Abstract
BACKGROUND:
Interindividual variability in pain perception and analgesic response is a major problem in perioperative practice. Adult studies suggest pain management is influenced by patient’s race. The objective of this study is to evaluate the influence of race on perioperative pain treatment in children.
METHODS:
Prospective observational study evaluating effect of race on analgesia and opioid related adverse effects after tonsillectomy in African American and Caucasian children. A sample of 194 healthy children between 6 and 15 years of age were included. Race was self-identified by parents. All participants received standard perioperative care with a standard anesthetic and an intraoperative dose of morphine. Analgesia outcomes included maximum postoperative pain scores, postoperative opioid requirement, and analgesic interventions. Safety outcomes included incidences of opioid related adverse effects.
RESULTS:
African American children experienced significantly more postoperative pain than Caucasian children as measured by postoperative opioid requirement (P = .0011), maximum postoperative pain scores (P < .0001), and analgesic interventions (P < .0001) in the recovery room. Although Caucasian children received relatively less opioids perioperatively, they had significantly higher opioid related adverse effects (P = .039). African American children with obstructive sleep apnea were more likely to have prolonged post anesthesia recovery unit stay due to inadequate pain control.
CONCLUSIONS:
After similar uses of intraoperative morphine for tonsillectomy, there was an unequal burden of increased pain in African American children and increased opioid adverse effects in Caucasian children in the recovery room. Though Caucasian children received relatively less opioids perioperatively, they had higher incidences of opioid related adverse effects than African American children.
KEY WORDS: pain management, pain response, pain sensitivity, race, safety
What’s Known on This Subject:
Disparities are known to exist in the prescription of opioid analgesics among racial and ethnic groups in the management of postoperative, cancer, and emergency department pain in patients across all ages, including children.
What This Study Adds:
Race is associated with an unequal burden of perioperative pain and opioid adverse effects in children. Relatively, African American children had higher postoperative pain, and Caucasian children had higher incidences of opioid related adverse effects.
Inadequately treated pain is a major public health problem and is a continuing problem in the perioperative setting in children.1 Interindividual variability in analgesic response and adverse effects of analgesic drugs is a major problem in perioperative practice, especially for drugs with narrow therapeutic indices such as opioids.
Pain is a highly complex, predominantly subjective, dynamic phenomenon that involves biological, psychological, and social factors. Pain sensitivity appears to differ among races/ethnicities. Specifically, considerable evidence exists that African American and Hispanic patients report lower tolerance for experimentally induced pain compared with Caucasian adults.2–4 In addition, several studies have revealed evidence of disparities in the prescription of opioid analgesics among racial and ethnic groups in the management of postoperative,5 cancer,6 and emergency department7–9 pain in patients across all ages, including children.
If racial disparities in pain management are to be eliminated, and care individualized, we need to better understand the role of race in opioid effects. No authors have examined the effect of race in surgical pain perception and opioid management in children. The purpose of this study was to investigate the influence of race on postoperative pain perception, opioid requirements, and opioid related side-effects in children. Such knowledge will advance the ultimate goal of individualizing perioperative pain management in children.
Methods
Study Design and Setting
This was a prospective clinical observational study with standard of perioperative anesthetic, surgical, and nursing care with a single group. This study was approved by the institutional review board, and written informed consents, and assents when appropriate, were obtained from all parents and participating children. In this study, we chose to focus on African American and Caucasian children because they represent 90% of the US population under 18 years of age: Caucasian (75.58%) and African American (15.13%).10
Participants
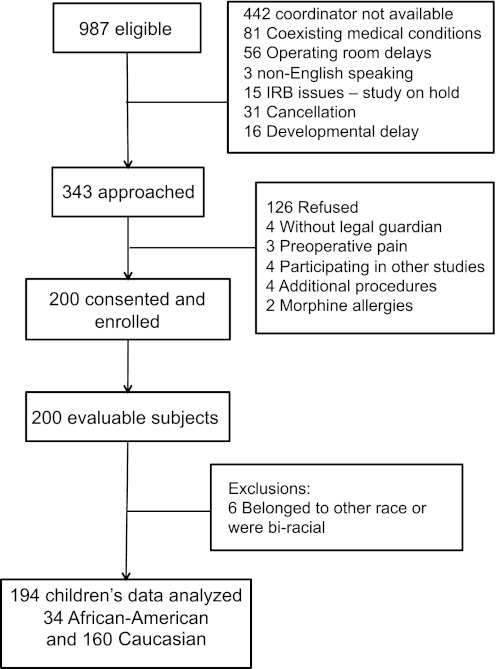
Children 6 to 15 years undergoing elective outpatient tonsillectomy or adenotonsillectomy were eligible for the study and recruited the day of surgery. Race was self-reported by parent or child; self-report is well accepted for identifying race.11,12 Sample inclusion criteria were children designated to have an American Society of Anesthesiologists physical status 1 or 2 scheduled for tonsillectomy or adenotonsillectomy because of recurrent tonsillitis, adenotonsillar hypertrophy, or obstructive sleep apnea (OSA). Children with sleep disordered breathing with history of snoring plus sleep pauses lasting more than 10 seconds or daytime drowsiness were considered as clinical symptoms of OSA; as documented in preoperative surgical note, the indication for tonsillectomy in these children was clinical diagnosis of OSA. Children were excluded if they or their parents were non-English speaking. Children allergic to study medications or who had developmental delay, liver or renal diseases, or preoperative pain requiring analgesics (eg, chronic tonsillitis) were excluded. Due to limited availability of research coordinators for this study, we were not able to recruit all eligible subjects, which resulted in convenient sampling (Fig 1).
