experimental
Materials and Instruments
All chemicals were purchased from Acros, Sigma-Aldrich, Matrix Scientific, EMD or Frinton labs and used without further purification. 1H NMR and 13C NMR spectra were recorded on Varian DRX400. The mass spectra of respective final compounds were recorded on Waters-Micromass ZQ quadupole located in the central instrumentation facility at Wayne State University, Detroit, MI. For the MTS and hemolysis assays, absorbance was collected using Synergy 2 multi-mode plate reader from Biotek Instruments Inc. Winooski, VT.
2-benzyl-4-isopropylcyclohexanone (8)
To a cooled (−20 °C) solution of diisopropylamine (0.160 g, 1.57 mmols) in dry toluene, n-butyllithium (1.6 M in hexanes ,0.95 mL, 1.5 mmols) was added and stirred for 15 min. To the solution, isopropyl cyclohexanone (7) (0.200 g, 1.43 mmols), dissolved in 5 mL of dry toluene, was added drop wise. The reaction was stirred at −20°C for 30 min and then cooled to −78 °C. To the chilled solution, benzylbromide (0.270 g, 1.57 mmols), dissolved in 5 mL dry toluene, was added drop wise and the reaction mixture was warmed to −45 °C and stirred at this temperature for 18h. The reaction was quenched with 0.5N HCl (10mL) and extracted with ether (150 mL). The combined organic layers were washed with brine, H2O and then dried over anhydrous Na2SO4 before being concentrated in vacuuo. The crude product was purified by flash silica column chromatography (1:9 ethyl acetate:hexanes) to yield pure 10 (0.26, 80%). 1H NMR (400 MHz, CD2Cl2) δ 0.85, (d, J=2.8Hz, 3H, CH3), 0.86, (d, J=2.4Hz, 3H, CH3), 1.44–1.59, (m, 4H, CH2, CH2), 2.31–2.37 (m, 4H, CH2, CH2) 2.38–2.65, (m, 2H, CH, CH), 3.2 (dd, Ja=14Hz, Jb=5.2Hz, 1H, CH), 7.15–7.28, (m, 5H, Ph); 13C NMR (100 MHz, CD2Cl2) δ 19.47, 20.00, 30.61, 32.22, 35.64, 37.13, 41.71, 43.16, 51.50, 125.96, 128.34, 129.29, 141.02, 212.38.
Ethyl 2-amino-4-benzyl-6-isopropyl-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate (9)
To a 50 mL round bottom flask, 8 (0.341 g, 1.48 mmols), sulfur (0.048 g, 1.48 mmols), ethylcyanoacetate (0.167 g, 1.48 mmols) and morpholine (0.129 g, 1.48 mmols) were added along with 15mL of absolute ethanol. The resulting solution was refluxed for 48h, cooled and then evaporated to dryness in vacuuo. The resulting crude product was purified by flash silica column chromatography (1:9 ethyl acetate: hexanes) to generate 0.21 g (39%) of 9. 1H NMR (400 MHz, CD2Cl2) δ 0.85, (d, J=7.2Hz, 3H, CH3), 0.89, (d, J=6.8Hz, 3H, CH3), 1.35, (t, J=5.2Hz, 3H, CH3), 1.78–1.89 (m, 2H, CH, CH), 2.45, (t, J=2.8Hz, 2H, CH2), 2.56–2.62, (m, 2H, CH2, CH2), 3.15 (dd, Ja=13.6Hz, Jb=2.8Hz, 1H, CH), 4.41, (q, J=8.4Hz, 2H, CH2), 6.03, (s, 2H, NH2), 7.20–7.32, (m, 5H, Ph); 13C NMR (100 MHz, CD2Cl2) δ 14.93, 19.56, 20.10, 30.64, 32.27, 40.54, 41.75, 43.19, 51.55, 59.74, 118.50, 126.03, 128.41, 129.35, 136.61, 141.05, 141.94, 162.80, 165.63. HRMS (ES+) m/z (M+H) calcd for C21H27NO2S 358.1839, found 358.1837 (M+H).
Ethyl 4-benzyl-6-isopropyl-2-((4-nitrophenoxy)carbonylamino)-4,5,6,7-tetrahydrobenzo[b]-thiophene-3-carboxylate (10)
A solution of dry pyridine (0.16 g, 2.0 mmols) and 9 (0.358 g, 1.0 mmols) in 20 mL of dry CH2Cl2 was stirred at room temperature for 30 min. To the reaction, a solution of p-nitrophenyl chloroformate (0.303g, 1.5 mmols), in 5 mL of dry CH2Cl2, was added drop wise over a period of 15 min. The reaction was stirred at room temperature for 12h and then evaporated in vacuuo to yield the crude product which was purified by flash silica column chromatography (3:7 ethyl acetate : hexanes) to give 0.41 g (77%) of the desired product. 1H NMR (400 MHz, CD2Cl2) δ 0.86, (d, J=6.8Hz, 3H, CH3), 0.91, (d, J=6.8Hz, 3H, CH3),1.46, (t, J=7.6Hz, 3H, CH3), 1.83–1.88 (m, 2H, CH, CH), 2.50–2.82, (m, 4H, CH2, CH2), 3.14–3.17, (m, 2H, CH2), 3.59–3.62 (m, 1H, CH), 4.47, (q, J=7.2Hz, 2H, CH2), 7.16–7.27, (m, 5H), 7.48, (d, J=8.0Hz, 2H, Ph), 8.32, (d, J=8.0Hz, 2H, Ph), 11.09, (s, 1H, NH); 13C NMR (100 MHz, CD2Cl2) δ 14.67, 19.62, 19.99, 28.32, 28.54, 32.36, 35.19, 40.85, 61.25, 111.58, 122.21, 125.37, 126.12, 127.81, 128.35, 129.30, 135.87, 141.30, 145.48, 148.67, 150.12, 155.50, 166.09. HRMS could not be obtained because of decomposition in the mass spectrometer.
