Abstract
OBJECTIVE:
Because of concerns about the safety and environmental impact of mercury, aneroid sphygmomanometers have replaced mercury-filled devices for blood pressure (BP) measurements. Despite this change, few studies have compared BP measurements between the 2 devices.
METHODS:
The SEARCH for Diabetes in Youth Study conducted a comparison of aneroid and mercury devices among 193 youth with diabetes (48% boys, aged 12.9 ± 3.7 years; 89% type 1). Statistical analyses included estimating Pearson correlation coefficients, Bland-Altman plots, paired t tests, and fitting regression models, both overall and stratified by age (<10 vs ≥10–18 years).
RESULTS:
Mean mercury and aneroid systolic and diastolic BPs were highly correlated. For the entire group, there was no significant difference in mean systolic BP using the aneroid device, but there was a −1.53 ± 5.06 mm Hg difference in mean diastolic BP. When stratified by age, a lower diastolic BP (−1.78 ± 5.2 mm Hg) was seen in those ≥10 to 18 years using the aneroid device. No differences in systolic BP were observed, and there were no differences in BP by device in individuals <10 years. Regression analyses did not identify any explanatory variables.
CONCLUSIONS:
Although a small discrepancy between diastolic BP measurements from aneroid versus mercury devices exists, this variation is unlikely to be clinically significant, suggesting that either device could be used in research or clinical settings.
KEY WORDS: pediatrics, blood pressure
What’s Known on This Subject:
As a result of safety and environmental concerns about mercury, aneroid sphygmomanometers have replaced mercury-filled devices for blood pressure measurements. Despite this change, few studies have compared the 2 devices.
What This Study Adds:
Little clinical variation exists between blood pressure measurements obtained from an aneroid or mercury device, suggesting that either device could be used in a research or clinical setting.
Auscultation measurements using a mercury sphygmomanometer are the gold standard for documenting blood pressure (BP).1,2 However, because of concerns about the environment and personal health, regulatory agencies have recommended removal of these devices.3 Thus, despite a lack of studies documenting the comparability of other devices, clinical programs and research studies have been required to use electronic and aneroid devices.
Only 1 study has previously compared BP measurements obtained by mercury and aneroid devices in pediatrics. NHANES reported a small mean difference in systolic BP (+1.1 mm Hg) in youth aged 8 to 17 years with the aneroid sphygmomanometer.4 Therefore, the SEARCH for Diabetes in Youth Study (SEARCH) sought to validate these results and to determine whether differences between the 2 devices were present by age.
Methods
SEARCH
SEARCH is a multicenter, population-based, observational study of diabetes mellitus in youth. Detailed study methods have been described previously.5 In brief, SEARCH began conducting population-based ascertainment of cases of diabetes in youth <20 years at diagnosis in 2001 and continues through the present. Cases are identified in geographically defined populations in Colorado, Ohio, South Carolina, and Washington; among health plan enrollees in California and Hawaii; and Indian Health Service beneficiaries from 4 American Indian populations. Cases are considered valid if they were diagnosed with diabetes by a health care provider.
Youth with diabetes were identified and asked to complete a survey that collected information on date of birth, race/ethnicity, type of diabetes, date of diagnosis, and current diabetes treatment. All participants who completed this survey (except those with known secondary diabetes) were invited to participate in an in-person study visit while metabolically stable (no episode of diabetic ketoacidosis during the previous month). At the beginning of the visit, consent was obtained from participants or their parents (if <18 years of age), and assent was obtained from younger subjects as directed by local institutional review boards. Information collected at the visit included questionnaires for demographics, blood and urine samples, and a brief physical examination that included height, weight, and BP data.
