Abstract
OBJECTIVE:
We examined whether metabolic conditions (MCs) during pregnancy (diabetes, hypertension, and obesity) are associated with autism spectrum disorder (ASD), developmental delays (DD), or impairments in specific domains of development in the offspring.
METHODS:
Children aged 2 to 5 years (517 ASD, 172 DD, and 315 controls) were enrolled in the CHARGE (Childhood Autism Risks from Genetics and the Environment) study, a population-based, case-control investigation between January 2003 and June 2010. Eligible children were born in California, had parents who spoke English or Spanish, and were living with a biological parent in selected regions of California. Children’s diagnoses were confirmed by using standardized assessments. Information regarding maternal conditions was ascertained from medical records or structured interview with the mother.
RESULTS:
All MCs were more prevalent among case mothers compared with controls. Collectively, these conditions were associated with a higher likelihood of ASD and DD relative to controls (odds ratio: 1.61 [95% confidence interval: 1.10–2.37; odds ratio: 2.35 [95% confidence interval: 1.43–3.88], respectively). Among ASD cases, children of women with diabetes had Mullen Scales of Early Learning (MSEL) expressive language scores 0.4 SD lower than children of mothers without MCs (P < .01). Among children without ASD, those exposed to any MC scored lower on all MSEL and Vineland Adaptive Behavior Scales (VABS) subscales and composites by at least 0.4 SD (P < .01 for each subscale/composite).
CONCLUSIONS:
Maternal MCs may be broadly associated with neurodevelopmental problems in children. With obesity rising steadily, these results appear to raise serious public health concerns.
KEY WORDS: autism, developmental delay, diabetes, epidemiology, hypertension, obesity
What’s Known on This Subject:
Diabetes during pregnancy has been associated with general development impairments in offspring; however, associations between autism and maternal diabetes have been inconsistent. Few studies have examined related conditions accompanied by underlying increased insulin resistance and their association with developmental outcomes.
What This Study Adds:
This population-based study in young children provides evidence that maternal metabolic conditions are a risk factor for autism, developmental delay without autistic symptoms, and impairments in several domains of development, particularly expressive language, after adjusting for sociodemographic and other characteristics.
Autism spectrum disorders (ASDs) are neurodevelopmental disorders characterized by impairments in social interaction, communication deficits, and stereotyped behaviors.1 Approximately 1 in 110 children has ASD, and the cumulative incidence of this disorder seems to be increasing.2,3 Moreover, 1 in 83 children has other developmental delays (DDs).4 Language and cognitive delays are also seen in a majority of children with ASD,5 suggesting that common exposures may be contributing to the pathology of both of these developmental disorders. To date, the etiology of ASD is unknown; however, several studies suggest that its pathogenesis most likely begins in utero.6–8 Similarly, the causes of cognitive impairment remain unknown for most children.9 An association between general developmental impairments and maternal diabetes has been previously observed. Studies involving women with diabetes found correlations between gestational measures of maternal lipid and glucose metabolism and poorer performance of the offspring on standardized IQ tests10 and motor development assessments.11,12 Dionne et al13 reported significant expressive language impairments in young children born to mothers with gestational diabetes (GDM) compared with children of women without diabetes. Moreover, 2 small studies14,15 demonstrated mild deficits in recognition memory performance in infants of mothers with diabetes, suggesting aberrations in hippocampal function. Abnormalities within the limbic system have also been documented in children with ASD, and language deficits are among the core features of this disorder. However, the association between ASD and maternal diabetes has not been consistently reported in population-based studies,16–18 highlighting the need for further investigation.
Insulin resistance and chronic inflammation in type 2 diabetes (T2D) and related conditions, including obesity and hypertension, have been well established.19–21 In addition, because sensitivity to insulin naturally decreases during gestation, women with impaired glucose tolerance before pregnancy may develop GDM when their insulin production becomes insufficient to maintain euglycemia.22,23 In the United States, nearly 60% of women of childbearing age (20–39 years) are overweight, one-third are obese, and 16% have metabolic syndrome.24,25 Moreover, recent studies found that 1.1% of US pregnancies were complicated by chronic hypertension,26 and in California, 1.3% of pregnant women had T2D and another 7.4% had GDM.27 To date, no studies involving humans have examined the relationship between these metabolic conditions (MCs) collectively and developmental outcomes in children. The aims of this study were to describe the prevalence of diabetes (T2D/GDM), hypertension, and obesity during the index pregnancy in mothers of children with ASD, DD, and typical development (TD) and to investigate whether these conditions were associated with impairments in specific developmental domains in the offspring.
