Abstract
OBJECTIVE:
The aim of the current study was to examine demographic and contextual correlates of voluntariness in parents making research or treatment decisions for their children with cancer.
METHODS:
Participants included 184 parents of children with cancer who made a decision about enrolling the child in a research or treatment protocol within the previous 10 days. Parents completed questionnaires that assessed voluntariness, external influence by others, concern that the child’s care would be negatively affected if the parent did not agree, time pressure, information adequacy, and demographics.
RESULTS:
Lower perceived voluntariness was associated with lower education, male gender, minority status, and not having previous experience with a similar decision. Parents who reported lower voluntariness also perceived more external influence and time pressure, had more concern about the child’s care being negatively affected if they declined, and perceived that they had either too much or not enough information about the decision. In a multivariate regression, education, minority status, gender, external influence, and too little information remained significantly associated with voluntariness.
CONCLUSIONS:
Several groups of parents appear to be at risk for decreased voluntariness when making research or treatment decisions for their seriously ill children, including fathers, nonwhite parents, and those with less education. Parental voluntariness may be enhanced by helping parents to mitigate the effects of unhelpful or unwanted influences by others and ensuring that their information needs are met.
Keywords: informed consent, parental permission, decision-making, pediatrics, oncology, ethics
What’s Known on This Subject:
Valid parental permission requires that the decision be both informed and voluntary. Previous research has focused on the informational components of decision-making (eg, disclosure and understanding), with little empirical attention to the voluntariness of decisions.
What This Study Adds:
We address this gap by examining the voluntariness of parents making research or treatment decisions in pediatric oncology. We identify demographic and contextual correlates of voluntariness and highlight the clinical implications of the findings for physicians and investigators.
Valid consent requires that the decision be both informed and voluntary. Previous research related to parental permission* to research or treatment in pediatric settings has focused largely on disclosure and understanding1–3 and motivations for agreeing to or declining research participation.4,5 Little empirical attention has been paid to the voluntariness of such decisions. There has been debate about the extent to which life-threatening illness may constrain voluntariness. Desperation or hopelessness may compel patients to agree to an intervention, despite lack of understanding or concerns about potential risks.6,7 Proxy decision makers, such as parents, face the stress and uncertainty of decision-making, while also caring for the medical and psychological needs of the child. In prior qualitative research parents of children with cancer reported feelings of shock and distress that impaired their decision-making about treatment8 and research participation.9,10 Parents reported feeling pressured to agree with physicians regarding treatment8 and perceived few alternatives with respect to treatment11,12 or clinical trial enrollment.9
A number of demographic and contextual factors may relate to voluntariness, including demographic characteristics such as gender,13 race,14 education,15 and income;2,16 previous experience with a similar decision; time since diagnosis; parents’ concern that the child’s care could be negatively affected or the medical team would be upset if the parent declines;4,17 others’ attempts to coerce, pressure, or manipulate the decision;18 time pressure;8,13,19 and the amount of information provided (too much or too little).19 However, previous research related to parental voluntariness has either been qualitative or focused on parental understanding of the voluntary nature of research participation and the right to withdraw. Previous studies have lacked a careful definition of the concept of voluntariness, and none have used a quantitative assessment tool. The use of such a measure, based on a clear and conceptually justified definition,20–22 can shed light on the various factors that may constrain voluntariness in parents making medical decisions for their children. Such information is important, especially because parents’ experiences of informed permission may provide the foundation for the parent-physician relationship,9 particularly in the context of life-threatening or chronic illness.
The study reported here is based on the data set for a larger project that was designed to develop a measure of voluntariness in parents making research and treatment decisions for their children with life-threatening illnesses.20–22 Previous reports based on this study include a description of the psychometrics and validity of the new measure of voluntariness.21,22 Validity was tested by examining associations with trust, self-efficacy, and distress. Another previous report described a secondary analysis that tested the relationships between perceptions of external influence and parental distress.23 The aim of the current study was to examine demographic and contextual correlates of voluntariness, which were not examined in previous reports, in a subset of parents of children with cancer. We determined whether demographic characteristics such as education, income, race, gender; previous experience with a similar decision; and time since diagnosis were associated with voluntariness. We also examined associations of voluntariness with contextual aspects of decision-making, including perceptions of external influence from others, concern that the child’s care would be negatively affected if the parent did not agree, time pressure, and the amount of information that was provided.
