Abstract
Background
Mechanisms underlying esophageal remodeling with subepithelial fibrosis in eosinophilic esophagitis (EoE) have not been delineated.
Objectives
To explore a role for Epithelial Mesenchymal Transition (EMT) in EoE, and whether EMT resolves with treatment.
Methods
Esophageal biopsies from 60 children were immunostained for epithelial (cytokeratin) and mesenchymal (vimentin) EMT biomarkers, and EMT quantified. Subjects studied had EoE (n=17), EoE-indeterminate (n=15), GERD (n=7) or normal esophagus (n=21). EMT was analyzed for relationships to diagnosis, eosinophils, and indices of subepithelial fibrosis, eosinophil peroxidase (EPX) and TGF-β immunostaining. EMT was assessed in pre- and post-treatment biopsies from 18 EoE subjects treated with elemental diet, six-food elimination diet, or topical corticosteroids (n=6/group).
Results
TGF-β1 treatment of esophageal epithelial cells in vitro for 24hrs induced upregulation of mesenchymal genes characteristic of EMT including N-cadherin (3.3-fold), vimentin (2.1-fold) and fibronectin (7.5-fold). EMT in esophageal biopsies was associated with EoE (or indeterminate EoE), but not GERD or normal esophagus, and was correlated to eosinophils (r=0.691), EPX (r=0.738) and TGF-β (r=0.520) immunostaining, and fibrosis (r=0.644) indices. EMT resolved with EoE treatments that induced clinicopathologic remission with reduced eosinophils. EMT decreased significantly post-treatment by 74.1% overall in the 18 treated EoE subjects; pre- vs. post-treatment EMT scores–3.17±0.82 vs. 0.82±0.39 (p<0.001), with similar decreases within treatment groups. Pre-/post-treatment EMT was strongly correlated with eosinophils for combined (r=0.804, p< 0.001) and individual treatment groups.
Conclusions
EMT likely contributes to subepithelial fibrosis in EoE, resolves with treatments that decrease esophageal inflammation, and its resolution correlates with decreased numbers of esophageal eosinophils.
Keywords: eosinophil, esophagitis, epithelium, remodeling, fibrosis, mesenchymal, vimentin, cytokeratin
INTRODUCTION
Eosinophilic esophagitis (EoE) has emerged as an increasingly recognized immune-mediated, food-allergy or aeroallergen associated, chronic inflammatory disorder of the esophagus.1, 2 Prolonged unbridled esophageal inflammation may lead to structural and functional changes including thickening of the mucosa and muscularis, dysmotility, decreased compliance, food impaction and strictures.3–5 A variety of clinical presentation patterns ranging from feeding difficulties in toddlers to solid food dysphagia and food impaction in adolescents and adults suggest that structural and functional changes may be part of the natural history of EoE.6 This is further supported by pediatric studies showing that subepithelial fibrosis occurs in greater than 50% of children with EoE.7, 8 Understanding mechanisms leading to subepithelial fibrosis in EoE could lead to identification of novel therapeutic targets.
Epithelial-Mesenchymal Transition (EMT) describes a series of events during which epithelia lose many epithelial characteristics including polarity, expression of epithelial markers and tight junctions, and acquire properties of mesenchymal cells, including motility, loose cell adhesion via N-cadherin, and de-polarized cytoskeletal arrangements such as vimentin.9 EMT facilitates development of tissue fibrosis in different organ systems in response to injury and chronic inflammation, and is associated with the development of fibrosis in kidney, lung (idiopathic pulmonary fibrosis, asthma), liver, heart (cardiac fibrosis) and gastrointestinal tract (Crohn’s disease).10–12 Whether EMT occurs in the esophagus and contributes to subepithelial fibrosis and remodeling in EoE has not been explored.
The purposes of this study were to determine whether EMT occurs in children with EoE and, if successful treatment of EoE (symptoms, histological remission) results in resolution of EMT. Results demonstrate that EMT occurs to a significantly greater degree in the esophageal tissues of children with EoE compared to those of children with gastroesophageal reflux disease (GERD) or those with normal esophageal tissue. The degree of EMT correlates with traditional measures of esophageal inflammation and remodeling in EoE including eosinophil number, expression of remodeling factors (TGF-β) and extent of subepithelial fibrosis. EMT resolves in EoE in response to treatments that decrease esophageal inflammation as characterized by decreases in eosinophil burden.
METHODS
Cell culture and induction of EMT in vitro
Human esophageal epithelial HET-1A cells (ATCC, Manassas, VA) were maintained in Bronchial Epithelial Growth Media (Lonza/Clonetics, Walkersville, MD). For mRNA analysis, cells at confluence in 6-well plates were cultured an additional 24 or 48 hours in fresh media ±5ng/ml TGF-β1 (R&D Systems, Minneapolis, MN). Expression of mRNAs encoding adhesion and cytoskeletal proteins representative of epithelial cells (E-cadherin, Cytokeratin-8 and Cytokeratin-14) and mesenchymal cells (N-cadherin, Vimentin and Fibronectin) as biomarkers of EMT were analyzed in total RNA by RT-Q-PCR.
Real-time reverse-transcription quantitative polymerase chain reaction (RT-Q-PCR)
Total RNA was prepared using QIAshredder columns, RNeasy Mini RNA Isolation Kit (Qiagen, Valencia, CA) and cDNA synthesized using a High Capacity cDNA Archive Kit (Applied BioSystems, Foster City, CA).13 Briefly, from 500ng RNA, gene transcripts were assessed using Taqman Gene Expression Assay FAM™ dye-labeled TaqMan® MGB probes (Table E1 in the Online Repository) (Applied Biosystems) and ABsolute™ Blue QPCR ROX MasterMix (Thermo Scientific, Surrey, UK). Thermocycling and analysis was performed with an ABI-7300 system. Data was normalized to 18S expression and calculated as RQ (Relative Quantity; 2−DDCt, where Ct is cycle threshold).
Study populations and design: Clinical biopsy specimens
Retrospective analysis of 890 archived esophageal biopsies from pediatric subjects at Children’s Hospital Colorado from 2006 was performed. Of these, tissue sections from 60 subject’s biopsies with >2 mm lamina propria were analyzed based on: (1) availability of sufficient formalin-fixed, paraffin-embedded tissue, and (2) subject diagnosis of EoE, EoE-indeterminate, gastroesophageal reflux disease (GERD) or normal esophagus. Diagnostic criteria were: active EoE–symptomatic with ≥15 eosinophils/high power field (HPF) and other causes excluded, indeterminate EoE–symptomatic with <15 eosinophils/HPF and clinical features suggestive of EoE or clinical features of EoE and ≥15 eosinophils/HPF without documented treatment with PPIs or pH probe to exclude GERD,1, 14 GERD–PPI-responsive esophagitis with <15 eosinophils/HPF, and normal controls–subjects undergoing clinically indicated endoscopy, but with endoscopically and histologically normal esophagus.