Methods
Study Population
This study was conducted by using data from the CHARGE (Childhood Autism Risks from Genetics and the Environment) study, an ongoing, population-based, case-control study.28 Participants were selected from 3 strata: ASD, DD without ASD, and general population (GP). Eligible children were between the ages of 24 and 60 months, born in California, living with at least 1 biological parent who spoke English or Spanish, and residing in the catchment areas of a specified list of regional centers in California. Children with major motor and sensory impairments (eg, blindness, deafness) that would preclude a valid developmental assessment were excluded. No other exclusions were made on the basis of genetics or family phenotype. Children with ASD or DD were identified through regional centers, providers/clinics, self-referrals, and general public outreach. GP children were identified from state birth files, and a stratified random sample was generated by frequency-matching to a projected distribution of ASD cases on age, gender, and regional center catchment area. Children with DD were not frequency-matched to either group. The CHARGE study protocol was approved by institutional review boards of the University of California in Davis and Los Angeles and the State of California Committee for the Protection of Human Subjects. Written informed consent was obtained before participation.
Diagnostic Validation
All children referred for the study with a diagnosis of autism/ASD were reevaluated with the Autism Diagnostic Interview, Revised (ADI-R)29 and the Autism Diagnostic Observation Schedule (ADOS)30 by trained clinicians at the UC Davis MIND (Medical Investigation of Neurodevelopmental Disorders) Institute to confirm the diagnosis by using criteria described by Risi et al.31 The Social Communication Questionnaire,32 designed to screen for ASD, was administered to parents of DD and GP children; children with scores above the ASD cutoff (≥15) were assessed with the ADOS and ADI-R, and reclassified to ASD if criteria were satisfied.
Mullen Scales of Early Learning (MSEL)33 and Vineland Adaptive Behavior Scales (VABS)34 were administered to all children to determine cognitive and adaptive development, respectively, and diagnostic groups for children without ASD were defined on the basis of these assessments. The DD group consisted of children with composite scores <70 on the MSEL and/or VABS. The TD group only included GP children with no previous diagnosis of ASD or DD, Social Communication Questionnaire score <15, and composite scores ≥70 on both the MSEL and VABS. All CHARGE study clinical assessment personnel had attained research reliability on the developmental assessments they administered (ADI-R, ADOS, MSEL, and VABS). Bilingual study staff were available to administer informed consent and all instruments/questionnaires in Spanish.
Maternal Conditions and Potential Confounders
Demographic and medical information was obtained from the CHARGE Environmental Exposure Questionnaire (EEQ; available for 97.6% of participants), birth files, and medical records (available for 57.7% of participants). The EEQ is a structured telephone-administered interview with the biological mother and includes questions about demographic characteristics, maternal medical history, and various environmental exposures. Trained study staff extracted data from medical records.
The primary MC of interest was maternal T2D or GDM in the index pregnancy. Other conditions of interest were hypertension and obesity, defined as BMI ≥30, with onset before the index pregnancy. BMI (kilograms per meter squared) was calculated by using the height and prepregnancy weight recorded in the medical records (when available) or from the EEQ. BMI obtained from self-reported measurements was validated against BMI calculated from medical record measurements in a subset of 346 (34.5%) women for whom both data sources were available. The intraclass correlation coefficient between these BMI calculations was 0.912, indicating strong agreement.
Diabetes and hypertension (with or without preeclampsia) were considered present if they were noted on the medical history form in the prenatal medical record (when available) or if mothers answered “yes” to “During this [index] pregnancy were you ever told by a physician or nurse that you had gestational diabetes?” or “At any time before you became pregnant with [index child], were you ever told by a doctor that you had [diabetes, high blood pressure]?” in the EEQ. Discrepancies between medical records and self-report were verified. Self-reported diabetes and hypertension were validated in a subset of 560 (55.8%) women. Agreement between self-report and medical records was excellent for diabetes (κ = 0.79) and fair for hypertension (κ = 0.38). Women who misreported having hypertension were more likely to be multiparous and to have a history of gestationally induced hypertension. Because obesity, hypertension, and diabetes (T2D/GDM) are closely linked clinical manifestations of insulin resistance, an “any metabolic condition” variable was created as another proxy measure of insulin resistance. For all analyses, these predictor variables were categorized as follows: (1) had condition of interest; (2) did not have condition of interest but had another MC or was overweight (BMI ≥25); and (3) did not have any MCs and had a BMI <25. For each MC variable, we present results for level 1 versus level 3 to maximize the contrast and simplify the interpretation of findings.
Covariates selected a priori included mother’s age at delivery, race/ethnicity (non-Hispanic white, other non-Hispanic, or Hispanic), education level (high school or less, some college, or bachelor degree or higher), delivery payer (government program or private insurance), calendar time defined as number of years from the first participant’s enrollment date, the child’s gender and age in years at study enrollment, and catchment area; the latter 3 factors were frequency-matching variables. An indicator for known chromosomal/genetic (eg, trisomy 21, Angelman syndrome), metabolic/mitochondrial (eg, Leigh syndrome, carnitine deficiency), or neurologic (eg, cerebral palsy, epilepsy, hydrocephalus) disorders was created by using parent-report data from the child’s medical history completed by a study physician.