FIGURE 1.
The consort diagram illustrates the flow of study participants through this clinical trial. Eligible participants, reasons for exclusions, and enrolled patients are reported. IRB, institutional review board.
Outcome Measures
Subjective pain assessments were done by using a 0 to 10 Numerical Rating Scale (NRS).13 Objective pain assessments were done with 0 to 10 FLACC (facial expression, leg movement, activity, cry, and consolability) scale.14 The analgesic effectiveness outcomes included maximum postoperative pain scores, prolonged post anesthesia recovery unit (PACU) stay (>90 minutes) due to inadequate pain control, postoperative morphine dose (mg/kg), need for an intravenous analgesic intervention, and the number of total analgesic interventions. Two binary safety outcomes were examined: opioid related side effects and prolonged PACU stay secondary to opioid related side effects. Opioid related side effect in the PACU was generated according to whether the patient had at least 1 of the following: opioid related respiratory depression (yes/no), nausea/vomiting (yes/no), excessive sedation (University of Michigan Sedation scores 3 and 4),15,16 or pruritus (yes/no).
Standard Care and Study Procedures
All participants received uniform perioperative care, including a standardized surgical technique and anesthesia with an intraoperative dose of 0.2 mg/kg of morphine, except children with OSA, who received a morphine dose of 0.1 mg/kg.
Standardized Anesthesia and PACU Protocol
It is standard practice that all tonsillectomy children get prophylactic ondansetron and dexamethasone intraoperatively. The anesthetic technique was similar in study subjects in terms of sevoflurane inhalational induction, propofol to facilitate endotracheal intubation, and sevoflurane inhalational anesthesia maintenance without use of neuromuscular relaxants, along with a standardized initial dose of morphine. A standardized initial dose of morphine was given intraoperatively before surgical pain. Children with history of OSA received 0.1 mg/kg, and children without OSA received 0.2 mg/kg. After surgical incision and with cauterization, if there were any signs suggestive of pain (clinically significant increase in heart rate and blood pressure), the clinical anesthesia team provided additional morphine intraoperatively.
Significant postoperative pain (NRS or FLACC ≥4/10) was managed in the PACU with rescue doses of morphine (0.05 mg/kg). Analgesic interventions and total morphine requirements (calculated by using the sum of the intraoperative dose and postoperative analgesic interventions) along with opioid adverse effects in PACU including respiratory depression were collected. Clinical respiratory depression is defined as respiratory rates <10 per minute or persistent oxygen desaturation <92% requiring supplemental oxygen to maintain pulse oxygen saturation >92% in the absence of clinically obvious upper airway obstruction.
Statistical Analysis
Statistical analyses were performed by using SAS, version 9.2 (SAS Institute, Inc, Cary, NC). Before analyses, the quality of the data was examined. Outcome measurements were compared between African American and Caucasian children overall and stratified by OSA.