To assess the impact of treatment on EMT, 18 pediatric subjects with EoE were randomly selected, from the eosinophilic esophagitis patient database at Children’s Memorial Hospital in Chicago, who had achieved histological remission following treatment with elemental diet (ED), empiric six-food elimination diet (SFED) or topical corticosteroid (TC) (n=6/group). Diagnostic criteria for EoE were as above, with histological remission defined as ≤10 eosinophils/HPF. Post-treatment biopsies were obtained from mid and distal esophagus after at least 6 weeks of treatment, and pre- and post-treatment tissue sections immunostained for EMT.
Assessment of epithelial mesenchymal transition: EMT index
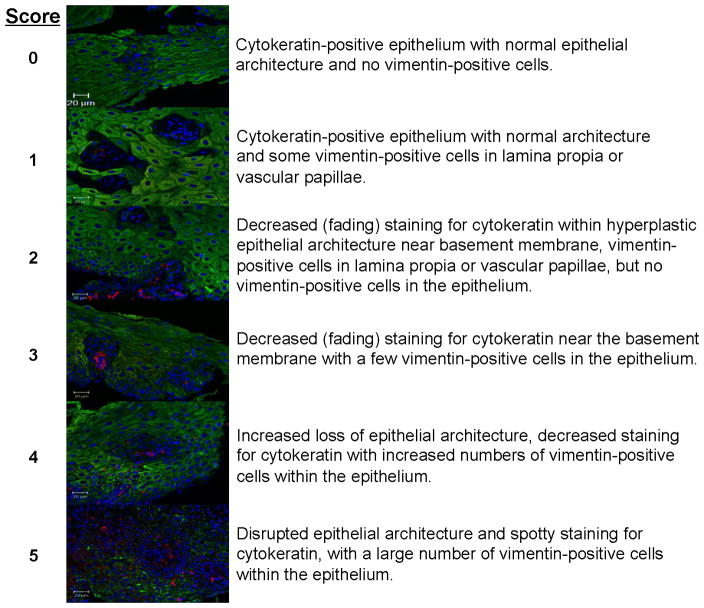
Three-color immunofluorescence and confocal microscopy were utilized to identify and evaluate epithelial and mesenchymal cells using cytokeratin (epithelial), vimentin (mesenchymal), and DAPI (nuclear) stains in esophageal biopsy tissue sections. A 6-point scale was developed to score the amount of EMT, assessing the presence, location and degree of vimentin positive mesenchymal cells and loss of cytokeratin staining of epithelial cells in the context of hyperplastic changes in epithelial architecture (Figure 2). Confocal microscopy was used to acquire fluorescent images of 18–25 HPFs covering the entirety of each tissue section. Confocal images of stained sections were analyzed in a blinded manner by two independent observers (NA, KRP), and scored for EMT; mean EMT indices/HPF were calculated.
Figure 2. Six point EMT assessment scale for quantitation in esophageal biopsies.
Representative merged confocal images of immunofluorescent staining for cytokeratin (green) and vimentin (red), with DAPI-stained nuclei (blue). The EMT score is indicated on the left, with descriptions of the characteristics of EMT biomarker staining relative to changes in epithelial architecture on the left. The score incorporates: (1) location and amount of vimentin-positive (mesenchymal marker) cell staining within the epithelium, and (2) decreased cytokeratin (epithelial marker) staining in the hyperplastic epithelium.
Assessment of eosinophil counts in biopsies
Eosinophils in hematoxylin-eosin (H&E)-stained slides were quantified in HPFs (area=0.26mm2) by counting the five most densely populated regions of the tissue, and peak (highest in single section) and mean values recorded.
Anti-Eosinophil Peroxidase Immunohistochemistry: EPX index
Sections from esophageal biopsies were stained using anti-eosinophil peroxidase monoclonal antibody (EPX-mAb, hybridoma MM25-82.2.1, Mayo Clinic, AZ).15 Based on the presence of eosinophils, evidence of eosinophil degranulation, and extent of eosinophil infiltration and/or degranulation, an EPX index was assigned (CP) to each subject’s biopsies as previously described.15
Fibrosis Index
H&E stained sections were utilized to assess the degree of fibrosis in esophageal biopsies. A fibrosis score from 0–2 was assigned by three independent blinded observers (SW, VM, JCM) based on the number of fibroblasts, thickness and character of collagen bundles and collagen accumulation as previously described.8 A fibrosis score of 0 indicated loose, lacy, individual collagen fibrils, a score of 1 more densely packed collagen fibrils along the basal lamina with loss of individual lacy-ness, but further away from the basal lamina normalized to lacy, individual fibrils, and a fibrosis score of 2 indicated tightly packed collagen fibrils with individual fibrils no longer evident (Figure E1 in the Online Repository).
Transforming growth factor β1 immunohistochemistry: TGF-β1 Index
Sections from esophageal biopsies were stained with anti-TGF-β1 antibody (Peprotech, Cat#500-M66, Rocky Hill, NJ) as previously described16 and scored by three independent blinded observers (SW, VM, JCM). A three point scoring system was used based on staining of the epithelium. A score of 1 (mild staining) corresponded to blue/light brown epithelium, 2 (moderate staining) to darker brown epithelium, and 3 (severe staining) to dark brown staining throughout the entire epithelium.
Statistical analyses
Data were analyzed by ANOVA with Bonferroni’s multiple comparisons test, or 2-tailed Student’s t-test. Differences between means were considered significant at the p<0.05 level, and are noted as *p<0.05, **p<0.01, ***p<0.001. Relationships between EMT index, eosinophil counts and staining indices for EPX, fibrosis and TGF-β were analyzed using Pearson’s test; correlation coefficients (r-values) were considered significant at p<0.05.