For this study, 1004 children (517 ASD, 172 DD, and 315 TD), consisting of 964 singletons and 20 sibling pairs, were included from a pool of 1317 participants who completed a clinic visit between January 2003 and June 2010. Excluded were 245 children who either did not complete the necessary assessments to confirm diagnosis or did not satisfy the diagnostic criteria for the groups considered in this study, and 68 children for whom data regarding maternal diabetes, hypertension, and obesity were unavailable.
Statistical Analyses
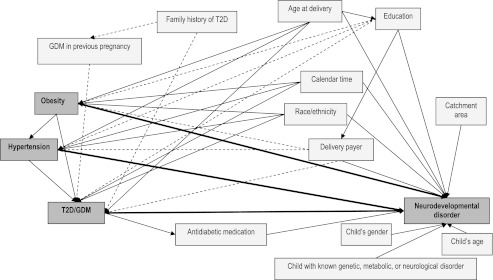
All analyses were performed by using SAS version 9.2 (SAS Institute Inc, Cary, NC). A directed acyclic graph35 (Fig 1) was constructed to represent the underlying causal relationships and used as guidance in evaluating associations among predictors, covariates, and outcomes. All variables with arrows pointing to the predictor (obesity, hypertension, or T2D/GDM) and the outcome (neurodevelopmental disorder) were defined as potential confounders and evaluated further. To determine whether MCs during pregnancy were associated with an increased risk of having a child with ASD or DD relative to TD, 4 multinomial logistic regression models were fitted and corrected for family clusters, 1 for each condition and 1 for the “any metabolic condition” predictor. Odds ratios (ORs) and 95% confidence intervals (CIs) were used as estimates of relative risk. Final models were adjusted for mother’s age at delivery, race/ethnicity, education, payer, calendar time, child’s age and gender, and catchment area.
FIGURE 1.
Directed acyclic graph. All relationships represented in this graph were based on review of the literature, with the exception of the frequency-matching variables (child’s age and gender, and catchment area). Solid arrows indicate stronger associations, and dashed arrows denote weaker associations.
To evaluate children’s developmental scores in association with maternal MCs, linear regression models corrected for family clusters were fitted. Age-standardized scores from MSEL visual reception, fine motor, receptive language, and expressive language subscales (mean ± SD: 50 ± 10) and from VABS communication, socialization, and motor skills domains (mean ± SD: 100 ± 15) were examined; MSEL and VABS composite scores (mean ± SD: 100 ± 15) were also considered. Covariates in these models included mother’s age, race/ethnicity, education, payer, calendar time, child’s age and gender, and catchment area. These models compared mean assessment scores of children whose mothers had an MC of interest (diabetes, hypertension, obesity, or any MC) versus those whose mothers did not have any of these MCs and had a BMI <25; comparisons were done separately among children with and those without ASD. Least squares (LS) means and SEs were used to measure association between MCs and developmental scores. The developmental domains examined were selected a priori because of their biological relevance to MCs, and adjustment for multiple comparisons was not performed. In the non-ASD subset, DD and TD groups were combined because the mean differences in developmental scores of children born to mothers with MCs compared with no MCs were similar in magnitude between these diagnostic groups. In addition, the combined sample provided more power to detect an association, through not only a larger sample but also the full range of variation in cognitive and adaptive scores.36
Results
Mothers of children with ASD were similar to controls in terms of race/ethnicity, education, and delivery payer (Table 1). Mothers of DD children were more likely to be Hispanic and to have lower education and less likely to have had private insurance compared with controls. Multiparas in both case groups were more likely to have a history of GDM compared with controls. As expected, because of frequency-matching, the ASD and TD groups were similar with respect to child’s age and gender; the DD group, which was not matched, had a higher proportion of girls compared with the other 2 groups. Discrepancies between the ASD and TD groups regarding the Los Angeles catchment area arose because of delayed implementation of the recruitment protocol for controls in the initial year of the study at the Los Angeles site, leading to some imbalance in the case-control ratio.
TABLE 1.