Study Population
The study population for this analysis consisted of all individuals who participated in an in-person research visit between September 1, 2007, and June 30, 2008, at 3 of the 6 SEARCH clinical sites: Hawaii, Ohio, and Washington. Approval for this study was obtained from local institutional review boards. Research personnel were trained in BP measurements by using a standard procedure derived from the National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (Fourth Report).1
BP Equipment and Measurements
Mercury measurements were performed by using either a portable or wall-mounted Baumometer sphygmomanometer Kompak Model-260 mm Hg (WA Baum, Copiague, NY). Aneroid measurements were performed by using the Welch Allyn Tycos 767-Series Mobile Aneroid with a weighted base (model 7670-04; Welch Allyn, Skaneateles Falls, NY). Before initial use, calibration checks of the aneroid sphygmomanometers were performed by using the Netech DigiMano 1000 digital pressure-vacuum meter (part number 200-2000 IN). Calibration checks were repeated every 6 months and whenever the device was transported in a motor vehicle from 1 location to another.
Before the first BP measurement, all participants were seated with both feet on the floor after resting quietly for a minimum of 5 minutes. The right arm was used for all BP measurements unless there was a contraindication. The subject’s arm was supported on a table at heart level. The examiner selected an appropriate-sized cuff based on the circumference of the upper arm.1 The pulse pressure was determined by locating the radial pulse and inflating the cuff until the radial pulse disappeared. The maximum inflation level was then recorded as the pulse pressure plus 30 mm Hg. All participants then had 4 BP measurements performed with the same sized cuff. The first and fifth Korotkoff sounds were recorded for each of the 4 measurements. Subjects rested quietly for a minimum of 30 seconds between measurements.
The first 2 measurements were always taken with an aneroid sphygmomanometer. The third and fourth measurements were measured by using either aneroid then mercury, or mercury then aneroid. The order of these third and fourth measurements was randomly assigned by the SEARCH Coordinating Center. Each of the 3 clinical sites were sent a list specifying the order (mercury then aneroid or aneroid then mercury) for performing the third and fourth BP measurements for each participant. This list was generated by using Proc Plan, a statistical procedure in SAS (version 9.2; SAS Institute, Cary, NC) for generating randomization plans. Only the third and fourth BP measurements were used in these analyses.
Statistical Analysis
Statistical analyses were performed by using SAS software (version 9.2; SAS Institute, Cary NC). All data were analyzed for the entire cohort and then by age group, <10 years and ≥10 to 18 years. Pearson correlation coefficients for systolic and diastolic BP measurements from the 2 BP devices (aneroid and mercury) were calculated. In addition, we fit Bland-Altman plots to provide a visual comparison between the 2 methods. Next, mean differences in systolic and diastolic BP obtained from each device were compared by using paired t tests to determine if they were different from zero. This was used to estimate the difference in millimeters of mercury observed between devices and to generate a correction factor between devices. Thus, if we assume that the observed average difference between devices represents the “shift” in blood pressure measurements due to the different devices, then adding this difference as a “correction factor” to the aneroid measures would allow for the new (aneroid) measures to have the same observed BP values as the mercury measures. Finally, general linear models were fit to determine if the reason for the observed differences in BP measures between devices could be identified. Models were adjusted for age, gender, race/ethnicity, BMI, clinic site, or type of diabetes. P values of < .05 were deemed significant in these analyses.
Results
One hundred ninety-three youth (48% male youth) with a mean age of 12.9 ± 3.7 years (age range 3.9–18.9 years) who had a mean duration of diabetes of 9.2 ± 5.6 months participated in the comparison of the 2 BP devices (Table 1). Of these participants, 171 had a clinical diagnosis of type 1 diabetes, and 22 had type 2 diabetes. A majority of the participants were non-Hispanic white. When stratified by age (aged <10 and ≥10–18 years), there were no significant differences between the groups except for diabetes type and BMI because the youth with type 2 diabetes were exclusively in the ≥10- to 18-year age category.
TABLE 1.