(E)-ethyl 4-benzyl-2-(3-(3-(4-(tert-butyldimethylsilyl)phenyl)allyl)ureido)-6-isopropyl-4,5,-6,7-tetrahydrobenzo[b]thiophene-3-carboxylate (11a)
Compound 5 (0.250 g, 0.95 mmols) was dissolved in 20 mL of dry THF followed by the addition of pyridine (0.090 g, 1.14mmols) and DMAP (0.017 g, 0.14 mmols). The resulting solution was stirred for 20 min. A solution of 10 (0.496 g, 0.95 mmols) in 15 mL of dry THF was added drop wise to the reaction mixture and the reaction was stirred for an additional 12–24 h. The reaction was then evaporated to dryness in vacuuo and the residue was purified by flash silica column chromatography (2:8 ethyl acetate: hexanes) to yield 0.49 g (79%) of the expected products. 1H NMR (400 MHz, CD2Cl2) δ 0.86, (d, J=6.8Hz, 3H, CH3), 0.91, (d, J=6.8Hz, 3H, CH3),1.46, (t, J=7.6Hz, 3H, CH3), 1.83–1.88 (m, 2H, CH, CH), 2.50–2.82, (m, 4H, CH2, CH2), 3.14–3.17, (m, 2H, CH2), 3.59–3.62 (m, 1H, CH), 4.47, (q, J=7.2Hz, 2H, CH2), 7.16–7.27, (m, 5H), 7.48, (d, J=8.0Hz, 2H, Ph), 8.32, (d, J=8.0Hz, 2H, Ph), 11.09, (s, 1H, NH); 13C NMR (100 MHz, CD2Cl2) δ 14.67, 19.62, 19.99, 28.32, 28.54, 32.36, 35.19, 40.85, 61.25, 111.58, 122.21, 125.37, 126.12, 127.81, 128.35, 129.30, 135.87, 141.30, 145.48, 148.67, 150.12, 155.50, 166.09. HRMS could not be obtained because of decomposition in the mass spectrometer.
(E)-ethyl 4-benzyl-6-isopropyl-2-(3-(3-(4-methoxyphenyl)allyl)ureido)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate (11b)
Compound 6 (0.097 g, 0.60 mmols) was dissolved in 15 mL of dry THF followed by the addition of pyridine (0.052g, 0.65mmols) and DMAP (0.008g, 0.06 mmols). To the reaction, which was stirred for 20 min, a solution of 10 (0.310g, 0.60 mmols) in 10 mL of dry THF was added drop wise. The reaction was stirred at room temperature for 12–24 h and evaporated to dryness in vacuuo. The expected product (0.16 g, 69%) was obtained from the residue after purification by flash silica column chromatography (3:7 ethyl acetate: hexanes). 1H NMR (400 MHz, CD2Cl2) δ 0.85, (d, J=6.8Hz, 3H, CH3), 0.90, (d, J=6.8Hz, 3H, CH3), 1.19–1.26 (m, 1H, CH), 1.37, (t, J=7.6Hz, 3H, CH3), 1.41–1.48 (m, 1H, CH), 1.79–1.85 (m, 1H, CH), 2.28 (t, J=5.6Hz, 2H, CH2), 2.55 (d, J=6.2Hz, 2H), 3.13, (d, J=6.0Hz, 2H, CH2), 3.76 (s, 3H, CH3), 4.05 (t, J=6.0Hz, 2H, CH2) 4.41, (q, J=6.8Hz, 2H, CH2), 5.71 (t, J=4.8Hz, 1H, NH), 6.08–6.17 (sextet, J = 15.6Hz, 1H, CH=CH), 6.53 (d, J = 15.6Hz, 1H, CH=CH), 6.84 (d, J=8.4Hz, 2H, Ph), 7.19–7.33, (m, 7H, Ph), 10.80, (s, 1H, NH); 13C NMR (100 MHz, CD2Cl2) δ 14.77, 19.66, 19.81, 28.48, 32.83, 35.23, 36.72, 40.87, 42.96, 55.41, 60.68, 108.39, 114.15, 123.76, 125.72, 126.00, 127.75, 128.40, 129.16, 129.44, 131.52, 134.52, 141.62, 152.25, 153.85, 159.60, 166.76., HRMS (ES+) m/z (M+H) calcd for C32H38N2O4S 547.2632, found 547.2631 (M+H)
(E)-ethyl 4-benzyl-6-isopropyl-2-(3-(3-(4-methoxyphenyl)allyl)theino[2,3-d]pyrimidine-2,4-dione (3)
Compound 11b (0.050 g, 0.09 mmols) was refluxed in sodium methoxide (prepared from NaOCH3 (0.005 g, 0.10 mmols) and 10 mL of methanol) for 3h. The reaction was cooled and neutralized with Dowex 50 H+ resin which resulted in a white precipitate. The precipitate was dissolved in hot methanol and filtered through a sintered glass funnel. The resulting filtrate [NOTE: check on this] was dried and purified using flash silica column chromatography (1:1 ethyl acetate: hexane) to yield 0.035 g (76%) of the final product. 1H NMR (400 MHz, DMSO) δ 0.86, (d, J=6.8Hz, 3H, CH3), 0.91, (d, J=6.4Hz, 3H, CH3), 1.46–1.58, (m, 1H, CH), 1.81–2.12, (m, 1H, CH), 2.26–2.43 (m, 4H, CH2, CH2) 2.73, (dd, Ja=11.6Hz, Jb=4.8Hz,1H,CH), 3.18 (d, J=12.0Hz, 2H, CH2), 3.73 (s, 3H, CH3), 4.61 (d, J=8.0Hz, 2H, CH2), 6.15–6.19 (sextet, J = 16Hz, 1H, CH=CH), 6.48 (d, J = 16Hz, 1H, CH=CH), 6.85 (d, J=8.8Hz, 2H, Ph), 7.18–7.38, (m, 7H, Ph), 12.18, (s, 1H, NH); 13C NMR (100 MHz, CD2Cl2) δ 20.25, 20.41, 27.31, 28.84, 32.61, 35.65, 37.46, 42.09, 55.75, 112.18, 114.66, 122.72, 126.57, 126.82, 128.19, 128.84, 129.65, 129.78, 131.73, 135.63, 141.86, 150.87, 151.07, 158.99, 159.50; HRMS (ES+) m/z (M+H) calcd. for C30H32N2O3S 501.2212, found 501.2210 (M+H).