To account for covariate effects, statistical modeling was then performed. Binary outcomes, including opioid related side effects, prolonged PACU stay due to pain, and intervention need, were analyzed by using logistic regression. Maximum pain score was analyzed by using a zero-inflated negative binomial model because of the inflated frequency of score 0. The total number of analgesic interventions followed a Poisson distribution and was analyzed by using a generalized linear model. Postoperative morphine dose was analyzed in patients who needed intervention by using a linear regression. We included age, sex, weight, BMI z-scores OSA, intraoperative morphine requirement (mg/kg), or total morphine requirement (mg/kg) as important covariates in our statistical models when significant effects were detected. Prolonged PACU stay due to pain, intervention need, maximum pain score, total number of analgesic interventions, and postoperative morphine dose were all used to measure the analgesic effect, and thus are highly correlated with each other (mean Spearman’s ρ = .72). Similarly, moderate correlation between opioid related side effects and other side effect outcomes was detected (mean Spearman’s ρ = .35). Opioid related side effects were also weakly correlated with analgesic effects outcomes (mean Spearman’s ρ = .16). Given the correlations among outcome variables, we classed all the outcomes into 2 outcome families, and adjusted the family wise error rate to 0.025 by using Bonferroni correction.
Results
Demographics
A consort diagram illustrates eligible, approached, and enrolled study subjects (Fig 1). Data collected from 194 Caucasian and African American children were analyzed (Fig 1). No significant differences were detected in age, weight, gender ratio, BMI, BMI z score, or BMI percentile between African American and Caucasian children (Table 1). The proportions of OSA differed by race; African American children had a higher proportion of OSA than Caucasian children. Therefore, outcomes in different races were compared with and without history of OSA stratified.
TABLE 1.
Demographic Data
| African American (N = 34) | Caucasian (N = 160) | P | |
|---|---|---|---|
| Age, y, median (IQR) | 8.75 (6.96 to 10.69) | 8.34 (7.15 to 11.04) | .67 |
| Weight, kg, median (IQR) | 35.25 (24.85 to 54.30) | 35.40 (25.90 to 46.80) | .72 |
| BMI z score, median (IQR) | 1.27 (0.07 to 2.06) | 0.90 (−0.15 to 1.63) | .26 |
| BMI percentile, median (IQR) | 89.75 (52.60 to 98.01) | 81.67 (43.98 to 94.83) | .26 |
| Gender, N (%) | |||
| Boy | 14 (41) | 77 (48) | .46 |
| OSA, N (%) | |||
| Yes | 25 (74) | 69 (43) | .001 |
BMI z score and percentile were calculated by using the Centers for Disease Control and Prevention growth charts. Differences in continuous data were tested by using the Wilcoxon rank sum test. Differences in proportions were tested by using the χ2 test.
Efficacy/Analgesia Outcome Measures
To compare postoperative pain and analgesic measures in different races, we first summarized the means or proportions of maximum FLACC pain scores in the PACU, postoperative morphine use, incidence of prolonged PACU stay due to inadequate pain control, postoperative analgesic intervention need, and number of postoperative analgesic interventions (Table 2). The comparisons of means and proportions suggested that African American children had significantly higher pain than Caucasian children and needed more analgesic interventions, especially in those with a history of OSA (Table 2). After adjusting for potential confounders in a statistical model, African American children showed higher FLACC scores (P < .0001), higher odds of intervention need (P = .0029), more interventions (P < .0001), and a greater postoperative morphine requirement (P = .0011). No OSA or OSA by race effects were detected in these outcomes. However, a significant race-specific OSA effect was detected (P = .0064) for prolonged stay in PACU due to inadequate pain control. Our statistical model demonstrated that while OSA revealed no effect in Caucasian children, African American children with OSA were more likely to have prolonged PACU stay due to inadequate pain control.
TABLE 2.
