Abstract
There is increased serum CRH with decreased lesional skin CRHR-1 gene expression in psoriasis and atopic dermatitis, suggesting possible involvement in stress-induced worsening of symptoms.
Keywords: Atopic dermatitis, corticotropin-releasing hormone, inflammation, mast cells, psoriasis, stress
To the Editor:
Autoimmune skin diseases, such as psoriasis (Ps) and atopic dermatitis (AD), are chronic inflammatory skin disorders mediated primarily by T cells 1, but mast cells are also implicated 2. Symptoms of both Ps and AD worsen with stress 3. Acute stress leads to increased skin vascular permeability and inflammation, through mast cell activation by corticotropin-releasing hormone (CRH) both in rodents 4 and humans 5. CRH and CRHR-1 are both expressed in human skin 6, leading to the hypothesis that CRH may be involved in the pathophysiology of skin diseases 7.
Full length 3 mm3 punch skin biopsies were collected for diagnostic purposes from subjects with Ps and AD who had not received any medication for 15 days prior to biopsy and were free from any systemic allergic or inflammatory disease. Serum was also collected from patients and controls. The Medical Ethics Committee approved this protocol. Psoriasis Area and Severity Index (PASI) score was recorded for some of the Ps patients; no severity index was obtained from the AD patients Also, some Ps and AD patients filled the State-Trait Anxiety Inventory (STAI), which has been validated for the Greek population 8, to investigate the extent of stress. The STAI measures separate constructs of psychosocial stress and clearly differentiates between the temporary condition of “state anxiety” (STATE now) and the more general and long-standing quality of “trait anxiety” (STATE trait).
Serum level and skin gene expression results from patients were compared to those of controls using the Mann-Whitney non parametric U-test. There was no statistical difference in the mean age between controls and patients. All subject characteristics and the summary of the results are presented in Table 1.
Table 1.
Summary of subject characteristics and results
| Sex; No; Age | Serum CRH | Serum VEGF | Skin CRHR-1 | Skin VEGF | |
|---|---|---|---|---|---|
| AD | F; n=13; 41±23 M; n=7; age: 28±9 |
F; n=8; 42±23 M; n=5; 28±ll |
F; n=12; 43±22 M; n=6; 27±9 |
F; n=10; 40±24 M; n=6; 27±9 |
F; n=10; 40±24 M; n=6; 27±9 |
| Results | 31±19.5 pg/ml (p<0.0001) |
291.4±280.1 pg/ml | Lower than control (p<0.0001)) |
Lower than control (p<0.0001) |
|
| Ps | F; n=30; 40±15 M;n=36;49±18 |
F; n=23; 41±14 M;n=25;42±15 |