Characteristics of the Study Sample According to Diagnostic Group: CHARGE Study, January 2003–June 2010 (N = 1004)
| Characteristic | ASD | DD | TD | ASD Versus TD | DD Versus TD | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | P | P | |
| Race/ethnicity | .49 | .002 | ||||||
| White, non-Hispanic | 304 | 58.8 | 82 | 47.7 | 199 | 63.2 | ||
| Hispanic (any race) | 131 | 25.3 | 60 | 34.9 | 68 | 21.6 | ||
| Other race, non-Hispanic | 82 | 15.9 | 30 | 17.4 | 48 | 15.2 | ||
| Education | .10 | <.001 | ||||||
| High school or less | 78 | 15.1 | 50 | 29.1 | 47 | 14.9 | ||
| Some college | 208 | 40.2 | 66 | 38.4 | 104 | 33.0 | ||
| Bachelor degree or higher | 231 | 44.7 | 56 | 32.5 | 164 | 52.1 | ||
| Delivery payer | .12 | <.001 | ||||||
| Government program | 84 | 16.3 | 50 | 29.1 | 39 | 12.4 | ||
| Private insurance | 433 | 83.7 | 122 | 70.9 | 276 | 87.6 | ||
| Multipara | 281 | 54.4 | 111 | 64.5 | 176 | 55.9 | .63 | .06 |
| Maternal parent with diabetes | 111 | 22.0 | 39 | 23.1 | 64 | 20.8 | .63 | .41 |
| Missing | 13 | 3 | 7 | |||||
| GDM in previous pregnancy (multiparas only) | 19 | 6.8 | 6 | 5.4 | 5 | 2.8 | .06 | .28 |
| BMI (kg/m2) | .05 | .003 | ||||||
| <18.5 (underweight) | 19 | 3.7 | 6 | 3.5 | 10 | 3.2 | ||
| 18.5–24.9 (normal) | 269 | 52.0 | 65 | 37.8 | 172 | 54.6 | ||
| 25.0–29.9 (overweight) | 118 | 22.8 | 60 | 34.9 | 88 | 27.9 | ||
| ≥30.0 (obese) | 111 | 21.5 | 41 | 23.8 | 45 | 14.3 | ||
| Hypertension before pregnancy | 19 | 3.7 | 6 | 3.5 | 4 | 1.3 | .03 | .11 |
| Diabetes in index pregnancy | ||||||||
| Type 1 | 1 | 0.2 | 1 | 0.6 | 0 | 0.0 | .99 | .36 |
| T2D | 4 | 0.8 | 1 | 0.6 | 1 | 0.3 | .65 | .30 |
| GDM | 44 | 8.5 | 19 | 11.0 | 19 | 6.1 | .18 | .16 |
| T2D or GDM | 48 | 9.3 | 20 | 11.6 | 20 | 6.4 | .13 | .10 |
| Any type | 49 | 9.5 | 21 | 12.2 | 20 | 6.4 | .11 | .07 |
| Took antidiabetic medication | 10 | 1.9 | 10 | 5.8 | 5 | 1.6 | .69 | .01 |
| Hypertension, obesity, or diabetes (T2D or GDM) | 148 | 28.6 | 60 | 34.9 | 61 | 19.4 | .003 | <.001 |
| Child’s gender, malea | 436 | 85.8 | 121 | 68.0 | 256 | 81.3 | .08 | .001 |
| Child with a known genetic, metabolic, or neurologic disorder | 12 | 2.3 | 56 | 32.9 | 0 | 0.0 | <.001 | <.001 |
| Missing | 1 | 2 | ||||||
| Regional Center catchment area at enrollmenta | <.001 | .01 | ||||||
| Alta, Far Northern, and Redwood Coast | 185 | 35.8 | 87 | 50.6 | 132 | 41.9 | ||
| North Bay | 70 | 13.6 | 18 | 10.4 | 48 | 15.2 | ||
| East Bay, San Andreas, and Golden Gate | 88 | 17.0 | 17 | 9.9 | 63 | 20.0 | ||
| Valley Mountain, Central Valley, and Kern | 88 | 17.0 | 38 | 22.1 | 51 | 16.2 | ||
| All Los Angeles, Orange, San Diego, Tri-counties, and Inland | 86 | 16.6 | 12 | 7.0 | 21 | 6.7 | ||
| Continuous variables | Mean | SD | Mean | SD | Mean | SD | P | P |
| Age at delivery, y | 31.11 | 5.48 | 30.81 | 6.52 | 31.08 | 5.65 | .94 | .63 |
| Child’s age at study enrollment, ya | 3.65 | 0.80 | 3.79 | 0.76 | 3.54 | 0.80 | .06 | .001 |
| Time from earliest consent date, y | 3.16 | 1.93 | 4.26 | 1.81 | 3.92 | 1.77 | <.001 | .05 |
Children from the GP were frequency matched on age, gender, and catchment area (regional centers) to ASD cases.
The proportions of T2D/GDM in the ASD (9.3%) and DD (11.6%) groups were higher compared with controls (6.4%); after adjustment for covariates, mothers with diabetes were 2.3 times more likely to have a child with DD (OR: 2.33 [95% CI: 1.08–5.05]), but the association between diabetes and ASD did not reach statistical significance (Table 2). The prevalence of hypertension was low in all groups but more common among case mothers than controls (ASD: 3.7%; DD: 3.5%; TD: 1.3%); after adjusting for covariates, the association between hypertension and ASD or DD was not significant. The risk of having a child with ASD or DD, relative to TD, was significantly increased among obese women (ASD, OR: 1.67 [95% CI: 1.10–2.56]; DD, OR: 2.08 [95% CI: 1.20–3.61]); >20% of case mothers were obese compared with 14.3% of controls. The prevalence of any MC was higher in the ASD (28.6%) and DD (34.9%) groups compared with controls (19.4%), with respective adjusted ORs of 1.61 (95% CI: 1.10–2.37) and 2.35 (95% CI: 1.43–3.88). Analyses restricted to children without known genetic, metabolic, or neurologic disorders conferred similar or slightly stronger associations (data not shown).
