Abstract
Background
This prospective, randomized controlled clinical trial determined whether an optimal exercise program length exists to efficaciously change claudication onset time (COT) and peak walking time (PWT) in patients with peripheral artery disease and claudication.
Methods
142 patients were randomized to either supervised exercise (n=106) or usual care control (n=36), with 80 completing exercise and 27 completing control. The exercise program consisted of intermittent walking to near maximal claudication pain three days per week. COT and PWT were the primary outcomes obtained from a treadmill exercise test at baseline and bi-monthly during the study.
Results
Following exercise, changes in COT (p<0.001) and PWT (p<0.001) were consistently greater than changes following control. In the exercise program, COT and PWT increased from baseline to month 2 (p<0.05), and from month 2 to month 4 (p<0.05), but did not significantly change from month 4 to month 6 (p>0.05). When changes were expressed per mile walked during the first 2 months, middle 2 months, and final 2 months of exercise, COT and PWT only increased during the first 2 months (p<0.05).
Conclusions
Exercise-mediated gains in COT and PWT occur rapidly within the first two months of exercise rehabilitation, and are maintained with further training. The clinical significance is that a relatively short two-month exercise program may be preferred to a longer program to treat claudication because adherence is higher, costs associated with personnel and utilization of facilities are lower per patient, and more patients can be trained for a given amount of personnel time and resource utilization.
INTRODUCTION
Peripheral artery disease (PAD) is prevalent in more than 12% of the US population aged 65 years and above,1 and is associated with elevated rates of mortality.2, 3 Claudication, a common manifestation of PAD, is prevalent in more than 6% of those in this age group, and leads to ambulatory dysfunction4, 5 and a physically inactive lifestyle.6 Exercise rehabilitation is a widely recognized conservative approach for the treatment of claudication, as medically supervised programs efficaciously increase claudication onset time (COT) and peak walking time (PWT).1, 7 Consequently, supervised exercise rehabilitation has been given a Class I recommendation by the American College of Cardiology and the American Heart Association indicating general agreement for effectiveness of treatment, supported by Level A evidence derived from multiple randomized controlled trials and meta-analyses.1
Although treatment of claudication with exercise rehabilitation is well documented, the importance of various elements that comprise the exercise prescription remains less clear. A recent meta-analysis concluded that an optimal exercise regimen, such as the optimal intensity and duration of exercise, could not be determined.8 We previously report in a randomized trial that increases in COT and PWT following six months of exercise were not different between those who trained at 40% and 80% of maximal intensity, provided that they completed a similar volume of exercise.9 The optimal length of an exercise program to increase COT and PWT is not clear, which has major implications related to the efforts of patients, staff, and utilization of resources at medical and research facilities. Determining the shortest length of exercise to elicit the majority of improvements in COT and PWT is a necessary step to optimally treat more patients with less expense and fewer resources.
This prospective, randomized controlled clinical trial determined whether an optimal exercise program length exists to efficaciously change COT and PWT in patients with PAD and claudication. We hypothesized that COT and PWT improve most rapidly during the early phase of a 6-month exercise rehabilitation program, and less so by the end.
METHODS
PATIENTS
IRB Approval and Informed Consent
The procedures used were approved by the Institutional Review Board at the University of Maryland and the Research and Development Committee at the Baltimore MVAHCS. Written informed consent was obtained from each patient prior to investigation.
Patient Recruitment
Patients were evaluated in the Geriatrics, Research, Education, and Clinical Center, Maryland Veterans Affairs Health Care System (MVAHCS) at Baltimore. Patients were recruited from the Vascular Clinic in the Baltimore MVAHCS and from newspaper and radio advertisements for possible enrollment. The study was designed to assess the efficacy of exercise at intermediate time points during a 6-month exercise program. Statistical power analyses were performed on the primary outcome measures of COT and PWT. Exercise and control group sample sizes of 75 and 25, respectively, achieved 80% power to detect a group difference in change scores from baseline of 120 seconds for COT and 150 seconds for PWT, using a two-sided two-sample t-test and a significance level (alpha) of 0.05. To allow for a drop out rate of 30%, we targeted randomizing at least 100 and 35 patients to the groups. A consecutive series of 241 individuals were evaluated for study eligibility.
Medical Screening through History, Physical Examination, and Anthropometry
Demographic information, height, weight, body mass index (BMI), waist and hip circumferences,10 cardiovascular risk factors, co-morbid conditions, claudication history, blood samples, and a list of current medications were obtained from a medical history and physical examination at the beginning of the study. Medication regimen of each patient was held constant during the study.
Inclusion and Exclusion Criteria
Patients with claudication secondary to vascular insufficiency were included if they met the following criteria: (a) a history of claudication, defined as reporting leg pain upon exertion, (b) ambulation during a graded treadmill test limited by claudication,4 and (c) an ankle-brachial index (ABI) ≤ 0.90 at rest,11 or a 20% decrease in ABI after exercise.7 Patients were excluded from this study for the following conditions: (a) absence of PAD (ABI > 0.90 at rest and < 20% decrease after exercise)7, 11 (n=17), (b) asymptomatic PAD12 determined from the medical history and verified during the graded treadmill test (n=2), (c) rest pain PAD (n=5), (d) inability to obtain an ABI measure due to non-compressible vessels (i.e., systolic blood pressure could be heard as the sphygmomanometer was inflated to the maximal value of 300 mm Hg) (n=4) , (e) use of cilostazol and pentoxifylline within three months of investigation (n=4), lower extremity revascularization within three months prior to investigation (n=6), (f) exercise tolerance limited by any disease process other than PAD, such as angina, dyspnea, or evidence of myocardial ischemia or rhythm changes from a 12-lead ECG (n=25), (g) uncontrolled hypertension, uncontrolled diabetes, active cancer, renal insufficiency, or abnormal liver function (n=15), and (h) non-compliant with baseline testing (n=21).