Race Associations With Analgesia and Opioid Adverse Effect Outcomes
| OSA | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | All | ||||||
| Median (IQR) or N (%) | P | Median (IQR) or N (%) | P | Median (IQR) or N (%) | P | |||
| Intraoperative morphine, mg/kg | AA | 0.20 (0.20–0.24) | .36 | 0.19 (0.13–0.20) | .15 | 0.20 (0.14–0.20) | .72 | |
| Caucasian | 0.20 (0.19–0.23) | 0.17 (0.10–0.20) | 0.19 (0.16–0.21) | |||||
| Postoperative morphine, mg/kg | AA | 0.08 (0–0.13) | .22 | 0.10 (0.05–0.19) | <.0001 | 0.10 (0.04–0.18) | <.0001 | |
| Caucasian | 0.04 (0–0.09) | 0.04 (0–0.08) | 0.04 (0–0.09) | |||||
| Maximum FLACC score | AA | 4 (0–5.5) | .48 | 6 (4–8) | <.0001 | 5 (3.75–8) | <.0001 | |
| Caucasian | 2 (0–4.25) | 2 (0–5) | 2 (0–5) | |||||
| No. of analgesic interventions | AA | 1 (0–2.5) | .27 | 1 (1–2.5) | .0003 | 1 (1–2.25) | <.0001 | |
| Caucasian | 1 (0–1) | 1 (0–1) | 1 (0–1) | |||||
| Prolonged PACU stay due to pain | No | AA | 7 (78) | .49 | 7 (28) | <.0001 | 14 (41) | .0054 |
| Caucasian | 58 (64) | 51 (74) | 109 (68) | |||||
| Yes | AA | 2 (22) | 18 (72) | 20 (59) | ||||
| Caucasian | 33 (36) | 18 (26) | 51 (32) | |||||
| Analgesic intervention need in PACU | No | AA | 3 (33) | .73 | 2 (8) | .0012 | 5 (15) | .0010 |
| Caucasian | 41 (45) | 30 (43) | 71 (44) | |||||
| Yes | AA | 6 (67) | 23 (92) | 29 (85) | ||||
| Caucasian | 50 (55) | 39 (57) | 89 (56) | |||||
| Opioid related side effects | No | AA | 1 (13) | .99 | 7 (29) | .06 | 8 (25) | .12 |
| Caucasian | 13 (15) | 8 (12) | 21 (14) | |||||
| Yes | AA | 7 (87) | 17 (71) | 24 (75) | ||||
| Caucasian | 73 (85) | 59 (88) | 132 (86) | |||||
| Prolonged PACU stay due to opioid side effects | No | AA | 4 (80) | .99 | 17 (89) | .21 | 21 (88) | .29 |
| Caucasian | 58 77) | 42 (74) | 100 (76) | |||||
| Yes | AA | 1 (20) | 2 (11) | 3 (12) | ||||
| Caucasian | 17 (23) | 15 (26) | 32 (24) | |||||
| RD | No | AA | 6 (67) | .39 | 20 (80) | .99 | 26 (76) | .64 |
| Caucasian | 73 (80) | 56 (81) | 129 (81) | |||||
| Yes | AA | 3 (33) | 5 (20) | 8 (24) | ||||
| Caucasian | 18 (20) | 13 (19) | 31 (19) | |||||
| PONV | No | AA | 9 (100) | .35 | 22 (88) | .55 | 31 (91) | .30 |
| Caucasian | 77 (85) | 56 (81) | 133 (83) | |||||
| Yes | AA | 0 (0) | 3 (12) | 3 (9) | ||||
| Caucasian | 14 (15) | 13 (19) | 27 (17) | |||||
| Pruritus | No | AA | 3 (38) | .69 | 11 (44) | .07 | 14 (42) | .09 |
| Caucasian | 25 (28) | 16 (24) | 41 (26) | |||||
| Yes | AA | 5 (63) | 14 (56) | 19 (58) | ||||
| Caucasian | 64 (72) | 51 (76) | 115 (74) | |||||
Continuous variables were shown as median (interquartile range [IQR]) and were compared by using the Wilcoxon rank-sum test; categorical variables were shown as number (proportion) and were compared by using Fisher’s exact test. AA, African-American; RD, respiratory depression; PONV, postoperative nausea and vomiting.
Safety Outcome Measures
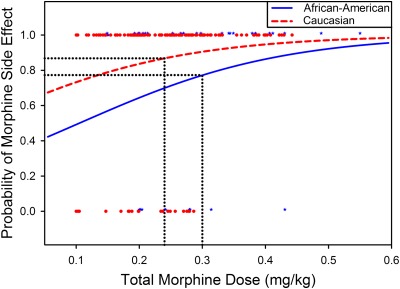
Overall incidences of opioid related side effects in the PACU and incidences of prolonged PACU stay due to side effects were relatively higher in Caucasian children compared with African American children, especially in the setting of relatively lower total perioperative opioid use in Caucasian children compared with African American children (Tables 2 and 3; Fig 2). After adjusting for total morphine administered, though no significant race effect was detected for respiratory depression alone, overall opioid related adverse effects were significantly higher in Caucasian patients (odds ratio = 2.8 [95% Confidence Intervals: 1.1–7.6], P = .039). Prolonged PACU stay due to side effects also tended to be higher in Caucasian children (odds ratio = 3.47 [95% CIs: 0.9–13.9], P = .079).