TABLE 2.
OR for Autism/ASD or Other Delays in Relation to Diabetes and Related Conditions: CHARGE Study, January 2003–June 2010 (N = 1004)
| Conditions in Index Pregnancy | ASD | DD | TD | ASD Versus TD | DD Versus TD | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ORa | 95% CI | ORa | 95% CI | |
| Diabetesb | 48 | 9.3 | 20 | 11.6 | 20 | 6.4 | 1.52 | 0.82–2.83 | 2.33 | 1.08–5.05 |
| Hypertension | 19 | 3.7 | 6 | 3.5 | 4 | 1.3 | 2.84 | 0.94–8.56 | 3.58 | 0.93–13.78 |
| Obesity | 111 | 21.5 | 41 | 23.8 | 45 | 14.3 | 1.67 | 1.10–2.56 | 2.08 | 1.20–3.61 |
| Any MC(s) | 148 | 28.6 | 60 | 34.9 | 61 | 19.4 | 1.61 | 1.10–2.37 | 2.35 | 1.43–3.88 |
Adjusted for mother’s age at delivery, race/ethnicity, education level, delivery payer, calendar time, child’s age at enrollment and gender, and catchment area. Comparison group had no hypertension or diabetes (T2D or GDM) and also had BMI <25; this group included 267 in ASD, 64 in DD, and 172 in TD groups.
T2D or GDM only.
Within the ASD group, children of mothers with diabetes performed 0.37 SD lower on the MSEL expressive language scale compared with children of nondiabetic mothers (P = .01; Table 3); MSEL receptive language and VABS communication scores were also lower among children of diabetic mothers, with differences approaching statistical significance. No significant differences in MSEL or VABS scores were observed regarding MCs collectively among children with ASD.
TABLE 3.
Assessment Scores of Children of Mothers With and Without Diabetes (T2D or GDM),a Stratified According to ASD Status
| Assessment | ASD (n = 315) | No ASD (n = 276) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes | No Conditions | Pb | Diabetes | No Conditions | Pb | |||||
| LS Mean | SE | LS Mean | SE | LS Mean | SE | LS Mean | SE | |||
| MSEL | ||||||||||
| Visual reception T score | 27.98 | 2.62 | 28.29 | 1.04 | .91 | 44.35 | 2.94 | 46.63 | 1.25 | .47 |
| Fine motor T score | 26.16 | 1.94 | 27.81 | 1.00 | .39 | 39.89 | 2.49 | 44.33 | 1.24 | .10 |
| Receptive language T score | 22.88 | 1.71 | 25.98 | 0.86 | .07 | 36.95 | 2.31 | 42.36 | 1.11 | .03 |
| Expressive language T score | 21.51 | 1.36 | 25.19 | 0.84 | .01 | 36.99 | 2.27 | 42.23 | 1.10 | .03 |
| Composite Standard score | 56.16 | 3.00 | 59.53 | 1.45 | .26 | 81.90 | 3.99 | 90.12 | 1.95 | .06 |
| VABS | ||||||||||
| Communication standard score | 61.74 | 2.19 | 66.07 | 1.24 | .05 | 87.08 | 3.25 | 92.53 | 1.55 | .12 |
| Socialization standard score | 64.43 | 1.82 | 66.52 | 1.04 | .25 | 87.84 | 2.70 | 95.13 | 1.43 | .01 |
| Motor skills standard score | 74.86 | 2.57 | 74.37 | 1.57 | .85 | 88.50 | 4.00 | 92.05 | 1.84 | .40 |
| Composite standard score | 60.82 | 1.95 | 62.89 | 1.21 | .28 | 85.36 | 3.68 | 91.69 | 1.78 | .11 |
Diabetes group includes mothers with T2D or GDM; no conditions group consists of mothers with no diabetes (T2D or GDM) or hypertension and who have BMI<25; this comparison group consisted of 267 in ASD and 236 in non-ASD strata.
Adjusted for mother’s age at delivery, race/ethnicity, education level, delivery payer, calendar time, child’s age at enrollment and gender, and catchment area.
Among children without ASD, MSEL receptive and expressive language scores were ∼0.5 SD lower among children of mothers with diabetes compared with nondiabetic mothers (P = .03 for both subscales; Table 4); MSEL composite scores were borderline lower among children of mothers with diabetes. VABS socialization scores were 0.49 SD lower among children from diabetic pregnancies (P = 0.01). The presence of any MC was associated with lower scores on all MSEL subscales and composite (visual: –0.51 SD [P = 0.01]; motor: –0.53 SD [P = 0.01]; receptive: –0.50 SD [P = .004]; expressive: –0.59 SD [P = .0004]; composite: –0.65 SD [P = .001]) and all VABS domains and composite (communication: –0.43 SD [P = .01]; socialization: –0.50 SD [P = 0.0004]; motor: –0.39 SD [P = 0.04]; composite: –0.51 SD [P = 0.005]). Findings from restricted analyses were nearly identical (data not shown).