Randomization and Flow of Patients
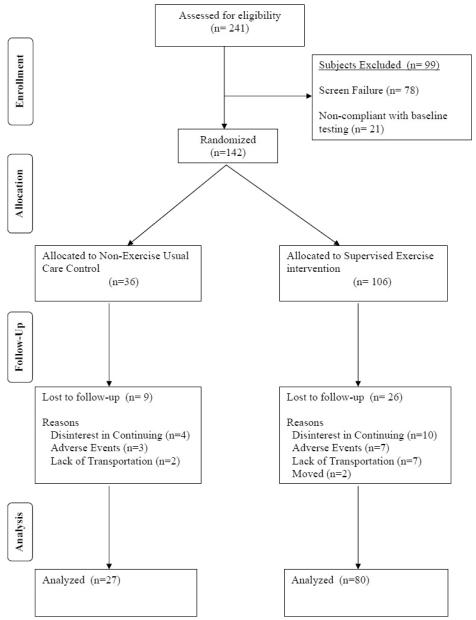
Patients were randomized to either supervised exercise (n=106) or to usual care control (n=36) in a 3:1 ratio. Patients were randomized to the groups using a random number program with blocking to assure that the 3:1 randomization scheme was maintained throughout study allocation. Study personnel were allowed access to the allocation list only after patient eligibility was determined and baseline data was completed. Flow of patients in the study is shown in Figure 1.
Figure 1.
Consolidated standards of reporting trials (CONSORT) flow diagram of patients through each stage of the trial.
INTERVENTION AND CONTROL GROUPS
Exercise Rehabilitation Program
The exercise rehabilitation program was designed to elicit increases in COT and PWT according to our previous studies.13-15 This program consisted of 6 months of supervised, intermittent treadmill walking to near maximal claudication pain 3 days per week. Walking duration and intensity of the sessions were progressively increased during the program. Walking duration began at 15 minutes for the first month of the program, and increased by 5 minutes per month until a total of 40 minutes of walking was accomplished by the sixth month of rehabilitation. Walking intensity began at an initial grade of 50% of the final work load attained during the baseline graded treadmill test, and was increased by 10% every 6 weeks up to 80% during the final 6 weeks of the exercise program. During each exercise session, patients walked at a speed of approximately 2 mph until their claudication pain reached a level of “3” on a 0-4 pain scale (0=no pain, 1=onset of pain, 2=moderate pain, 3=intense pain, and 4=maximal pain),13 after which they rested until claudication pain completely resolved. This pattern of intermittent walking and rest continued until the prescribed number of minutes of walking was accomplished. Five minutes of cycling on a stationary bicycle ergometer served as a warm-up and cool-down during each exercise session. The program was supervised by exercise physiologists and nurses.
To quantify volume of exercise performed in the supervised exercise program, MET-minutes were calculated as the product of exercise intensity, expressed as metabolic equivalents, and exercise duration expressed in minutes.16 Calculation of MET-minutes is clinically useful to summarize the total amount of exercise performed during training because it provides a summative score for exercise duration, intensity, and frequency. In this manner, the amount of exercise performed can be better compared with other studies. The metabolic intensity of each exercise training session was determined from objectively measured oxygen uptake during the most recent graded treadmill test of each patient. Oxygen uptake corresponding to the training grade was then divided by 3.5 to convert to MET’s, and this value was multiplied by duration of each supervised exercise session to yield a MET-minute value. Patients in the supervised program were not given advice or instructions to perform additional exercise away from our research center.
Non-Exercise, Usual Care Contol Group
Patients randomized to this group were encouraged to walk more on their own but they did not receive specific recommendations regarding an exercise program during the study. This approach is similar to advice typically given by clinicians during routine follow-up vascular appointments. No other risk factor management, lifestyle modification, or educational programming interventions were provided to patients in either group. The following measurements were obtained at baseline, 2 months, 4 months, and 6 months during the study.
MEASUREMENTS
Primary Outcome Measures
COT and PWT Obtained During the Graded Treadmill Test
Patients performed a progressive, graded treadmill protocol (walking speed of 2 mph beginning at 0% grade, which then increased by 2% every 2 minutes) to determine study eligibility, as well as to obtain outcome measures related to exercise performance.4 The claudication onset time (COT), defined as the walking time at which the patients first experienced pain, and the peak walking time (PWT), defined as the walking time at which ambulation could not continue due to maximal pain, were both recorded to quantify the severity of claudication. Peak oxygen uptake was measured by oxygen uptake obtained during the peak exercise work load with a Medical Graphics VO2000 metabolic system (Medical Graphics Inc, St. Paul, MN). Using these procedures, the test-retest intraclass reliability coefficient is R=0.89 (95% CI; 0.86 to 0.91) for COT,4 R=0.93 (95% CI: 0.91 to 0.94) for PWT,4 and R=0.88 (95% CI; 0.83 to 0.92) for peak oxygen uptake.17 The final grade attained during this test at baseline was used to calculate the training intensity of the exercise group during the first two months, and the final grade attained during this test at month 2 and month 4 was used to determine the intensity during the subsequent 4 months.
