Abstract
J Clin Hypertens (Greenwich). 2012; 14:322–329. ©2012 Wiley Periodicals, Inc.
Clinical inertia is a major contributor to poor blood pressure (BP) control. The authors tested the effectiveness of an intervention targeting physician, patient, and office system factors with regard to outcomes of clinical inertia and BP control. A total of 591 adult primary care patients with elevated BP (mean systolic BP ≥140 mm Hg or mean diastolic BP ≥90 mm Hg) were randomized to intervention or usual care. An outreach coordinator raised patient and provider awareness of unmet BP goals, arranged BP‐focused primary care clinic visits, and furnished providers with treatment decision support. The intervention reduced clinical inertia (−29% vs −11%, P=.001). Nonetheless, change in BP did not differ between intervention and usual care (−10.1/−4.1 mm Hg vs −9.1/−4.5 mm Hg, P=.50 and 0.71 for systolic and diastolic BP, respectively). Future primary care–focused interventions might benefit from the use of specific medication titration protocols, treatment adherence support, and more sustained patient follow‐up visits.
Hypertension is the most common condition treated in the ambulatory environment. 1 , 2 While more than 20% of the US population has a diagnosis of hypertension, only 50% of patients with this diagnosis have acceptable blood pressure (BP) control, which leads to tens of thousands of premature deaths from cardiovascular disease annually in the United States. 1 An important contributing factor is clinical inertia, defined as the failure of medical providers to initiate or intensify therapy when treatment goals are unmet. 3 , 4 , 5 Clinical inertia may be present in as many as two thirds of hypertension clinic visits, 3 , 6 , 7 , 8 , 9 and recent reviews have identified clinical inertia as a key intervention target for improving BP control. 4 , 10
To overcome clinical inertia, prior studies have not demonstrated that provider education by itself is an effective strategy. 4 , 10 Instead, clinical inertia arises from a combination of medical provider, patient, and office system factors 4 and addressing these factors simultaneously is the most effective way to overcome the problem. 4 , 10 , 11 Nurse‐ and pharmacist‐run care models, for example, focus exclusively on hypertension treatment and use standardized medication titration protocols. 10 , 11 , 12 , 13 These approaches simultaneously address provider factors of poor recognition of elevated BP, nonhypertension‐related patient concerns, and clinical uncertainty about how to modify therapy; patient factors of poor medication adherence and poor awareness of disease severity; and office system factors related to variable BP measurement methods. Although promising, nurse‐ and pharmacist‐based programs remain uncommon because of inadequate reimbursement, human resource requirements, and logistical complexity.
Because primary care providers (PCPs) continue to have major responsibility for managing hypertension, it is important to improve the effectiveness of traditional clinic visits in real‐world settings. 14 , 15 Prior studies have not specified the components of a minimally intense but effective intervention that is feasible to deploy in real‐world clinical practice, nor have they generally used a study population of primary care patients who were not consented for a research study. 4 , 10 With this in mind, we designed a pragmatic trial to test the effects of an intervention that incorporated the following elements: a hypertension registry to track BP control for adult patients in our primary care setting (office system factors); ancillary staff to educate patients and PCPs about suboptimal BP control (office system, patient and provider factors); patient recall for up to 2 BP‐specific clinic visits (office system and patient factors); and treatment decision support for PCPs (provider factors). Disease‐specific registries, patient outreach and recall by nonclinician team members, and electronic health record (EHR)–based reminders and alerts are key elements of patient‐centered medical home practices. We hypothesized that, compared with usual care, this type of practice redesign would be associated with improvements in clinical inertia and BP.
Methods
Principal Hypothesis and Primary Outcome
Using a primary care population with elevated BP, we carried out a pragmatic randomized controlled trial (RCT) to evaluate the hypothesis that, compared with usual care, an intervention targeting physician, patient, and office system factors would result in at least a 5‐mm Hg greater improvement in systolic BP.
Study Setting and Population
This study was carried out within the University of Colorado Hospital primary care system using a diverse patient population that received care in two general internal medicine clinics, 4 family medicine clinics, and a women’s health clinic. All 7 clinics shared an EHR (Allscripts Touchworks, version 10, Chicago, IL) and were staffed by approximately 60 PCPs (physicians, nurse practitioners, and physicians’ assistants) caring for more than 6000 adults with hypertension.