F; n= 26; 40±14 M; n=30;48±19 |
F; n=19; 42±16 M;n=21;48±18 |
F; n=19; 42±16 M;n=21;48±18 |
| Results | 22.5±13.7 pg/ml (p<0.0001) ┼ |
411.9±280 pg/ml ╪ |
Lower than control (p<0.0001) |
Lower than control (p=0.121) |
|
| Control | F; n=22; 49±16 M; n=26; 54±14 |
F; n=10; 10±5 M; n=9; 9±3 |
F; n=14; 53±16 M; n=19; 49±19 |
F; n=22; 52±18 M; n=8; 40±16 |
F; n=22; 52±18 M; n=8; 40±16 |
| Results | 9.7±4.2 pg/ml | 257.2±123.8 pg/ml |
mean±SD
For Ps patients with PASI>10 serum CRH levels were statistically significant increased (27.8±10.4, p=0.0001)
For Ps patients with PASI>10 serum VEGF levels were significantly increased (369.8±197.5, p=0.0286)
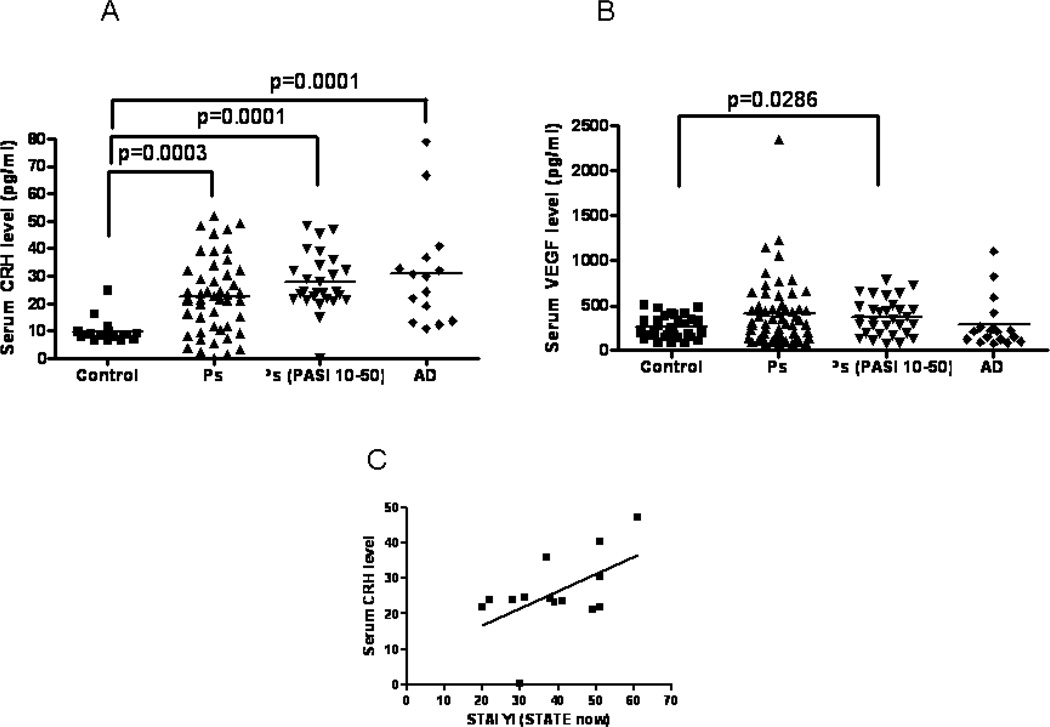
For CRH serum measurements in Ps patients (n=48, 27 of which had PASI=10-50), the mean age was 46±17 (23 women and 25 men), while the mean age for controls (n=19) was 47±16 (10 women and 9 men). Serum CRH levels were higher (p=0.0001) in Ps patients (22.5±13.7 pg/ml) as compared to controls (9.7±4.2 pg/ml) (Fig. 1A). When Ps patients were separated in those with PASI scores <10>, there was a statistically significant increase (p=0.0001) in Ps patients with PASI >10 (Fig. 1A), but not in those with <10 (data not shown). For CRH serum measurements in AD patients (n=15), the mean age was 36±18 (9 women and 6 men), while the mean age for control (n=19) was 47±16 (10 women and 9 men). Serum CRH levels were higher (p=0.0001) in AD patients (31.0±19.5 pg/ml) as compared to controls (9.7±4.2 pg/ml) (Fig. 1A).
Figure 1.
CRH and VEGF serum levels in Ps and AD patients and controls (A–B), as well as correlation of serum CRH levels in severe Ps patients with STAI (STATE now), (C).
For cytokine serum measurements, the mean age for Ps patients (n=56, 31 of which were Ps patients with PASI 10–50) was 40±14 (26 women and 30 men), while the mean age for controls (n=33) was 48±18 (14 women and 19 men). Serum cytokines and VEGF were evaluated using Milliplex microbead technology and the measurements were performed by Millipore (St. Charles, MI). Serum levels of IL-6, IL-9, IL-33, TNFβ, and TSLP in Ps patients were undetectable, except for VEGF and IL-8. Serum VEGF levels were not different in Ps patients (411.9±280 pg/ml) as compared to controls (257±123.8 pg/ml), (Fig. 1B). However, serum VEGF levels in Ps patients with PASI score >10 were significantly increased (p=0.0286) (Fig. 1B), while in Ps patients with PASI score <10 were not (data not shown). There was no difference between serum IL-8 levels in Ps patients (25.7±27.5 pg/ml) as compared to controls (31.3±31.2 pg/ml).