RESULTS
EMT is induced by TGF-β in cultured esophageal epithelial cells
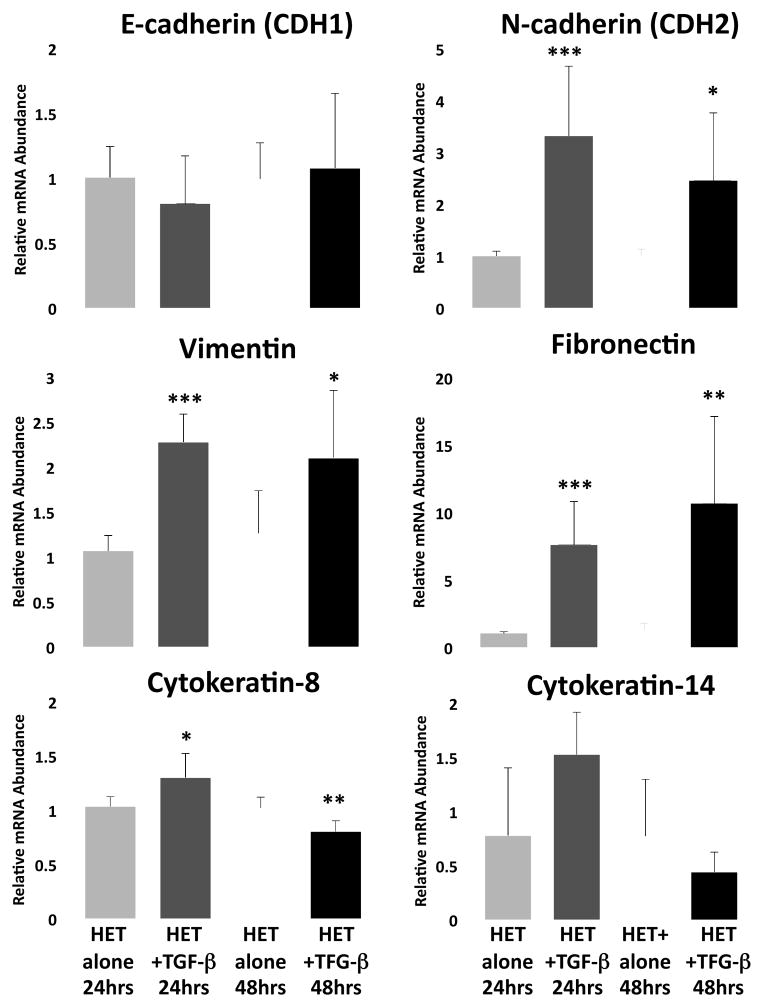
To determine whether esophageal epithelium has the capacity to undergo EMT, we analyzed the ability of TGF-β1 to induce EMT in vitro in the HET-1A esophageal epithelial cell line. Culture of HET-1A cells with TGF-β1 decreased gene transcription for epithelial biomarkers including adhesion proteins and cytoskeletal components representative of the epithelial phenotype, cytokeratin-8 (22% decrease, p<0.01) and cytokeratin-14 (44% decrease, p=0.27) (Figure 1). Correspondingly, increased mRNA expression for a number of biomarkers representative of the mesenchymal phenotype including N-cadherin (adhesion) (3.3-fold, p<0.001), vimentin (cytoskeletal) (2.1-fold, p<0.001) and fibronectin (extracellular matrix) (7.5-fold, p<0.001) was detected (Figure 1), all gene expression changes characteristic of metastable EMT.17–19
Figure 1. Induction of mesenchymal genes in esophageal epithelial cells in culture: Evidence for epithelial-mesenchymal transition (EMT) in vitro.
Analysis of adhesion molecules and cytoskeletal component expression representative of epithelial cells (E-cadherin, Cytokeratin-8 and Cytokeratin-14), and mesenchymal cells (N-cadherin, Vimentin and Fibronectin) in HET-1A cells following 24 and 48 hours of culture with TGF-β1 (5ng/ml). Data are expressed as mean (±SD) relative mRNA abundance compared to untreated controls as determined by real-time RT-Q-PCR. Statistical significance was assessed by Students t-test compared to untreated controls at 24 and 48 hours; *p<0.05, **p<0.01, ***p<0.001 (n=5–7/group).
EMT is present in esophageal tissue from subjects with active EoE
Clinical characteristics of the 60 subjects studied based on diagnostic criteria are shown in Table I. Subjects ranged from 8 months to 22 years old, with duration of symptoms from 2 months to 7 years. Treatment histories for active EoE subjects included proton pump inhibitors (PPIs), topical corticosteroid (TC) and elimination of allergenic foods, whereas subjects with normal esophagus, GERD and indeterminate EoE had only been treated with PPIs. To determine the impact of treatments for EoE on EMT, pre- and post-treatment biopsies from an additional 18 randomly selected pediatric EoE subjects were analyzed. Patients ranged in age from 6–13, and had been treated with ED, SFED or TC (n=6/treatment). Subjects’ clinical symptoms had resolved, and histopathologic remission of their EoE was defined as ≤10 eosinophils/HPF with normalization of epithelial hyperplasia.
Table I. Clinicopathologic Characteristics of Patient Study Groups.
Demographic and clinical features of the 60 subjects evaluated for EMT, fibrosis, TGF-β, EPX index and eosinophil counts. The subject groups include patients undergoing a clinically indicated endoscopy with biopsy, but with an otherwise histologically normal esophagus, and those with recorded diagnoses of GERD, indeterminate EoE and EoE.
