Abstract
Because multiple symptoms associated with “sickness behavior” have a negative impact on functional status and quality of life, increased information on the mechanisms that underlie inter-individual variability in this symptom experience is needed. The purposes of this study were to determine: if distinct classes of individuals could be identified based on their experience with pain, fatigue, sleep disturbance, and depression; if these classes differed on demographic and clinical characteristics; and if variations in pro- and anti- inflammatory cytokine genes were associated with latent class membership.
Self-report measures of pain, fatigue, sleep disturbance, and depression were completed by 168 oncology outpatients and 85 family caregivers (FCs). Using latent class profile analysis (LCPA), three relatively distinct classes were identified: those who reported low depression and low pain (83%), those who reported high depression and low pain (4.7%), and those who reported high levels of all four symptoms (12.3%). The minor allele of IL4 rs2243248 was associated with membership in the “All high” class along with younger age, being White, being a patient (versus a FC), having a lower functional status score, and having a higher number of comorbid conditions.
Findings suggest that LPCA can be used to differentiate distinct phenotypes based on a symptom cluster associated with sickness behavior. Identification of distinct phenotypes provides new evidence for the role of IL4 in the modulation of a sickness behavior symptom cluster in oncology patients and their FCs.
Keywords: fatigue, pain, sleep disturbance, depression, cancer, genetics, cytokines, symptom clusters, single nucleotide polymorphism, sickness behavior
INTRODUCTION
Oncology patients and their family caregivers (FC) report experiencing, with the same frequency and severity, pain, fatigue, sleep disturbance, and depression.[1–5] While these symptoms can occur singly, they often co-occur as a cluster[6–12] and have significant deleterious effects on an individual’s functional status and quality of life (QOL).[3,9,13–17] In addition, several studies have identified distinct subgroups of individuals based on their experiences with these four symptoms.[14,17–20] Across these studies, a consistent finding was a subgroup of individuals who reported high levels of pain, fatigue, sleep disturbance, and depression. These individuals may represent a high risk group with a distinct phenotype.
Recent reviews suggest that inter-individual variability in symptom experiences may result from an individual’s genetically determined ability to respond to physical and psychological stressors through changes in pro- and anti-inflammatory cytokines.[10,21,22] In fact, in studies that induced “sickness behavior” through the administration of inflammatory agents,[23–29] individuals reported the co-occurrence of lethargy, anorexia, depression, anxiety, sleepiness, and hyperalgesia. For oncology patients and their FCs, both physical and psychosocial stressors may modulate the release of pro-inflammatory cytokines.[30] Therefore, the sickness behavior model provides a framework for evaluation of four common symptoms (i.e., pain, fatigue, sleep disturbance, and depression) in oncology patients and their FCs.[7]
The associations between common symptoms and specific pro-inflammatory cytokines were the focus of a number of studies.[31] For example, in animal models, pro-inflammatory cytokines and chemokines are associated with pain hypersensitivity.[32,33] In humans, individuals with a painful neuropathy had twofold higher mRNA levels for the pro-inflammatory cytokines, interleukin (IL)-2 and tumor necrosis factor (TNF)-α and twofold lower mRNA levels for the anti-inflammatory IL-10 than individuals with a painless neuropathy.[34] In another study, the preoperative use of pentoxifylline, which inhibits TNF-α production and leukotriene synthesis in immune cells, decreased the release of pro-inflammatory cytokines and reduced post-surgical morphine consumption in patients with colorectal cancer.[35]
In addition, in patients with a variety of cancer diagnoses, increased levels of fatigue severity were associated with increased levels of IL6 and IL1 receptor antagonists,[36] as well as higher levels of tumor growth factor (TGF-α).[37] More recently, associations between single nucleotide polymorphisms (SNPs) in the promoter regions of IL6 and TNF-α and increased levels of fatigue and sleep disturbance were shown in a sample of oncology patients and their FCs.[38,39] Additionally, a SNP in the promoter region of IL1β was found to be associated with persistent fatigue in breast cancer survivors.[40]
In terms of depressive symptoms, several studies and a meta-analysis found significant elevations in circulating levels of pro-inflammatory cytokines, particularly IL6 and TNF-α, in patients with major depression.[41–47] Furthermore, depressive behaviors and mood alterations including sadness, inability to feel, depressed mood, and suicidal ideation were observed in patients who received repeated injections of recombinant cytokines, for the treatment of autoimmune diseases, viral infections, and cancer.[48,49]
While knowledge of the mechanisms through which pro-inflammatory cytokines contribute to inter-individual variability in single symptoms continues to grow, less is known about the association between cytokines and multiple symptoms or symptom clusters.[10,11] In addition, little is known about the role of anti-inflammatory cytokines in this phenomenon. While Reyes-Gibby et al [50] advanced a hypothesis-driven, pathway-based approach that assessed the contributions of both pro- and anti-inflammatory cytokine genes to cancer symptoms, no studies have evaluated this hypothesis using a comprehensive panel of pro- and anti-inflammatory cytokine genes in participants who were categorized based on their experiences with the symptom cluster of pain, fatigue, sleep disturbance, and depression. Therefore, the purposes of this study were to determine if distinct latent classes of oncology patients and their FCs could be identified based on their experience with the symptom cluster of pain, fatigue, sleep disturbance, and depression and to determine if these classes differed on demographic and clinical characteristics. In addition, the study sought to determine if genetic variations in a number of pro- and anti- inflammatory cytokines were associated with latent class membership.
METHODS
Participants and Settings
This descriptive, correlational study is part of a larger, longitudinal study that evaluated multiple symptoms in both patients who underwent primary or adjuvant radiotherapy (RT) and their FCs. Although it is difficult to determine when a family member assumes the role of a caregiver, in most studies of symptoms in FCs, the caregiver role is linked to the trajectory of the patient’s treatment. Therefore, to obtain a “baseline” assessment of symptoms, FCs were recruited with patients before the initiation of RT. Patients and their FCs were recruited from two RT departments located in a Comprehensive Cancer Center and a community-based oncology program at the time of the patient’s simulation visit.
Patients were eligible to participate if they: were ≥18 years of age; were scheduled to receive primary or adjuvant RT for one of four cancer diagnoses (i.e., breast, prostate, lung, brain); were able to read, write, and understand English; gave written informed consent; and had a Karnofsky Performance Status (KPS) score of ≥ 60. Patients were excluded if they had: metastatic disease; more than one cancer diagnosis; or a diagnosed sleep disorder.
FCs were eligible to participate if they: were an adult (≥18 years of age); were able to read, write, and understand English; gave written informed consent; had a KPS ≥ 60; were living with the patient; and did not have a diagnosed sleep disorder.
Instruments
A demographic questionnaire obtained information on age, gender, marital status, living arrangements, education, ethnicity, employment status, and the presence of a number of comorbid conditions.
Pain was evaluated using a modified version of the Brief Pain Inventory.[51] Participants who responded yes to the question of having pain were asked to indicate the cause of their pain and to rate its intensity (i.e., now, least, average, and worst) using 0 (no pain) to 10 (worst pain imaginable) numeric rating scales (NRS).[52]
Fatigue was evaluated using the Lee Fatigue Scale that consists of 13 items designed to assess physical fatigue.[53] Each item was rated on a 0 to 10 NRS. A total fatigue score was calculated as the mean of the 13 fatigue items, with higher scores indicating greater fatigue severity. Respondents were asked to rate each item based on how they felt “right now,” within 30 minutes of going to bed (evening fatigue). The LFS has been used with healthy individuals [53,54] and with patients with cancer and HIV.[20,55–57] A cutoff score of ≥5.6 indicates clinically significant levels of evening fatigue.[3] The LFS was chosen for this study because it is relatively short, easy to administer, and has well-established validity and reliability. In this study, Cronbach’s alphas for evening fatigue for patients and FCs were 0.96 and 0.95, respectively.
Sleep disturbance was evaluated using the General Sleep Disturbance Scale (GSDS) that consists of 21 items designed to assess the quality of sleep in the past week. Each item was rated on a 0 (never) to 7 (everyday) NRS. The GSDS total score is the sum of the 21 items that can range from 0 (no disturbance) to 147 (extreme sleep disturbance). A GSDS total score of ≥43 indicates a significant level of sleep disturbance.[3] The GSDS has well-established validity and reliability in shift workers, pregnant women, and patients with cancer and HIV.[56,58–60] In the current study, the Cronbach’s alphas for the GSDS total score for patients and FCs were 0.84 and 0.79, respectively.
Depressive symptoms were assessed using the Center for Epidemiological Studies-Depression (CES-D) scale that consists of 20 items selected to represent the major symptoms in the clinical syndrome of depression. Scores can range from 0 to 60. Scores of ≥16 are considered clinically significant and indicate the need for individuals to seek clinical evaluation for major depression. The CES-D has well-established concurrent and construct validity.[61–63] In the current study, the Cronbach’s alphas for the CES-D for patients and FCs were 0.88 and 0.84, respectively.