For serum measurements, the mean age for AD patients (n=18) was 38±20 (12 women and 6 men), while mean age for control (n=33) was 48±18 (14 women and 19 men). Serum levels of IL-6, IL-9, IL-33, TNFβ, and TSLP were undetectable, except for VEGF and IL-8. Serum VEGF levels were not different in AD patients (291.4±280.1 pg/ml) as compared to controls (257.2±123.8 pg/ml) (Fig. 1B). Serum IL-8 levels were lower (p=0.0171) in AD patients (19.7±17.2 pg/ml) as compared to controls (31.3±32.2 pg/ml) (data not shown). There was a reasonable correlation between serum CRH levels and STAI (STATE now) scores (Pearson r=0.55, p=0.04, n=14) for Ps patients with PASI score>10, but there was no correlation with STAI (STATE trait). There was no correlation between STAI scores and CRH serum measurements or VEGF gene expression for the AD patients (Fig. 1C).
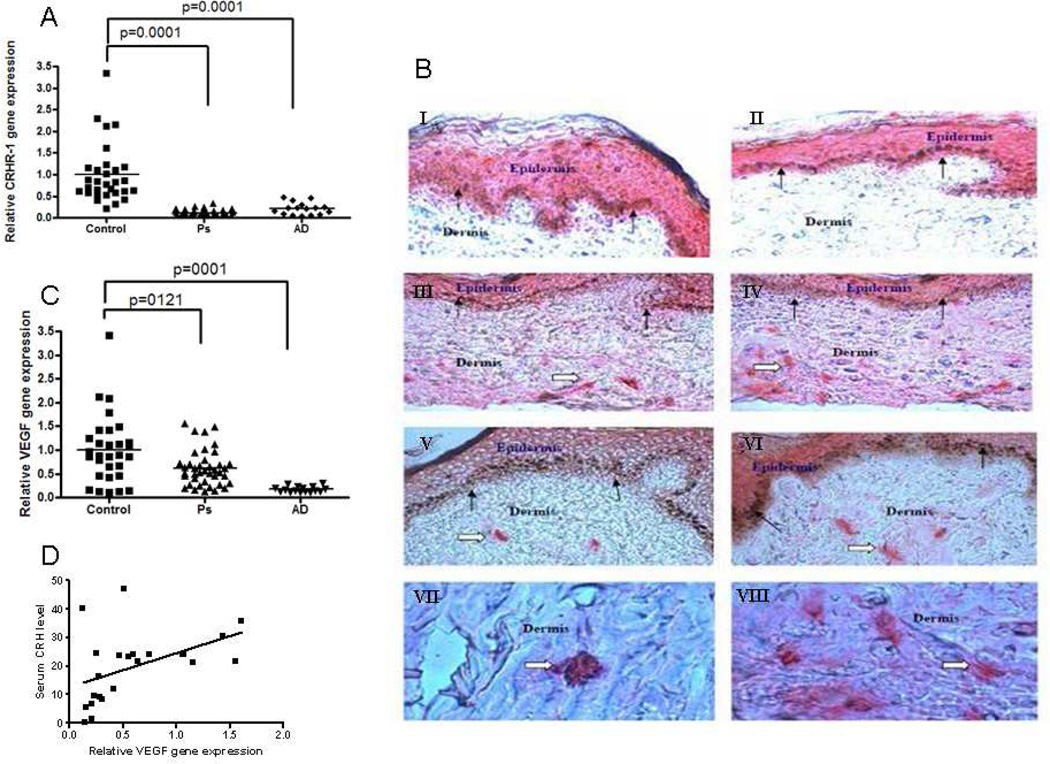
For gene expression assays, the mean age for Ps patients (n=40) was 46±17 (19 women and 21 men), while mean age for controls (n=30) was 43±15 (22 women and 8 men). Skin CRHR-1 gene expression was lower (p<0.0001) in affected skin samples from Ps patients (0.1±0.1) as compared to controls (1±0.7) (Fig. 2A). CRHR-1 expression was documented in lesional Ps skin by immunohistochemistry and appeared to co-localize with mast cells (Fig. 2B) There was also statistically significant lower (p=0.0001) CRHR-1 gene expression in the samples obtained from affected versus unaffected Ps skin, while there was no statistically significant difference in CRHR-1 gene expression between the control and the non-lesion samples (data not shown). Lesional skin CRH gene expression was undetectable in Ps (data not shown). For CRHR-1 gene expression assays, the mean age for AD patients (n=16) was 35±21 (10 women and 6 men), while mean age for controls (n=30) was 43±15 (22 women and 8 men). Skin CRHR-1 gene expression was lower (p=0.0001) in affected samples from AD patients (0.2±0.1) as compared to controls (1±0.7) (Fig. 2A).