Methods
Recruitment
Parents for the larger study on which this analysis is based were recruited from January 2007 through June 2008 at an urban, tertiary care pediatric hospital in the northeastern United States. Parents were eligible if they had a seriously ill child receiving care at the hospital and made a decision about enrolling the child in a research or treatment protocol within the previous 10 days. Potential participants were identified from oncology, the cardiac and pediatric intensive care units, and the clinical trials office.
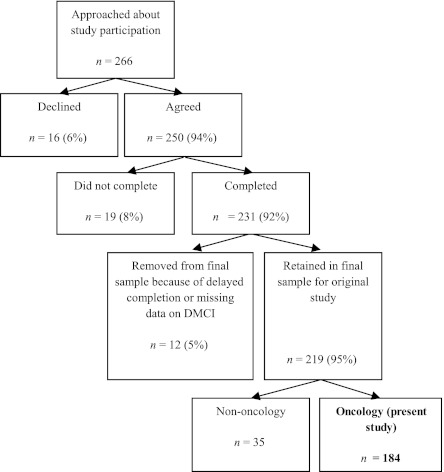
Two hundred sixty-six parents were invited to participate, and the final sample for the original study consisted of 219 participants (see Fig 1). A comparison of the final sample to the 47 parents who declined (n = 16), did not return the questionnaires (n = 19), or were removed from the analysis (n = 12) showed that the samples did not differ in terms of parent gender [χ2(1) = 2.63, P = .105] or medical unit [χ2(3) = 4.73, P = .193]. The present analysis is limited to the 184 parents from oncology.
FIGURE 1.
Recruitment and enrollment.
Procedures
The study was approved by the hospital’s institutional review board. Potential participants were approached by the study coordinator as soon as possible after they made a decision about a research or treatment protocol. In the majority of cases (n = 162, 88%), parents were approached on the inpatient oncology units; others were approached in the outpatient clinic. The study coordinator addressed the elements of informed consent (eg, procedures, risks, and benefits) and explained that the purpose of the study was to develop a survey to measure whether a choice is voluntary. After parents provided verbal informed consent, they completed the questionnaires, usually in the child’s room or in a clinic room. They received $20 after completing the questionnaires.
Measures
Voluntariness
The development of the Decision-Making Control Instrument (DMCI)21 was the primary objective of the larger study on which this analysis is based. Potential items were generated from existing measures of related constructs and a qualitative study. Final items were chosen on the basis of factor analysis and conceptual clarity. The DMCI contains 9 parent-report items that assess perceived voluntariness, defined as control over the decision about whether to agree to a research or treatment protocol (eg, “I was powerless in the face of this decision,” reverse scored). The response format was a 6-point Likert scale ranging from strongly disagree to strongly agree. We did not include a neutral response option because of concern that respondents might choose that option to avoid making a commitment in either direction. Items were summed to create a total score; higher scores indicate greater voluntariness. Analyses with the larger sample on which this study is based support the reliability and validity of the DMCI.21 Cronbach’s α was 0.83 in this subsample of parents from oncology.
External Influence
External influence was assessed with 6 parent-report items that were developed for the study23 and that captured the degree to which others tried to influence, persuade, manipulate, and/or coerce the decision (eg, “Others tried to manipulate me into making a particular decision”). The response format was the same as for the DMCI. Items were summed to create a total score; higher scores indicate greater external influence. Cronbach’s α was 0.93 in the present sample.
Concern That Care Would Be Negatively Affected
Parents’ concern that the child’s care would be negatively affected and the medical team would be upset if they did not agree was assessed with 3 items (eg, “I was concerned that my child’s care would be negatively affected if I didn’t agree to the protocol”). The response format was the same as for the DMCI. Items were summed to create a total score; higher scores indicate greater concern. Cronbach’s α was 0.77 in the present sample.
Time Pressure
Parents’ perception of time pressure was assessed with 1 item: “I had to make a decision quickly.” The response format was the same as for the DMCI. A higher score indicates more time pressure.