TABLE 4.
Assessment Scores of Children of Mothers With and Without any MCs,a Stratified According to ASD Status
| Assessment | ASD (n = 315) | No ASD (n = 276) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any MC | No Conditions | Pb | Any MC | No Conditions | Pb | |||||
| LS Mean | SE | LS Mean | SE | LS Mean | SE | LS Mean | SE | |||
| MSEL | ||||||||||
| Visual reception T score | 26.98 | 1.41 | 28.31 | 1.03 | 0.37 | 41.57 | 1.72 | 46.66 | 1.25 | .01 |
| Fine motor T score | 27.11 | 1.18 | 27.79 | 1.00 | 0.58 | 38.98 | 1.72 | 44.32 | 1.24 | .01 |
| Receptive language T score | 24.68 | 1.09 | 25.92 | 0.86 | 0.28 | 37.34 | 1.48 | 42.32 | 1.11 | .004 |
| Expressive language T score | 23.72 | 0.96 | 25.16 | 0.84 | 0.16 | 36.33 | 1.38 | 42.21 | 1.10 | <.001 |
| Composite Standard score | 57.59 | 1.79 | 59.49 | 1.44 | 0.31 | 80.35 | 2.61 | 90.11 | 1.94 | .001 |
| VABS | ||||||||||
| Communication standard score | 63.71 | 1.47 | 66.04 | 1.23 | 0.13 | 85.87 | 2.11 | 92.38 | 1.55 | .01 |
| Socialization standard score | 67.16 | 1.32 | 66.50 | 1.04 | 0.62 | 87.63 | 1.78 | 95.10 | 1.43 | <.001 |
| Motor skills standard score | 75.00 | 1.83 | 74.35 | 1.57 | 0.73 | 86.15 | 2.51 | 92.06 | 1.84 | .04 |
| Composite standard score | 62.62 | 1.55 | 62.87 | 1.20 | 0.86 | 84.09 | 2.32 | 91.68 | 1.77 | .005 |
Any MCs group includes mothers with diabetes (T2D or GDM), hypertension, or a BMI ≥30; no conditions group consists of mothers with no diabetes (T2D or GDM) or hypertension and who have a BMI <25; this comparison group consisted of 267 in ASD and 236 in non-ASD strata.
Adjusted for mother’s age at delivery, race/ethnicity, education level, delivery payer, calendar time, child’s age at enrollment and gender, and catchment area.
Discussion
In this study, we observed that diabetes, hypertension, and obesity were more common among mothers of children with ASD and DD compared with controls. Furthermore, diabetes, in particular, was associated with statistically significantly greater deficits in expressive language among children with ASD, although the magnitude of the deficits was relatively small. Among children without ASD, MCs collectively were associated with impairments in visual reception, motor skills, and receptive and expressive language, as well as adaptive communication and socialization.
Our findings relating diabetes to impairments in cognitive and language development are consistent with some10,13,16 but not all previous studies.17,18 Hultman et al17 included numerous maternal and pregnancy characteristics in their multivariable model; as such, the temporal and causal interrelationships among these risk factors were effectively ignored. Hence, it is plausible that the association with ASD may have been attenuated as a consequence of including complications downstream of diabetes. Furthermore, although Dodds et al18 reported higher proportions of diabetes (preexisting and GDM) among mothers of ASD children compared with controls in unadjusted analyses that only neared statistical significance, key confounders were not considered. Interestingly, prepregnancy obesity (≥90 kg) and excessive weight gain (≥18 kg) during pregnancy were significantly associated with ASD; however, the final model also included intermediary maternal characteristics (eg, labor type) potentially on the causal pathway between these risk factors and the outcome, thus limiting the interpretability of these findings. Nevertheless, obesity is a significant risk factor for both hypertension and diabetes (T2D and GDM) and is characterized by increased insulin resistance and chronic inflammation, as are the other 2 MCs we examined.19,20,22 Therefore, in our study, we constructed a causal diagram to investigate the interrelationships among maternal MCs, covariates, and the outcome. We also compared the risk of an adverse outcome in children whose mothers had a given condition (eg, diabetes) relative to those whose mothers had neither that condition nor the risk factors for it (eg, no hypertension and with BMI <25). This approach was applied to account for increased insulin resistance due to other MCs.
Reliance on self-reported medical conditions was a limitation of this study. However, in the 56% of participants for whom medical records were available, we found the 2 sources to be in good agreement. Thus, despite this limitation, we can have confidence in our results. Furthermore, although biological measurements (eg, glucose, insulin, lipids, immune biomarkers) before and during pregnancy would have been ideal, we chose conditions (T2D/GDM, hypertension, and obesity) highly indicative of increased insulin resistance as proxy measures of dysregulated metabolism and chronic inflammation because we lacked these biological measurements for most of the participants.