(E)-ethyl 4-benzyl-2-(3-(3-(4-(hydroxy)phenyl)allyl) theino[2,3-d]pyrimidine-2,4-dione (1)
Compound 11a (0.100 g, 0.15 mmols) was refluxed in sodium methoxide (prepared from NaOCH3 (0.010 g, 0.19 mmols) and 15 mL of methanol) for 3h. The reaction was cooled and neutralized with Dowex 50 H+ resin which resulted in a white precipitate. The precipitate was dissolved in hot methanol and filtered through a sintered glass funnel. The resulting filtrate [NOTE: check on this] was dried and purified using flash silica column chromatography (1:1 ethyl acetate: hexane) to yield 0.058 g (77%) of the final product. 1H NMR (400 MHz, DMSO) δ 0.86, (d, J=6.8Hz, 3H, CH3), 0.90, (d, J=6.8Hz, 3H, CH3), 1.44–1.60, (m, 2H, CH, CH), 2.25–2.42 (m, 4H, CH2, CH2) 2.70–2.75, (m, 1H, CH), 3.16 (d, J=7.8Hz, 2H, CH2), 4.59 (d, J=7.0Hz, 2H, CH2), 6.04–6.14 (sextet, J = 16Hz, 1H, CH=CH), 6.44 (d, J = 16Hz, 1H, CH=CH), 6.67 (d, J=8.8Hz, 2H, Ph), 7.18–7.38, (m, 7H, Ph), 9.47 (s, 1H, OH), 12.18, (s, 1H, NH); 13C NMR (100 MHz, DMSO) δ 20.26, 20.42, 27.31, 28.85, 32.62, 35.66, 37.47, 42.15, 60.44, 112.21, 116.04, 121.49, 126.58, 126.86, 128.04, 128.25, 128.86, 129.79, 132.29, 135.65, 141.86, 150.82, 150.90, 157.78, 158.97; HRMS (ES+) m/z (M+H) calcd for C29H30N2O3S 487.2055, found 487.2046 (M+H).
Ethyl 3-oxo-5-phenylpentanoate (13)
Sodium ethylacetoacetate (12) (5 g, 32.87 mmols) was dissolved in 50 mL of dry THF and cooled to 0°C under argon. Once cooled, a solution (1.6 M in hexane) of n-butyl lithium (21.88 mL, 34.51 mmols) was added drop wise and the reaction was stirred for 30 min at 0° C to generate the enolate. Benzyl bromide (5.62 g, 32.87 mmols) was then added and the reaction continued stirring for another 90 min at 0 °C. Upon completion, the reaction was poured into a cold solution of saturated potassium hydrogen phosphate and the resulting aqueous solution was extracted with ethyl ether (3 × 100 mL). The combined organic layers were washed with water, dried over anhydrous Na2SO4 and concentrated in vacuuo. The resulting material was purified by flash silica column chromatography (1:1:8 CH2Cl2: ethyl acetate: hexanes) to obtained 4.75 g (66%) of the final product as eluent. 1H NMR (400 MHz, CD2Cl2) δ 1.25, (t, J = 7.2Hz, 3H, CH3), 2.87–2.92, (m, 4H, CH2, CH2), 3.42, (s, 2H, CH2) 4.15, (q, J = 6.8Hz, 2H, CH2), 7.19–7.31, (m, 5H, Ph); 13C NMR (100 MHz, CD2Cl2) δ 14.1, 29.52, 44.56, 49.58, 61.46, 126.31, 128.5, 128.64, 141.05, 167.26, 202.11.
Diethyl 5-amino-3-phenethylthiophene-2,4-dicarboxylate (14)
Compound 13 (3.04 g, 13.8 mmols), sulfur (0.443 g, 13.8 mmols), ethylcyanoacetate (1.56 g, 13.8 mmols) and morpholine (1.2 g, 13.8 mmols) were refluxed in 60 mL of absolute ethanol for 48h. Upon completion, the solvent was evaporated in vacuuo and the resulting material was purified by flash silica column chromatography (1:2:7 ethyl acetate: CH2Cl2: hexanes) to yield 1.78 g (38%) of the final product. 1H NMR (400 MHz, CD2Cl2) δ 1.31, (t, J = 7.2Hz, 3H, CH3), 1.37, (t, J = 7.2Hz, 3H, CH3), 2.82, (t, J = 8.4Hz, 2H, CH2) 3.52, (t, J = 8.4Hz, 2H, CH2), 4.24, (q, J = 7.2Hz, 2H, CH2), 4.34, (q, J = 7.2Hz, 2H, CH2), 6.58, (s, 2H, br, NH2), 7.19 (q, J = 4.8Hz, 1H, Ph), 7.29, (d, J = 4.4Hz, 4H, Ph); 13C NMR (100 MHz, CD2Cl2) δ 14.47, 14.54, 31.25, 36.73, 60.44, 60.73, 107.79, 109.02, 125.96, 128.42, 128.63, 142.64, 151.57, 162.51, 165.91, 166.83. HRMS (ES+) m/z (M+Na) calcd for C18H21NO4SNa 370.1079, found 370.1083 (M+Na).
Diethyl 5-((4-nitrophenoxy)carbonylamino)-3-phenethylthiophene-2,4-dicarboxylate (15)
A mixture of dry pyridine (0.12 g, 1.5 mmols) and 14 (0.300 g, 0.86 mmols) in 20 mL of dry CH2Cl2 was placed under argon and stirred for 30 min at room temperature. To the solution, p-nitrophenyl chloroformate (0.186 g, 0.93 mmols) dissolved in 10 mL of dry CH2Cl2 was added drop wise over a period of 15 min and the resulting mixture was stirred at room temperature for 18h. The solvent was evaporated in vacuuo and the residue was purified by flash silica column chromatography (2:2:6 ethyl acetate: CH2Cl2: hexanes) to give 0.34 g (77%) of the final, pure product. 1H NMR (400 MHz, CD2Cl2) δ 1.35, (t, J = 6.8Hz, 3H, CH3), 1.43, (t, J = 7.2Hz, 3H, CH3), 2.86, (t, J = 8.4Hz, 2H, CH2) 3.61, (t, J = 8.4Hz, 2H, CH2), 4.3, (q, J = 7.2Hz, 2H, CH2), 4.42, (q, J = 7.2Hz, 2H, CH2), 7.19–7.34, (m, 5H, Ph), 7.47 (d, J = 9.2Hz, 2H, Ph), 8.31, (d, J = 9.2Hz, 2H, Ph), 11.36 (s, 1H, NH); 13C NMR (100 MHz, CD2Cl2) δ 14.33, 30.63, 36.85, 61.2, 61.86, 114.15, 118.61, 122.19, 125.48, 126.1, 128.46, 128.59, 142.17, 145.73, 148.85, 150.24, 153.67, 155.17, 162.25, 166.15. HRMS could not be obtained because of decomposition in the mass spectrometer.