Figure 2.
CRHR-1 and VEGF skin gene expression in Ps and AD patients and controls (A,C), and CRHR-1 immunostaining in Ps skin (B). Cryostat sections were prepared and fixed with acetone for 2–3 min and incubated with normal blocking serum for 20 min. Sections were then incubated with primary antibodies (Goat polyclonal antibody to CRHR-1; Abcam Inc, Cambridge, MA, Cat # ab59023) diluted to 1:100 for 30 min and then immunostained with Vectastain ABC AP kit (Vectastain Lab, Burlingame, CA) and Vector Red Alkaline Phosphatase Substrate kit (Vector Lab) as per the kit’s directions. Presence of red color indicate the positive reaction for CRHR-1. (A&B). epidermis and mast cells in dermis (C,D,E,F) and only mast cells (G&H). Solid arrow show epidermollow arrow show mast cells. Correlation of serum CRH levels with VEGF gene expression in severe Ps patients (D).
Skin VEGF gene expression was lower (p=0.0121) in affected samples from Ps patients (0.6±0.4) as compared to controls (1±0.7) (Fig 2C). There was statistically significant lower VEGF gene expression (p=0.0009) among the samples obtained from affected and from unaffected psoriatic skin (data not shown). Skin VEGF gene expression was also lower (p=0.0001) in affected samples from AD patients (0.2±0.06) as compared to controls (1.0±0.7) (Fig. 2C).
There was a positive correlation between CRH serum levels and VEGF gene expression in Ps patients with PASI score >10 (Pearson r=0.44, n=22, p=0.04, Fig. 2D), which is known to be association with increased skin vascularization. (This finding imply that high serum CRH correlates best only in severe Ps patients.
Our findings suggest that overstimulation by the increased serum levels of CRH and VEGF, possibly in response to stress, leads to decreased gene expression of skin CRH and CRHR-1, as well as skin VEGF gene expression, respectively. The positive correlation between serum CRH levels and the STAI-now scores in severe Ps patients supports this possibility. The lack of similar correlation in AD patients may be because we did not have access of severity index scores for these patients, unlike in Ps patients, in order to carry out a subgroup analysis.
The present findings suggest that high serum CRH, possibly in response to stress, stimulates skin mast cells to release VEGF and contribute to skin inflammatory evident in severe Ps patients. Continuous or repeated stimulation may lead to decreased expression of skin CRHR-1 as we showed recently with cultured mast cells 9, and hence decreased VEGF as was evident in patients with mild disease. CRH and CRHR-1 may therefore participate in the pathogenesis of Ps and AD, especially when worsened with stress, through mast cell activation to release VEGF. Mast cell blockers may provide novel treatment approaches.
Acknowledgements
We thank Dr. C. D. Spielberger (University of South Florida, Tampa, FL) for assistance with his STAI, as well as Dr. James Marchand (Tufts University) and Duraisamy Kempuraj (University of Iowa) with the immunohistochemistry. This work was supported in part from NIH grant AR47652 awarded to T. C. T.