Nonetheless, our study offers several strengths. First, it includes a representative population of cases and controls with well-defined and consistently applied diagnoses; the lack of these design characteristics was a limitation in previously published population-based studies investigating maternal risk factors and ASD.16–18 Secondly, whereas previous studies have examined maternal T2D or GDM in relation to neurodevelopmental disorders or developmental measures, to our knowledge, this is the first to also examine a broader group of conditions highly predictive of insulin resistance and these forms of diabetes.
In a diabetic and possibly prediabetic pregnancy, poorly regulated maternal glucose can result in adverse fetal development. Prolonged fetal exposure to elevated glucose levels results in chronic fetal hyperinsulinemia, which in turn triggers the fetus to increase oxygen consumption and metabolism, inducing chronic intrauterine tissue hypoxia.37 Further biological responses may result in fetal iron deficiency.38 Both fetal hypoxia and iron deficiency can profoundly affect neurodevelopment in humans, including alterations in myelination and cortical connectivity and aberrations in hippocampal neurons.39 Fetal iron deficiency has also been associated with reduced recognition memory as well as behavioral and developmental problems.40 In addition, increased maternal levels of cytokine interleukin-6, which can cross the placenta, have been shown to disrupt normal fetal brain development in experimental studies involving animal models, resulting in onset of seizures, impairments in spatial learning, increases in hypothalamic-pituitary activity, disturbances in cholinergic input to the hippocampus, and reduced neurogenesis in adult hippocampus.41,42 Increased levels of this interleukin and other proinflammatory cytokines are also produced in the presence of MCs such as diabetes and obesity.
Conclusions
The prevalence of obesity and diabetes among US women of childbearing age is 34% and 8.7%, respectively.24,27 Our findings raise concerns that these maternal conditions may be associated with neurodevelopmental problems in children and therefore could have serious public health implications.
Glossary
- ADI-R
Autism Diagnostic Interview, Revised
- ADOS
Autism Diagnostic Observation Schedule
- ASD
autism spectrum disorder
- CI
confidence interval
- DD
developmental delays
- EEQ
Environmental Exposure Questionnaire
- GDM
gestational diabetes
- GP
general population
- LS
least squares
- MC
metabolic condition
- MSEL
Mullen Scales of Early Learning
- OR
odds ratio
- T2D
type 2 diabetes
- TD
typical development
- VABS
Vineland Adaptive Behavior Scales
Footnotes
Ms Krakowiak developed the concept for this article with substantial contribution from Drs Walker, Bremer, and Hertz-Picciotto; Ms Baker extracted medical records data and contributed extensively to data verification; Ms Krakowiak performed data validation and statistical analysis and prepared the manuscript; Dr Ozonoff and Dr Hansen provided input regarding diagnoses and participated in the interpretation of findings; and Dr Hertz-Picciotto, as principal investigator, obtained funding for the CHARGE study and supervised all stages of the study. All authors participated in the revision of the manuscript and gave final approval of the version to be published.
FINANCIAL DISCLOSURE: Dr Hansen receives grant support from Autism Speaks; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This research was supported by the National Institutes of Health (P01 ES11269 and R01 ES015359), the US Environmental Protection Agency through the Science to Achieve Results program (R829388 and R833292), and by the MIND Institute, University of California Davis. Funded by the National Institutes of Health (NIH).