Information Adequacy
Parents’ perception of the amount of information that was provided was assessed separately with 3 items (eg, “At the time, I felt I had too little information about this decision”). The response format was the same as for the DMCI. A higher score for each item indicates greater agreement.
Analytic Plan
Ordinal variables were recoded into dichotomous variables for the analysis. Parent race was recoded into a dichotomous variable that reflected whether the parent was from a minority racial group, defined as nonwhite. Spearman ρ correlations and t tests were used to determine if demographic variables and contextual factors were associated with parental voluntariness. Variables that were significant at P < .05 were entered into a multivariate regression to determine which variables remained significant predictors of voluntariness when all other variables were included in the model.
Results
Participants and Decision Characteristics
Demographic and illness characteristics are presented in Table 1. The average time since diagnosis in children was 9.78 months (SD = 22.41), with a range of 0 to 123 months. Approximately half (n = 90) of children had been diagnosed for <1 month. Fifty-seven percent of parents made a decision about enrolling the child in a research protocol versus standard treatment; 37% made a decision about agreeing to a treatment protocol (these data are missing for 6% of participants). The average number of days from decision to study participation was 4.18 (SD = 2.54). Of the parents who made a research decision, 98% enrolled their children in the research protocol, and 2% declined the research protocol and chose standard treatment. Of the parents who made a treatment decision, 100% agreed to the treatment of their children. Forty-three percent of parents made a similar decision for the child in the past.
TABLE 1.
Demographic and Illness Characteristics (N = 184)
| Variable | n (%) or M (SD) |
|---|---|
| Parent age, y | 38.02 (7.82) |
| Parent gender, female | 138 (75%) |
| Parent race | |
| Black or African American | 42 (23%) |
| American Indian/Alaskan Native | 1 (< 1%) |
| Asian | 9 (5%) |
| White | 120 (65%) |
| Other | 12 (7%) |
| Marital status | |
| Married/living with partner | 133 (72%) |
| Single | 29 (16%) |
| Separated | 10 (5%) |
| Divorced | 9 (5%) |
| Widowed | 2 (1%) |
| Other | 1 (1%) |
| Highest education | |
| Some high school | 8 (4%) |
| Completed high school | 30 (16%) |
| Vocational or some college | 66 (36%) |
| College degree | 43 (23%) |
| Some postgraduate education | 7 (4%) |
| Professional or graduate degree | 29 (16%) |
| Unknown/missing | 1 (< 1%) |
| Income | |
| <$19 999 | 20 (11%) |
| $20 000–39 999 | 37 (20%) |
| $40 000–59 999 | 28 (15%) |
| $60 000–79 999 | 33 (18%) |
| $80 000–99 999 | 25 (14%) |
| >$100 000 | 38 (21%) |
| Unknown/missing | 3 (2%) |
| Child’s illness | |
| Leukemia | 65 (35%) |
| Lymphoma | 19 (10%) |
| Solid tumor | 91 (50%) |
| Unspecified cancer | 9 (5%) |
| Time since diagnosis | |
| <1 mo | 90 (49%) |
| 1–3 mo | 42 (23%) |
| 3–6 mo | 4 (2%) |
| 6–12 mo | 13 (7%) |
| 1–2 y | 13 (7%) |
| >2 y | 22 (12%) |
Demographic Characteristics and Voluntariness
In bivariate analyses lower perceived voluntariness was associated with lower education, male gender, minority status, and not having previous experience with a similar decision. Income and time since diagnosis were not associated with parental voluntariness (Table 2).
TABLE 2.
Bivariate Relationships Between Demographic Factors and Voluntariness
| Variable | Test Statistic | P Value |
|---|---|---|
| Education (less than college vs college and above) | t(181) = −2.76 | .006 |
| Income (<$60K vs ≥$60K) | t(179) = −1.49 | .877 |
| Minority (yes vs no) | t(182) = 3.17 | .002 |
| Parent gender | t(182) = −2.24 | .026 |
| Prior experience with similar decision | t(182) = −2.74 | .007 |
| Time since diagnosis (<1 mo ago vs ≥1 mo) | t(182) = −0.06 | .951 |
Contextual Factors and Voluntariness
In bivariate analyses lower perceived voluntariness was associated with making a treatment decision (versus research), more external influence, more concern that the child’s care would be negatively affected if the parent declined, more time pressure, less agreement with having enough information about the decision, and more agreement with having too little or too much information (Table 3).