| Normal | GERD | EoE (indeterminate)a | EoE | |
|---|---|---|---|---|
| n (= 60) | 21 | 7 | 15 | 17 |
| Gender | ||||
| Male/Female | 10/11 | 7/0 | 8/7 | 10/7 |
| Age Range | 8 months – 15 yrs | 1 – 18 yrs | 22 months – 21 yrs | 1–15 yrs |
| Duration of Symptoms | 3 months – 6 yrs | 2 months – 2 yrs | 1 day – 3 yrs | 1 – 7 yrs |
| Previous Treatments | Zantac, Prevacid, Prilosec, Reglan (metoclopromide), Zyrtec (cetirizine), Miralax (polyethylene glycol) | Zantac, Prevacid, Prilosec, Protonix | none, unknown Zantac, TUMS, Prevacid | Zantac (ranitidine), Prevacid (lansoprazole), Prilosec (Omeprazole), swallowed Flovent (fluticasone), Singulair, Elimination of foods identified as allergic |
| Number of Eosinophils (peak/hpf range) | 0–2 | 0–30 | 0–87 | 7–123 |
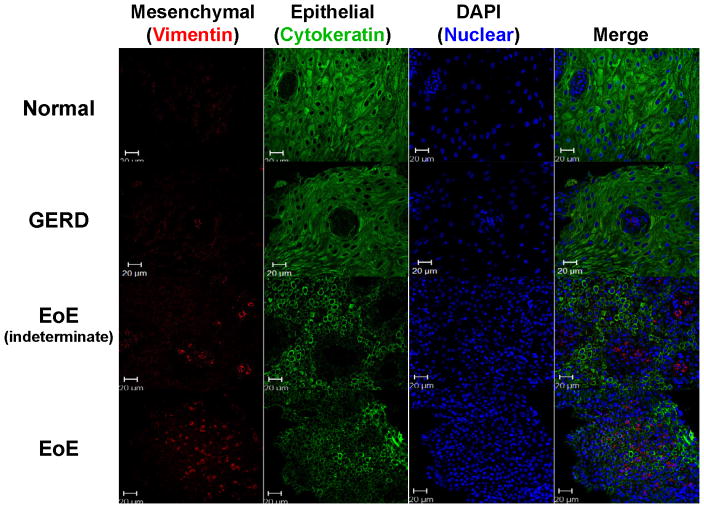
To determine whether EMT occurred in the esophageal mucosa of subjects with EoE, tissue sections were dual-stained for the EMT biomarkers cytokeratin (epithelial) and vimentin (mesenchymal) (Figure 2). Vimentin-positive cells were present within the lamina propria, consistent with fibroblasts and/or myofibroblasts. Importantly, vimentin-positive cells were visualized within the hyperplastic epithelium (Figure 2), consistent with the process of EMT.17, 19 A small number vimentin-positive cells were also cytokeratin-positive, suggesting cells in transition between epithelial and mesenchymal phenotypes, a characteristic feature of EMT, but these were infrequent and more difficult to visualize (Figure E2 in the Online Repository). Epithelium-localized vimentin-positive mesenchymal-like cells were found most commonly in tissues from subjects with active EoE–12/17 (70.6%) and indeterminate EoE–11/15 (73.3%) compared to subjects with GERD–1/8 (12.5%) or normal esophagus (0/21) (Figure 3). Tissues from subjects with active EoE or indeterminate EoE, but not GERD or normal esophagus had markedly decreased epithelial staining for cytokeratins, coupled with vimentin-positive cells within the epithelium, a characteristic appearance of EMT (Figure 3), and representative of a score of 5 on the 6-point EMT scale (Figure 2). EMT scores were significantly higher in tissues of subjects with active EoE (3.08±0.25) and indeterminate EoE (2.70±0.31) compared to GERD (1.71±0.33) or normal esophagus (1.28±0.15) (Figure 4, Table E2 in the Online Repository).
Figure 3. Immunofluorescent staining for EMT in subject study groups.
Immunofluorescent staining for cytokeratin (epithelial marker – green) and vimentin (mesenchymal marker – red) in the epithelium, with DAPI nuclear counterstain (blue). Representative confocal images from esophageal biopsies of subjects with EoE, indeterminate EoE, GERD and normal esophagus are shown. Quantitative assessment of the EMT index for these subject groups is shown in Figure 4.
Figure 4. Quantitative assessment of EMT in subjects with EoE, GERD and normal esophagus.
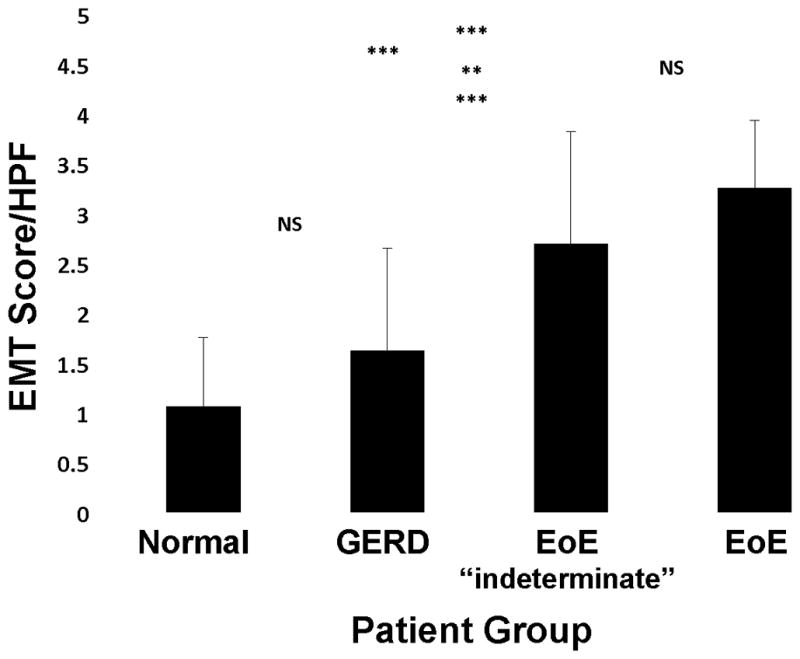
Mean (±SD) EMT scores for the four subject groups are shown. The highest EMT index was associated with EoE, followed by EoE-indeterminate > GERD > normal. The mean for the normal esophagus “control” group was right skewed, i.e., more values were closer to an EMT score of zero, whereas the mean for EoE was slightly left-skewed. The level of EMT in EoE was not significantly different from the EoE-indeterminate group, whereas both these subject groups had significantly greater EMT scores than GERD and normal esophagus. Comparative EMT scores for the two independent observers are shown in Table E2 in the Online Repository. (NS= not significant, **p<0.01, ***p<0.001)
EMT correlates with measures of eosinophilic inflammation
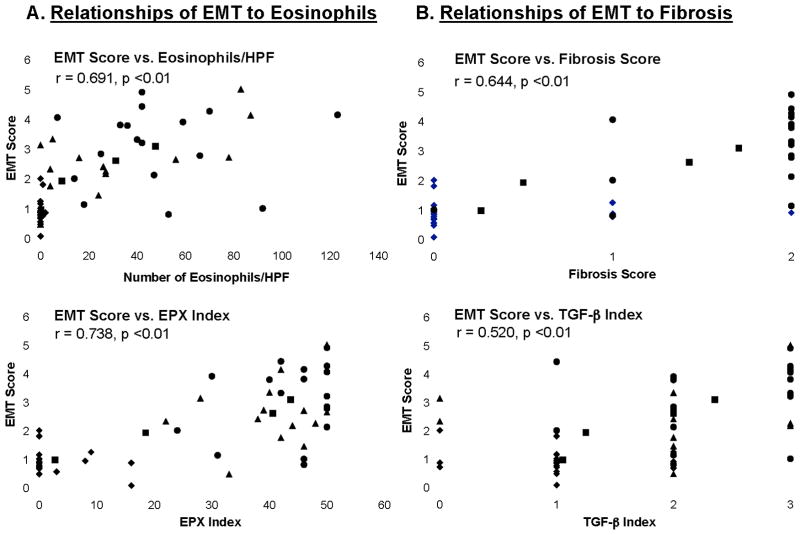
Since eosinophils are potent sources of remodeling factors associated with EMT, e.g. TGF-β, we quantitated the eosinophil burden associated with active EoE and correlated it with EMT scores. Consistent with previous studies, mean eosinophil counts and EPX scores (±SEM) from tissues of subjects with active EoE (46.2±6.9 eos/HPF and 43.4±1.9) and indeterminate EoE (31.5±7.9 eos/HPF and 40.9±2.1) were significantly greater than those from subjects with GERD (5.0±4.2 eos/HPF and 14.6±5.9) and normal esophagus (0.2±0.1 eos/HPF and 2.7±1.2) (Table E2 in the Online Repository). Comparison of subjects’ EMT scores to peak eosinophil counts/HPF (Figure 5A, top) and EPX index (Figure 5A, bottom) identified significant correlations for both eosinophils (r=0.691, p<0.01) and EPX index (r=0.738, p<0.01).