Abbreviations used:
- CRH
corticotropin-releasing hormone
- CRHR-1
CRH receptor 1
- Ps
psoriasis
- STAI
State-Trait Anxiety Inventory
- VEGF
Vascular endothelial growth factor
Biographies
Magdalini Vasiadi, BSc
Graduate Student, Sackler School of Graduate Biomedical Sciences, Department of Molecular Physiology and Pharmacology, Tufts University, Boston, MA, USA
Graduate student, Allergy Clinical Research Center, Attikon Hospital, Athens University Medical School, Athens, Greece
Anastasia Therianou, MD
Graduate Student, First Department of Dermatology, A. Sygros Hospital, Athens University Medical School, Athens, Greece
Kyriaki Sideri, MD
Graduate Student, Allergy Clinical Research Center, Attikon Hospital, Athens University Medical School, Athens, Greece
Marilena Smyrnioti, BSc, MA
Clinical Psychologist, Pain Relief Clinic, Second Department of Anesthesiology, Attikon Hospital, Athens University Medical School, Athens, Greece
Nikolaos Sismanopoulos, MD
Graduate Student, Allergy Clinical Research Center, Attikon Hospital, Athens University Medical School, Athens, Greece
Research Fellow, Molecular Immunopharmacology and Drug Discovery Laboratory, Department of Molecular Physiology and Pharmacology, Tufts University School of Medicine, Boston, MA, USA
Danae A Delivani, MD
Research Fellow, Molecular Immunopharmacology and Drug Discovery Laboratory, Department of Molecular Physiology and Pharmacology, Tufts University School of Medicine, Boston, MA, USA
Present Address: Resident, Department of Internal Medicine, University of Connecticut Medical Center, Hartford, CT, USA
Shahrzad Asadi, PharmD
Research Fellow, Molecular Immunopharmacology and Drug Discovery Laboratory, Department of Molecular Physiology and Pharmacology and Department of Pharmacy, Tufts Medical Center Tufts University School of Medicine, Boston, MA, USA
Alexandra Katsarou-Katsari, MD, PhD
Associate Professor, First Department of Dermatology, A. Sygros Hospital, Athens University Medical School, Athens, Greece
Theodora Petrakopoulou, MD
Attending Physician, Aghios Savvas, Oncology Hospital Athens, Greece
Athanasios Theoharides, MD, PhD
Staff ENT surgeon, Ano Toumba IKA Polyclinic, Thessaloniki, Greece
Christina Antoniou, MD, PhD
Professor and Director, First Department of Dermatology, A. Sygros Hospital, Athens University Medical School, Athens, Greece
Evaggelia Papadavid, MD, PhD
Assistant Professor, Second Department of Dermatology, Attikon Hospital, Athens University Medical School, Athens, Greece
Nikolaos Stavrianeas, MD, PhD
Professor and Director, Second Department of Dermatology, Attikon Hospital, Athens University Medical School, Athens, Greece
Dimitrios Kalogeromitros, MD, PhD, deseased
Associate Professor and Director, Allergy Clinical Research Center, Attikon Hospital, Athens University Medical School, Athens, Greece (deceased)
Theoharis C. Theoharides, MS, PhD, MD
Professor and Director, Molecular Immunopharmacology and Drug Discovery Laboratory, Department of Molecular Physiology and Pharmacology, and Departments of Biochemistry and Internal Medicine, and Sackler School of Graduate Biomedical Sciences, Tufts University, Boston, MA, USA
Visiting Professor, Allergy Clinical Research Center, Attikon Hospital, Athens University Medical School, Athens, Greece
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflict of interest.
References
- 1.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis-Part I: Clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127(5):1110–1118. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 2.Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–328. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 3.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its pro-inflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 5.Crompton R, Clifton VL, Bisits AT, Read MA, Smith R, Wright IM. Corticotropin-releasing hormone causes vasodilation in human skin via mast celldependent pathways. J Clin Endocrinol Metab. 2003;88:5427–5432. doi: 10.1210/jc.2003-030377. [DOI] [PubMed] [Google Scholar]
- 6.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 7.Slominski A. On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. Br J Dermatol. 2009;160(2):229–232. doi: 10.1111/j.1365-2133.2008.08958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fountoulakis KN, Papadopoulou M, Kleanthous S, Papadopoulou A, Bizeli V, Nimatoudis I, et al. Reliability and psychometric properties of the Greek translation of the State-Trait Anxiety Inventory form Y: Preliminary data. Ann Gen Psychiatry. 2006;5:2. doi: 10.1186/1744-859X-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asadi S, Alysandratos KD, Angelidou A, Miniati A, Sismanopoulos N, Vasiadi M, et al. Substance P (SP) induces expression of functional corticotropin-releasing hormone receptor-1 (CRHR-1) in human mast cells. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]