(E)-diethyl 3-phenethyl-5-(3-(3-(4-(trimethylsilyloxy)phenyl)allyl)ureido)thiophene-2,4-dicarboxylate (16a)
Compound 5 (0.170 g, 0.65 mmols) was dissolved in 15 mL of dry THF and to the resulting solution, pyridine (0.061 g, 0.78mmols) and DMAP (0.012 g, 0.10 mmols) were added. The amine solution was stirred at room temperature for 20 min followed by a drop wise addition of a solution of 15 (0.331 g, 0.65 mmols) in 15 mL of dry THF. The reaction was stirred for an additional 12 h and then evaporated in vacuuo. The residue was purified by flash silica column chromatography (2:8 ethyl acetate: hexanes) to yield 0.33 g (80%) of pure 16a. 1H NMR (400 MHz, CD2Cl2) δ 0.19, (s, 6H, CH3), 0.98, (s, 9H, tBu) 1.30–1.39, (m, 6H, CH3), 2.82 (t, J = 8.0Hz, 2H, CH2), 3.57 (t, J = 8.0Hz, 2H, CH2), 4.07 (t, J = 5.8Hz, 2H, CH2), 4.26–4.37, (m, 4H, CH2, CH2), 5.49, (t, J = 5.2Hz, 1H, NH), 6.12 (sextet, J = 15.6Hz, 1H, CH=CH), 6.55 (d, J = 16.4Hz, 1H, CH=CH), 6.80 (d, J = 8.8Hz, 2H, Ph), 7.25–7.30, (m, 7H, Ph), 11.04 (s, 1H, NH); 13C NMR (100 MHz, CD2Cl2) δ -4.49, 14.44, 25.66, 30.69, 31.91, 32.44, 36.87, 53.67, 60.86, 61.35, 120.15, 120.44, 123.54, 126.02, 127.75, 128.45, 128.59, 129.42,130.56, 131.89, 142.50, 148.59, 153.86, 155.77, 162.88, 166.78. HRMS (ES+) m/z (M+H) calcd for C34H44N2O6SSi 637.2755, found 637.2715 (M+H).
(E)-diethyl 5-(3-(3-(4-methoxyphenyl)allyl)ureido)-3-phenethylthiophene-2,4-dicarboxylate (16b)
To a solution of 6 (0.173 g, 1.06 mmols), dissolved in 20 mL of dry THF, pyridine (0.092 g, 1.17mmols) and DMAP (0.013 g, 0.106 mmols) were added and the resulting solution was stirred at room temperature for 20 min. To the amine solution, a solution of 15 (0.543 g, 1.06 mmols) in 15 mL of dry THF was added drop wise and the resulting reaction was allowed to stir for an additional 12 h. Upon completion, the solvent was evaporated in vacuuo and the resulting mixture was purified by flash silica column chromatography (3:7 ethyl acetate: hexanes) to generate 0.49 g (85%) of 16b. 1H NMR (400 MHz, CD2Cl2) δ 1.32, (t, J = 7.2Hz, 3H, CH3), 1.37, (t, J = 7.2Hz, 3H, CH3), 2.83, (t, J = 8.4Hz, 2H, CH2) 3.60, (t, J = 8.4Hz, 2H, CH2), 3.8 (s, 3H, CH3), 4.10 (t, J = 5.6Hz, 2H, CH2), 4.28, (q, J = 7.2Hz, 2H, CH2), 4.37, (q, J =7.6Hz, 2H, CH2), 5.57 (t, J = 5.2Hz, 1H, NH), 6.07–6.14 (sextet, J = 15.6Hz, 1H, CH=CH), 6.56 (d, J = 15.6Hz, 1H, CH=CH), 6.84 (d, J = 8.4Hz, 2H, Ph), 6.95, (d, J = 8.8Hz, 2H, Ph), 7.17–7.31 (m, 3H, Ph), 8.13 (d, J = 9.2, 2H, Ph), 11.14 (s, 1H); 13C NMR (100 MHz, CD2Cl2) δ 14.35, 30.58, 36.82, 55.47, 61.26, 61.57, 114.20, 115.94, 126.07, 126.32, 127.79, 128.46, 128.55,129.31, 142.27, 148.87, 154.18, 156.30, 159.70, 162.61, 163.32, 166.82. HRMS (ES+) m/z (M+Na) calcd for C29H32N2O6SNa 559.1869, found 559.1872 (M+Na).
(E)-ethyl 3-(3-(4-methoxyphenyl)allyl)-2,4-dioxo-5-phenethyl-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidine-6-carboxylate (4)
Compound 16b (0.385 g, 0.72 mmols) was refluxed in sodium methoxide (prepared from NaOCH3 (0.043 g, 0.79 mmols) and 30 mL methanol) for 3h. The reaction was cooled and neutralized with Dowex 50 H+ resin which resulted in a white precipitate. The precipitate was dissolved in hot methanol and filtered through a sintered glass funnel. The resulting filtrate [NOTE: check on this] was dried and purified using flash silica column chromatography (3:7 ethyl acetate: hexane) to yield 0.31 g (88%) of the final product. 1H NMR (400 MHz, DMSO-d6) δ 1.28, (t, J = 6.8Hz, 3H, CH3), 2.78, (t, J = 7.6Hz, 2H, CH2) 3.55, (t, J = 8.0Hz, 2H, CH2), 3.73 (s, 3H, CH3), 4.25, (q, J = 7.2Hz, 2H, CH2), 4.60, (d, J = 6.0Hz, 2H, CH2), 6.11–6.18 (sextet, J = 16Hz, 1H, CH=CH), 6.52 (d, J = 16Hz, 1H, CH=CH), 6.87 (d, J = 8.8Hz, 2H, Ph), 7.19 (t, J = 6.8Hz, 1H, Ph), 7.25–7.32 (m, 4H, Ph), 7.36 (d, J = 8.8Hz, 2H, Ph), 12.56 (s, 1H, NH); 13C NMR (100 MHz, DMSO-d6) δ 14.91, 30.51, 36.47, 42.17, 55.76, 61.33, 114.68, 116.51, 123.02, 126.53, 126.88, 128.17, 128.91,128.97, 129.77, 131.79, 140.27, 142.27, 149.31, 159.48, 160.03, 162.55, 164.69; HRMS (ES+) m/z (M+Na) calcd for C27H26N2O5SNa 513.1460, found 513.1453 (M+Na).