TABLE 3.
Number of Children With Side Effects in Different Races and Morphine Groups
| Total Morphine Dose, mg/kg | |||||||
|---|---|---|---|---|---|---|---|
| 0–0.2 | 0.2–0.3 | >0.3 | |||||
| N | Proportion, % | N | Proportion, % | N | Proportion, % | ||
| Side effect | |||||||
| AA | Yes | 4 | 100 | 9 | 60 | 11 | 85 |
| No | 0 | 0 | 6 | 40 | 2 | 15 | |
| Caucasian | Yes | 44 | 80 | 55 | 85 | 30 | 100 |
| No | 11 | 20 | 10 | 15 | 0 | 0 | |
In the contingency table, the raw adverse outcome data for each of the children is described as categorical “yes” or “no” instead of probabilities for overall total perioperative morphine use (in mg/kg). AA, African-American.
FIGURE 2.
Racial differences in morphine related adverse events and perioperative morphine use; total morphine dose is plotted in the x axis and the probabilities of morphine related adverse effects are plotted in the y axis for African American and Caucasian children. The red dots and blue stars represent raw opioid side effect data (yes side effect as 1, and no side effect as 0). Overall incidences of opioid related side effects in the PACU were relatively higher in Caucasian children compared with African American children, despite a relatively lower mean total perioperative opioid use in Caucasian children (0.24 mg/kg) compared with African American children (0.3 mg/kg).
BMI z score revealed some effect on postoperative morphine requirement (P = .028) and prolonged PACU stay due to side effects (P = .017). After accounting for the effect of BMI z score, the conclusion of race effect on these outcomes remains unchanged.
Discussion
This study demonstrates that African American and Caucasian children exhibit significant differences in postoperative pain responses, postoperative opioid requirements, and opioid adverse effects after tonsillectomy or adenotonsillectomy. African American race is associated with higher maximum postoperative pain scores and greater postoperative morphine requirements, whereas Caucasian race is associated with higher incidence of opioid related adverse effects despite lower morphine requirement. Importantly, these differences were independent of OSA.
In our study, African American children had significantly higher postoperative pain scores and received more morphine than Caucasian children. The higher pain scores in African American children are consistent with adult studies.17–21 An experimental study in children examined the influence of race on children’s pain sensitivity and revealed a differential moderating effect of pain coping and pain sensitivity between African American and Caucasian children.22 Authors of previous studies have demonstrated that African American patients have disproportionally inadequate pain control than Caucasian patients.6,9,23 We showed that African American and Caucasian children receiving similar doses of morphine during surgery have different postoperative pain experience for the same surgical procedure.
Racial disparities in pain management have been reported in emergency department,9 cancer,6 and postsurgical pain management.5 Though these studies6,9,23 suggest that there is under treatment of African American patients leading to inadequate pain control, our study indicates that African American children have intrinsically higher morphine requirements for comfort after surgery. African American children had higher postoperative pain scores in the PACU after receiving identical morphine doses to Caucasian children.
We also found that African American children had lower levels of side effects and increased tolerance of higher morphine doses than Caucasian children. Overall incidences of opioid related side effects in the PACU and incidences of prolonged PACU stay due to side effects were higher in Caucasian children compared with African American children, despite Caucasian children receiving relatively lower total perioperative opioid dose than African American children (Fig 2). Though a few children had clinical respiratory depression with higher doses of opioid, in our monitored PACU setting, none of the participants had serious harm or required re-intubation, naloxone use, or extended ventilator support.
Tonsillectomy and adenotonsillectomy are a common surgical treatment of recurrent tonsillitis and OSA in children.24 OSA is a common indication for adenotonsillectomy in children. African Americans have higher incidence of OSA than Caucasian patients,25 which was observed in this study’s pediatric population as well. Postsurgical administration of opioids in patients with OSA has recently been linked to an increased risk for respiratory complications.26 It is recommended that the total opioid dose to ensure adequate analgesia is about half in children with OSA compared with those with no OSA history.24 Paradoxically, in our study we observed significantly higher incidence of opioid related adverse effects in Caucasian children than African American children.