Characteristics of the Study Population
| Total | <10 y | ≥10 to 18 y | |
|---|---|---|---|
| No. of participants | 193 | 45 | 148 |
| Male, n (%) | 93(48) | 21 (47) | 72 (49) |
| Female, n (%) | 100 (52) | 24 (53) | 76 (51) |
| Age, y, mean ± SD | 12.85 ± 3.7 | 7.8 ± 1.8 | 14.4 ± 2.6 |
| BMI, kg/m2 | 21.7 ± 7.4 | 16.7 ± 2.3 | 23.1 ± 7.7 |
| Diabetes duration, mo, mean ± SDa | 9.2 ± 5.6 | 10.1 ± 6.1 | 9.0 ± 5.4 |
| Type 1 diabetes, n (%) | 171 (89) | 45 (100) | 126 (85) |
| Non-Hispanic white, % | 75 | 69 | 77 |
| African American, % | 9 | 9 | 9 |
| Hispanic/Asian Pacific Islander/Other, % | 16 | 22 | 14 |
| Type 2 diabetes, n (%) | 22 (11) | 0 (0) | 22 (15) |
| Non-Hispanic white, % | 23 | 0 | 23 |
| African American, % | 27 | 0 | 27 |
| Hispanic/Asian Pacific Islander/Other, % | 50 | 0 | 50 |
Duration of diabetes indicates duration of diabetes at time of study visit.
The randomization of the sequence of BP measurements (aneroid then mercury or mercury then aneroid) resulted in 92 subjects randomized to receive aneroid then mercury and 101 individuals to mercury then aneroid for the third and fourth BP measurements.
Mercury and aneroid systolic and diastolic BP measures were significantly correlated for the total sample and by age group (Table 2). For systolic BP, the overall correlation was r = .94, P < .0001. By age, r = .90, P < .0001 for <10 years and r = .94, P < .0001 for ≥10 to 18 years. Likewise, for diastolic BP, the overall correlation was very high: r = .82, P < .0001 and r = .83, P < .0001 and r = .81, P < .0001 for <10 years and ≥10 to 18 years, respectively.
TABLE 2.
Comparison of Mercury versus Aneroid BP Measurements
| Systolic BP, (mm Hg) | Pearson Correlation | Diastolic BP, mm Hg | Pearson correlation | Systolic BP Different From zero, mm Hga | Diastolic BP Different From Zero, mm Hga | |||
|---|---|---|---|---|---|---|---|---|
| Mercury | Aneroid | Mercury | Aneroid | |||||
| All | 105.0 ± 13.2 | 104.5 ± 13.3 | r = .94, P < .0001 | 65.6 ± 11.1 | 64.4 ± 9.1 | r = .82, P < .0001 | −0.48 ± 4.41, P = .13 | −1.25 ± 6.35, P = .007 |
| ≤10 y | 96.0 ± 10.4 | 96.1 ± 10.4 | r = .90, P < .0001 | 60.6 ± 7.8 | 59.5 ± 7.2 | r = .83, P < .0001 | 0.13 ± 4.66, P = .85 | −0.71 ± 4.45, P = .29 |
| ≥10–18 y | 107.7 ± 12.8 | 107.0 ± 13.0 | r = .94, P < .0001 | 67.1 ± 11.6 | 65.7 ± 9.3 | r = .81, P < .0001 | −0.67 ± 4.33, P = .062 | −1.78 ± 5.22, P < .0001 |
Data are mean ± SD.
Reference device is the mercury sphygmomanometer.
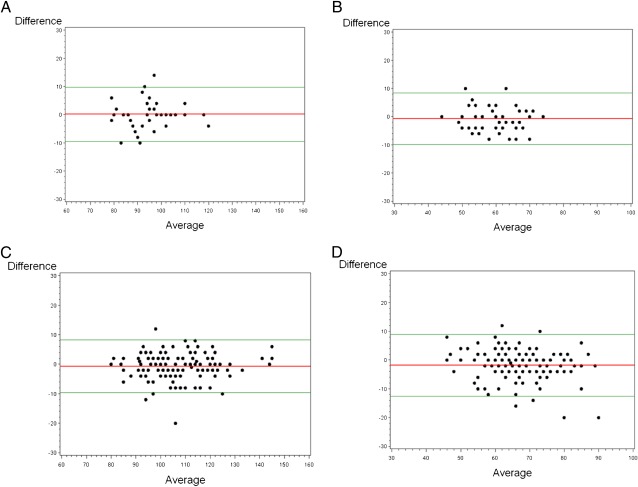
We examined Bland-Altman plots to further assess agreement between the 2 methods. These plots are presented in Fig 1 with 4 panels, 2 for each measure (systolic and diastolic BP) and 2 for each age group (<10 years and ≥10–18 years). For the older group (Fig 1d) larger differences in diastolic BP seemed to occur for diastolic blood pressures >65 mm Hg; however, there is no clear trend in these data to suggest a specific cutpoint where the 2 methods become discordant.