(E)-ethyl 3-(3-(4-hydroxyphenyl)allyl)-2,4-dioxo-5-phenethyl-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidine-6-carboxylate (2)
Compound 16a (0.100 g, 0.16 mmols) was refluxed in sodium methoxide (prepared from NaOCH3 (0.009 g, 0.17 mmols) in 30 mL methanol) for 3h. The reaction was cooled and neutralized with Dowex 50 H+ resin which resulted in a white precipitate. The precipitate was dissolved in hot methanol and filtered through a sintered glass funnel. The resulting filtrate was dried and purified using flash silica column chromatography (3:7 ethyl acetate:hexane) to yield 0.083 g (90%) of the final product. 1H NMR (400 MHz, DMSO-d6) δ 1.28, (t, J = 7.2Hz, 3H, CH3), 2.78, (t, J = 8.0Hz, 2H, CH2) 3.55, (t, J = 7.8Hz, 2H, CH2), 4.26, (q, J = 6.8Hz, 2H, CH2), 4.58, (d, J = 6.0Hz, 2H, CH2), 6.06 (sextet, J = 15.6Hz, 1H, CH=CH), 6.47 (d, J = 16Hz, 1H, CH=CH), 6.69 (d, J = 8.4Hz, 2H, Ph), 7.17–7.32 (m, 7H, Ph), 9.48 (s, 1H, OH), 12.55 (s, 1H, NH); 13C NMR (100 MHz, DMSO-d6) δ 14.62, 30.30, 36.33, 42.31, 61.60, 114.01, 115.89, 117.27, 120.99, 126.50, 128.05, 128.20,128.83, 128.90, 132.69, 141.99, 148.87, 150.47, 154.81, 157.67, 159.20, 162.20. HRMS HRMS (ES+) m/z (M+Na) calcd for C26H24N2NaO4S 499.1304, found 499.1306 (M+Na)
(E)-3-(4-(tert-butyldimethylsilyloxy)phenyl)acrylic acid (18)
p-hydroxycinnmic acid (17) (5.0 g, 30.46 mmols) and imidazole (5.2 g, 76.15 mmols) were dissolved in 25 mL of dry DMF and the solution was cooled to 0 °C. To the chilled solution, t-butyldimethylsilylchloride (11.5 g, 76.15 mmols) was added and the reaction was stirred at room temperature for 3h. The resulting reaction mixture was poured into 125 mL of water. The white, cloudy solution was extracted with ethyl acetate (3 × 100 mL) and the combined organic layers were washed with brine and dried with sodium sulfate. The organic layer was evaporated to dryness and the residue was purified by flash silica column chromatography (3:7 ethyl acetate:hexanes) to yield 8.32 g (98%) of the final product. 1H NMR (400 MHz, CDCl3) δ 0.23, (s, 6H, CH3), 0.99, (s, 9H, tBu) 6.33 (d, J = 16Hz, 1H, CH=CH), 6.85 (d, J = 8.4Hz, 2H, Ph), 7.46 (d, J = 8.4Hz, 2H, Ph), 7.74 (d, J = 16Hz, 1H, CH=CH); 13C NMR (CDCl3) δ -4.16, 18.46, 25.83, 114.77, 120.80, 127.59, 130.24, 146.94, 158.49, 171.23.
(E)-3-(4-(tert-butyldimethylsilyloxy)phenyl)prop-2-en-1-ol (19)
Compound 18 (5.0 g, 17.98 mmols) was dissolved in 25 mL of dry CH2Cl2 and the solution was cooled to -78 °C. To the chilled solution, DIBAL (20% solution in toluene, 61 mL, 71.91 mmol) was added drop wise and the reaction was stirred at −78 °C for 8h. Upon com pletion, 75 mL of ether was added to the reaction mixture and the mixture was slowly warmed to 0 °C. Once the solution was equilibrated to 0 °C, 2.5 mL of H 2O was added drop wise, followed by 5 mL of 1N NaOH and an additional 5 mL of H2O. The solution was then was warmed to room temperature and stirred for an additional 15 min and anhydrous MgSO4 was added slowly with stirring another 15 min until no hydrated MgSO4 aggregates formed. The salts were filtered and filtrate evaporated to obtain the pure product in 2.43 g (59%) yield. 1H NMR (400 MHz, CD2Cl2) δ 0.22 (s, 6H, CH3), 1.01 (s, 9H, tBu) 2.13 (s, br, 1H, OH), 4.25 (t, J = 4.8Hz, 2H, CH2), 6.24 (quintet, J = 16Hz, 1H, CH=CH), 6.54 (d, J = 16Hz, 1H, CH=CH), 6.81 (d, J = 8.8Hz, 2H, Ph), 7.28 (d, J = 8.4Hz, 2H, Ph); 13C NMR (100 MHz, CD2Cl2) δ -4.48, 18.34, 25.67, 63.74, 120.43, 127.14, 127.77, 130.44, 130.51, 155.64.
(E)-(4-(3-azidoprop-1-enyl)phenoxy)(tert-butyl)dimethylsilane (20)
Compound 19 (0.300 g, 1.13 mmols) was dissolved in 10 mL of dry THF followed by the addition of triethylamine (0.172 g, 1.7 mmol). Methanesulfonyl chloride (0.195 g, 1.7 mmols) was added drop wise to the reaction and the reaction was stirred at room temperature for 3 h. The reaction was then quenched by the addition of 20 mL H2O and the resulting solution was extracted with ethyl acetate (3 × 25mL). The combined organic layers were then washed with brine and dried over anhydrous Na2SO4. The organic layer was evaporated in vacuuo to obtain the crude product which was used immediately in the next reaction.
The crude mesylated compound was dissolved in dry DMF and sodium azide (0.067 g, 1.64 mmol) was added. The reaction was stirred at room temperature for 5h and then poured into 50 mL H2O to generate a white, cloudy solution. The solution was ethyl acetate (3 × 50mL) and the combined organic layers were washed with brine and dried over anhydrous Na2SO4. The organic layer was evaporated in vacuuo to dryness and the crude product was purified by flash silica column chromatography (1:9 ethyl acetate:hexanes) to give 0.25 g (82%) of the final product. 1H NMR (400 MHz, CDCl3) δ 0.22 (s, 6H, CH3), 1.01 (s, 9H, tBu) 3.93 (d, J = 6.8Hz, 2H, CH2), 6.12 (quintet, J = 16Hz, 1H, CH=CH), 6.60 (d, J = 16Hz, 1H, CH=CH ), 6.83 (d, J = 8.4Hz, 2H, Ph), 7.30 (d, J = 8.4Hz, 2H, Ph); 13C NMR (100 MHz, CDCl3) δ -4.17, 18.47, 53.45, 120.40, 120.52, 128.08, 129.53, 134.58, 156.12.