Figure 5. EMT scores correlate with measures of esophageal eosinophils and staining for EPX, and with subepithelial fibrosis and staining for TGF-β.
EMT scores/HPF were analyzed for relationships to subjects’ (A) peak numbers of esophageal eosinophils/HPF (top) and staining index for EPX (bottom) and, (B) fibrosis index (top) and TGF-β staining index (bottom) for all 60 individuals in the subject groups. Pearson’s correlation coefficient (r) and its associated statistical significance are shown for all 60 subjects (dot plot with symbols) comprising the subject groups including EoE (●), EoE-indeterminate (▲), GERD (○) and normal esophagus (◆) (dot plots with symbols). The trend line for the mean indices (■) is also shown (solid line).
EMT correlates with esophageal subepithelial fibrosis
We analyzed correlations between EMT scores and indices of subepithelial fibrosis and TGF-β1 expression in esophageal biopsies from the EoE and other study groups. The mean (±SEM) fibrosis index was significantly higher in tissues from subjects with EoE (1.67±0.14) and indeterminate EoE (1.47±0.19) compared to GERD (0.29±0.3) and normal esophagus (0.26±0.13) (EoE vs. GERD, p<0.001 and vs. normal, p< 0.001; indeterminate EoE vs. GERD (p<0.01) and vs. normal, p<0.001; Table E2 in the Online Repository). Of note, EMT scores were significantly correlated with the fibrosis index (r=0.644, p<0.01) (Figure 5B, top). Similarly, the TGF-β index was significantly higher in active EoE (2.33±0.16) and indeterminate EoE (2.0±0.24) compared to GERD (1.14±0.34) or normal esophagus (1.09±0.14) (EoE vs. GERD, p<0.01 and vs. normal, p< 0.001; indeterminate EoE vs. GERD, ns and vs. normal, p<0.01; Table E2 in the Online Repository). As well, EMT scores were significantly correlated with the TGF-β index (r= 0.520, p<0.01) (Figure 5B, bottom).
Treatment of EoE resolves EMT
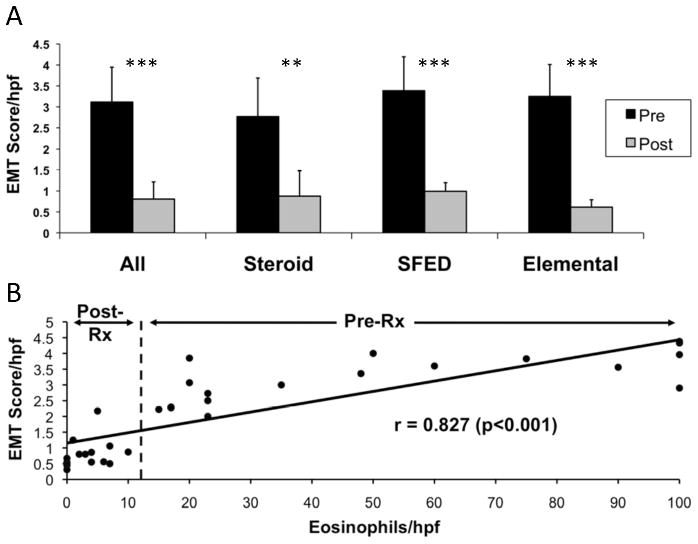
To determine if treatment impacts esophageal EMT in children with EoE, EMT was quantitated in esophageal biopsies before and after treatments known to induce clinicopathological remission (Figure 6A, Table E3 in the Online Repository). Following treatment, EMT scores decreased significantly in all subjects (pre-treatment, 3.17±0.17 vs. post-treatment, 0.82±0.09). Analysis of EMT with respect to individual treatments (n=6 subjects/group) showed similarly decreased EMT scores–TC (pre-treatment, 2.77±0.92 vs. post-treatment, 0.88±0.61, a 68.2% decrease, p<0.001), SFED (pre-treatment, 3.49±0.71 vs. post-treatment, 0.99±0.25, a 72.8% decrease, p<0.001), and ED (pre-treatment, 3.25±0.76 vs. post-treatment, 0.61±0.18, an 81.2% decrease, p<0.001).
Figure 6. Resolution of esophageal EMT in EoE subjects following treatment: correlation with eosinophil load.
A. The presence/amount of EMT was scored in EoE subjects treated with three different modalities that reduce epithelial eosinophilic inflammation. Mean EMT score (±SD) pre- and post-treatment is shown (A) for all treated subjects combined (n=18), and for individual treatment groups, TC, SFED, or ED (n=6/group) (see Table E3 in the Online Repository for % reduction in EMT). B. Resolution of esophageal EMT was directly correlated with the number of esophageal eosinophils pre- and post-treatments that reduced the esophageal eosinophil burden. The correlation coefficient (r) and associated significance is shown in (B) for the relationship between all 18 EoE subjects’ pre- and post-treatment EMT scores and peak eosinophil counts (combined n=36). The vertical dashed line delineates pre- from post-treatment eosinophil counts/EMT scores (see Table E4 and Figure E3 in the Online Repository for correlations within each of the individual treatment groups). NS=not significant, *p<0.05, **p<0.01, ***p<0.001.