Because of this increased sensitivity to morphine related adverse effects in children with OSA, children in our study who presented for tonsillectomy due to OSA had lower intraoperative morphine doses compared with those presented for recurrent tonsillitis. Despite controlling for OSA in the study design, a history of OSA appears to be associated with increased pain and analgesic requirement in African American children. Our results indicate that reducing morphine dose is reasonable practice for Caucasian patients with OSA but may be leading to more pain related complications postoperatively in African American children with OSA.
In our follow-up and related study, we observed that African American children have higher morphine clearance than Caucasian children. Common UGT2B7 genetic variations (−161C>T and 802C>T) were not associated with observed racial differences in morphine’s clearance although the wild type of the UGT2B7 isozyme is more prevalent in the African American patients. Race of the child is an important factor in perioperative intravenous morphine’s clearance, and its potential role in personalizing analgesia with morphine needs further investigation (S.S., personal communication 2012).
There are a few limitations in our study. Although our study revealed an association between race and postoperative pain perception and responses to perioperative opioid, it is not possible to say whether these differences are related to race per se or to some unknown or not measurable variables that are highly associated with race, such as socioeconomic status, pain coping skills, and unknown genetic factors. Secondly, due to local demographics, we enrolled mainly African American and Caucasian children. Our current study does not address other races, especially the growing Hispanic population. Inclusion of other races/ethnicities is necessary to generalize and personalize perioperative outcomes based on race and ethnicity. Lastly, variations among clinical care providers such as PACU nurses and their subjective bias toward children’s pain perception and comfort level with opioid doses and responses might have influenced the results because of the observational nature of this prospective study. Findings from a large national study to describe pediatric nurses’ projected responses to children’s pain suggest that most nurses would make appropriate decisions relating to the treatment of children’s pain.27 Because intraoperative doses of morphine in both races were the same, only postoperative doses differed between races. The differences in dosing are based on postoperative pain scores; thus we believe the data to be free of racial bias.
Accurate measures of pain intensity are needed if postoperative pain is to be adequately managed while avoiding potentially harmful side effects. Difficulties in communication with children especially after surgery while recovering from anesthesia can compromise subjective pain assessment. The NRS is a self-reported pain score. In addition to subjective pain scores, we used an objective pain score, FLACC score, to measure pain in children. The FLACC scale uses behavioral cues to evaluate the degree of pain, and it is unknown if children of different ethnic backgrounds behave differently in response to pain.
Conclusions
Our statistically significant association between race, pain perception, and clinical responses to morphine used to manage perioperative pain does not by itself establish causality. Yet, our results demonstrate that race, with unknown differences in underlying genetic and associated nongenetic factors, influences surgical pain and opioid responses in children. When managing children’s pain, clinicians need to anticipate potentially higher opioid requirement in African American patients and consider increased opioid dosing in response to early signs of inadequate pain control. To advance personalized pain management, more studies are needed to understand the causes of racial differences and quantify doses of analgesics needed to optimally manage postoperative pain with minimal or no adverse effects.
Glossary
- FLACC
facial expression, leg movement, activity, cry, and consolability
- NRS
Numerical Rating Scale
- OSA
obstructive sleep apnea
- PACU
post anesthesia recovery unit
Footnotes
This pharmacogenetic study was designed and undertaken by the authors. The authors directed and had access to all the analyses and the full clinical and genetic database, wrote all drafts of the report, decided to publish the results, and attest for the accuracy and completeness of the data.
This trial reflects a portion of a larger study, Personalizing Perioperative Morphine Analgesia in Children, registered at www.clinicaltrials.gov (identifier NCT01140724).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The sponsor of this study, the Cincinnati Children’s Hospital Medical Center, provided funding support for the genetic analyses and supported salary of the research team. This work was supported in part by US Public Health Service grant UL1 RR026314 from the National Center for Research Resources, NIH, and with the Place Outcomes Research Award and Translational Research Award, Cincinnati Children’s Hospital Medical Center. Additional research funding support was provided by the Department of Anesthesia, Cincinnati Children’s Hospital Medical Center. No financial support except departmental salary support for the authors was provided. Funded by the National Institutes of Health (NIH).