(E)-3-(4-(tert-butyldimethylsilyloxy)phenyl)prop-2-en-1-amine (5)
Compound 20 (0.200 g, 0.69 mmols) was dissolved in 8 mL of ethanol and Lindlar’s catalyst (Pd/CaCO3, 0.043 g, 0.21 mmol) was added to the solution. The reaction was stirred under 1 atm of H2 for 5 h, filtered through celite and evaporated to dryness in vacuuo. The resulting product (0.17 g, 93%) was used without any additional purification. 1H NMR (400 MHz, CD2Cl2) δ 0.19 (s, 6H, CH3), 0.98 (s, 9H, tBu), 1.19 (br, 2H, NH2), 3.40 (d, J = 5.6Hz, 2H, CH2), 6.19 (quintet, J = 15.6Hz, 1H, CH=CH), 6.42 (d, J = 16Hz, 1H, CH=CH), 6.78 (d, J = 8.4Hz, 2H, Ph), 7.25 (d, J = 8.4Hz, 2H, Ph); 13C NMR (100 MHz, CDCl3) δ -4.54, 18.29, 25.63, 44.50, 120.35, 127.41, 128.68, 130.12, 130.99, 155.27.
((E)-3-(4-methoxyphenyl)acrylonitrile (22)
A mixture of 4-iodo anisole (21) (20 g, 85.46 mmols), acrylonitrile (18.14 g, 342 mmols), Pd(OAc)2 (1.92 g, 8.55 mmols), Bu4NBr (5.51 g, 17.1 mmols) and NaHCO3 (14.36 g, 171 mmols) were vigorously stirred in 80 mL of distilled water at 80 °C for 10 hrs under argon. The reaction mixture was cooled to room temperature and extracted with diethyl ether (3 × 150 mL). The combined organic layer was washed with distilled water, brine and then dried over anhydrous Na2SO4. The organic layer was evaporated in vacuuo and the resulting material purified by silica column chromatography (7:3 CH2Cl2 : hexane) yield 4.89 g (48%) of the product as a white solid. 1H NMR (400 MHz, CD2Cl2) δ 3.83, (s, 3H, CH3), 5.75, (d, J = 16.8Hz, 1H, CH=CH), 6.92, (d, J = 8.8Hz, 2H, Ph), 7.35, (d, J = 16.4Hz, 1H, CH=CH), 7.42, (d, J = 8.4 Hz, 2H, Ph); 13C NMR (100 MHz, CD2Cl2) δ 30.24, 93.91, 114.97, 119.2, 126.93, 129.61, 150.43, 162.64.
(E)-3-(4-methoxyphenyl)prop-2-en-1-amine (6)
Lithium aluminum hydride (0.751 g, 19.79 mmols) was added to 25 mL of anhydrous ethyl ether and the resulting solution was stirred at room temperature for 20 min. To a solution of the reductant, 22 (3.15g, 19.79 mmols), dissolved in 15 mL of anhydrous ethyl ether, was added drop wise over a 10 min period. The reaction was stirred at room temperature for an additional 30 min and then was quenched by the addition of (2.5 mL) of15% NaOH. The reaction was extracted with ethyl ether (3 × 100mL) and the combined organic layers were washed with brine before being dried with anhydrous Na2SO4. The organic layer was evaporated in vacuuo and the resulting crude residue was purified by flash silica column chromatography (1:10:89 NH4OH: CH3OH: CH2Cl2)to generate the desired pure product (1.95 g, 61%) in good yield. 1H NMR (400 MHz, CDCl3) δ 1.40, (s, 2H, br, NH2), 3.47, (s, 2H, br, CH2), 3.81, (s, 3H, CH3) 6.17–6.22, (quintet, J = 20 Hz, 1H, CH=CH), 6.45, (d, J = 16 Hz, 1H, CH=CH), 6.85, (d, J = 8.4 Hz, 2H, Ph), 7.31, (d, J = 8.4 Hz, 2H, Ph); 13C NMR (100 MHz, CDCl3) δ 29.93, 44.64, 55.51, 114.2, 127.58, 129.16, 130.2, 159.22.
MTS assay
The MTS assay was performed using the Cell Titer 96 aqueous cell proliferation assay (Promega). Fibroblast cells derived from a NIH Swiss mouse embryo (NIH-3T3) were obtained from ATCC and maintained in Dulbecco's Modified Eagles Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The cells were grown to 3 passages and approximately 10,000 cells were seeded into each well of a 96-well plate and allowed to incubate overnight to allow cells to attach to the substrate. Individual wells were treated with various concentrations of compounds 1–4 (10, 25, 50 and 100 mg/L in DMSO) in 100 µL of DMEM/FBS media and incubated for 24 h. To analyze the viability of the cells, the media was removed and replaced with 100 µL of fresh DMEM media and 20 µL of MTS reagent solution. The resulting assay mixture was incubated for 1 h at 37 °C in a CO 2 incubator and the absorbance of each well was measured at 490 nm. The assay was conducted in four replicants using DMEM/FBS media as negative control and 5% DMSO as positive control. The results are expressed as the mean percentage of cell viability relative to untreated cells. LD50 values were determined by Prism software using non-linear regression.
Hemolysis assay
Sheep whole blood (Lampire Biological Laboratories, 1 mL) was centrifuged at 3000 rpm for 10 min and the seperated plasma and white blood cells were discarded. The remaining red blood cells were washed twice with 2–3 mL of TBS buffer (10 mM Tris, 150 mM NaCl, pH 7.0)and diluted to 1% in TBS. The hemolysis assay was performed, in duplicate, in a 96-well microplate plate using DMSO as the negative control and 1% Triton-X as the positive control. In each well, 120 µL of the 1% red blood cell stock was treated with increasing concentrations of 1 or 2, dissolved in DMSO) and the final volume was adjusted to 150 µL by the addition of TBS. The plate was incubated at 37 °C for 1 h.. After incubation, the plate was centrifuged at 3800 rpm for 5 min to collect the red blood cells and the supernantant was analyzed by diluting 20 µL of the supernatent with TBS buffer until the final volume reached 120 µL. The absorbance at 414 nm was measure to determine the release of hemoglobin. The absorbance of negative control solution was set to 0% hemolysis while the absorbance of the 1% Triton X was set to 100% hemolysis.