Secondary Outcome Measures
ABI and Ischemic Window
As previously described, ABI measures were obtained from the more severely diseased lower extremity before and 1, 3, 5, and 7 minutes after the treadmill test.4 The reduction in ankle systolic blood pressure after treadmill exercise from resting baseline was quantified by calculating the area under the curve, referred to as the ischemic window.18 Because the ischemic window is a function of both PAD severity and the amount of exercise performed, the ischemic window was normalized per meter walked.
6-Minute Walk Test
A trained technician administered the over ground, 6-minute walk test in which two cones were placed 100 feet apart in a marked corridor.19 Patients were instructed to walk as many laps around the cones as possible. During the test, patients indicated if and when they experienced the onset of claudication pain. The pain-free and total distances walked during the test were recorded. The test-retest intraclass reliability coefficient is R=0.75 (95% CI; 0.70 to 0.81) for the distance to onset of claudication pain, and R=0.94 (95% CI; 0.92 to 0.95) for the total 6-minute walking distance.19
Walking Impairment Questionnaire (WIQ)
Self-reported ambulatory ability was assessed using a validated questionnaire for PAD patients that assesses ability to walk at various speeds and distances, and to climb stairs.20
Daily Physical Activity
Physical activity level was monitored over two consecutive weekdays by a Caltrac accelerometer (Muscle Dynamics, Torrance, CA) attached to the belt of each patient.21 The accelerometer assessed daily physical movements by converting vertical accelerations of the body into caloric expenditure during the 48-hour monitoring period. The accelerometer measure of physical activity has a test-retest intraclass reliability coefficient of R=0.84 (95% CI; 0.77 to 0.90),22 and provides a valid estimate of daily physical activity assessed by the gold standard technique of doubly labeled water.21
Calf Blood Flow
Calf blood flow was obtained under resting, reactive hyperemic, and maximal hyperemic conditions in the more severely diseased leg using venous occlusion mercury strain-gauge plethysmography.23 The test-retest intraclass reliability coefficient is R=0.86 (95% CI; 0.83 to 0.88) for calf blood flow.23
STATISTICAL ANALYSES
Independent t test was used for comparison of baseline means and single degree of freedom Chi Square test for proportions of two groups. Subsequent comparisons were performed as Intent-to-Treat analyses by first imputing missing values before proceeding. A Group by Time ANOVA with repeated measure on Time factor was initial procedure for comparison of means. The Time simple effect within each group with Tukey-Kramer Multiple-Comparison Test was used for comparisons among the means at each time, and was performed for all variables for which a significant Interaction was observed. For each two-month time period after baseline, the regression of miles walked on change in PWT from the preceding test was used to examine the relationship of these two variables in the exercise group. This relationship was further examined by computing the ratio of the change for each individual to the miles walked since the preceding test, and graphically displaying the means for each interval. A similar examination was made for COT. All analyses and imputation procedures were performed using NCSS software. Statistical significance was set at p < 0.05.
RESULTS
Randomization resulted in similar baseline clinical characteristics between groups, except that the exercise group weighed less than the control group (p=0.023) (Table I). One hundred seven patients completed the study, whereas 35 did not (Figure 1). Of the 35 patients who did not complete, 26 were from the exercise group and 9 were controls. The primary reasons for discontinuing were lack of interest, serious adverse events, and lack of transportation. The serious adverse events which were exclusionary for study continuation consisted of cerebral vascular accidents (n=3), uncontrolled hypertension (n=2), myocardial infarction (n=1), coronary artery bypass graft (n=1), below-knee amputation (n=1), deep vein thrombosis (n=1), and a broken ankle (n=1). Three additional serious adverse events occurred which did not result in study exclusion, consisting of hospitalization for pneumonia (n=1), hospitalization for hernia surgery (n=1), and a back injury (n=1).
Table I.
Baseline clinical characteristics. Values are means (SD) or percentage of patients.
| Variables | Control Group (n = 36) |
Exercise Group (n = 106) |
P Value |
|---|---|---|---|
| Age (years) | 68 (8) | 68 (8) | 0.651 |
| Weight (kg) | 87.1 (16.5) | 80.6 (14.2) | 0.023 |
| Body Mass Index (kg/m2) | 29.5 (4.6) | 27.9 (4.7) | 0.077 |
| Ankle/Brachial Index | 0.70 (0.18) | 0.64 (0.21) | 0.124 |
| Race (% Caucasian) | 69 | 53 | 0.082 |
| Sex (% men) | 83 | 86 | 0.713 |
| Current Smoking (% yes) | 39 | 46 | 0.444 |
| Diabetes (% yes) | 20 | 26 | 0.458 |
| Hypertension (% yes) | 64 | 64 | 0.978 |
| Dyslipidemia (% yes) | 60 | 58 | 0.829 |
| Obesity (% yes) | 33 | 25 | 0.361 |
| Metabolic Syndrome (% yes) | 54 | 56 | 0.878 |
| Prior Revascularization (% yes) | 17 | 13 | 0.509 |
Adherence to the Exercise Program
Adherence to exercise was 74±33% during the entire study, as patients completed an average of 53±24 sessions out of a possible 72 scheduled (Table II). Exercise adherence progressively declined from the first two months (86±25%) to the final two months (63±42%). The total duration of exercise performed was 1370±704 minutes, the total walking distance was 46.8±25.5 miles, and the total exercise volume was 4815±2705 MET-min. A substantial training effect was evident, as progressive increases in walking grade during the program did not elicit increases in heart rate and blood pressure during exercise training sessions. Furthermore, walking became more economical during the training sessions, as the estimated oxygen uptake remained relatively constant even though the treadmill grade was progressively increased.