To determine whether resolution of EMT was associated with a decline in eosinophils, EMT scores were compared to peak eosinophil counts in biopsies obtained from all 18 subjects before and after treatment. There was a strong positive correlation of EMT with subjects’ peak eosinophils/HPF for the combined EoE treatment groups (r=0.804, p<0.001, n=36) (Figure 6B) and within individual treatment groups (TC, r=0.868; SFED, r=0.857; ED, r=0.820; all p<0.001, all n=12) (Figure E3 and Table E4 in the Online Repository).
DISCUSSION
Since esophageal tissue may demonstrate significant epithelial basal zone hyperplasia and subepithelial fibrosis in EoE, we hypothesized that EMT may be one of the processes associated with these remodeling events. Our results identified the presence of EMT in children with active EoE, and showed that treatments that resolve eosinophilic inflammation and epithelial hyperplasia reverse EMT and subepithelial fibrosis. We also showed that the degree of EMT and its resolution in EoE is strongly correlated with the load of tissue eosinophils within the esophagus.
Esophageal remodeling with subepithelial fibrosis occurs in both children and adults with EoE.6–8, 20, 21 Histologically, the subepithelial space is occupied by increased collagen deposition, and utilizing endoscopic ultrasonography (EUS), several studies demonstrated significant thickening of the mucosa, submucosa and muscularis, suggestive of fibrosis.4, 22, 23 Most recently, Straumann et al24 used EUS to show that in adolescents and adults with long-term EoE (symptoms for 9.3±5.2 years), there was marked thickening of these esophageal layers, leading to speculation that in some patients, chronic unbridled inflammation in EoE results in fibrosis and remodeling. Molecular support for remodeling arises from studies showing that in association with subepithelial fibrosis and esophageal stricture formation, there is increased expression of TGF-β1 and its downstream signaling molecules phospoSMAD2/3.8, 25, 26 TGF-β1 expressing cells include eosinophils8 and mast cells.25 Thus, current evidence suggests that increased expression of and signaling by profibrotic TGF-β1 is key to induction of esophageal fibrosis in EoE.6
The origins of mesenchymal cells (fibroblasts, myofibroblasts) participating in tissue repair during chronic inflammation and tissue damage, notably fibrosis, are still poorly understood. Emerging evidence from fields including allergic diseases and asthma suggests EMT contributes to the genesis of disease-related fibroblasts, myofibroblasts and development of tissue fibrosis, representing a significant source of these fibrogenic cells.9, 11, 27, 28 We demonstrated the presence of vimentin-positive mesenchymal-like cells in the context of loss of normal epithelial architecture and decreased expression of cytokeratins within hyperplastic epithelium in a majority of our subjects with active EoE compared to those with GERD or normal esophagus. We also showed that these changes, characteristic of EMT, are directly proportional to the eosinophil load in esophageal biopsies.
EMT participates in the genesis of tissue and organ fibrosis in the kidney, liver and lung in response to chronic injury and repair by contributing to the population of disease-related fibroblasts and myofibroblasts that overproduce extracellular matrix.9, 29, 30 These responses are regulated in part by exogenous and/or autocrine sources and signaling by TGF-β in epithelial cells.18, 29 The presence of EMT in subjects with EoE was highly correlated with the index of subepithelial fibrosis, eosinophil presence and state of activation (determined by expression of EPX, both cell-associated and secreted), and the presence of TGF-β1 in the biopsies, thus providing compelling support that EMT contributes to the genesis of subepithelial fibrosis in EoE. A number of growth factors induce or regulate the development of EMT, primarily TGF-β, with others more variable and context dependent, e.g. FGF2, EGF, IGF-II and HGF (reviewed in18, 31). Of these, TGF-β, either induced autocrine expression within epithelial cells themselves in response to tissue damage or a paracrine inflammatory cell source, plays a key role in inducing EMT and is vital to expression of the EMT proteome.18, 19, 32
We showed here that TGF-β1 induces changes in gene expression in vitro in an esophageal epithelial cell, HET-1A, in a manner consistent with EMT. Importantly, TGF-β1 potently induced transcription of mesenchymal genes (N-cadherin, vimentin, fibronectin) and down-regulated expression of cytokeratins in HET-1A esophageal cells, characteristic findings for induction of EMT in primary and epithelial-derived cell lines from other tissues and organs.18, 29, 33, 34 Previous studies of TGF-β-family induced EMT in culture showed that while p-Smad signaling occurs rapidly, induction of transcriptional repressors associated with induction of EMT does not occur until 48hrs, and subsequent repression of epithelial markers such as E-cadherin at both mRNA and protein levels takes even longer, up to 72hrs.35 Loss of epithelial phenotype with dissolution of tight polarized cell-cell adhesion may be a gradual or even reversible process,36 or may be regulated post-translationally by proteases, including metalloproteases, as shown for EMT-processes associated with tumorigenesis.37 A number of studies showed simultaneous expression of both epithelial and mesenchymal adherens junction proteins in primary and metastatic tumors, but functional studies suggest the metastatic invasive phenotype of mesenchymal N-cadherin prevails over stable polarized E-cadherin when they are co-expressed.38, 39 Thus, while HET-1A cells co-expressed epithelial and mesenchymal markers in the current study, the mesenchymal phenotype may prevail, allowing this gradual EMT process to contribute to development of subepithelial fibrosis in the esophagus in EoE.
Eosinophils, in addition to direct contribution of TGF-β for induction of EMT, may induce expression of EMT- and fibrosis-relevant remodeling factors in epithelial cells themselves, including TGF-β and others (endothelin-1, TGF-α, PDGF-AB, EGFR, MMP-9, IL-6, IL-11, fibronectin and tenascin), through secretion of their granule cationic proteins (MBP1 and EPX)40, 41 or cytokines (IL-13).42, 43 Increased TGF-β1 expression, previously shown in pediatric EoE,8, 44 was also demonstrated in the current study, providing further evidence for its role and EMT in inducing esophageal fibrosis in EoE. Importantly, the relevant cellular sources of TGF-β (eosinophils, mast cells, epithelial cells, fibrocytes), the mechanisms by which TGF-β in the esophagus in EoE becomes activated from its latent form (via integrin αvβ6-mediated11, 45 or proteolytic pathways), and the down-stream signaling pathways relevant to induction of EMT9, 18 and its resolution46 remain to be determined in EoE.