Study Procedures
This study was approved by the Committee on Human Research at the University of California, San Francisco and at the second site. At the time of the simulation visit (i.e., approximately one week prior to the initiation of RT), patients were approached by a research nurse to discuss participation in the study. After obtaining written informed consent, patients completed the study questionnaires. After recruitment, patients were asked to identify the person most involved in their care (i.e., their FC). If the FC was with the patient, the research nurse explained the study and obtained written informed consent from the FC. FCs who were not with the patient were contacted by phone to determine their interest in study participation. The research nurse visited those FCs at home, obtained written informed consent, and had FCs complete the study questionnaires. Data from these enrollment questionnaires were used in the subsequent analyses. Both patients and FCs had blood drawn for genomic analyses. In addition, patients’ medical records were reviewed for disease and treatment information.
Methods of Analysis for Clinical Data
Descriptive statistics and frequency distributions were generated for the sample characteristics and symptom data. All calculations used actual values. Adjustments were not made for missing data or multiple testing.[64] Therefore, the cohort for each analysis was dependent on the largest set of available data across groups. A p-value of <.05 is considered statistically significant.
Latent class analysis (LCA; sometimes called latent class cluster analysis), a type of finite mixture model,[65,66] was used to identify participants with similar experiences with the symptom cluster of pain, fatigue, sleep disturbance, and depression (i.e., latent classes). Conceptually similar to cluster analysis,[67] LCA identifies latent classes based on an observed response pattern.[68,69]
LCA has several advantages over cluster analysis. LCA is model-based and generates probabilities for group membership. In addition, statistical fit indices are used to assess model fit and to determine the number of classes. The final number of latent classes is identified by evaluating the Bayesian Information Criterion (BIC), the Vuong-Lo-Mendell-Rubin likelihood ratio test (VLMR), the parametric bootstrapped likelihood ratio test (BLRT), and entropy (the consistency between model-based latent classes and the classes to which observations are assigned). The model that fits the data best has the lowest BIC and a VLMR and/or BLRT that indicates that the estimated model is a better fit than the model with one fewer class.[70] In addition, better-fitting models should produce higher entropy values.[71] Well fitting models have loglikelihood values that are replicated in analyses with multiple “random starts,” which indicates that the solution is not based on a local maximum for the loglikelihood. Finally, well-fitting models “make sense” conceptually and the estimated classes differ as might be expected on variables not used in the generation of the model.[70]
Latent class models often use categorical variables.[72,73] However, as in this study, when continuous variables are analyzed (i.e., pain fatigue, sleep disturbance, and depression scores), LCA is called latent class profile analysis (LCPA). However, in this study one of the continuous variables, namely “worst pain,” which was reported on a 0 to 10 NRS, had a large number of zeros because a number of the participants did not report any pain. Therefore, the number of zeros was accommodated by modeling worst pain as a “two part” variable. In this type of model, the variable is examined with one “part” representing the difference between those who reported no pain compared to those who reported any pain, and with the second “part” differentiating among those who reported any pain on the remaining portion of the NRS (i.e., the 1 to 10 part of the NRS).[74,75]
An additional consideration in this analysis was that 65% of the participants were in patient-caregiver dyads. Although no differences were found in the severity of symptoms between patients and FCs within dyads, we chose to accommodate even minor dependency due to dyads by carrying out our analyses, treating the sample as a “complex” sample, clustered by dyads. Due to the small number of respondents within clusters (only one for singletons and two for dyads); because no differences were found between the models with and without the inclusion of the dyadic term; and because dyads constituted only 65% of the sample, the results of the LCPA are reported without dyadic status.
The LCPA was performed using Mplus™ Version 6.[75,76] Estimation was carried out with robust Maximum-Likelihood (MLR) and the Expectation- Maximization (EM) algorithm.[65] Due to the inclusion of a categorical variable (i.e., the binary variable for the occurrence of pain versus no pain), Gauss-Hermite adaptive numerical integration with 20 integration points was employed. Subsequent analyses of differences among the identified classes were carried out with SPSS Version 18 for Windows™.[77]
Methods of Analysis for Genomic Data
Gene Selection
Cytokines and their receptors are classes of polypeptides that exercise a major influence on the inflammatory process. Their dysregulation is hypothesized to induce symptoms associated with “sickness behavior”.[22,78] These polypeptides are divided into pro- and anti-inflammatory cytokines. Pro-inflammatory cytokines promote systemic inflammation and include: interferon (IFN) gamma 1 receptor (IFNGR1), IL1R1, IL2, IL8, IL17A, nuclear factor kappa beta (NFKB1), NFKB2, and TNF-α Anti-inflammatory cytokines suppress the activity of pro-inflammatory cytokines and include: IL1R2, IL4, IL10, and IL13. Of note, INFG1, IL1β, and IL6 possess pro- and anti-inflammatory functions.[31]
Blood collection and genotyping
Genomic DNA was extracted from archived buffy coats maintained by the UCSF Genomic Markers of Symptoms Tissue Bank using the PUREGene DNA Isolation System (Invitrogen, Carlsbad, CA). Of the 287 participants recruited, DNA could be recovered from the archived buffy coats of 253 (i.e., 168 patients and 85 FCs). No differences were found in any demographic and clinical characteristics between participants who did and did not choose to participate in the study or in those participants for whom DNA could not be recovered from archived specimens.
Genotyping was performed blinded to clinical status and positive and negative controls were included. DNA samples were quantitated with a Nanodrop Spectrophotometer (ND-1000) and normalized to a concentration of 50 ng/µL (diluted in 10 mM Tris/1 mM EDTA). Samples were genotyped using the GoldenGate genotyping platform (Illumina, San Diego, CA) and processed according to the standard protocol using GenomeStudio (Illumina, San Diego, CA). Signal intensity profiles and resulting genotype calls for each SNP were visually inspected by two blinded reviewers. Disagreements were adjudicated by a third reviewer.
SNP Selection
Because no studies of the association between a specific symptom cluster and candidate genes has evaluated a comprehensive panel of pro-and anti-inflammatory cytokine genes, a combination of tagging SNPs and literature driven SNPs (i.e., SNPs reported as being associated with altered function and/or symptoms) were selected for analysis. Tagging SNPs were required to be common (defined as having a minor allele frequency ≥ 0.05) in public databases (e.g., HapMap). In order to ensure robust genetic association analyses, quality control filtering of SNPs was performed. SNPs with call rates of <95% or Hardy-Weinberg p-values of <.001 were excluded.
As shown in Table 1, a total of 104 SNPs among the 15 candidate genes (IFNG1: 6 SNPs, IFNGR1: 1SNP; IL1β: 12 SNPs; IL1R1: 5 SNPs; IL1R2: 3 SNPs; IL2: 5 SNPs; IL4: 9 SNPs; IL6: 12 SNPs; IL8: 3 SNPs; IL10: 8 SNPs; IL13: 5 SNPs; IL17A: 6 SNPs; NFKB1: 15 SNPs; NFKB2: 4 SNPs; TNF-α: 10 SNPs) passed all quality control filters and were included in the genetic association analyses. Potential functional roles of SNPs associated with specific symptoms were examined using PUPASuite 2.0,[79] a comprehensive search engine that tests a series of functional effects (i.e., nonsynonymous changes, altered transcription factor binding sites, exonic splicing enhancing or silencing, splice site alterations, microRNA target alterations).
Table 1.