TABLE 3.
Bivariate Relationships Between Contextual Factors and Voluntariness
| Variable | Test Statistic | P Value |
|---|---|---|
| Research decision (vs treatment) | t(171) = −2.35 | .02 |
| External influence | r = −.62 | <.0001 |
| Concern that care would be negatively affected | r = −.44 | <.0001 |
| Time pressure | r = −.24 | .001 |
| Enough information | r = .54 | <.0001 |
| Too little information | r = −.56 | <.0001 |
| Too much information | r = −.29 | <.0001 |
Multivariate Regression
In the regression model including the demographic and contextual variables that were significant in the bivariate analyses, education, minority status, gender, external influence, and perception of enough and too little information remained significantly associated with parental voluntariness (Table 4). The model accounted for 53% of the variance in voluntariness scores. Previous experience with a similar decision, research versus treatment decision, concern that the child’s care would be negatively affected, time pressure, and the perception of too much information were no longer associated with voluntariness.
TABLE 4.
Multivariate Regression Predicting Voluntariness
| Variable | B | P Value | 95% CI for B | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Education (less than college vs college and above) | 2.37 | .003 | 0.802 | 3.93 |
| Minority (yes vs no) | −2.38 | .005 | −4.03 | −0.73 |
| Parent gender | 2.40 | .007 | 0.674 | 4.13 |
| Prior experience with similar decision | 0.51 | .524 | −1.06 | 2.07 |
| Research decision (vs treatment) | 1.23 | .116 | −0.31 | 2.76 |
| External influence | −0.62 | <.0001 | −0.80 | −0.44 |
| Concern that care would be negatively affected | 0.16 | .334 | −0.16 | 0.48 |
| Time pressure | −0.02 | .937 | −0.49 | 0.45 |
| Enough information | 1.12 | .016 | 0.21 | 2.02 |
| Too little information | −1.02 | .039 | −1.99 | −0.05 |
| Too much information | −0.47 | .224 | −1.24 | 0.29 |
Discussion
We found that voluntariness was associated with a number of demographic and contextual variables, but only some remained significant in the multivariate regression. Parent perception of too little information was associated with lower voluntariness, whereas perception of enough information was associated with greater voluntariness. Having too little information may deprive parents of a key means through which to cope with a stressful situation.24 In contrast, the perception of having enough information may enhance coping ability and self-efficacy, a concept closely related to control. These findings support the idea that tailoring information to the needs of each parent may be an important aspect of effective informed permission.13,24
Parents making treatment decisions experienced lower voluntariness compared with parents making research decisions, but this finding was no longer significant in the multivariate regression. When making a decision about a treatment protocol in pediatric oncology, parents are faced with a tragic, unwanted choice between potentially lifesaving and toxic treatment or death.11,12 This situation is likely to produce feelings of powerlessness, which may translate into decreased control over specific decisions. In contrast, parents making a decision about a research protocol usually (but not always) have at least 2 options: the research protocol or standard treatment. In making this decision, parents may experience a sense of empowerment, and, therefore, enhanced voluntariness. Although the relationship between type of decision and voluntariness was only significant in the bivariate analysis, the effect of this and other decision features (eg, type of intervention; risks and benefits) warrants additional study.
One issue not addressed in this study is that some parents may not want control over decisions having to do with their child’s medical care. Previous research suggests that there is variability in the extent to which parents prefer decision-making autonomy.24–26 Tait et al26 found that African American parents were more likely than white parents to prefer a passive role in decisions about the child’s anesthetic care. Thus, parents who report lower levels of control may prefer less control. Research suggests that outcomes, such as satisfaction and adherence, may be enhanced when the individual’s expectations for control are matched by the situation.26,27 An examination of these complex relationships is an important next step, because the ethical imperative of beneficence may be best achieved by helping parents to reach their desired level of control.