FIGURE 1.
Agreement between aneroid and mercury devices. The red line indicates the mean difference between readings, and the green line is the 95% confidence intervals of agreement. Bland-Altman Plots: A, systolic BP, age 0–9. B, diastolic BP, age 0–9. C, systolic BP, age 10–18. D, diastolic BP, age 10–18.
We then tested whether the mean difference in systolic and diastolic BPs obtained by the mercury and aneroid devices was significantly different from zero. This was used to estimate a correction factor between devices. Among individuals <10 years, the mean difference in systolic and diastolic BPs was not different from zero (P = .85 and P = .29, respectively). In individuals ≥10 to 18 years of age, the mean difference in systolic BP was not significant (−.67 ± 4.33, P = .06), but the mean difference for diastolic BP was significantly different from zero (−1.78 ± 5.22, P < .0001). Thus, to equate BPs between devices a correction factor of +1.8 could be added to an aneroid BP measurement in individuals ≥10 years.
Regression models were used to seek for the reason for the differences in diastolic BP by device. Models were only created for diastolic BP as systolic BP was not statistically different by device. Univariate analysis revealed race/ethnicity and BMI z score to be significantly associated with the mean difference in diastolic BP. Nonwhites were found to have a larger difference in diastolic BP (1.71 mm Hg, P = .03), and a 1-unit change in BMI z score corresponded to a 0.85 mm Hg (P = .014) difference in diastolic BP. In the fully adjusted models, race/ethnicity and BMI z score were no longer significant, and no other predictors to explain the difference in the diastolic BP measures were identified.
Discussion
This study demonstrates that systolic and diastolic BPs measured by mercury and aneroid devices are highly correlated for the entire sample and by age group. In individuals ≥10 to 18 years, a significantly lower mean diastolic BP was found when using the aneroid sphygmomanometer. On the basis of our analyses, this corresponds to a mean correction of +1.8 mm Hg that could be added to an aneroid BP measurement to equate the 2 devices if both are used in a single study. However, given the good agreement between devices, this correction is unlikely to result in clinically significant differences in BP, suggesting that either device could be used in a clinical or research setting.
Two previous studies have compared aneroid to mercury devices with only 1 performed in a pediatric population. In middle-aged adults, the Diabetes Prevention Program Outcomes Study (DPPOS) found no difference in mean systolic BP and a small but statistically significant lower diastolic BP when comparing aneroid and mercury devices.6 This pattern is identical to the findings in our study. These results are in contrast to those reported by NHANES in which a statistically higher systolic BP among 8- to 17-year-olds was found using an aneroid sphygmomanometer.4
Discrepant results between NHANES and our study may be due to several factors, including participant differences, different analysis techniques, and the presence of diabetes. First, it is possible the study subjects in the 2 cohorts were of different age, gender, race/ethnicity, and BMI distribution. Although the study population was not described in the NHANES article, the DPPOS found “participant” differences explained 8% to 10% of the variation in BPs between the 2 devices.6 Second, we stratified our subjects by age to separate those prepubertal (<10 years) from those who were pubertal or postpubertal (≥10–18 years). Subjects in the NHANES cohort were analyzed as a single group (8–17 years).4 Thus, it is possible changes occur in BP measurements during or after puberty that were not accounted for in NHANES report. Finally, there may be differences in BP related to the presence of diabetes, although to our knowledge, this has not been previously described. Thus, it is not clear why there are differences between our study and NHANES. Multivariate analyses did not identify any factors that contributed to the differences in diastolic BP by device. Thus, the reasons for the differences in blood pressure by device can only be speculated.