Summary of Single Nucleotide Polymorphisms Analyzed for Pro- and Anti-inflammatory Cytokine Genes
| Gene | SNP* | Position | Chr | MAF | Alleles | Chi square |
p-value | Model |
|---|---|---|---|---|---|---|---|---|
| IFNG1 | rs2069728 | 66834051 | 12 | .079 | G>A | 5.70 | .222 | A |
| IFNG1 | rs2069727 | 66834490 | 12 | .411 | A>G | 4.76 | .313 | A |
| IFNG1 | rs2069718 | 66836429 | 12 | .442 | C>T | 0.41 | .982 | A |
| IFNG1 | rs1861493 | 66837463 | 12 | .264 | A>G | 1.38 | .848 | A |
| IFNG1 | rs1861494 | 66837676 | 12 | .279 | T>C | 1.53 | .821 | A |
| IFNG1 | rs2069709 | 66839970 | 12 | .008 | G>T | 0.78 | .677 | A |
| IFNG1 | HapA3 | n/a | 12 | 1.38 | .848 | |||
| IFNG1 | HapA5 | n/a | 12 | 4.76 | .313 | |||
| IFNGR1 | rs9376268 | 137574444 | 6 | .246 | G>A | 1.70 | .719 | A |
| IL1B | rs1071676 | 106042060 | 2 | .198 | G>C | 5.48 | .242 | A |
| IL1B | rs1143643 | 106042929 | 2 | .331 | G>A | 4.97 | .290 | A |
| IL1B | rs1143642 | 106043180 | 2 | .095 | C>T | 2.40 | .663 | A |
| IL1B | rs1143634 | 106045017 | 2 | .196 | C>T | 5.37 | .252 | A |
| IL1B | rs1143633 | 106045094 | 2 | .345 | G>A | 3.33 | .505 | A |
| IL1B | rs1143630 | 106046282 | 2 | .103 | C>A | 0.67 | .955 | A |
| IL1B | rs3917356 | 106046990 | 2 | .432 | A>G | 5.64 | .228 | A |
| IL1B | rs1143629 | 106048145 | 2 | .353 | T>C | 5.81 | .214 | A |
| IL1B | rs1143627 | 106049014 | 2 | .390 | T>C | 3.12 | .538 | A |
| IL1B | rs16944 | 106049494 | 2 | .380 | G>A | 4.15 | .386 | A |
| IL1B | rs1143623 | 106050452 | 2 | .248 | G>C | 4.69 | .321 | A |
| IL1B | rs13032029 | 106055022 | 2 | .428 | C>T | 5.41 | .248 | A |
| IL1B | HapA1 | n/a | 1.49 | .829 | ||||
| IL1B | HapA3 | n/a | 2.16 | .339 | ||||
| IL1B | HapA4 | n/a | 5.03 | .284 | ||||
| IL1B | HapA5 | n/a | 5.38 | .251 | ||||
| IL1B | HapB1 | n/a | 3.16 | .531 | ||||
| IL1B | HapB7 | n/a | 4.01 | .405 | ||||
| IL1B | HapB9 | n/a | 5.50 | .240 | ||||
| IL1B | HapB11 | n/a | 0.47 | .976 | ||||
| IL1R1 | rs949963 | 96533648 | 2 | .213 | G>A | 2.28 | .685 | A |
| IL1R1 | rs2228139 | 96545511 | 2 | .066 | C>G | 8.75 | .013 | R |
| IL1R1 | rs3917320 | 96556738 | 2 | .068 | A>C | 2.45 | .294 | A |
| IL1R1 | rs2110726 | 96558145 | 2 | .333 | C>T | 5.06 | .281 | A |
| IL1R1 | rs3917332 | 96560387 | 2 | .124 | T>A | 1.22 | .875 | A |
| IL1R2 | rs4141134 | 96370336 | 2 | .401 | T>C | 3.09 | .543 | A |
| IL1R2 | rs11674595 | 96374804 | 2 | .233 | T>C | 2.10 | .718 | A |
| IL1R2 | rs7570441 | 96380807 | 2 | .393 | G>A | 2.74 | .602 | A |
| IL1R2 | HapA1 | n/a | 7.69 | .104 | ||||
| IL1R2 | HapA2 | n/a | 7.31 | .120 | ||||
| IL1R2 | HapA4 | n/a | 2.93 | .569 | ||||
| IL2 | rs1479923 | 119096993 | 4 | .302 | C>T | 1.64 | .802 | A |
| IL2 | rs2069776 | 119098582 | 4 | .244 | T>C | 3.03 | .552 | A |
| IL2 | rs2069772 | 119099739 | 4 | .238 | A>G | 6.75 | .150 | A |
| IL2 | rs2069777 | 119103043 | 4 | .054 | C>T | 3.94 | .139 | A |
| IL2 | rs2069763 | 119104088 | 4 | .287 | T>G | 3.29 | .511 | A |
| IL2 | HapA1 | n/a | 8.23 | .084 | ||||
| IL2 | HapA2 | n/a | 6.81 | .146 | ||||
| IL2 | HapA3 | n/a | 2.93 | .570 | ||||
| IL2 | HapA5 | n/a | 1.64 | .802 | ||||
| IL4 | rs2243248 | 127200946 | 5 | .101 | T>G | 9.85 | .0073>1 | D |
| IL4 | rs2243250 | 127201455 | 5 | .260 | C>T | 1.62 | .806 | A |
| IL4 | rs2070874 | 127202011 | 5 | .219 | C>T | 2.58 | .631 | A |
| IL4 | rs2227284 | 127205027 | 5 | .399 | C>A | 8.09 | .088 | A |
| IL4 | rs2227282 | 127205481 | 5 | .401 | C>G | 8.01 | .091 | A |
| IL4 | rs2243263 | 127205601 | 5 | .124 | G>C | 1.20 | .549 | A |
| IL4 | rs2243266 | 127206091 | 5 | .203 | G>A | 2.85 | .583 | A |
| IL4 | rs2243267 | 127206188 | 5 | .205 | G>C | 2.86 | .581 | A |
| IL4 | rs2243274 | 127207134 | 5 | .262 | G>A | 0.50 | .974 | A |
| IL4 | HapA1 | n/a | 5.78 | .216 | ||||
| IL4 | HapA10 | n/a | 2.71 | .608 | ||||
| IL6 | rs4719714 | 22643793 | 7 | .196 | A>T | 3.91 | .419 | A |
| IL6 | rs2069827 | 22648536 | 7 | .071 | G>T | 1.35 | .853 | A |
| IL6 | rs1800796 | 22649326 | 7 | .095 | G>C | 5.55 | .235 | A |
| IL6 | rs1800795 | 22649725 | 7 | .355 | C>G | 5.34 | .254 | A |
| IL6 | rs2069835 | 22650951 | 7 | .066 | T>C | 0.98 | .613 | A |
| IL6 | rs2066992 | 22651329 | 7 | .091 | G>T | 6.76 | .149 | A |
| IL6 | rs2069840 | 22651652 | 7 | .308 | C>G | 0.87 | .929 | A |
| IL6 | rs1554606 | 22651787 | 7 | .405 | T>G | 3.54 | .472 | A |
| IL6 | rs2069845 | 22653229 | 7 | .405 | G>A | 3.54 | .472 | A |
| IL6 | rs2069849 | 22654236 | 7 | .039 | C>T | 8.46 | .076 | A |
| IL6 | rs2069861 | 22654734 | 7 | .083 | C>T | 2.77 | .251 | A |
| IL6 | rs35610689 | 22656903 | 7 | .242 | A>G | 3.56 | .469 | A |
| IL6 | HapA4 | n/a | 0.85 | .932 | ||||
| IL6 | HapA6 | n/a | 5.62 | .229 | ||||
| IL8 | rs4073 | 70417508 | 4 | .498 | T>A | 3.36 | .500 | A |
| IL8 | rs2227306 | 70418539 | 4 | .366 | C>T | 7.20 | .0272>1 | R |
| IL8 | rs2227543 | 70419394 | 4 | .374 | C>T | 6.44 | .0402>1 | R |
| IL8 | HapA1 | n/a | 1.30 | .861 | ||||
| IL8 | HapA3 | n/a | 8.93 | .063 | ||||
| IL8 | HapA4 | n/a | 3.36 | .500 | ||||
| IL10 | rs3024505 | 177638230 | 1 | .138 | C>T | 6.00 | .199 | A |
| IL10 | rs3024498 | 177639855 | 1 | .236 | A>G | 2.36 | .670 | A |
| IL10 | rs3024496 | 177640190 | 1 | .459 | T>C | 1.06 | .901 | A |
| IL10 | rs1878672 | 177642039 | 1 | .452 | G>C | 1.12 | .891 | A |
| IL10 | rs3024492 | 177642438 | 1 | .207 | A>T | 4.44 | .350 | A |
| IL10 | rs1518111 | 177642971 | 1 | .267 | G>A | 3.24 | .519 | A |
| IL10 | rs1518110 | 177643187 | 1 | .267 | G>T | 3.24 | .519 | A |
| IL10 | rs3024491 | 177643372 | 1 | .448 | T>G | 0.69 | .952 | A |
| IL10 | HapA5 | n/a | 1.08 | .898 | ||||
| IL10 | HapA6 | n/a | 3.51 | .476 | ||||
| IL10 | HapA8 | n/a | 3.89 | .421 | ||||
| IL10 | HapA9 | n/a | 5.95 | .203 | ||||
| IL13 | rs1881457 | 127184713 | 5 | .192 | A>C | 1.84 | .766 | A |
| IL13 | rs1800925 | 127185113 | 5 | .227 | C>T | 2.31 | .678 | A |
| IL13 | rs2069743 | 127185579 | 5 | .021 | A>G | 0.58 | .750 | A |
| IL13 | rs1295686 | 127188147 | 5 | .252 | G>A | 5.56 | .235 | A |
| IL13 | rs20541 | 127188268 | 5 | .174 | C>T | 3.53 | .473 | A |
| IL13 | HapA1 | n/a | 4.63 | .328 | ||||
| IL13 | HapA4 | n/a | 3.10 | .542 | ||||
| IL17A | rs4711998 | 51881422 | 6 | .293 | G>A | 3.20 | .526 | A |
| IL17A | rs8193036 | 51881562 | 6 | .255 | T>C | 2.93 | .569 | A |
| IL17A | rs3819024 | 51881855 | 6 | .374 | A>G | 5.92 | .205 | A |
| IL17A | rs2275913 | 51882102 | 6 | .345 | G>A | 5.12 | .275 | A |
| IL17A | rs3804513 | 51884266 | 6 | .027 | A>T | 1.28 | .865 | A |
| IL17A | rs7747909 | 51885318 | 6 | .225 | G>A | 3.69 | .450 | A |
| NFKB1 | rs3774933 | 103645369 | 4 | .444 | T>C | 1.29 | .864 | A |