Antibacterial activity
Eight bacterial strains (five Gram-positive and 3 Gram-negative pathogens) were selected from the Anti-Infective Research Laboratory collection. Tested isolates included 3 Staphylococcus aureus (VISA Mu50, MRSA 494, and VRSA MI) strains,Streptococcus pneumoniae (ATCC 49619), Enterococcus faecium (VRE 7303), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Enterobacter aerogenes (CEF 3978) and Klebsiella pneumoniae (CEF 3978). Antibacterial activity of the thieno[2,3-d]pyrimidinedione compounds 1–4, 11a, 16a, and 16b was evaluated by determining minimum inhibitory concentration (MIC) values using the broth microdilution method [34] Briefly, strains were grown overnight at 35°C on Tryptic soy agar plates (TSA, Difco, Detroit, MI). Bacterial inocula (0.5 Mac Farland, i.e. 108 CFU/mL) were prepared by suspending few colonies grown onto a TSA plate in sterile normal saline solution. Resulted bacterial suspensions were diluted in Mueller-Hinton broth (MHB, Difco, Detroit, MI) in order to reach an inoculum of 1–2 × 106 CFU/mL. A volume of 100 µL of this inoculum (final titer 5 × 105 CFU/mL) was added to 100 µL of twofold serial dilutions of compounds 1–4 (final concentrations of 0.06 to 32 mg/L) in Mueller-Hinton broth. Inoculated plates were incubated at 35°C for 18–24h, after which the MIC values were visually read as the lowest concentration of compound that prevented bacterial growth.
Four unique thieno[2,3-d]pyrimidinediones were synthesized by a unique route
Two compounds displayed potent activity against antibiotic-resistant Gram-positive bacteria
One compound was selectively cytotoxic to bacteria and displayed no hemolytic activity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Appelbaum PC. MRSA - the tip of the iceberg. Clin Microbiol Infec. 2006;12:3–10. doi: 10.1111/j.1469-0691.2006.01402.x. [DOI] [PubMed] [Google Scholar]
- 2.Bishai WR, Zetola N, Francis JS, Nuermberger EL. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005;5:275–286. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- 3.Williams DH, Bardsley B. The vancomycin group of antibiotics and the fight against resistant bacteria. Angew Chem Int Edit. 1999;38:1173–1193. doi: 10.1002/(SICI)1521-3773(19990503)38:9<1172::AID-ANIE1172>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406:775–781. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- 5.Ashalatha BV, Narayana B, Raj KKV, Kumari NS. Synthesis of some new bioactive 3-amino-2-mercapto-5,6,7,8-tetrahydro 1 benzothieno 2,3-d pyrimidin-4(3H)-one derivatives. European Journal of Medicinal Chemistry. 2007;42:719–728. doi: 10.1016/j.ejmech.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Rashad AE, Shamroukh AH, Abdel-Megeid RE, El-Sayed WA. Synthesis, Reactions, and Antimicrobial Evaluation of Some Polycondensed Thienopyrimidine Derivatives. Synthetic Communications. 2010;40:1149–1160. [Google Scholar]
- 7.Chambhare RV, Khadse BG, Bobde AS, Bahekar RH. Synthesis and preliminary evaluation of some N-[5-(2-furanyl)-2-methyl-4-oxo-4H-thieno[2,3-d]pyrimidin-3-yl]-carboxamide and 3-substituted-5-(2-furanyl)-2-methyl-3H-thieno[2,3-d]pyrimidin-4-ones as antimicrobial agents. European Journal of Medicinal Chemistry. 2003;38:89–100. doi: 10.1016/s0223-5234(02)01442-3. [DOI] [PubMed] [Google Scholar]
- 8.Elsherbeny MA, Elashmawy MB, Elsubbagh HI, Elemam AA, Badria FA. SYNTHESIS, ANTIMICROBIAL AND ANTIVIRAL EVALUATION OF CERTAIN THIENOPYRIMIDINE DERIVATIVES. European Journal of Medicinal Chemistry. 1995;30:445–449. [Google Scholar]
- 9.Rashad AE, Ali MA. Synthesis and antiviral screening of some thieno 2,3-d pyrimidine nucleosides. Nucleosides Nucleotides & Nucleic Acids. 2006;25:17–28. doi: 10.1080/15257770500377730. [DOI] [PubMed] [Google Scholar]
- 10.Alagarsamy V, Meena S, Ramseshu KV, Solomon VR, Thirumurugan K, Dhanabal K, Murugan M. Synthesis, analgesic, anti-inflammatory, ulcerogenic index and antibacterial activities of novel 2-methylthio-3-substituted-5,6,7,8-tetrahydrobenzo(b)thieno 2,3-d pyrimi din-4(3H)-ones. European Journal of Medicinal Chemistry. 2006;41:1293–1300. doi: 10.1016/j.ejmech.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Deng JF, Peng L, Zhang GC, Lan XB, Li CF, Chen FX, Zhou YY, Lin ZX, Chen L, Dai RK, Xu HJ, Yang L, Zhang XQ, Hu WH. The highly potent and selective dipeptidyl peptidase IV inhibitors bearing a thienopyrimidine scaffold effectively treat type 2 diabetes. European Journal of Medicinal Chemistry. 2011;46:71–76. doi: 10.1016/j.ejmech.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Horiuchi T, Nagata M, KitagawaB M, Akahane K, Uoto K. Discovery of novel thieno 2,3-d pyrimidin-4-yl hydrazone-based inhibitors of Cyclin D1-CDK4: Synthesis, biological evaluation and structure-activity relationships. Part 2. Bioorganic & Medicinal Chemistry. 2009;17:7850–7860. doi: 10.1016/j.bmc.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Aly AA, Brown AB, Ramadan M, Gamal-Eldeen AM, Abdel-Aziz M, Abuo-Rahma GE-DAA, Radwan MF. Thieno 2,3-d pyrimidines in the Synthesis of Antitumor and Antioxidant Agents. Archiv Der Pharmazie. 2010;343:301–309. doi: 10.1002/ardp.200900245. [DOI] [PubMed] [Google Scholar]