The findings should be interpreted in light of several limitations. First, our sample consisted of parents who agreed to participate in a study about decision-making, and such parents may differ from parents who did not participate. For example, parents who participated may have perceived the permission process more positively than those who declined. A related point is that the sample consisted primarily of women, and almost half held a college degree or higher. Additional work needs to be done to understand predictors of voluntariness in fathers and in potentially vulnerable groups. Second, our definition of voluntariness focuses on the individual’s subjective perception, but there may be controlling influences in the environment that the parent is unaware of (eg, intentional deception). A decision in the presence of such an influence should be considered nonvoluntary from a regulatory and ethical standpoint, even if the decision maker perceives a high degree of control. Third, our measure of external influence did not distinguish between sources of influence (eg, physician; family member), which may have different associations with voluntariness. Fourth, this study was cross-sectional, so it is impossible to determine the direction of effects between voluntariness and the other variables. Fifth, because the variables were based on parent report, shared method variance cannot be ruled out as an explanation for the findings. In other words, the significant correlations could have resulted because the same method was used to measure all variables. Sixth, some items used to assess voluntariness and external influence may have been difficult for less educated parents to understand, a point that should be addressed in future research using the DMCI. Finally, these findings are based on a secondary analysis of an existing data set. The intent of the original study was to develop the DMCI; it was not designed to answer the specific questions that are addressed here. Additional studies with new samples should be undertaken to replicate and extend the current findings.
The findings have clinical implications for physicians and investigators. It may be helpful to assess parents’ perceptions of information adequacy, because too little or too much information may impair decision-making ability and voluntariness. Another strategy would be to assist parents in identifying unhelpful or unwanted influences on the decision and help them to mitigate these influences. One simple way to do this is to emphasize that the decision is up to them. This recommendation is consistent with what was found in previous research with parents of children with cancer, who suggested that physicians emphasize the voluntary nature of trial participation and use the word “choice” at least 3 times during the protocol discussion.19 Another strategy would be to talk to parents about the extent to which individuals they have talked to about the decision have or have not been helpful. Although the physician who conducted the protocol discussion could engage in this dialogue with parents, nurses may be more accessible, and parents may be more willing to open up to them regarding the decision-making process. Previous research demonstrated that the presence of a nurse at the consent conference for pediatric leukemia was associated with parental understanding; the authors suggest that nurses may provide emotional support and promote an environment in which parents are encouraged to participate.1 Finally, there are several potentially vulnerable subgroups of parents in terms of voluntariness: fathers, less educated parents, and nonwhite parents. Additional research is needed to understand the reasons for decreased voluntariness among these parents.
The informed permission process provides an opportunity to establish a trusting and respectful relationship with families,7 which is critical in the context of a life-threatening or chronic disease. Future research should incorporate longitudinal designs to examine whether aspects of informed permission, including voluntariness, predict the quality of the parent-physician relationship. Perceived voluntariness may also predict other outcomes that are important to clinicians and investigators, such as adherence to the treatment or research protocol, attrition and follow-up, and parental adjustment. Research on parental voluntariness that incorporates objective measurement of the informed permission process and physician or researcher behaviors is also needed. For example, Kodish and colleagues1,28 have conducted numerous studies related to informed permission in pediatric leukemia. In those studies they used an observer checklist that provides an objective assessment of elements of the protocol discussion. Future research should also determine whether different interventions to enhance parental decision-making, such as anticipatory guidance,29 physician training,28 and other modifications such as enhanced consent forms and the use of multimedia,30 affect voluntariness.
Conclusions
Parental permission for a child to undergo a medical treatment or research intervention should be informed and voluntary. We found that fathers, nonwhite parents, and less educated parents were at risk for decreased voluntariness. Lower voluntariness was also associated with the perception of more influence from others and having too little information at the time of the decision. Parental voluntariness may be enhanced by helping parents to mitigate the effects of unhelpful or unwanted influences and ensuring that their information needs are met. Additional research is needed to understand longitudinal outcomes of voluntariness and whether interventions to improve informed permission also impact parental voluntariness.
Acknowledgments
We thank Drs Tom Beauchamp, Diana Harris, Richard Ittenbach, Mary Frances Luce, and William Reynolds for their contributions to the larger project on which this study is based, which included study conceptualization and design, data collection, and data analysis and interpretation.