| NFKB1 | rs170731 | 103667933 | 4 | .397 | T>A | 2.89 | .576 | A |
| NFKB1 | rs17032779 | 103685279 | 4 | .023 | T>C | 4.28 | .118 | A |
| NFKB1 | rs230510 | 103695201 | 4 | .366 | T>A | 5.21 | .267 | A |
| NFKB1 | rs230494 | 103706005 | 4 | .477 | A>G | 4.64 | .326 | A |
| NFKB1 | rs4648016 | 103708706 | 4 | .017 | C>T | 1.04 | .594 | A |
| NFKB1 | rs4648018 | 103709236 | 4 | .025 | G>C | 0.38 | .827 | A |
| NFKB1 | rs3774956 | 103727564 | 4 | .479 | C>T | 4.70 | .319 | A |
| NFKB1 | rs10489114 | 103730426 | 4 | .025 | A>G | 0.38 | .827 | A |
| NFKB1 | rs4648068 | 103737343 | 4 | .366 | A>G | 6.29 | .179 | A |
| NFKB1 | rs4648095 | 103746914 | 4 | .052 | T>C | 0.69 | .709 | A |
| NFKB1 | rs4648110 | 103752867 | 4 | .205 | T>A | 5.30 | .258 | A |
| NFKB1 | rs4648135 | 103755716 | 4 | .060 | A>G | 0.36 | .835 | A |
| NFKB1 | rs4648141 | 103755947 | 4 | .188 | G>A | 6.54 | .162 | A |
| NFKB1 | rs1609798 | 103756488 | 4 | .337 | C>T | 3.04 | .551 | A |
| NFKB1 | HapA1 | n/a | 5.47 | .242 | ||||
| NFKB1 | HapA9 | n/a | 2.58 | .631 | ||||
| NFKB2 | rs12772374 | 104146901 | 10 | .157 | A>G | 2.87 | .580 | A |
| NFKB2 | rs7897947 | 104147701 | 10 | .229 | T>G | 5.87 | .209 | A |
| NFKB2 | rs11574849 | 104149686 | 10 | .085 | G>A | 0.31 | .989 | A |
| NFKB2 | rs1056890 | 104152760 | 10 | .317 | C>T | 5.23 | .265 | A |
| TNFA | rs2857602 | 31533378 | 6 | .360 | T>C | 1.60 | .809 | A |
| TNFA | rs1800683 | 31540071 | 6 | .388 | G>A | 1.81 | .772 | A |
| TNFA | rs2239704 | 31540141 | 6 | .370 | G>T | 1.73 | .785 | A |
| TNFA | rs2229094 | 31540556 | 6 | .256 | T>C | 5.96 | .202 | A |
| TNFA | rs1041981 | 31540784 | 6 | .388 | C>A | 1.81 | .772 | A |
| TNFA | rs1799964 | 31542308 | 6 | .202 | T>C | 5.99 | .200 | A |
| TNFA | rs1800750 | 31542963 | 6 | .019 | G>A | 0.62 | .961 | A |
| TNFA | rs1800629 | 31543031 | 6 | .157 | G>A | 3.89 | .422 | A |
| TNFA | rs1800610 | 31543827 | 6 | .105 | C>T | 3.64 | .458 | A |
| TNFA | rs3093662 | 31544189 | 6 | .072 | A>G | 2.77 | .598 | A |
| TNFA | HapA1 | n/a | 3.91 | .419 | ||||
| TNFA | HapA5 | n/a | 4.69 | .321 | ||||
| TNFA | HapA8 | n/a | 1.75 | .783 |
A = additive model, Chr = chromosome, D = dominant model, Hap = haplotype, IFNG, interferon gamma, IL = interleukin, n/a = not applicable, MAF = minor allele frequency, R = recessive model, NFKB, nuclear factor kappa beta, SNP= single nucleotide polymorphism, TNFA = tumor necrosis factor alpha
Haplotypes are listed as the last variables for each gene.
Statistical Analyses
Allele and genotype frequencies were determined by gene counting. Hardy-Weinberg equilibrium was assessed by the Chi-square exact test. Measures of linkage disequilibrium (i.e., D’ and r2) were computed from the participants’ genotypes with Haploview 4.2. LD-based haplotype block definition was based on the D’ confidence interval method.[80]
For SNPs that were members of the same haploblock, haplotype analyses were conducted in order to localize the association signal within each gene and to determine if haplotypes improved the strength of the association with the phenotype. Haplotypes were constructed using the program PHASE version 2.1.[81] In order to improve the stability of haplotype inference, the haplotype construction procedure was repeated five times using different seed numbers with each cycle. Only haplotypes that were inferred with probability estimates of ≥ 0.85 across the five iterations were retained for downstream analyses. Only inferred haplotypes that occurred with a frequency estimate of ≥15% were included in the association analyses, assuming a dosage model (i.e., analogous to the additive model).
For association tests, three genetic models were assessed for each SNP: additive, dominant, and recessive. Barring trivial improvements (delta <10%), the genetic model that best fit the data, by maximizing the significance of the p-value, was selected for each SNP. Logistic regression analysis, that controlled for significant covariates as well as race/ethnicity, was used to evaluate the association between genotype and latent class group membership. Only those genetic associations identified as significant in the post hoc contrasts from the univariate analyses were evaluated in the multivariate analyses. A backwards stepwise approach was used to create the most parsimonious model. Except for race/ethnicity, only predictors with a p-value of <0.05 were retained in the final model. Genetic model fit and both unadjusted and covariate-adjusted odds ratios were estimated using the STATA software package, version 9 (STATA Corp).
Ancestry informative markers (AIMs) can be used as a tool to minimize confounding due to population stratification in case-control association studies.[82–84] Homogeneity in ancestry among participants was verified by principal component analysis (PCA),[85] using HelixTree (GoldenHelix, Bozeman, MT). Briefly, the number of principal components (PCs) was sought which distinguished the major racial/ethnic groups in the sample by visual inspection of scatter plots of orthogonal PCs (i.e., PC 1 versus PC2, PC2 versus PC3). This procedure was repeated until no discernable clustering of participants by their self-reported race/ethnicity was possible (data not shown). The first three PCs were selected to adjust for potential confounding due to population substructure (i.e., race/ethnicity) by including them in all logistic regression models (described in the preceding paragraph). One hundred and six ancestry informative markers were included in the analysis.
RESULTS
Participant characteristics
As summarized in Table 2, the majority of the participants were Caucasian, well educated, and married/partnered. Patients made up 66.4% of the total sample. The mean age of the total sample was 61.5 years. The average participant had greater than four comorbid conditions and a mean KPS score of 92. Gender was evenly represented within the total sample with 46.2% male and 53.8% female participants. The majority of the FCs (91%) were the patients’ spouses. Approximately 38% of the patients had breast cancer, 49% had prostate cancer, 7% had brain cancer, and 6% had lung cancer. No significant differences were found between patients and FCs in age (60.9 (± 11.6) years versus 62.5 (± 10.5) years), KPS score (91.1 (± 11.9) versus 93.7 (± 10.6)), and number of comorbidities (4.8 (± 2.6) versus 4.2 (± 2.9)). In addition, no significant differences were found between patients and FCs in their ratings of worst pain (2.0 (± 3.2) versus 1.5 (± 3.1)), fatigue (4.2 (± 2.0) versus 4.5 (± 2.0)), sleep disturbance (38.9 (± 19.6) versus 38.7 (± 16.7)), and depression (9.1 (± 8.7) versus 8.3 (± 7.2)).