As mentioned earlier, a correction factor of 1.8 could be applied to diastolic BP measurement obtained by using an aneroid device in adolescents aged ≥10 to 18 years. This correction factor is only applicable to equate BPs in clinical or research settings where both devices have been used or there has been a change of devices due to more recent regulations preventing mercury BP measurements. In settings where aneroid only technology has been used, we cannot conclude from this study that this correction factor should apply.
A potential limitation of our report is that this study was performed in youth who have diabetes. As previously mentioned, the presence of diabetes should not affect the measurements of BP obtained by using the mercury or aneroid devices. Given that our results coincide with those published by the DPPOS, it appears reasonable to conclude that the data presented here are applicable to all youth. It should also be noted that youth with hypertension were not studied, and thus it is unclear whether larger discrepancies between devices exist at higher BPs.
Conclusions
This study found no significant difference in mean systolic BP but a slightly significantly lower mean diastolic BP in youth using the aneroid device. Although the differences described are statistically significant, as suggested by others,4,6 the differences in BP measurements between aneroid and mercury sphygmomanometers are small and are unlikely to be clinically significant. In conclusion, either device could be used.
Acknowledgments
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their health care providers, whose participation made this study possible.
Site Contract Numbers: Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Kuakini Medical Center (U58CCU919256 and U01 DP000245), Children’s Hospital Medical Center (Cincinnati) (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708-01), University of Washington School of Medicine (U58/CCU019235-4, U01 DP000244, and U18DP002710-01), Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200-2010-35171). We acknowledge the involvement of General Clinical Research Centers at the South Carolina Clinical & Translational Research (SCTR) Institute, at the Medical University of South Carolina (National Institute of Health/National Center for Research Resources [NIH/NCRR] grant UL1RR029882); Children’s Hospital and Regional Medical Center (grant M01RR00037); Colorado Pediatric General Clinical Research Center (grant M01 RR00069), and the Barbara Davis Center at the University of Colorado at Denver (Diabetes and Endocrinology Research Center/National Institutes of Health grant P30 DK57516); and the Institutional Clinical and Translational Science Award, NIH/NCRR at the University of Cincinnati (grant 1UL1RR026314-01).
Glossary
- BP
blood pressure
- DPPOS
Diabetes Prevention Program Outcomes Study
- SEARCH
SEARCH for Diabetes in Youth Study
Footnotes
Dr Shah aided in the concept and study design, interpreted the data analysis, and wrote the manuscript; Dr Dolan aided in the concept and study design, interpreted the data analysis, and revised the manuscript for intellectual content; Dr D’Agostino aided in the concept and study design, conducted the analysis, and helped in the interpretation of the data; Ms Standiford aided in the concept and study design and revised the manuscript for intellectual content; Mr Davis aided in the concept and study design and conducted the analysis; Ms Testaverde revised the manuscript for intellectual content; Dr Pihoker revised the manuscript for intellectual content; Dr Daniels revised the manuscript for intellectual content; and Dr Urbina aided in the concept and study design, interpreted the data analysis, and revised the manuscript for intellectual content.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Funded by the National Institutes of Health (NIH).
References
- 1.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004(suppl 2, 4th report);114:555–576. [PubMed]
- 2.Chobanian AV, Bakris GL, Black HR. The seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6)1206–1252. [DOI] [PubMed]
- 3.Messelbeck J, Sutherland L. Applying environmental product design to biomedical products research. Environ Health Perspect. 2000;108(suppl 6):997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostchega Y, Prineas RJ, Nwankwo T, Zipf G. Assessing blood pressure accuracy of an aneroid sphygmomanometer in a national survey environment. Am J Hypertens. 2011;24(3):322–327 [DOI] [PubMed] [Google Scholar]
- 5.SEARCH Study Group . SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25(5):458–471 [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Temprosa M, Fowler S, et al. Diabetes Prevention Program Research Group . Evaluating the accuracy of an aneroid sphygmomanometer in a clinical trial setting. Am J Hypertens. 2009;22(3):263–266 [DOI] [PMC free article] [PubMed] [Google Scholar]