Table II.
Measures from the exercise group during their exercise rehabilitation sessions. Values are means (SD).
| Variables | Months 1 and 2 (N = 106) |
Months 3 and 4 (N = 88) |
Months 5 and 6 (N = 80) |
Months 1 through 6 (N = 106) |
|---|---|---|---|---|
| Exercise Sessions Completed (n) |
21 (6) | 17 (9) | 15 (10) | 53 (24) |
| Exercise Sessions Completed (%) |
86 (25) | 73 (39) | 63 (42) | 74 (33) |
| Exercise Duration (min / exercise session) |
17 (1) | 26 (2) | 36 (3) | 24 (5) |
| Total Exercise Time (min) | 354 (111) | 467 (256) | 549 (374) | 1370 (704) |
| Total Distance Walked (miles)* |
12.0 (4.3) | 19.5 (6.4) | 25.0 (9.0) | 46.8 (25.5) |
| Estimated Oxygen Uptake (ml.kg−1.min−1) |
12.3 (2.7) | 12.2 (2.6) | 12.5 (2.6) | 12.3 (2.3) |
| Exercise Intensity (MET / min) |
3.5 (0.8) | 3.5 (0.7) | 3.6 (0.7) | 3.5 (0.7) |
| Total Volume of Exercise (MET-minutes) † |
1239 (491) | 1949 (695) | 2595 (1010) | 4815 (2705) |
| Walking Speed (mph) | 2.0 (0.3) | 2.1 (0.3) | 2.1 (0.4) | 2.1 (0.4) |
| Walking Grade (%) | 3.7 (2.6) | 5.3 (3.0) | 6.4 (3.4) | 5.5 (3.4) |
| Heart Rate (b/min) | 103 (13) | 103 (13) | 103 (13) | 103 (14) |
| Systolic Blood Pressure (mm Hg) |
167 (17) | 165 (15) | 166 (15) | 166 (17) |
| Diastolic Blood Pressure (mm Hg) |
83 (8) | 82 (8) | 81 (8) | 82 (9) |
MET = metabolic equivalent.
Mileage determined by multiplying walking speed (expressed in mph) and total exercise time.
MET-minutes of exercise determined by multiplying exercise intensity (expressed in MET’s) and total exercise time.
Primary Outcome Measures
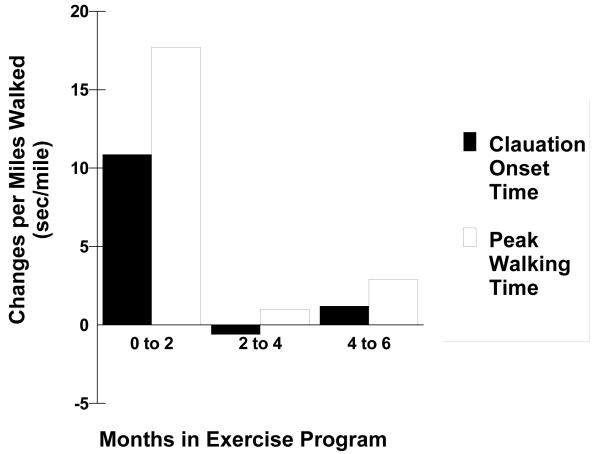
COT and PWT during the graded treadmill test increased in the exercise group (p<0.001) and in the control group (p<0.001), but changes were consistently greater in exercise group (interaction p<.001) (Table III). COT and PWT in the exercise group progressively increased from baseline to month 2 (p<0.05), and from month 2 to month 4 (p<0.05), but there was no significant change from month 4 to month 6 (p>0.05). When change scores in COT and PWT were expressed per mile completed during exercise rehabilitation, the large gains seen during the first two months of training dissipated during the final four months (Figure 2). The regression estimates for linear trend of changes in COT and PWT regressed on miles walked is shown in Table IV.. During the first 2 months of exercise, the regression estimates of change in seconds per mile walked for COT and PWT were 13.1 (p = 0.035) and 17.2 (p = 0.046), respectively (Table IV). The changes in COT and PWT during the final 4 months of exercise were minimal (p>0.05) and did not exceed 1.4 seconds per mile walked (Table IV).
Table III.
Treadmill test exercise performance measures of patients in the usual care control group (n = 36) and exercise group (n = 106). Values are means (SD).
| Variables | Baseline | Month 2 | Month 4 | Month 6 | ANOVA p values ITT |
||
|---|---|---|---|---|---|---|---|
| G | T | GxT | |||||
| Claudication Onset Time (sec) |
|||||||
| Control Group | 146 (112) | 225 (136) * | 213 (158) *† | 218 (159) * | <.001 | <.001 | <.001 |
| Exercise Group | 189 (142) | 333 (188) * | 382 (210) *† | 411 (232) *† | |||
| Peak Walking Time (sec) | |||||||
| Control Group | 386 (226) | 469 (155) | 436 (236) | 446 (308) | <.001 | <.001 | <.001 |
| Exercise Group | 431 (243) | 651 (267) * | 711 (286) *† | 746 (297) *† | |||
| Peak Oxygen Uptake (ml.kg−1.min−1) |
|||||||
| Control Group | 14.2 (3.3) | 14.9 (4.6) | 14.5 (3.3) | 14.5 (3.8) | ns | <.01 | <.001 |
| Exercise Group | 13.8 (3.4) | 14.4 (3.6) | 14.8 (2.9) * | 15.4 (3.6) *† | |||
| Ischemic Window (AUC) | |||||||
| Control Group | 0.75 (0.82) | 0.64 (0.54) | 0.49 (1.53) | 0.69 (0.91) | ns | <.001 | <.001 |
| Exercise Group | 0.81 (0.66) | 0.39 (0.47) * | 0.45 (0.35) * | 0.40 (0.29) * | |||
Change from Baseline (p < .05),
Change from Month 2 (p < 0.05).