Results of LCPA
Using LCPA, three distinct latent classes of individuals were identified, based on their experiences with the symptoms of pain, fatigue, sleep disturbance, and depression. The fit indices for the candidate models are shown in Table 3. As summarized in Table 4, the largest percentage of participants (83%) was classified in the “Low depression and low pain” class and had mean scores for all four symptoms that were below clinically meaningful cutoff scores. A second group, that comprised 4.7% of participants, was classified as the “High depression and low pain” class. High levels of depression, average levels of fatigue, and low levels of pain and sleep disturbance characterized this class. The third class, comprised of 12.3% of participants was classified as the “All high” class. Clinically meaningful levels of all four symptoms characterized this group.
Table 3.
Latent class solutions and fit indices for two through four class solutions
| Model | LL | BIC | BICSSAdj | VLMR | Entropy |
|---|---|---|---|---|---|
| 2 Class | −1683.05 | 3490.60 | 3420.84 | 96.67** | .90 |
| 3 Class | −1666.38 | 3496.88 | 3404.92 | 33.34* | .92 |
| 4 Class | −1651.93 | 3507.60 | 3393.44 | 28.09ns | .86 |
p < .05;
p < .01;
Not significant
LL = log-likelihood; BIC= Bayesian Information Criterion; BICSSAdj = sample-size adjusted BIC; VLMR= the Vuong-Lo-Mendel-Rubin likelihood ratio test for the K vs. K-1 model
Table 4.
Symptom severity scores for the total sample and differences in symptom severity scores among the latent classes
| Symptom scores at enrollment | Total sample N=253 |
Low Depression & Low Pain (1) N=210 83.0% |
High Depression & Low Pain (2) N=12 4.7% |
All High (3) N=31 12.3% |
p-value post-hoc contrasts |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| CES-D scores | 8.8 (8.2) | 5.9 (4.7) | 20.6 (6.8) | 24.5 (5.0) | p<.0001 3>1; p<.0001 |
| GSDS scores | 38.9 (18.6) | 35.3 (15.4) | 27.5 (12.0) | 68.1 (14.6) | p<.0001 3>1 and 2; p<.0001 |
| LFS scores for evening fatigue | 4.3 (2.0) | 4.1 (2.0) | 4.4 (2.6) | 5.6 (1.3) | p<.0001 3>1; p<.0001 |
| Worst pain intensity scores | 1.9 (3.2) | 1.5 (2.9) | 0.8 (2.7) | 4.7 (3.7) | p<.0001 3>1 and 2; p=.001 |
CES-D = Center for Epidemiologic Studies-Depression Scale; GSDS = General Sleep Disturbance Scale; LFS = Lee Fatigue Scale; SD= standard deviation
Differences in demographic and clinical characteristics among the three latent classes
Significant differences among the three latent classes were found for several characteristics including age, gender, patient/FC status, KPS score, number of comorbid conditions, and cancer diagnoses (patients only) (Table 2). Participants in the “All high” class were significantly younger (p=.011) and had a lower KPS score (p<.0001) than participants in the “Low depression and low pain” class. The average number of comorbid conditions of participants in the “All high” class was significantly higher than for the other two classes (p=.04). Compared to the “Low depression and low pain” class, the “All high” class had a higher percentage of female participants (p<.05). Compared to the other two classes, participants in the “All high” class were more likely to be patients than FCs (p<.05). Finally, the “All high” class was composed primarily of patients with breast cancer compared to the “Low depression and low pain” class that was composed primarily of patients with prostate cancer (p=.001). No significant differences were found among the latent classes in education level, ethnicity, employment status, living arrangements, or marital status.
Table 2.
Demographic and clinical characteristics of total sample and differences in characteristics among the latent classes
|
Characteristics |
Total sample N=253 |
Low Depression & Low Pain (1) N=210 83.0% |
High Depression & Low Pain (2) N=12 4.7% |
All High (3) N=31 12.3% |
p-value post-hoc contrasts |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 61.5 (11.3) | 62.5 (11.3) | 57.0 (11.1) | 56.2 (9.3) | p=.005 3<1; p=.011 |
| Education (years) | 16.0 (3.0) | 15.9(3.0) | 15.4 (3.9) | 16.4 (2.6) | p=.586 |
| Karnofsky Performance Status (KPS) score |
92.0 (11.5) | 93.7 (9.9) | 86.4 (17.5) | 81.4 (13.3) | p<.0001 3<1; p<.0001 |
| Number of comorbid conditions | 4.6 (2.7) | 4.4 (2.6) | 3.8 (2.0) | 6.6 (2.9) | p=.004 3>1 and 2; p=.04 |
| % | % | % | % | ||
| Gender Male Female |
46.2 53.8 |
50.5 49.5 |
25.0 75.0 |
25.8 74.2 |
p= .012 1<3; p<.05 |
| Ethnicity White Asian/Pacific Islander Black Hispanic/Mixed/Other |
74.6 6.3 13.5 5.6 |
76.6 5.3 13.4 4.8 |
50.0 16.7 25.0 8.3 |
71.0 9.7 9.7 9.7 |
p=.328 |
| Participant Patient Family caregiver |
66.4 33.6 |
62.9 37.1 |
66.7 33.3 |
90.3 9.7 |
p=.01 3>1 and 2; p<.05 |
| Lives alone Yes No |
32.1 67.9 |
29.5 70.5 |
50.0 50.0 |
39.3 60.7 |
p=.327 |
| Married/ partnered? Yes No |
69.3 30.7 |
71.4 28.6 |
58.3 41.7 |
58.6 41.4 |
p=.262 |
| Work for pay? Yes No |
46.4 53.6 |
48.3 51.7 |
41.7 58.3 |
34.5 65.5 |
p=.356 |
SD = standard deviation
Differences in mean symptom scores among the three latent classes
As summarized in Table 4, the mean CES-D score was significantly higher for the “All high” class compared to the “Low depression and low pain” class (p<.0001). The mean CES-D scores for both the “All high” and the “High depression and low pain” classes were above the clinically meaningful cutoff score (≥16) for depressive symptoms. The mean GSDS score was significantly higher for the “All high” class compared to the other two classes (both, p<.0001). The “All high” class had clinically meaningful levels of sleep disturbance (≥43). The mean LFS score was significantly higher for the “All high” class compared to the “Low depression and low pain” class (p<.0001). The mean worst pain score was significantly higher for the “All high” class compared to the other two classes (both, p=.001).
Candidate gene analyses of the three LCPA classes
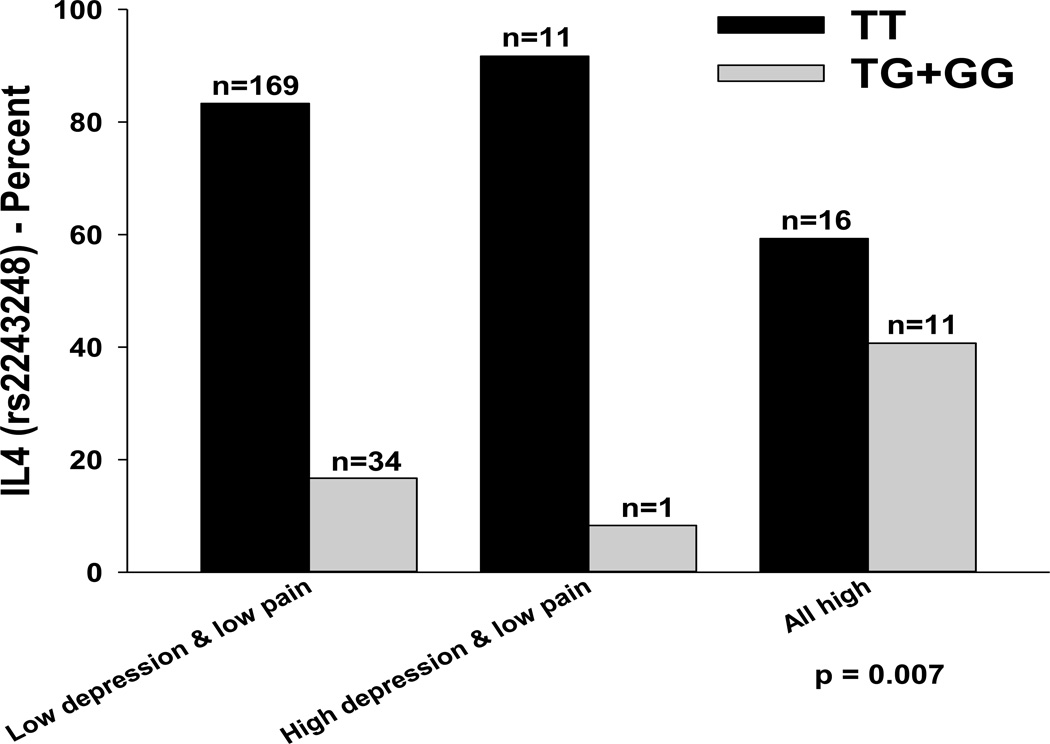
As summarized in Table 1, the minor allele frequency was significantly different among the latent classes for four SNPs: IL1R1 rs2228139, IL4 rs2243248, IL8 rs2227306, and IL8 rs2227543. For IL1R1 rs2228139, a recessive model fit the data best (p=.013). However, post-hoc contrasts of IL1R1 rs2228139 were unable to determine which classes drove the differences among the latent classes.