References
- 1.Berde CB, Sethna NF. Analgesics for the treatment of pain in children. N Engl J Med. 2002;347(14):1094–1103 [DOI] [PubMed] [Google Scholar]
- 2.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63(2):316–323 [DOI] [PubMed] [Google Scholar]
- 3.Edwards RR, Moric M, Husfeldt B, Buvanendran A, Ivankovich O. Ethnic similarities and differences in the chronic pain experience: a comparison of African American, Hispanic, and white patients. Pain Med. 2005;6(1):88–98 [DOI] [PubMed] [Google Scholar]
- 4.Rahim-Williams FB, Riley JL, III, Herrera D, Campbell CM, Hastie BA, Fillingim RB. Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain. 2007;129(1-2):177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng B, Dimsdale JE, Rollnik JD, Shapiro H. The effect of ethnicity on prescriptions for patient-controlled analgesia for post-operative pain. Pain. 1996;66(1):9–12 [DOI] [PubMed] [Google Scholar]
- 6.Bernabei R, Gambassi G, Lapane K, et al. Management of pain in elderly patients with cancer. SAGE Study Group. Systematic assessment of geriatric drug use via epidemiology. JAMA. 1998;279(23):1877–1882 [DOI] [PubMed] [Google Scholar]
- 7.Rupp T, Delaney KA. Inadequate analgesia in emergency medicine. Ann Emerg Med. 2004;43(4):494–503 [DOI] [PubMed] [Google Scholar]
- 8.Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70–78 [DOI] [PubMed] [Google Scholar]
- 9.Todd KH, Deaton C, D’Adamo AP, Goe L. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35(1):11–16 [DOI] [PubMed] [Google Scholar]
- 10.US Census Bureau. National population estimates. Available at: www.census.gov/popest/national/asrh/NC-EST2009-asrh.html. Accessed November 12, 2011
- 11.Boehmer U, Kressin NR, Berlowitz DR, Christiansen CL, Kazis LE, Jones JA. Self-reported vs administrative race/ethnicity data and study results. Am J Public Health. 2002;92(9):1471–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Office of Management and Budget. Appendix: Directive No. 15. Race and ethnic standards for federal statistics and administrative reporting. In: Office of Management and Budget (US). Available at: www.house.gov/omb/fedreg/race-ethnicity.html. Accessed November 12, 2011
- 13.Voepel-Lewis T, Burke CN, Jeffreys N, Malviya S, Tait AR. Do 0-10 numeric rating scores translate into clinically meaningful pain measures for children? Anesth Analg. 2011;112(2):415–421 [DOI] [PubMed] [Google Scholar]
- 14.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23(3):293–297 [PubMed] [Google Scholar]
- 15.Ramsay MA. Anesthesia and pain management at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2000;13(2):151–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;2(5920):656–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastie BA, Riley JL, Fillingim RB. Ethnic differences and responses to pain in healthy young adults. Pain Med. 2005;6(1):61–71 [DOI] [PubMed] [Google Scholar]
- 18.Green CR, Baker TA, Sato Y, Washington TL, Smith EM. Race and chronic pain: A comparative study of young black and white Americans presenting for management. J Pain. 2003;4(4):176–183 [DOI] [PubMed] [Google Scholar]
- 19.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113(1-2):20–26 [DOI] [PubMed] [Google Scholar]
- 20.Mechlin MB, Maixner W, Light KC, Fisher JM, Girdler SS. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosom Med. 2005;67(6):948–956 [DOI] [PubMed] [Google Scholar]
- 21.Cano A, Mayo A, Ventimiglia M. Coping, pain severity, interference, and disability: the potential mediating and moderating roles of race and education. J Pain. 2006;7(7):459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans S, Lu Q, Tsao JC, Zelter LK. The role of coping and race in healthy children’s experimental pain responses. J Pain Manag. 2008;1(2):151–162 [PMC free article] [PubMed] [Google Scholar]
- 23.Todd KH. Pain assessment and ethnicity. Ann Emerg Med. 1996;27(4):421–423 [DOI] [PubMed] [Google Scholar]
- 24.Brown KA, Laferrière A, Lakheeram I, Moss IR. Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology. 2006;105(4):665–669 [DOI] [PubMed] [Google Scholar]
- 25.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155(1):186–192 [DOI] [PubMed] [Google Scholar]
- 26.Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104(5):1081–1093; quiz 1117–1118 [DOI] [PubMed] [Google Scholar]
- 27.Griffin RA, Polit DF, Byrne MW. Nurse characteristics and inferences about children’s pain. Pediatr Nurs. 2008;34(4):297–305 [PubMed] [Google Scholar]