Abstract
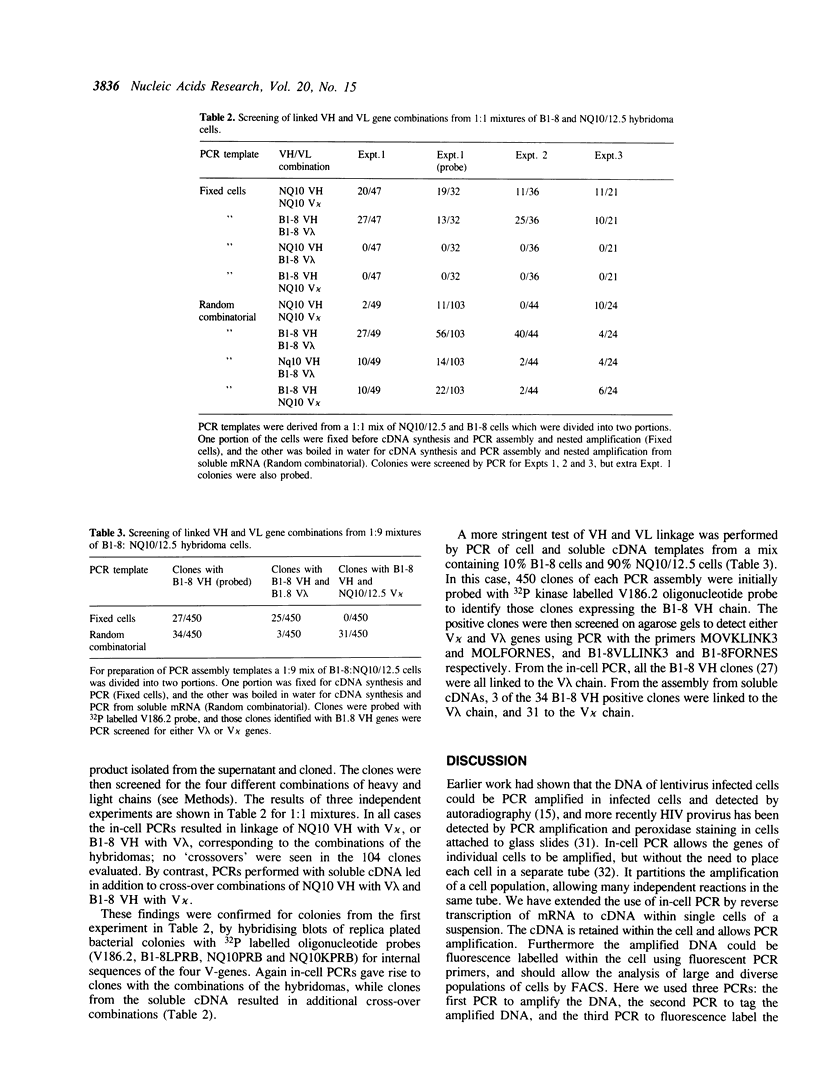
We describe a process for the identification of mRNAs within single cells, as demonstrated with the immunoglobulin (Ig) variable region (V) genes of two mouse hybridoma cell lines and the bcr-abl fusion gene of the human K562 myeloid leukaemia line. The cells were fixed and permeabilised, the mRNA reverse transcribed to cDNA and the cDNA amplified by the polymerase chain reaction (PCR). After using fluorescent PCR primers, the amplified DNA could be detected within the cells as demonstrated by confocal fluorescence microscopy and flow cytometry. Furthermore the amplified Ig VH and VL DNA could be assembled within the same cell using suitable PCR primers. We detected no cross-contamination of amplified DNA between cells: the DNA isolated from mixtures of two hybridoma cell lines (B1-8 and NQ10/12.5) treated to in-cell PCR and assembly, was shown by cloning to correspond to the combinations of VH and VL genes of the parent hybridomas. We forsee diverse applications of in-cell assembly by PCR, especially for the analysis of the combinations of chains of rearranged Ig or T cell receptor (TCR) V-genes in a population of cells, and the construction of human antibodies from the V-genes of immune B-lymphocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Fenyö E. M. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 in clinical specimens by polymerase chain reaction with nested primers. J Clin Microbiol. 1990 Jul;28(7):1560–1564. doi: 10.1128/jcm.28.7.1560-1564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L. C., Nilsson K., Gahmberg C. G. K562--a human erythroleukemic cell line. Int J Cancer. 1979 Feb;23(2):143–147. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- Bagasra O., Hauptman S. P., Lischner H. W., Sachs M., Pomerantz R. J. Detection of human immunodeficiency virus type 1 provirus in mononuclear cells by in situ polymerase chain reaction. N Engl J Med. 1992 May 21;326(21):1385–1391. doi: 10.1056/NEJM199205213262103. [DOI] [PubMed] [Google Scholar]
- Barber K. E., Crosier P. S., Purdie K. J., Buchanan J. M., Cattermole J. A., Watson J. D., Gillis S. Human interleukin 3: effects on normal and leukemic cells. Growth Factors. 1989;1(2):101–114. doi: 10.3109/08977198909029120. [DOI] [PubMed] [Google Scholar]
- Better M., Chang C. P., Robinson R. R., Horwitz A. H. Escherichia coli secretion of an active chimeric antibody fragment. Science. 1988 May 20;240(4855):1041–1043. doi: 10.1126/science.3285471. [DOI] [PubMed] [Google Scholar]
- Buluwela L., Forster A., Boehm T., Rabbitts T. H. A rapid procedure for colony screening using nylon filters. Nucleic Acids Res. 1989 Jan 11;17(1):452–452. doi: 10.1093/nar/17.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab F. F., Kan Y. W. Detection of specific DNA sequences by fluorescence amplification: a color complementation assay. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9178–9182. doi: 10.1073/pnas.86.23.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S., Scharpf S., Franko M., Vermeulen C. W. Effect of iso-propyl-thio-beta-D-galactoside concentration on the level of lac-operon induction in steady state Escherichia coli. Biochem Biophys Res Commun. 1985 May 16;128(3):1268–1273. doi: 10.1016/0006-291x(85)91077-0. [DOI] [PubMed] [Google Scholar]