For IL4 rs2243248, a dominant model fit the data best (p=.007). Post-hoc contrasts of IL4 rs2243248 revealed that differences among the three classes in terms of carriers of the minor allele was driven by the “All high” class as compared with the “Low depression and low pain” class (p=.008). For IL8 rs2227306 (p=.027) and IL8 rs2227543 (p=.04), a recessive model fit the data best. Post-hoc contrasts of IL8 rs2227306 and IL8 rs2227543 suggested that differences among the three classes in terms of rare allele homozygotes was driven by the “High depression and low pain” class as compared with the “Low depression and low pain” class (p=.020 and .026, respectively). No significant differences were found among the latent classes for any of the haplotypes analyzed.
Regression analyses of IL4 and IL8 genotypes and symptom experience classification
In order to better estimate the magnitude (i.e., odds ratio, OR) and precision (95% confidence interval, CI) of genotype on LCPA group membership, multivariate logistic regression models were fit in a pairwise fashion between the LCPA groups that were identified through post hoc contrasts in the univariate analyses. In addition to genotype, the phenotypic variables evaluated in the model were age, gender, ethnicity (i.e., White, Asian/Pacific Islander, Black, Hispanic/Mixed ethnic background/Other), patient/caregiver status, functional status (i.e., KPS score), and number of comorbid conditions.
The only genetic association that remained significant in the multivariate logistic regression analyses was for IL4 rs2243248 that compared the “Low depression and low pain” class with the “All high” class (Figure 1 and Table 5). In this model, IL4 rs2243248 genotype, functional status, number of comorbid conditions, age, ethnicity (White versus Black), and patient/FC status were the predictors retained in the final model (p<.0001). The overall model explained 34.1% of the variance in LCPA group membership. Controlling for KPS score, number of comorbid conditions, age, ethnicity, and patient/FC status, carrying a minor allele (i.e., TG + GG) was associated with over a six-fold increase in the odds of belonging to the “All high” class (OR: 6.02, 95% CI: 1.874, 19.366, p=.003).
Figure 1.
Differences among the latent classes in the percentages of participants who were homozygous for the common allele (TT) or heterozygous or homozygous for the minor allele (TG + GG) for rs223248 in IL4.
Table 5.
Multiple Logistic Regression Analyses for IL4 rs2243248
| LCPA Class Comparison |
Predictor | Odds Ratio |
Standard Error | 95% CI | Z | p-value |
|---|---|---|---|---|---|---|
| Class 1 versus Class 3 (n=224) |
Genotype | 6.02 | 3.589 | 1.873, 19.366 | 3.01 | .003 |
| Functional status | 0.48 | 0.092 | 0.330, 0.697 | −3.85 | <.001 | |
| # of comorbidities | 1.35 | 0.134 | 1.109, 1.638 | 3.00 | .003 | |
| Age | 0.78 | 0.093 | 0.619, 0.986 | −2.08 | .037 | |
| White versus Black | 0.04 | 0.058 | 0.003, 0.647 | −2.27 | .023 | |
| Patient/FC | 0.21 | 0.160 | 0.045, 0.946 | −2.03 | .042 | |
| Overall model fit: χ2 = 54.80, p < .0001 R2 = 0.3407 | ||||||
Pair-wise multiple logistic regression analysis of Latent Class Profile Analysis (LCPA) groups. Class 1: “Low depression and low pain”, Class 2: “High depression and low pain”, and Class 3: “All high”. For each model, the first three principle components identified from the analysis of ancestry informative markers were retained to adjust for potential confounding due to race or ethnicity (data not shown). For Table 5, predictors evaluated in the model included IL4 rs2243248 genotype (TT versus TG+GG), age (years), gender (female versus male), self-reported ethnicity (white, Asian/Pacific Islander, Black, Hispanic/Mixed race/Other), patient versus family caregiver (FC) status, functional status estimated using Karnofsly Performance Status (KPS) score, and number of comorbid conditions.
DISCUSSION
This study is the first to use LCPA to characterize a sample of oncology patients and their FCs using a cluster of symptoms associated with “sickness behavior” and to identify an association between these latent classes and one anti-inflammatory cytokine (i.e., IL4). The identification of distinct subgroups of individuals with different symptom experiences is consistent with previous reports.[14,17–20] However, only one cytokine gene was associated with differences in the severity of this “sickness behavior” symptom cluster.
In this sample, three relatively distinct classes of participants were identified, namely those who reported low depression and low pain (83%), those who reported high depression and low pain (4.7%), and those who reported high levels of all four symptoms (12.3%). While our previous studies identified four distinct latent classes using the same symptom cluster,[18–20] a consistent finding across all four studies is that the “All high” class constituted between 10% and 15% (mean 13.0%) of the sample. This finding suggests that a subset of individuals share some common biological mechanisms that influence their experience with the multiple symptoms associated with sickness behavior. Identification of these mechanisms could lead to the development of targeted interventions for this high-risk group.
Because LCPA is an exploratory analytic procedure that facilitates the emergence of distinct latent classes based on similarities in some dependent variables (in this study – differences in participants’ ratings of pain, fatigue, sleep disturbance, and depression), group membership can change based on sample characteristics as well as timing of the symptom assessments. Therefore, differences across studies[14,17–20] in the number of latent classes, as well as in the symptom characteristics of the various latent classes, may be related to differences in demographic and clinical characteristics of the samples; differences in inclusion and exclusion criteria, as well as differences in some unidentified phenotypic and environmental characteristics. Because blood samples were not obtained from participants in our previous studies,[18–20] the genetic association identified in this study awaits verification in future studies.
In this cohort, carrying the minor allele for IL4 rs2243248 was associated with membership in the “All high” class along with younger age, being White, being a patient (versus a FC), having a lower functional status, and having a higher number of comorbid conditions. Similar associations between higher symptom severity scores and various demographic and clinical characteristics were reported in previous studies. [14,17–20] However, it is important to note that the genetic association was not confounded by any of these demographic or clinical characteristics. These findings are particularly interesting because IL4 was either not evaluated or identified as a candidate gene in previous research on symptoms. Previous studies found genetic associations between IL1β and IL6 and severity of fatigue,[40] as well as associations between IL6 and TNF-α and severity of fatigue and sleep disturbance.[38,39] The discrepancy in study findings may be related to differences in symptom phenotypes (i.e. single symptoms versus a symptom cluster). Given the fact that a number of reviews suggested that alterations in pro-inflammatory cytokines contributed to the symptoms associated with sickness behavior,[10,21] additional research is warranted to evaluate associations between single symptoms and symptom clusters and pro- and anti- inflammatory cytokine genes.
The anti-inflammatory cytokine IL4 blocks the action of a number of pro-inflammatory cytokines (i.e., IL1-β, IL6, IL8, and TNF-α. [86] The IL4 SNP identified in this study (rs2243248) is known to occur in an evolutionarily conserved region. While the functional effects of this SNP are not known, findings from this study suggest that carrying the minor allele may result in alterations in the regulation of several pro-inflammatory cytokines. This dysregulation in IL4 function places these individuals in a high-risk group for experiencing multiple symptoms related to “sickness behavior”.
In fact, the neuromodulatory effects of IL4 have been evaluated in animal models of sickness behavior. For example, in one study, cytokine-induced sickness behavior in rats was inhibited when IL4 was administered 12 hours prior to lipopolysaccharide (LPS) but was potentiated when IL4 was co-administered with LPS. This finding suggests that the regulation of sickness behavior by IL4 can be either inhibitory or stimulatory.[87] Interestingly, LPS–induced sickness behavior was more profound in IL4 (−/−) mice, which suggests a more protective role for IL4.[88]. Furthermore, Sherry and colleagues observed decreased sickness behavior in wild type mice fed a soluble fiber diet which induced the up regulation of IL4.[89] The protective effect of the soluble fiber diet was reduced in IL-4 (−/−) mice. While research on the association between IL4 and sickness behavior in humans is limited, one study found that an eight-week meditation program increased production of IL4 and decreased production of interferon (IFN)-γ and IL10 in individuals with early stage prostate or breast cancer.[90] These changes in serum cytokines were associated with reduced symptoms of stress (including depression), increased sleep quality, and increased QOL.