For the 18 children with active EoE who were successfully treated with three different treatment modalities (TC, SFED, ED), their decreased post-treatment EMT scores were significantly correlated with their reduced esophageal eosinophil load. However, we were unable to assess a corresponding post-treatment decrease in the levels of fibrosis in these EoE subjects, as there was insufficient lamina propria (<2mm) present in many of the esophageal biopsies to allow quantitation of the fibrosis index. However, several studies previously showed that TC treatment decreases or completely resolves esophageal fibrosis in children with EoE.6, 25 Thus, in addition to these earlier reports of decreased subepithelial fibrosis with steroid treatment, the present study shows a corresponding decrease in the amount of EMT that likely contributes to the fibrogenesis characteristic of EoE.
EoE is a chronic inflammatory disorder21 and remission of eosinophilic esophageal inflammation results in resolution of EMT (current study) and fibrosis.44 Current therapies, either pharmacologic with TCs or dietary restrictions (SFED, ED) are effective for inducing disease remission, but maintaining remission long-term is difficult since disease recurs once the treatment is discontinued. For example, TCs are effective in many but not all patients with EoE in inducing clinicopathologic remission, but relapse rates are high once the corticosteroid is discontinued.47 Currently, there are no recommendations for low-dose maintenance therapy with TCs that will maintain subjects in remission and thereby prevent fibrosis.1 A recent study in adults showed that low-dose maintenance treatment with budesonide was well tolerated, and 50% of patients were maintained in remission after a 50-week treatment period.24 However, after 50-weeks of low-dose budesonide, submucosal and muscularis propria thickening still persisted, fibrosis scores were increased slightly, and TGF-β and tenascin C were still elevated. Additionally, long-term low-dose budesonide therapy was associated with significant reductions in overall mucosal but not epithelial thickness, and esophageal remodeling showed only a trend toward normalization.24 Finally, one year of topical fluticasone treatment of adults with EoE lead to a non-significant reduction in subepithelial fibrosis.20 Taken together, these findings suggest that low-dose continuous TC may be unable to prevent the progression of esophageal fibrosis, supporting the need to explore alternative long-term treatment modalities to block fibrogenesis in the esophagus in EoE.
Finally, we analyzed a group of pediatric patients with an indeterminate diagnosis of EoE, and found their EMT scores to be virtually identical to those with confirmed EoE. Clinical experience is identifying an increasing number of these kinds of children and adults with features highly suggestive of EoE, but who do not reach the requisite eosinophil threshold number.1 Reasons for this may include limitations in biopsy sampling, a later more chronic stage in the inflammatory process, a different EoE phenotype, or a more fibrotic phenotype of GERD. Future studies that provide additional clinical and molecular characterization will help clarify this patient population more fully.
Conclusions
Correlations of EMT to esophageal eosinophils, their state of activation, and measures and mediators of fibrosis suggest that EMT contributes significantly to the subepithelial fibrosis characteristic of EoE. Thus, treatments impacting esophageal eosinophilia in EoE may alter the natural history of the disease in terms of reversing esophageal remodeling. Prospective studies are needed to extend these findings to further define the pro-fibrotic mediators and signaling cascades that propagate esophageal epithelial reactions in EoE leading to EMT, and factors such as bone morphogenic protein-7 (BMP-7) that may be involved in its resolution with treatment.48, 49
Key Messages.
Esophageal epithelial cells can undergo TGF-β-induced epithelial mesenchymal transition (EMT), a process associated with tissue and organ fibrosis, including the subepithelial fibrosis associated with airway remodeling in asthma
Fibrosis-associated EMT occurs in the esophagus of children with active EoE, but not in children with other esophageal diseases such as GERD
The degree of EMT in the esophagus in EoE is highly associated with the amount of subepithelial fibrosis, numbers and measures of activation of esophageal eosinophils, and levels of remodeling factors such as TGF-β
Esophageal EMT resolves with EoE treatments that significantly decrease the esophageal burden of eosinophils
EoE treatments that significantly reduce esophageal eosinophils are likely to alter the natural history of this food-allergic disease by reversing EMT-associated fibrogenesis
Acknowledgments
The authors thank Wendy Moore, Zachary Robinson and Mark Lovell at the Children’s Hospital Colorado and associated nurses and histology lab staff at Children’s Memorial Hospital in Chicago for their participation and support, and Christine Jun, M.D. at UIC COM for participation in preliminary development of the project and immunostaining methods for EMT.
Funding support: This work was supported in part by a translational research award from the AGA (to SJA, GTF), NIH grant R21AI079925 (to GTF/SJA), unrestricted gifts from the Campaign Urging Research on Eosinophilic Diseases (CURED) (to SJA, GTF, AFK) and the Buckeye Foundation (to AFK), and research funds from the UIC Department of Pediatrics (to AFK, SJA), and the Mayo Foundation and its NIH grant NCRR K26 RR0109709 (JJL). BR was supported in part by a Craig Medical Student Summer Research Fellowship from the UIC COM. The CURED and Buckeye Foundations did not play any role in development of the study or in the preparation of this manuscript aside from providing research funding. This project was also supported in part by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780 (GTF/SW/JM). Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views. Steven Ackerman and Glenn Furuta are members of the Medical Advisory Panel of the American Partnership for Eosinophilic Diseases.