Walking economy = oxygen uptake obtained during final minute of walking 2 mph at 0% grade.
Fractional utilization = walking economy oxygen uptake / peak oxygen uptake.
ITT = Intent-to-Treat, G = Group effect, T = Test effect, GxT = Group by Test interaction.
Figure 2.
Changes in claudication onset time and peak walking time per mile walked during different stages of an exercise rehabilitation program.
Table IV.
Regression estimates for linear trend of changes in claudication onset time (COT) and peak walking time (PWT) regressed on miles walked.
| Variables | Intercept (95% CI) (Δ sec/mile) |
Slope (95% CI) (Δ sec/mile) |
r | R2 | P Value |
|---|---|---|---|---|---|
| COT | |||||
| Months 1-2 of Exercise |
−32.96 (−196.16 to 130.24) |
13.07 (0.95 to 25.19) * |
0.233 | 0.054 | 0.035 |
| Months 3-4 of Exercise |
45.48 (−60.00 to 150.96) |
−0.18 (−5.16 to 4.81) |
−0.0088 | 0.0001 | 0.9434 |
| Months 5-6 of Exercise |
1.47 (−6.65 to 9.60) |
−0.0097 (−0.2886 to 0.2693) |
−0.0099 | 0.0001 | 0.9447 |
| PWT | |||||
| Months 1-2 of Exercise |
−0.1253 −226.1905 to 225.9399 |
17.1754 0.3548 to 33.9960 * |
0.2202 | 0.0485 | 0.0455 |
| Months 3-4 of Exercise |
16.6116 −152.0199 to 185.2430 |
1.3949 −6.5760 to 9.3657 |
0.0430 | 0.0018 | 0.7279 |
| Months 5-6 of Exercise |
−1.1184 −7.0578 to 4.8211 |
0.0496 −0.1542 to 0.2534 |
0.0690 | 0.0048 | 0.6268 |
p < 0.05.
Secondary Outcome Measures
Ischemic window following the treadmill test and peak oxygen uptake both improved in the exercise group (p<0.001), which was different than changes in the controls (p<0.001) (Table III). For self-paced exercise performance measurements, pain-free distance and total distance walked during the 6-minute walk test increased in the exercise group (p<0.001) (Table V), which was different than changes in the controls (p<0.001). WIQ speed score, daily physical activity, and calf blood flow under resting, reactive hyperemic, and maximal hyperemic conditions were increased in the exercise group (p<0.001) (Table V), which was different than changes in the controls (p<0.001). Although the group by test interaction was significant for WIQ speed score, the test main effect was not significant because the increases seen in the exercise group were countered by the decreases found in the control group. It should be further noted that the baseline WIQ measures, and other measures of baseline ambulation, such as COT, PWT, peak oxygen uptake, and 6-minute walk pain-free distance and total walking distance, were not different between the exercise and control groups. In fact, the exercise group had higher baseline values on some measures than the control group, and lower values on others.
Table V.
Self-paced ambulation, self-perceived exercise performance, physical activity, and calf blood flow measures of patients in the usual care control group (n = 36) and exercise group (n = 106). Values are means (SD).
| Variables | Baseline | Month 2 | Month 4 | Month 6 | ANOVA p values ITT |
||
|---|---|---|---|---|---|---|---|
| G | T | GxT | |||||
| 6-Minute Walk Pain-Free Distance (m) |
|||||||
| Control Group | 154 (86) | 184 (163) | 160 (95) | 180 (149) | ns | <.001 | <.001 |
| Exercise Group | 165 (92) | 180 (119) | 186 (88) | 210 (106) *† | |||
| 6-Minute Walk Distance (m) |
|||||||
| Control Group | 393 (74) | 404 (197) | 422 (128) | 383 (116) | ns | <.001 | <.001 |
| Exercise Group | 368 (88) | 404 (96) * | 402 (80) * | 396 (92) * | |||
| WIQ Distance Score (%) | |||||||
| Control Group | 34 (34) | 32 (32) | 43 (32) | 38 (30) | ns | <.001 | ns |
| Exercise Group | 34 (30) | 45 (30) * | 50 (33) * | 47 (34) * | |||
| WIQ Speed Score (%) | |||||||
| Control Group | 39 (27) | 34 (41) | 33 (27) | 35 (29) | ns | ns | <.05 |
| Exercise Group | 34 (24) | 43 (24) * | 42 (27) * | 42 (26) * | |||
| WIQ Stair Climbing Score (%) |
|||||||
| Control Group | 43 (29) | 49 (34) | 46 (38) | 45 (37) | ns | ns | ns |
| Exercise Group | 46 (29) | 54 (31) | 47 (31) | 55 (31) | |||
| Daily Physical Activity (kcal/day) |
|||||||
| Control Group | 435 (208) | 308 (288) | 469 (470) | 327 (233) | ns | <.001 | <.001 |
| Exercise Group | 332 (207) | 346 (205) | 413 (261) *† | 410 (272) *† | |||
| Calf Blood Flow: Rest (%/min) |
|||||||
| Control Group | 3.60 (1.17) | 3.02 (1.65) | 3.73 (1.75) † | 3.46 (1.32) | ns | <.001 | <.01 |
| Exercise Group | 3.22 (1.09) | 3.60 (1.29) * | 3.84 (1.29) * | 3.60 (1.06) * | |||
| Calf Blood Flow: PORH (%/min) |
|||||||
| Control Group | 10.42 (4.26) | 8.71 (5.21) | 10.42 (5.26) | 9.53 (5.44) | ns | <.01 | <.001 |
| Exercise Group | 8.50 (3.75) | 9.64 (4.30) * | 10.17 (3.86) * | 10.86 (3.72) *† |
|||
| Calf Blood Flow: Maximal (%/min) |
|||||||
| Control Group | 15.77 (6.56) | 15.53 (10.05) | 13.92 (6.39) | 14.59 (7.36) | ns | <.05 | <.001 |
| Exercise Group | 12.02 (5.03) | 12.89 (5.85) | 13.98 (6.37) * | 15.01 (6.73)*† | |||
Change from Baseline (p < .05),
Change from Month 2 (p < 0.05).