Previous work with this sample of patients and FCs identified associations between TNF-α (rs1800629) and sleep disturbance and morning fatigue[38] and between IL6 (rs4719714) and sleep disturbance, evening fatigue, and morning fatigue.[39] The lack of an association between these SNPs and the subgroups of participants found in this study may be explained by a number of factors. First, the symptom phenotype that was evaluated in this study (i.e., symptom cluster of pain, fatigue, sleep disturbance and depression) compared to previous studies (i.e., single symptoms) are distinctly different and may be associated with different cytokine genes. This hypothesis is supported by the fact that the p-values of the additive models for TNF-α rs1800629 (p=.422) and IL6 rs 4719714 (p=.419) in this study did not approach statistical significance (Table 1). An equally plausible hypothesis is that additional research, with larger samples might identify additional candidate genes. In addition, rather than polymorphisms in various cytokine genes being directly responsible for the symptoms associated with sickness behavior and elevations in serum levels of cytokines, polymorphisms in other gene pathways may be involved in activation or inhibition of cytokine genes. This hypothesis warrants investigation in future studies.
Several study limitations need to be acknowledged. The majority of participants were middle-aged, Caucasian, well educated, and married/ partnered, which limits the generalizability of these findings to individuals with similar demographic characteristics. The major reasons for enrollment refusal were being too overwhelmed with treatment or too busy which may have led to either underestimation or overestimation of symptoms in the individuals included in this study. In addition, the exact etiologies for and duration of each of the individual symptoms within the symptom cluster were not evaluated. While most studies of sickness behavior reported symptoms associated with an acute stimulus (e.g., administration of lipopolysaccharide),[22,91,92] it is possible that a symptom cluster that occurs because of one or more chronic conditions is associated with genetic variations in pro- and anti-inflammatory cytokines. Future studies need to evaluate the relationships between cytokine genes and individual symptoms as well as symptom clusters.
Due to the small sample sizes for the “All high” and the “High depression and low pain” classes, it is plausible that some genetic associations were not identified because of low minor allele frequency. For example, findings for several SNPs in IL1R1, IL2, IL8, IL10, IL17A, and TNF-α approached statistical significance and warrant investigation in future studies with larger sample sizes. It is plausible that other genetic associations with symptom clusters will emerge if the same analyses are conducted at several points over the trajectory of the patient’s treatment as latent class membership can change over time.[14] Finally, future studies may need to evaluate levels of pro- and anti-inflammatory cytokines in order to refine our understanding of the associations between genotype and self-reported symptom experiences.
In summary, the recognition of a distinct phenotype that may represent sickness behavior reveals new evidence for the role of IL4 in the modulation of this symptom cluster in oncology patients and their FCs. Using new statistical approaches like LCPA to identify distinct phenotypes may provide new information about the biologic mechanisms that underline this symptom experience. Indeed, this study uncovered a role for an anti-inflammatory cytokine in the modulation of symptom experience that was not described previously and warrants confirmation in future studies.
Highlights.
Distinct groups had high levels of pain, fatigue, sleep disturbance and depression.
Approximately 12% of patients reported high levels of all four symptoms.
New role for IL4 in the modulation of a sickness behavior symptom was identified.
ACKNOWLEDGEMENTS
This research was supported by a grant from the National Institute of Nursing Research (NR04835) and partially supported by a UCSF Academic Senate grant to Drs. Dunn and Aouizerat. Dr. Aouizerat was funded through the National Institutes of Health (NIH) Roadmap for Medical Research Grant (KL2 RR624130). Dr. Miaskowski is funded by the American Cancer Society as a Clinical Research Professor. Dr. Dhruva is funded through NIH Mentored Patient-Oriented Research Career Development Award (K23 AT005340).
ABBREVIATIONS
- AIMs
Ancestry informative markers
- BIC
Bayesian Information Criterion
- BLRT
Bootstrapped Likelihood Ratio Test
- CES-D
Center for Epidemiological Studies- Depression Scale
- CI
confidence interval
- DNA
deoxyribonucleic acid
- EM
expectation-maximization
- FC
family caregiver
- GSDS
General Sleep Disturbance Scale
- IFN
interferon
- IL
interleukin
- KPS
Karnofsky Performance status
- LCA
Latent class analysis
- LCPA
Latent class profile analysis
- LFS
Lee Fatigue Scale
- MLR
robust maximum likelihood
- PCA
Principal component analysis
- NFKB
nuclear factor kappa beta
- NRS
numeric rating scale
- OR
odds ratio
- QOL
quality of life
- RT
radiation therapy
- SNP
single nucleotide polymorphism
- TGF
tumor growth factor
- TNF
tumor necrosis factor
- VLMR
Vuong-Lo-Mendell-Rubin likelihood ratio test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fletcher BA, Miaskowski C, Dodd MJ, Schumacher KL. A review of the literature on the symptom experience of family caregivers of patients with cancer. Oncol Nurs Forum. 2008;35:E23–E44. doi: 10.1188/08.ONF.E23-E44. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher BA, Schumacher KL, Dodd M, Paul SM, Cooper BA, Lee K, et al. Trajectories of fatigue in family caregivers of patients undergoing radiation therapy for prostate cancer. Res Nurs Health. 2009;32:125–139. doi: 10.1002/nur.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher BS, Paul SM, Dodd MJ, Schumacher K, West C, Cooper B, et al. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol. 2008;26:599–605. doi: 10.1200/JCO.2007.12.2838. [DOI] [PubMed] [Google Scholar]
- 4.Gibbins J, McCoubrie R, Kendrick AH, Senior-Smith G, Davies AN, Hanks GW. Sleep-wake disturbances in patients with advanced cancer and their family carers. J Pain Symptom Manage. 2009;38:860–870. doi: 10.1016/j.jpainsymman.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Carney S, Koetters T, Cho M, West C, Paul SM, Dunn L, et al. Differences in sleep disturbance parameters between oncology outpatients and their family caregivers. J Clin Oncol. 2011;29:1001–1006. doi: 10.1200/JCO.2010.30.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barsevick AM. The concept of symptom cluster. Semin Oncol Nurs. 2007;23:89–98. doi: 10.1016/j.soncn.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Barsevick AM. The elusive concept of the symptom cluster. Oncol Nurs Forum. 2007;34:971–980. doi: 10.1188/07.ONF.971-980. [DOI] [PubMed] [Google Scholar]
- 8.Chen ML, Tseng HC. Symptom clusters in cancer patients. Support Care Cancer. 2006;14:825–830. doi: 10.1007/s00520-006-0019-8. [DOI] [PubMed] [Google Scholar]
- 9.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28:465–470. [PubMed] [Google Scholar]
- 10.Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Semin Oncol Nurs. 2007;23:99–105. doi: 10.1016/j.soncn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Miaskowski C, Aouizerat BE, Dodd M, Cooper B. Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. J Natl Cancer Inst Monogr. 2007;37:39–46. doi: 10.1093/jncimonographs/lgm003. [DOI] [PubMed] [Google Scholar]
- 12.Miaskowski C, Dodd M, Lee K. Symptom clusters: the new frontier in symptom management research. J Natl Cancer Inst Monogr. 2004:17–21. doi: 10.1093/jncimonographs/lgh023. [DOI] [PubMed] [Google Scholar]
- 13.Desai MJ, Kim A, Fall PC, Wang D. Optimizing quality of life through palliative care. J Am Osteopath Assoc. 2007;107:ES9–E14. [PubMed] [Google Scholar]
- 14.Dodd MJ, Cho MH, Cooper BA, Petersen J, Bank KA, Lee KA, et al. Identification of latent classes in patients who are receiving biotherapy based on symptom experience and its effect on functional status and quality of life. Oncol Nurs Forum. 2011;38:33–42. doi: 10.1188/11.ONF.33-42. [DOI] [PubMed] [Google Scholar]
- 15.Esther Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37:715–736. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granda-Cameron C, Viola SR, Lynch MP, Polomano RC. Measuring patient-oriented outcomes in palliative care: functionality and quality of life. Clin J Oncol Nurs. 2008;12:65–77. doi: 10.1188/08.CJON.65-77. [DOI] [PubMed] [Google Scholar]
- 17.Gwede CK, Small BJ, Munster PN, Andrykowski MA, Jacobsen PB. Exploring the differential experience of breast cancer treatment-related symptoms: a cluster analytic approach. Support Care Cancer. 2008;16:925–933. doi: 10.1007/s00520-007-0364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs. 2010;14:101–110. doi: 10.1016/j.ejon.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pud D, Ben Ami S, Cooper BA, Aouizerat BE, Cohen D, Radiano R, et al. The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manage. 2008;35:162–170. doi: 10.1016/j.jpainsymman.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33:E79–E89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 21.Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 22.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dantzer R, Bluthe RM, Gheusi G, Cremona S, Laye S, Parnet P, et al. Molecular basis of sickness behavior. Ann N Y Acad Sci. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- 24.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 25.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 26.Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- 27.Watkins LR, Maier SF. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- 28.Krabbe KS, Reichenberg A, Yirmiya R, Smed A, Pedersen BK, Bruunsgaard H. Low-dose endotoxemia and human neuropsychological functions. Brain Behav Immun. 2005;19:453–460. doi: 10.1016/j.bbi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brydon L, Walker C, Wawrzyniak A, Whitehead D, Okamura H, Yajima J, et al. Synergistic effects of psychological and immune stressors on inflammatory cytokine and sickness responses in humans. Brain Behav Immun. 2009;23:217–224. doi: 10.1016/j.bbi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 32.Maier SF, Watkins LR. Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatment. Brain Behav Immun. 2003;17(Suppl 1):S125–S131. doi: 10.1016/s0889-1591(02)00079-x. [DOI] [PubMed] [Google Scholar]