Glossary
- DMCI
Decision-Making Control Instrument
Footnotes
Dr Miller’s contributions include manuscript conceptualization, data analysis, interpretation of data, and writing and reviewing of manuscript drafts; and Dr Nelson’s contributions include study design, manuscript conceptualization, interpretation of data, and reviewing of manuscript drafts.
The work reported in this article was conducted before Dr Nelson joined the US Food and Drug Administration and does not represent the views and/or policies of the Food and Drug Administration or the Department of Health and Human Services.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Financial support was provided by grants from the National Science Foundation (SES-0527618; Drs Nelson, Luce, and Beauchamp) and the National Institutes of Health/National Cancer Institute (grant R21CA118377-01A1, Dr Nelson) and an Institutional Development Grant to the Center for Research Integrity, Department of Anesthesiology and Critical Care, The Children’s Hospital of Philadelphia. The funding agreements ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. Funded by the National Institutes of Health (NIH).
We use the word “permission” instead of “consent” because consent refers to decisions that a legally competent individual makes regarding his or her own medical care. Permission is the appropriate term when referring to decisions that parents make on behalf of children.
References
- 1.Kodish E, Eder M, Noll RB, et al. Communication of randomization in childhood leukemia trials. JAMA. 2004;291(4):470–475 [DOI] [PubMed] [Google Scholar]
- 2.Miller VA, Drotar D, Burant C, Kodish E. Clinician-parent communication during informed consent for pediatric leukemia trials. J Pediatr Psychol. 2005;30(3):219–229 [DOI] [PubMed] [Google Scholar]
- 3.Tait AR, Voepel-Lewis T, Malviya S. Do they understand? (part I): parental consent for children participating in clinical anesthesia and surgery research. Anesthesiology. 2003;98(3):603–608 [DOI] [PubMed] [Google Scholar]
- 4.Zupancic JA, Gillie P, Streiner DL, Watts JL, Schmidt B. Determinants of parental authorization for involvement of newborn infants in clinical trials. Pediatrics. 1997;99(1). Available at: www.pediatrics.org/cgi/content/full/99/1/e6. [DOI] [PubMed] [Google Scholar]
- 5.Rothmier JD, Lasley MV, Shapiro GG. Factors influencing parental consent in pediatric clinical research. Pediatrics. 2003;111(5 Pt 1):1037–1041 [DOI] [PubMed] [Google Scholar]
- 6.Stevens PE, Pletsch PK. Ethical issues of informed consent: mothers’ experiences enrolling their children in bone marrow transplantation research. Cancer Nurs. 2002;25(2):81–87 [DOI] [PubMed] [Google Scholar]
- 7.Jacoby LH, Maloy B, Cirenza E, Shelton W, Goggins T, Balint J. The basis of informed consent for BMT patients. Bone Marrow Transplant. 1999;23(7):711–717 [DOI] [PubMed] [Google Scholar]
- 8.Pyke-Grimm KA, Stewart JL, Kelly KP, Degner LF. Parents of children with cancer: factors influencing their treatment decision making roles. J Pediatr Nurs. 2006;21(5):350–361 [DOI] [PubMed] [Google Scholar]
- 9.Levi RB, Marsick R, Drotar D, Kodish ED. Diagnosis, disclosure, and informed consent: learning from parents of children with cancer. J Pediatr Hematol Oncol. 2000;22(1):3–12 [DOI] [PubMed] [Google Scholar]
- 10.Kupst MJ, Patenaude AF, Walco GA, Sterling C. Clinical trials in pediatric cancer: parental perspectives on informed consent. J Pediatr Hematol Oncol. 2003;25(10):787–790 [DOI] [PubMed] [Google Scholar]
- 11.Hinds PS, Oakes L, Quargnenti A, et al. An international feasibility study of parental decision making in pediatric oncology. Oncol Nurs Forum. 2000;27(8):1233–1243 [PubMed] [Google Scholar]
- 12.Benedict JM, Simpson C, Fernandez CV. Validity and consequence of informed consent in pediatric bone marrow transplantation: the parental experience. Pediatr Blood Cancer. 2007;49(6):846–851 [DOI] [PubMed] [Google Scholar]