WIQ = walking impairment questionnaire.
PORH = post-occlusive reactive hyperemia.
ITT = Intent-to-Treat, G = Group effect, T = Test effect, GxT = Group by Test interaction.
DISCUSSION
Novel Findings – Length of Exercise Program
The primary novel finding to this investigation is that exercise-mediated gains in COT and PWT occur rapidly within the first two months of exercise rehabilitation, and are maintained with further training. This is quite remarkable given that only a small percentage of the total mileage walked (26%) and the total MET-min of exercise completed (26%) were accomplished during the first two months. This finding has major implications for patients, exercise program personnel, and utilization of resources and facilities. From the patient’s viewpoint, claudication severity can be improved with relatively little effort by walking an average of 1.5 miles per week for two months. These realistic short-term targets may make an exercise program less daunting for many patients, and may be a motivating factor. From the viewpoint of the exercise program personnel and director, less time, effort, and resources are required per patient enrolled in a 2-month program, which translates to a cost savings to train a given number of patients per year. Alternatively, the productivity of the exercise program could increase by training more patients per year with similar time, effort, and utilization of resources. Other studies have found a more continuous increase in COT and PWT during an exercise program.24, 25 It should be noted, however, that if the continuous increase in COT and PWT in previous studies were expressed relative to the greater mileage walked and MET-min of exercise completed during training, the trend of diminishing returns for the outcome measures would be evident with each month in the program.
Secondary Outcome Measures
Peak oxygen uptake increased by 12% following the exercise program, which is consistent with most,9, 15, 25-27 but not all14 previous studies. Furthermore, performance during the 6-minute walk test improved, as we observed a 27% increase in pain-free walking distance and an 8% increase in total walk distance. These findings support our previous observations of a 26-44% increase in 6-minute walk pain-free distance and a 7-12% increase in total 6-minute walk distance following supervised exercise rehabilitation programs.9, 14, 15 In the current study, the 24% increase in self-perceived walking speed from the WIQ questionnaire following exercise rehabilitation agrees with previous studies,13, 28 and is consistent with the increase in objectively measured walking speed as evident by the increase in total walk distance during the 6-minute walk test. We also noted a 24% increase in daily physical activity following exercise rehabilitation, which supports previous observations of 31-44% increases9, 13, 15 and a 68% increase in the daily percentage of time spent in physical activity.28 Although there is a consistent increase in daily physical activity following exercise rehabilitation, it should be noted that there is high between-subject variability at any given time point of measurement. Finally, we observed significant increases in calf blood flow under resting, hyperemic, and maximal conditions, and improvements in the ischemic window, supporting the notion that exercise rehabilitation improves peripheral circulation. However, an increase in calf blood flow is not a consistent finding following exercise rehabilitation.29
Other Components of an Exercise Program to Consider in Treating Claudication
In addition to the program length, an exercise program consists of other key components which define the overall volume of exercise performed. These include intensity, duration, frequency, and mode of exercise, environment in which the exercise was completed (i.e., under medical supervision or home-based), and claudication pain endpoint used during individual exercise sessions to define when walking should be discontinued (i.e., walking to near-maximal, moderate, or onset of pain). Although numerous studies have examined the efficacy of exercise to treat claudication, including 15 randomized trials,30 systematic reviews,30-32 and many more uncontrolled trials,33 relatively few answers can be gleaned from the literature about how each component of exercise influence the increases in COT and PWT.