Abbreviations used
- EoE
Eosinophilic Esophagitis
- EMT
Epithelial Mesenchymal Transition
- EPX
Eosinophil Peroxidase
- HPF
High Power Field
- GERD
Gastroesophageal Reflux Disease
- ED
Elemental Diet
- SFED
Six-Food Elimination Diet
- TC
topical corticosteroids
- RT-Q-PCR
real-time reverse-transcription quantitative polymerase chain reaction
- PPI
Proton pump inhibitor
- EUS
Endoscopic ultrasonography
Footnotes
Disclosures: Steven Ackerman has served as a consultant for Sunovion. Glenn T. Furuta has served as a consultant to Nutricia and Meritage Pharma, and Amir Kagalwalla serves on the speaker’s bureau for Abbott Nutrition. The other authors have declared they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e6. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Straumann A, Aceves SS, Blanchard C, Collins MH, Furuta GT, Hirano I, et al. Pediatric and adult eosinophilic esophagitis: similarities and differences. Allergy. 2012 doi: 10.1111/j.1398-9995.2012.02787.x. [DOI] [PubMed] [Google Scholar]
- 3.Straumann A, Bussmann C, Zuber M, Vannini S, Simon HU, Schoepfer A. Eosinophilic esophagitis: analysis of food impaction and perforation in 251 adolescent and adult patients. Clin Gastroenterol Hepatol. 2008;6:598–600. doi: 10.1016/j.cgh.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Fox VL, Nurko S, Teitelbaum JE, Badizadegan K, Furuta GT. High-resolution EUS in children with eosinophilic “allergic” esophagitis. Gastrointest Endosc. 2003;57:30–6. doi: 10.1067/mge.2003.33. [DOI] [PubMed] [Google Scholar]
- 5.Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82–90. doi: 10.1053/j.gastro.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aceves SS, Ackerman SJ. Relationships between eosinophilic inflammation, tissue remodeling, and fibrosis in eosinophilic esophagitis. Immunol Allergy Clin North Am. 2009;29:197–211. xiii–xiv. doi: 10.1016/j.iac.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chehade M, Sampson HA, Morotti RA, Magid MS. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:319–28. doi: 10.1097/MPG.0b013e31806ab384. [DOI] [PubMed] [Google Scholar]
- 8.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–12. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–47. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 11.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–5. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bataille F, Rohrmeier C, Bates R, Weber A, Rieder F, Brenmoehl J, et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn’s disease. Inflamm Bowel Dis. 2008;14:1514–27. doi: 10.1002/ibd.20590. [DOI] [PubMed] [Google Scholar]
- 13.Masterson JC, McNamee EN, Jedlicka P, Fillon S, Ruybal J, Hosford L, et al. CCR3 Blockade Attenuates Eosinophilic Ileitis and Associated Remodeling. Am J Pathol. 2011;179:2302–14. doi: 10.1016/j.ajpath.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Protheroe C, Woodruff SA, de Petris G, Mukkada V, Ochkur SI, Janarthanan S, et al. A novel histologic scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:749–55. e11. doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Rodriguez M, et al. Immunostimulatory DNA inhibits transforming growth factor-beta expression and airway remodeling. Am J Respir Cell Mol Biol. 2004;30:651–61. doi: 10.1165/rcmb.2003-0066OC. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–9. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 19.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucendo AJ, Arias A, De Rezende LC, Yague-Compadre JL, Mota-Huertas T, Gonzalez-Castillo S, et al. Subepithelial collagen deposition, profibrogenic cytokine gene expression, and changes after prolonged fluticasone propionate treatment in adult eosinophilic esophagitis: A prospective study. J Allergy Clin Immunol. 2011;128:1037–46. doi: 10.1016/j.jaci.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11. 5 years. Gastroenterology. 2003;125:1660–9. doi: 10.1053/j.gastro.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Stevoff C, Rao S, Parsons W, Kahrilas PJ, Hirano I. EUS and histopathologic correlates in eosinophilic esophagitis. Gastrointest Endosc. 2001;54:373–7. doi: 10.1067/mge.2001.116569. [DOI] [PubMed] [Google Scholar]
- 23.Dalby K, Nielsen RG, Kruse-Andersen S, Fenger C, Durup J, Husby S. Gastroesophageal reflux disease and eosinophilic esophagitis in infants and children. A study of esophageal pH, multiple intraluminal impedance and endoscopic ultrasound. Scand J Gastroenterol. 2010;45:1029–35. doi: 10.3109/00365521.2010.487917. [DOI] [PubMed] [Google Scholar]
- 24.Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400–9. e1. doi: 10.1016/j.cgh.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126:1198–204. e4. doi: 10.1016/j.jaci.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 26.Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 27.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–32. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, et al. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. 2009;180:122–33. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 29.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–34. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 30.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006;3:377–82. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 32.Feighery R, Maguire P, Ryan MP, McMorrow T. A proteomic approach to immune-mediated epithelial-mesenchymal transition. Proteomics Clin Appl. 2008;2:1110–7. doi: 10.1002/prca.200780148. [DOI] [PubMed] [Google Scholar]
- 33.Molloy EL, Adams A, Moore JB, Masterson JC, Madrigal-Estebas L, Mahon BP, et al. BMP4 induces an epithelial-mesenchymal transition-like response in adult airway epithelial cells. Growth Factors. 2008;26:12–22. doi: 10.1080/08977190801987166. [DOI] [PubMed] [Google Scholar]
- 34.Galichon P, Hertig A. Epithelial to mesenchymal transition as a biomarker in renal fibrosis: are we ready for the bedside? Fibrogenesis Tissue Repair. 2011;4:11. doi: 10.1186/1755-1536-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masterson JC, Molloy EL, Gilbert JL, McCormack N, Adams A, O’Dea S. Bone morphogenetic protein signalling in airway epithelial cells during regeneration. Cell Signal. 2011;23:398–406. doi: 10.1016/j.cellsig.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185:7–19. doi: 10.1159/000101298. [DOI] [PubMed] [Google Scholar]
- 37.Masterson J, O’Dea S. Posttranslational truncation of E-cadherin and significance for tumour progression. Cells Tissues Organs. 2007;185:175–9. doi: 10.1159/000101318. [DOI] [PubMed] [Google Scholar]
- 38.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148:779–90. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–44. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pegorier S, Wagner LA, Gleich GJ, Pretolani M. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol. 2006;177:4861–9. doi: 10.4049/jimmunol.177.7.4861. [DOI] [PubMed] [Google Scholar]
- 41.Rochester CL, Ackerman SJ, Zheng T, Elias JA. Eosinophil-fibroblast interactions. Granule major basic protein interacts with IL-1 and transforming growth factor-beta in the stimulation of lung fibroblast IL-6-type cytokine production. J Immunol. 1996;156:4449–56. [PubMed] [Google Scholar]
- 42.Schmid-Grendelmeier P, Altznauer F, Fischer B, Bizer C, Straumann A, Menz G, et al. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol. 2002;169:1021–7. doi: 10.4049/jimmunol.169.2.1021. [DOI] [PubMed] [Google Scholar]
- 43.Blanchard C, Stucke EM, Burwinkel K, Caldwell JM, Collins MH, Ahrens A, et al. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol. 2010;184:4033–41. doi: 10.4049/jimmunol.0903069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aceves SS, Newbury RO, Chen D, Mueller J, Dohil R, Hoffman H, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy. 2010;65:109–16. doi: 10.1111/j.1398-9995.2009.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev. 2005;24:395–402. doi: 10.1007/s10555-005-5131-6. [DOI] [PubMed] [Google Scholar]
- 46.Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285:20202–12. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198–206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 48.Morrissey J, Hruska K, Guo G, Wang S, Chen Q, Klahr S. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol. 2002;13 (Suppl 1):S14–21. [PubMed] [Google Scholar]
- 49.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–8. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]