- Clackson T., Hoogenboom H. R., Griffiths A. D., Winter G. Making antibody fragments using phage display libraries. Nature. 1991 Aug 15;352(6336):624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- Cumano A., Rajewsky K. Structure of primary anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in normal and idiotypically suppressed C57BL/6 mice. Eur J Immunol. 1985 May;15(5):512–520. doi: 10.1002/eji.1830150517. [DOI] [PubMed] [Google Scholar]
- Davis G. T., Bedzyk W. D., Voss E. W., Jacobs T. W. Single chain antibody (SCA) encoding genes: one-step construction and expression in eukaryotic cells. Biotechnology (N Y) 1991 Feb;9(2):165–169. doi: 10.1038/nbt0291-165. [DOI] [PubMed] [Google Scholar]
- Gherardi E., Milstein C. Original and artificial antibodies. Nature. 1992 May 21;357(6375):201–202. doi: 10.1038/357201a0. [DOI] [PubMed] [Google Scholar]
- Güssow D., Clackson T. Direct clone characterization from plaques and colonies by the polymerase chain reaction. Nucleic Acids Res. 1989 May 25;17(10):4000–4000. doi: 10.1093/nar/17.10.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORWITZ J. P., CHUA J., CURBY R. J., TOMSON A. J., DAROOGE M. A., FISHER B. E., MAURICIO J., KLUNDT I. SUBSTRATES FOR CYTOCHEMICAL DEMONSTRATION OF ENZYME ACTIVITY. I. SOME SUBSTITUTED 3-INDOLYL-BETA-D-GLYCOPYRANOSIDES. J Med Chem. 1964 Jul;7:574–575. doi: 10.1021/jm00334a044. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Retzel E. F., Staskus K. A. Amplification and detection of lentiviral DNA inside cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4971–4975. doi: 10.1073/pnas.87.13.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstrom S., Shively J. E., Paxton R. J., Beatty B. G., Larsson A., Ghosh R., Bormer O., Buchegger F., Mach J. P., Burtin P. Antigenic sites in carcinoembryonic antigen. Cancer Res. 1989 Sep 1;49(17):4852–4858. [PubMed] [Google Scholar]
- Heisterkamp N., Stam K., Groffen J., de Klein A., Grosveld G. Structural organization of the bcr gene and its role in the Ph' translocation. 1985 Jun 27-Jul 3Nature. 315(6022):758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- Hoo W. F., Lacy M. J., Denzin L. K., Voss E. W., Jr, Hardman K. D., Kranz D. M. Characterization of a single-chain T-cell receptor expressed in Escherichia coli. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4759–4763. doi: 10.1073/pnas.89.10.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse W. D., Sastry L., Iverson S. A., Kang A. S., Alting-Mees M., Burton D. R., Benkovic S. J., Lerner R. A. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989 Dec 8;246(4935):1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- Kang A. S., Jones T. M., Burton D. R. Antibody redesign by chain shuffling from random combinatorial immunoglobulin libraries. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11120–11123. doi: 10.1073/pnas.88.24.