Formulating an Exercise Prescription
The current investigation focused on the changes in COT and PWT at different lengths of time in an exercise program up to a maximum of six months. Increases in COT and PWT occur rapidly during the first two months of training, and then begin to plateau afterwards, especially when expressing the change scores relatively to mileage walked and MET-min completed during training. We previously reported that intensity of exercise training is not a significant factor for increasing COT and PWT, provided that similar volume of exercise is achieved, as a higher-intensity, shorter duration program was not different than a lower-intensity, longer duration program.9 The lower intensity program may be the preferred method since it is well-tolerated, relatively safe, and is similar to daily ambulation performed in the community setting. Additionally, we recently reported that the efficacy of a monitored, home-based exercise program with periodic feedback approached the level seen with supervised exercise. Others have observed that non-ambulatory exercise, such as arm and leg ergometry34-36 and polestriding37 are efficacious in increasing COT and PWT. Thus, we believe that a more precise exercise prescription is beginning to emerge for treating claudication, consisting of training at relatively low intensity for longer duration per exercise session, and utilizing walking and/or non-ambulatory exercise for a period of at least two months. Furthermore, if this exercise program is monitored with periodic feedback, it does not need to be limited to a hospital-based, supervised setting, but rather this program can be exported to a home-based setting.16
Study Limitations
Although the results of this trial support the efficacy of exercise rehabilitation for PAD patients, several limitations exist. First, patients who participated in this trial were volunteers and therefore may represent those who were more interested in exercise, who had better access to transportation to the program, and who had relatively better health than PAD patients who did not volunteer. Second, the study patients were predominantly men. Consequently, the efficacy of exercise may not generalize to women. Third, the study patients were limited by intermittent claudication, and thus the efficacy of exercise rehabilitation to increase COT and PWT may not be representative of the response in patients with atypical claudication pain. Fourth, the study design was limited to obtaining change scores in outcome measures every two months. It is possible that most of the improvement in COT and PWT seen at the month 2 evaluation actually occurred sooner.
Summary, Conclusion, and Clinical Significance
This prospective, randomized controlled trial demonstrates that COT and PWT increase through the first four months of exercise rehabilitation, with minimal change occurring during the final two months. However, relative to the mileage walked during the program, increases in COT and PWT were only observed during the first two months of the exercise program. We conclude that exercise-mediated gains in COT and PWT occur rapidly within the first two months of exercise rehabilitation, and are maintained with further training. The clinical significance is that a relatively short two-month exercise program may be preferred to a longer program to treat claudication because adherence is higher, costs associated with personnel and utilization of facilities are lower per patient, and more patients can be trained for a given amount of personnel time and resource utilization.
Acknowledgments
Supported by grants from the National Institute on Aging (NIA) (R01-AG-24296 and K01-00657; AWG), a Claude D. Pepper Older Americans Independence Center grant from NIA (P60-AG12583), and a Geriatric, Research, Education, and Clinical Center grant from Department of Veterans Affairs and Veterans Affairs Medical Center Baltimore. Final peer-reviewed version is subject to NIH Public Access Policy, and will be submitted to PubMed Central.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration Information – URL: http://www.ClinicalTrial.Gov. Unique Identifier: NCT00654810.
REFERENCES
- 1.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 2.Brass EP, Hiatt WR. Review of mortality and cardiovascular event rates in patients enrolled in clinical trials for claudication therapies. Vasc.Med. 2006;11:141–5. doi: 10.1177/1358863x06069513. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N.Engl.J.Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 4.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med.Sci.Sports Exerc. 1991;23:402–8. [PubMed] [Google Scholar]
- 5.Hiatt WR, Nawaz D, Regensteiner JG, Hossack KF. The evaluation of exercise performance in patients with peripheral vascular disease. J Cardiopulmonary Rehabil. 1988;12:525–32. [Google Scholar]
- 6.McDermott MM, Liu K, O’Brien E, Guralnik JM, Criqui MH, Martin GJ, et al. Measuring physical activity in peripheral arterial disease: a comparison of two physical activity questionnaires with an accelerometer. Angiology. 2000;51:91–100. doi: 10.1177/000331970005100201. [DOI] [PubMed] [Google Scholar]
- 7.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc.Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Wind J, Koelemay MJ. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomised controlled trials. Eur J Vasc Endovasc Surg. 2007;34:1–9. doi: 10.1016/j.ejvs.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Gardner AW, Montgomery PS, Flinn WR, Katzel LI. The effect of exercise intensity on the response to exercise rehabilitation in patients with intermittent claudication. J.Vasc.Surg. 2005;42:702–9. doi: 10.1016/j.jvs.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 10.Lohman TC, Roche AF, Marubini E. Anthropometric standardization reference manual. Human Kinetics Books; Champaign, IL: 1988. pp. 39–70. [Google Scholar]