- 14.Al-Taisan KM, Al-Hazimi HMA, Al-Shihry SS. Synthesis, Characterization and Biological Studies of Some Novel Thieno 2,3-d pyrimidines. Molecules. 2010;15:3932–3957. doi: 10.3390/molecules15063932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rashad AE, Shamroukh AH, Abdel-Megeid RE, Mostafa A, El-Shesheny R, Kandeil A, Ali MA, Banert K. Synthesis and screening of some novel fused thiophene and thienopyrimidine derivatives for anti-avian influenza virus (H5N1) activity. European Journal of Medicinal Chemistry. 2010;45:5251–5257. doi: 10.1016/j.ejmech.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 16.Patil VD, Wise DS, Townsend LB. THE SYNTHESIS OF THIENO 2,3-D PYRIMIDINE NUCLEOSIDES RELATED TO THE NATURALLY-OCCURRING NUCLEOSIDES CYTIDINE AND URIDINE. Journal of the Chemical Society-Perkin Transactions. 1980;1:1853–1858. [Google Scholar]
- 17.Sabnis RW. The Gewald synthesis. Sulfur Reports. 1994;16:1–17. [Google Scholar]
- 18.Sabnis RW, Rangnekar DW, Sonawane ND. 2-aminothiophenes by the Gewald reaction. Journal of Heterocyclic Chemistry. 1999;36:333–345. [Google Scholar]
- 19.Bhattacharyya T, Sundin A, Nilsson UJ. Synthesis of chiral, amphiphilic, and water-soluble macrocycles via urea formation. Tetrahedron. 2003;59:7921–7928. [Google Scholar]
- 20.Patil VD, Wise DS, Townsend LB. The Synthesis of Thieno[2,3-D]Pyrimidine Nucleosides Related to the Naturally-Occurring Nucleosides Cytidine and Uridine. Journal of the Chemical Society-Perkin Transactions. 1980;1:1853–1858. [Google Scholar]
- 21.Cimino G, Gavagnin M, Sodano G, Spinella A, Strazzullo G, Schmitz FJ, Yalamanchili G. Revised Structure of Bursatellin. J Org Chem. 1987;52:2301–2303. [Google Scholar]
- 22.Corey EJ, Nicolaou KC, Balanson RD, Machida Y. Useful method for the conversion of azides to amines. Synthesis. 1975:590–591. [Google Scholar]
- 23.Matsuno M, Nagatsu A, Ogihara Y, Mizukami H. Synthesis of 2-O-(4-coumaroyl)-3-(4-hydroxyphenyl)lactic acid, an important intermediate of rosmarinic acid biosynthesis. Chem Pharm Bull. 2001;49:1644–1646. doi: 10.1248/cpb.49.1644. [DOI] [PubMed] [Google Scholar]
- 24.Servais A, Azzouz M, Lopes D, Courillon C, Malacria M. Radical cyclization of N-acylcyanamides: total synthesis of luotonin A. Angewandte Chemie, International Edition. 2007;46:576–579. doi: 10.1002/anie.200602940. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchida S, Kaneshige A, Ogata T, Baba H, Yamamoto Y, Tomioka K. Consecutive cyclization of allylaminoalkene by intramolecular aminolithiation-carbolithiation. Org Lett. 2008;10:3635–3638. doi: 10.1021/ol801397v. [DOI] [PubMed] [Google Scholar]
- 26.Young SD, Payne LS, Thompson WJ, Gaffin N, Lyle TA, Britcher SF, Graham SL, Schultz TH, Deana AA, Darke PL, Zugay J, Schleif WA, Quintero JC, Emini EA, Anderson PS, Huff JR. Hiv-1 Protease Inhibitors Based on Hydroxyethylene Dipeptide Isosteres - an Investigation into the Role of the P1' Side-Chain on Structure Activity. J Med Chem. 1992;35:1702–1709. doi: 10.1021/jm00088a004. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Cai MZ, Peng CY. Stereoselective synthesis of (E)-cinnamonitriles via Heck arylation of acrylonitrile and aryl iodides in water. Synthetic Communications. 2002;32:3419–3423. [Google Scholar]
- 28.Dumas J, Sibley R, Wood J. Preparation of thienopyrimidines as inhibitors of prolylpeptidase, inducers of apoptosis and cancer treatment agents, in, (Bayer Corporation, USA). Application: WO, ppf. 2003. p. 50. [Google Scholar]
- 29.Ekkati AR, Mandiyan V, Ravindranathan KP, Bae JH, Schlessinger J, Jorgensen WL. Aryl extensions of thienopyrimidinones as fibroblast growth factor receptor 1 kinase inhibitors. Tetrahedron Lett. 2011;52:2228–2231. doi: 10.1016/j.tetlet.2010.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClellan WJ, Dai YJ, Abad-Zapatero C, Albert DH, Bouska JJ, Glaser KB, Magoc TJ, Marcotte PA, Osterling DJ, Stewart KD, Davidsen SK, Michaelides MR. Discovery of potent and selective thienopyrimidine inhibitors of Aurora kinases. Bioorg Med Chem Lett. 2011;21:5620–5624. doi: 10.1016/j.bmcl.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 31.Ni YK, Gopalsamy A, Cole D, Hu YH, Denny R, Ipek M, Liu J, Lee J, Hall JP, Luong M, Telliez JB, Lin LL. Identification and SAR of a new series of thieno[3,2-d]pyrimidines as Tpl2 kinase inhibitors. Bioorg Med Chem Lett. 2011;21:5952–5956. doi: 10.1016/j.bmcl.2011.07.069. [DOI] [PubMed] [Google Scholar]
- 32.Deng Y, Zhou X, Kugel Desmoulin S, Wu J, Cherian C, Hou Z, Matherly LH, Gangjee A. Synthesis and Biological Activity of a Novel Series of 6-Substituted Thieno[2,3-d]pyrimidine Antifolate Inhibitors of Purine Biosynthesis with Selectivity for High Affinity Folate Receptors over the Reduced Folate Carrier and Proton-Coupled Folate Transporter for Cellular Entry. J. Med. Chem. 2009;52:2940–2951. doi: 10.1021/jm8011323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng YJ, Zhou XL, Desmoulin SK, Wu JM, Cherian C, Hou ZJ, Matherly LH, Gangjee A. Synthesis and Biological Activity of a Novel Series of 6-Substituted Thieno[2,3-d]pyrimidine Antifolate Inhibitors of Purine Biosynthesis with Selectivity for High Affinity Folate Receptors over the Reduced Folate Carrier and Proton-Coupled Folate Transporter for Cellular Entry. J Med Chem. 2009;52:2940–2951. doi: 10.1021/jm8011323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clinical Laboratory and Standard Institute. “Methods for Dilution Antimicrobial susceptibility Tests for bacteria that grow Aerobically; Approved Standard. 9th ed. Wayne, PA: CLSI.”. 2010. [Google Scholar]