- 33.Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uceyler N, Rogausch JP, Toyka KV, Sommer C. Differential expression of cytokines in painful and painless neuropathies. Neurology. 2007;69:42–49. doi: 10.1212/01.wnl.0000265062.92340.a5. [DOI] [PubMed] [Google Scholar]
- 35.Lu CH, Chao PC, Borel CO, Yang CP, Yeh CC, Wong CS, et al. Preincisional intravenous pentoxifylline attenuating perioperative cytokine response, reducing morphine consumption, and improving recovery of bowel function in patients undergoing colorectal cancer surgery. Anesth Analg. 2004;99:1465–1471. doi: 10.1213/01.ANE.0000132974.32249.C8. [DOI] [PubMed] [Google Scholar]
- 36.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 38.Aouizerat BE, Dodd M, Lee K, West C, Paul SM, Cooper BA, et al. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biol Res Nurs. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- 39.Miaskowski C, Dodd M, Lee K, West C, Paul SM, Cooper BA, et al. Preliminary evidence of an association between a functional interleukin-6 polymorphism and fatigue and sleep disturbance in oncology patients and their family caregivers. J Pain Symptom Manage. 2010;40:531–544. doi: 10.1016/j.jpainsymman.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: Early findings. Brain Behav Immun. 2008;22:1197–1200. doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522–2530. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- 42.Brambilla F, Maggioni M. Blood levels of cytokines in elderly patients with major depressive disorder. Acta Psychiatr Scand. 1998;97:309–313. doi: 10.1111/j.1600-0447.1998.tb10005.x. [DOI] [PubMed] [Google Scholar]
- 43.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 44.Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 45.Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. J Gerontol A Biol Sci Med Sci. 1999;54:M434–M439. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maes M, Bosmans E, Meltzer HY. Immunoendocrine aspects of major depression. Relationships between plasma interleukin-6 and soluble interleukin-2 receptor, prolactin and cortisol. Eur Arch Psychiatry Clin Neurosci. 1995;245:172–178. doi: 10.1007/BF02193091. [DOI] [PubMed] [Google Scholar]
- 47.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 48.Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 49.De La Garza R., 2nd Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci Biobehav Rev. 2005;29:761–770. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Reyes-Gibby CC, Wu X, Spitz M, Kurzrock R, Fisch M, Bruera E, et al. Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncol. 2008;9:777–785. doi: 10.1016/S1470-2045(08)70197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 52.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4:2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 53.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36:291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 54.Gay CL, Lee KA, Lee SY. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5:311–318. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee KA, Portillo CJ, Miramontes H. The fatigue experience for women with human immunodeficiency virus. J Obstet Gynecol Neonatal Nurs. 1999;28:193–200. doi: 10.1111/j.1552-6909.1999.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 56.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17:320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 57.Miaskowski C, Paul SM, Cooper BA, Lee K, Dodd M, West C, et al. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2008;35:632–643. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barsevick A, Beck SL, Dudley WN, Wong B, Berger AM, Whitmer K, et al. Efficacy of an intervention for fatigue and sleep disturbance during cancer chemotherapy. J Pain Symptom Manage. 2010;40:200–216. doi: 10.1016/j.jpainsymman.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15:493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- 60.Lee KA, DeJoseph JF. Sleep disturbances, vitality, and fatigue among a select group of employed childbearing women. Birth. 1992;19:208–213. doi: 10.1111/j.1523-536x.1992.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 61.Carpenter JS, Andrykowski MA, Wilson J, Hall LA, Rayens MK, Sachs B, et al. Psychometrics for two short forms of the Center for Epidemiologic Studies-Depression Scale. Issues Ment Health Nurs. 1998;19:481–494. doi: 10.1080/016128498248917. [DOI] [PubMed] [Google Scholar]
- 62.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 63.Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J Pers Assess. 1995;64:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- 64.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 65.Muthen B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 66.Vermunt JK, Magdison J. Latent class cluster analyses. New York: Cambridge University Press; 2002. [Google Scholar]
- 67.Everitt BS, Landau S, Leese M. Cluster Analysis. 4 ed. New York: Oxford University Press; 2001. [Google Scholar]
- 68.Collins LM, Lanza ST. Latent class and latent transition analysis: with applications in the Social, Behavioral, and Health Science. Hoboken, NJ: John Wiley & Sons; 2010. [Google Scholar]
- 69.Nylund K, Bellmore A, Nishina A, Graham S. Subtypes, severity, and structural stability of peer victimization: what does latent class analysis say? Child Dev. 2007;78:1706–1722. doi: 10.1111/j.1467-8624.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 70.Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–569. [Google Scholar]
- 71.Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification. 1996;13:195–212. [Google Scholar]
- 72.Collins LM, Wugalter SE. Latent class models for stage-sequential dynamic latent variables. Multivariate Behavioral Research. 1992;27:131–157. [Google Scholar]
- 73.Lanza ST, Flaherty BP, Collins LM. Latent class and latent transition analysis. In: Schinka JA, Velicer WF, editors. Handbook of Psychology: Research Methods in Psychology. Hoboken, NJ: John Wiley & Sons, Inc; 2003. pp. 663–685. [Google Scholar]
- 74.Olsen MK, Schafer JL. A two-part random effects model for semicontinuous longitudinal data. Journal of the American Statistical Association. 2001;96:730–745. [Google Scholar]
- 75.Muthen LK, Muthen BO. Mplus User's Guide (6th ed.) Los Angeles, CA: Muthen & Muthen; 1998–2010. [Google Scholar]
- 76.Muthen LK, Muthen BO. Mplus. 6 ed. Los Angeles, CA: Muthen & Muthen; 1998–2010. [Google Scholar]
- 77.SPSS. IBM SPSS for Windows (Version 19) Chicago, Illinois: SPSS, Inc; 2010. [Google Scholar]
- 78.Dantzer R, Capuron L, Irwin MR, Miller AH, Ollat H, Perry VH, et al. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrinology. 2008;33:18–29. doi: 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conde L, Vaquerizas JM, Dopazo H, Arbiza L, Reumers J, Rousseau F, et al. PupaSuite: finding functional single nucleotide polymorphisms for large-scale genotyping purposes. Nucleic Acids Res. 2006;34:W621–W625. doi: 10.1093/nar/gkl071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 81.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, et al. Control of confounding of genetic associations in stratified populations. Am J Hum Genet. 2003;72:1492–1504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications. Hum Mutat. 2008;29:648–658. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- 84.Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome-wide association studies. Hum Mol Genet. 2008;17:R143–R150. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 86.Hamblin A. Cytokines and Cytokine Receptors. 2nd ed. New York: Oxford University Press; 1993. [Google Scholar]
- 87.Parnet P, Kelley KW, Bluthe RM, Dantzer R. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J Neuroimmunol. 2002;125:5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 88.Lyons A, McQuillan K, Deighan BF, O'Reilly JA, Downer EJ, Murphy AC, et al. Decreased neuronal CD200 expression in IL-4-deficient mice results in increased neuroinflammation in response to lipopolysaccharide. Brain Behav Immun. 2009;23:1020–1027. doi: 10.1016/j.bbi.2009.05.060. [DOI] [PubMed] [Google Scholar]
- 89.Sherry CL, Kim SS, Dilger RN, Bauer LL, Moon ML, Tapping RI, et al. Sickness behavior induced by endotoxin can be mitigated by the dietary soluble fiber, pectin, through up-regulation of IL-4 and Th2 polarization. Brain Behav Immun. 2010;24:631–640. doi: 10.1016/j.bbi.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med. 2003;65:571–581. doi: 10.1097/01.psy.0000074003.35911.41. [DOI] [PubMed] [Google Scholar]
- 91.Tizard I. Sickness behavior, its mechanisms and significance. Anim Health Res Rev. 2008;9:87–99. doi: 10.1017/S1466252308001448. [DOI] [PubMed] [Google Scholar]
- 92.Myers JS. Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncol Nurs Forum. 2008;35:802–807. doi: 10.1188/08.ONF.802-807. [DOI] [PubMed] [Google Scholar]