- 11.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N.Engl.J Med. 2001;344:1608–21. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 12.Pentecost MJ, Criqui MH, Dorros G, Goldstone J, Johnston KW, Martin EC, et al. Guidelines for peripheral percutaneous transluminal angioplasty of the abdominal aorta and lower extremity vessels. A statement for health professionals from a special writing group of the Councils on Cardiovascular Radiology, Arteriosclerosis, Cardio-Thoracic and Vascular Surgery, Clinical Cardiology, and Epidemiology and Prevention, the American Heart Association. Circulation. 1994;89:511–31. doi: 10.1161/01.cir.89.1.511. [DOI] [PubMed] [Google Scholar]
- 13.Gardner AW, Katzel LI, Sorkin JD, Killewich LA, Ryan A, Flinn WR, et al. Improved functional outcomes following exercise rehabilitation in patients with intermittent claudication. J.Gerontol.A Biol.Sci.Med.Sci. 2000;55:M570–M7. doi: 10.1093/gerona/55.10.m570. [DOI] [PubMed] [Google Scholar]
- 14.Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, et al. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J.Am.Geriatr.Soc. 2001;49:755–62. doi: 10.1046/j.1532-5415.2001.49152.x. [DOI] [PubMed] [Google Scholar]
- 15.Gardner AW, Katzel LI, Sorkin JD, Goldberg AP. Effects of long-term exercise rehabilitation on claudication distances in patients with peripheral arterial disease: a randomized controlled trial. J.Cardiopulm.Rehabil. 2002;22:192–8. doi: 10.1097/00008483-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123:491–8. doi: 10.1161/CIRCULATIONAHA.110.963066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner AW. Reliability of transcutaneous oximeter electrode heating power during exercise in patients with intermittent claudication. Angiology. 1997;48:229–35. doi: 10.1177/000331979704800305. [DOI] [PubMed] [Google Scholar]
- 18.Feinberg RL, Gregory RT, Wheeler JR, Snyder SO, Jr., Gayle RG, Parent FN, III, et al. The ischemic window: a method for the objective quantitation of the training effect in exercise therapy for intermittent claudication. J.Vasc.Surg. 1992;16:244–50. doi: 10.1067/mva.1992.36947. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J.Am.Geriatr.Soc. 1998;46:706–11. doi: 10.1111/j.1532-5415.1998.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 20.Regensteiner JG, Steiner JF, Panzer RL, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–52. [Google Scholar]
- 21.Gardner AW, Poehlman ET. Assessment of free-living daily physical activity in older claudicants: validation against the doubly labeled water technique. J Gerontol.A Biol.Sci.Med.Sci. 1998;53:M275–M80. doi: 10.1093/gerona/53a.4.m275. [DOI] [PubMed] [Google Scholar]
- 22.Sieminski DJ, Cowell LL, Montgomery PS, Pillai SB, Gardner AW. Physical activity monitoring in patients with peripheral arterial occlusive disease. J.Cardiopulm.Rehabil. 1997;17:43–7. doi: 10.1097/00008483-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Gardner AW, Sieminski DJ, Killewich LA. The effect of cigarette smoking on free-living daily physical activity in older claudication patients. Angiology. 1997;48:947–55. doi: 10.1177/000331979704801103. [DOI] [PubMed] [Google Scholar]
- 24.Hiatt WR, Creager MA, Amato A, Brass EP. Effect of propionyl-L-carnitine on a background of monitored exercise in patients with claudication secondary to peripheral artery disease. J Cardiopulm Rehabil Prev. 2011;31:125–32. doi: 10.1097/HCR.0b013e3181f1fd65. [DOI] [PubMed] [Google Scholar]
- 25.Hiatt WR, Wolfel EE, Meier RH, Regensteiner JG. Superiority of treadmill walking exercise versus strength training for patients with peripheral arterial disease. Implications for the mechanism of the training response. Circulation. 1994;90:1866–74. doi: 10.1161/01.cir.90.4.1866. [DOI] [PubMed] [Google Scholar]
- 26.Regensteiner JG, Meyer TJ, Krupski WC, Cranford LS, Hiatt WR. Hospital vs home-based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology. 1997;48:291–300. doi: 10.1177/000331979704800402. [DOI] [PubMed] [Google Scholar]
- 27.Hiatt WR, Regensteiner JG, Hargarten ME, Wolfel EE, Brass EP. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation. 1990;81:602–9. doi: 10.1161/01.cir.81.2.602. [DOI] [PubMed] [Google Scholar]
- 28.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J.Vasc.Surg. 1996;23:104–15. doi: 10.1016/s0741-5214(05)80040-0. [DOI] [PubMed] [Google Scholar]
- 29.Parmenter BJ, Raymond J, Singh MA Fiatarone. The effect of exercise on haemodynamics in intermittent claudication: a systematic review of randomized controlled trials. Sports Med. 2010;40:433–47. doi: 10.2165/11531330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Wind J, Koelemay MJ. Exercise Therapy and the Additional Effect of Supervision on Exercise Therapy in Patients with Intermittent Claudication. Systematic Review of Randomised Controlled Trials. Eur.J.Vasc.Endovasc.Surg. 2007 doi: 10.1016/j.ejvs.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 31.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N.Engl.J.Med. 2002;347:1941–51. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 32.Stewart AH, Lamont PM. Exercise training for claudication. Surgeon. 2007;5:291–9. doi: 10.1016/s1479-666x(07)80028-x. [DOI] [PubMed] [Google Scholar]
- 33.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975–80. [PubMed] [Google Scholar]
- 34.Tew G, Nawaz S, Zwierska I, Saxton JM. Limb-specific and cross-transfer effects of arm-crank exercise training in patients with symptomatic peripheral arterial disease. Clin Sci (Lond) 2009;117:405–13. doi: 10.1042/CS20080688. [DOI] [PubMed] [Google Scholar]
- 35.Treat-Jacobson D, Bronas UG, Leon AS. Efficacy of arm-ergometry versus treadmill exercise training to improve walking distance in patients with claudication. Vasc Med. 2009;14:203–13. doi: 10.1177/1358863X08101858. [DOI] [PubMed] [Google Scholar]
- 36.Walker RD, Nawaz S, Wilkinson CH, Saxton JM, Pockley AG, Wood RF. Influence of upper- and lower-limb exercise training on cardiovascular function and walking distances in patients with intermittent claudication. J.Vasc.Surg. 2000;31:662–9. doi: 10.1067/mva.2000.104104. [DOI] [PubMed] [Google Scholar]
- 37.Collins EG, Langbein WE, Orebaugh C, Bammert C, Hanson K, Reda D, et al. Cardiovascular training effect associated with polestriding exercise in patients with peripheral arterial disease. J Cardiovasc Nurs. 2005;20:177–85. doi: 10.1097/00005082-200505000-00009. [DOI] [PubMed] [Google Scholar]