- 13.McKenna K, Collier J, Hewitt M, Blake H. Parental involvement in paediatric cancer treatment decisions. Eur J Cancer Care (Engl). 2010;19(5):621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon C, Zyzanski SJ, Eder M, Raiz P, Kodish ED, Siminoff LA. Groups potentially at risk for making poorly informed decisions about entry into clinical trials for childhood cancer. J Clin Oncol. 2003;21(11):2173–2178 [DOI] [PubMed] [Google Scholar]
- 15.Breese PE, Burman WJ, Goldberg S, Weis SE. Education level, primary language, and comprehension of the informed consent process. J Empir Res Hum Res Ethics. 2007;2(4):69–79 [DOI] [PubMed] [Google Scholar]
- 16.Nelson RM, Merz JF. Voluntariness of consent for research: an empirical and conceptual review. Med Care. 2002;40(suppl 9):V69–V80 [DOI] [PubMed] [Google Scholar]
- 17.Hewlett S. Consent to clinical research—adequately voluntary or substantially influenced? J Med Ethics. 1996;22(4):232–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 5th ed. New York, NY: Oxford University Press; 2001 [Google Scholar]
- 19.Eder ML, Yamokoski AD, Wittmann PW, Kodish ED. Improving informed consent: suggestions from parents of children with leukemia. Pediatrics. 2007;119(4). Available at: www.pediatrics.org/cgi/content/full/119/4/e849. [DOI] [PubMed] [Google Scholar]
- 20.Nelson RM, Beauchamp TL, Miller VA, Reynolds WW, Ittenbach RF, Luce MF. The concept of voluntary consent. Am J Bioeth. 2011;11(8):6–16 [DOI] [PubMed] [Google Scholar]
- 21.Miller VA, Ittenbach RF, Harris D, et al. The Decision Making Control Instrument to assess voluntary consent. Med Decis Making. 2011;31(5):730–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller VA, Reynolds WW, Ittenbach RF, Luce MF, Beauchamp TL, Nelson RM. Challenges in measuring a new construct: perception of voluntariness for research and treatment decision making. J Empir Res Hum Res Ethics. 2009;4(3):21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller VA, Luce MF, Nelson RM. Relationship of external influence to parental distress in decision making regarding children with a life-threatening illness. J Pediatr Psychol. 2011;36(10):1102–1112 10.1093/jpepsy/jsr033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pyke-Grimm KA, Degner L, Small A, Mueller B. Preferences for participation in treatment decision making and information needs of parents of children with cancer: A pilot study. J Pediatr Oncol Nurs. 1999;16(1):13–24 [DOI] [PubMed] [Google Scholar]
- 25.Gagnon EM, Recklitis CJ. Parents’ decision-making preferences in pediatric oncology: the relationship to health care involvement and complementary therapy use. Psychooncology. 2003;12(5):442–452 [DOI] [PubMed] [Google Scholar]
- 26.Tait AR, Voepel-Lewis T, Munro HM, Malviya S. Parents’ preferences for participation in decisions made regarding their child’s anaesthetic care. Paediatr Anaesth. 2001;11(3):283–290 [DOI] [PubMed] [Google Scholar]
- 27.Krantz DS, Baum A, Wideman M. Assessment of preferences for self-treatment and information in health care. J Pers Soc Psychol. 1980;39(5):977–990 [DOI] [PubMed] [Google Scholar]
- 28.Yap TY, Yamokoski A, Noll RB, Drotar D, Zyzanski S, Kodish ED, Multi-site Intervention Study to Improve Consent Research Team . A physician-directed intervention: teaching and measuring better informed consent. Acad Med. 2009;84(8):1036–1042 [DOI] [PubMed] [Google Scholar]
- 29.Yamokoski AD, Hazen RA, Kodish ED. Anticipatory guidance to improve informed consent: a new application of the concept. J Pediatr Oncol Nurs. 2008;25(1):34–43 [DOI] [PubMed] [Google Scholar]
- 30.Schenker Y, Fernandez A, Sudore R, Schillinger D. Interventions to improve patient comprehension in informed consent for medical and surgical procedures: a systematic review. Med Decis Making. 2011;31(1):151–173 [DOI] [PMC free article] [PubMed] [Google Scholar]