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Marchuk D., Drumm M., Saulino A., Collins F. S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991 Mar 11;19(5):1154–1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J. D., Hoogenboom H. R., Bonnert T. P., McCafferty J., Griffiths A. D., Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991 Dec 5;222(3):581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- McCafferty J., Griffiths A. D., Winter G., Chiswell D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990 Dec 6;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- Novotny J., Ganju R. K., Smiley S. T., Hussey R. E., Luther M. A., Recny M. A., Siliciano R. F., Reinherz E. L. A soluble, single-chain T-cell receptor fragment endowed with antigen-combining properties. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8646–8650. doi: 10.1073/pnas.88.19.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi R., Güssow D. H., Jones P. T., Winter G. Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 May;86(10):3833–3837. doi: 10.1073/pnas.86.10.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry L., Alting-Mees M., Huse W. D., Short J. M., Sorge J. A., Hay B. N., Janda K. D., Benkovic S. J., Lerner R. A. Cloning of the immunological repertoire in Escherichia coli for generation of monoclonal catalytic antibodies: construction of a heavy chain variable region-specific cDNA library. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5728–5732. doi: 10.1073/pnas.86.15.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerra A., Pfitzinger I., Plückthun A. The functional expression of antibody Fv fragments in Escherichia coli: improved vectors and a generally applicable purification technique. Biotechnology (N Y) 1991 Mar;9(3):273–278. doi: 10.1038/nbt0391-273. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Brodsky I., Yunis J. J. Molecular quantification of residual disease in chronic myelogenous leukemia after bone marrow transplantation. Blood. 1992 Mar 15;79(6):1629–1635. [PubMed] [Google Scholar]
- Waldmann T. A. Monoclonal antibodies in diagnosis and therapy. Science. 1991 Jun 21;252(5013):1657–1662. doi: 10.1126/science.2047874. [DOI] [PubMed] [Google Scholar]
- Wick M. R., Loy T., Mills S. E., Legier J. F., Manivel J. C. Malignant epithelioid pleural mesothelioma versus peripheral pulmonary adenocarcinoma: a histochemical, ultrastructural, and immunohistologic study of 103 cases. Hum Pathol. 1990 Jul;21(7):759–766. doi: 10.1016/0046-8177(90)90036-5. [DOI] [PubMed] [Google Scholar]
- Winter G., Milstein C. Man-made antibodies. Nature. 1991 Jan 24;349(6307):293–299. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]
- Winter G., Milstein C. Man-made antibodies. Nature. 1991 Jan 24;349(6307):293–299. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]
- Zebedee S. L., Barbas C. F., 3rd, Hom Y. L., Caothien R. H., Graff R., DeGraw J., Pyati J., LaPolla R., Burton D. R., Lerner R. A. Human combinatorial antibody libraries to hepatitis B surface antigen. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3175–3179. doi: 10.1073/pnas.89.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]