References
- 1.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 2.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2006 Principal Investigators. Centers for Disease Control and Prevention (CDC) . Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58(10):1–20 [PubMed] [Google Scholar]
- 3.Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology. 2009;20(1):84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhasin TK, Brocksen S, Avchen RN, Van Naarden Braun K. Prevalence of four developmental disabilities among children aged 8 years—Metropolitan Atlanta Developmental Disabilities Surveillance Program, 1996 and 2000. MMWR Surveill Summ. 2006;55(1):1–9 [PubMed] [Google Scholar]
- 5.Matson JL, Shoemaker M. Intellectual disability and its relationship to autism spectrum disorders. Res Dev Disabil. 2009;30(6):1107–1114 [DOI] [PubMed] [Google Scholar]
- 6.Newschaffer CJ, Croen LA, Daniels J, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–258 [DOI] [PubMed] [Google Scholar]
- 7.Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17(4):434–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry. 2009;195(1):7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeargin-Allsopp M, Murphy CC, Cordero JF, Decouflé P, Hollowell JG. Reported biomedical causes and associated medical conditions for mental retardation among 10-year-old children, metropolitan Atlanta, 1985 to 1987. Dev Med Child Neurol. 1997;39(3):142–149 [DOI] [PubMed] [Google Scholar]
- 10.Rizzo TA, Metzger BE, Dooley SL, Cho NH. Early malnutrition and child neurobehavioral development: insights from the study of children of diabetic mothers. Child Dev. 1997;68(1):26–38 [PubMed] [Google Scholar]
- 11.Ratzon N, Greenbaum C, Dulitzky M, Ornoy A. Comparison of the motor development of school-age children born to mothers with and without diabetes mellitus. Phys Occup Ther Pediatr. 2000;20(1):43–57 [PubMed] [Google Scholar]
- 12.Rizzo TA, Dooley SL, Metzger BE, Cho NH, Ogata ES, Silverman BL. Prenatal and perinatal influences on long-term psychomotor development in offspring of diabetic mothers. Am J Obstet Gynecol. 1995;173(6):1753–1758 [DOI] [PubMed] [Google Scholar]
- 13.Dionne G, Boivin M, Séguin JR, Pérusse D, Tremblay RE. Gestational diabetes hinders language development in offspring. Pediatrics. 2008;122(5). Available at: www.pediatrics.org/cgi/content/full/122/5/e1073. [DOI] [PubMed] [Google Scholar]
- 14.DeBoer T, Wewerka S, Bauer PJ, Georgieff MK, Nelson CA. Explicit memory performance in infants of diabetic mothers at 1 year of age. Dev Med Child Neurol. 2005;47(8):525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deregnier RA, Nelson CA, Thomas KM, Wewerka S, Georgieff MK. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. J Pediatr. 2000;137(6):777–784 [DOI] [PubMed] [Google Scholar]
- 16.Leonard H, de Klerk N, Bourke J, Bower C. Maternal health in pregnancy and intellectual disability in the offspring: a population-based study. Ann Epidemiol. 2006;16(6):448–454 [DOI] [PubMed] [Google Scholar]
- 17.Hultman CM, Sparén P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology. 2002;13(4):417–423 [DOI] [PubMed] [Google Scholar]
- 18.Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord. 2011;41(7):891–902 [DOI] [PubMed] [Google Scholar]
- 19.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246 [DOI] [PubMed] [Google Scholar]
- 20.Ferrannini E, Haffner SM, Stern MP. Essential hypertension: an insulin-resistant state. J Cardiovasc Pharmacol. 1990;15(suppl 5):S18–S25 [PubMed] [Google Scholar]
- 21.Sweet IR, Gilbert M, Maloney E, Hockenbery DM, Schwartz MW, Kim F. Endothelial inflammation induced by excess glucose is associated with cytosolic glucose 6-phosphate but not increased mitochondrial respiration. Diabetologia. 2009;52(5):921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zavalza-Gómez AB, Anaya-Prado R, Rincón-Sánchez AR, Mora-Martínez JM. Adipokines and insulin resistance during pregnancy. Diabetes Res Clin Pract. 2008;80(1):8–15 [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(32 suppl 1):S62–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235–241 [DOI] [PubMed] [Google Scholar]
- 25.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report. May 2009;(13):1–7 [PubMed] [Google Scholar]
- 26.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2007. Natl Vital Stat Rep. 2010;58(24):1–85 [PubMed] [Google Scholar]
- 27.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999-2005. Diabetes Care. 2008;31(5):899–904 [DOI] [PubMed] [Google Scholar]
- 28.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114(7):1119–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Couteur A, Lord C, Rutter M. Autism Diagnostic Interview–Revised (ADI-R). Los Angeles, CA: Western Psychological Services; 2003 [Google Scholar]
- 30.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223 [PubMed] [Google Scholar]
- 31.Risi S, Lord C, Gotham K, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1094–1103 [DOI] [PubMed] [Google Scholar]
- 32.Rutter M, Bailey A, Berument SK, Lord C, Pickles A. Social Communication Questionnaire (SCQ). Los Angeles, CA: Western Psychological Services; 2003 [Google Scholar]
- 33.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Services, Inc; 1995 [Google Scholar]
- 34.Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales Interview Edition Expanded Form Manual. Circle Pines, MN: American Guidance Services, Inc; 1984 [Google Scholar]
- 35.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48 [PubMed] [Google Scholar]
- 36.Winship C.Radbill L. Sampling weights and regression analysis. Sociol Methods Res. 1994;23(2):230–257 [Google Scholar]
- 37.Eidelman AI, Samueloff A. The pathophysiology of the fetus of the diabetic mother. Semin Perinatol. 2002;26(3):232–236 [DOI] [PubMed] [Google Scholar]
- 38.Georgieff MK. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc Trans. 2008;36(pt 6):1267–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Georgieff MK. The effect of maternal diabetes during pregnancy on the neurodevelopment of offspring. Minn Med. 2006;89(3):44–47 [PubMed] [Google Scholar]
- 40.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158–165 [DOI] [PubMed] [Google Scholar]
- 41.Jonakait GM. The effects of maternal inflammation on neuronal development: possible mechanisms. Int J Dev Neurosci. 2007;25(7):415–425 [DOI] [PubMed] [Google Scholar]
- 42.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24(6):881–897 [DOI] [PubMed] [Google Scholar]