We created a hypertension registry by querying the EHR to identify primary care patients aged 18 to 79 years who had elevated BP defined as an average systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg based on the 2 or 3 most recent clinic visits (whichever was greater) and a systolic BP ≥135 mm Hg or diastolic BP ≥85 mm Hg during the most recent clinic visit. The rationale for averaging BP in our study definition of “elevated BP” was to ensure participants met the diagnostic criteria for hypertension. 16 If multiple BP readings were recorded on a single visit, only the final BP reading on that date was extracted. Additional eligibility criteria, designed to identify patients currently receiving care within our system who did not have a recent or near‐future primary care clinic visit, included: (1) ≥1 primary care clinic visit in the past 18 months; (2) ≥2 BP readings from separate days in the past 18 months; (3) first PCP visit ≥6 months in the past; and (4) no primary care clinic visit in the past month and none scheduled in the next 6 weeks. Patients were excluded if they had serious comorbidities (eg, active cancer diagnosis, hospice care, end‐stage renal disease), diabetes mellitus, BP management by a nephrologist or other subspecialist, EHR notation of white coat hypertension, or EHR notation to monitor BP at home.
An outreach coordinator spent an average of 3 minutes per patient reviewing the EHR to exclude patients whose eligibility requirements could not be determined electronically (eg, those who were deceased, had left the clinic system, or whose hypertension was managed by a subspecialist). Patients who met final inclusion criteria were randomly assigned in a 1:1 ratio to usual care and intervention groups. Patients were also randomly selected to include 50% with and 50% without a formal diagnosis of hypertension. Patients in the latter group had ≥2 elevated BP readings during the prior 18 months but had not received a formal hypertension diagnosis in the EHR.
Intervention Description and Implementation
An outreach coordinator (OC) delivered the intervention during a 3‐month period. Registry records of intervention group patients were imported into an information management utility developed for this and other preventive and chronic disease outreach interventions. 17 , 18 The information management utility generated invitation letters that were mailed to patients’ homes. Each letter, bearing the name of a patient’s PCP on the signature line, explained that the patient’s BP was elevated, that BP control is important for preventing cardiovascular disease, and that a BP‐focused PCP visit was recommended. Letters referenced “hypertension” only if patients had this diagnosis on their problem list. Patients were invited to call the OC to schedule visits, but if they did not do so within 2 weeks, the OC made up to 3 attempts to contact the patient by telephone during the next 2‐week period. Patients in the usual care group were merely tracked; they did not receive mail or telephone contact nor did their providers receive any prompts in their EHR task list.
When telephone contact was made, the OC reiterated the letter’s message by explaining to patients that recent BP results were elevated and that BP control is important to prevent cardiovascular disease. The OC helped patients schedule a BP‐focused PCP appointment, encouraged them to focus on BP during the visit, noted “hypertension” or “elevated BP” as the visit reason in the EHR, and then mailed patients an appointment reminder postcard. Subsequently, the OC sent an electronic prompt to each PCP’s patient care task list to be delivered 2 days prior to patients’ BP‐focused appointments. These prompts summarized the patient’s recent BP readings, strongly encouraged a focus on BP management during the upcoming visit, and included a Web address for the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7) guidelines on hypertension diagnosis and treatment. 16 Prompts became part of the EHR and were visible during BP‐focused appointments near the top of a patient’s clinic notes. If BP was elevated during the index visit (first postrandomization clinic visit), the OC telephoned patients a second time to facilitate a 4‐week follow‐up BP‐focused clinic visit if one had not already been scheduled. The OC made up to 3 attempts to contact the patient by telephone during a 2‐week period. Patients were enrolled and tracked between November 3, 2009, and October 8, 2010.
Determination of Sample Size
Using effect sizes and standard deviations demonstrated in prior hypertension clinical trials, 19 we determined that 200 patients per group would be required for at least 80% power to demonstrate a clinically meaningful group difference in systolic BP of 5 mm Hg using a 2‐sided t test and α=0.05. A 5‐mm Hg difference is associated with a 7% reduction in all‐cause mortality at a population level 16 and has been achieved in other BP interventions. 10 , 11 , 20 , 21 Based on prior experience, we estimated that 50% of patients would be deemed ineligible for inclusion after manual chart review. Thus, to reach a target of at least 400 eligible patients, an initial sample of approximately 800 patients was required. In assembling an initial cohort, we oversampled African Americans, Latinos, and patients with greater hypertension severity (average systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg) in order to improve the study’s external validity for minority populations and patients with stage 2 hypertension.
Variables
The primary outcome was the between‐group difference in change in BP (ΔBP) from baseline to end of study. Baseline BP was defined as the average of the 2 or 3 (whichever was greater) most recent BPs recorded at any outpatient clinic visit during an 18‐month prerandomization period. The end‐of‐study BP was defined as the average of the 1 or 2 (whichever was greater) most recent BPs recorded at any outpatient clinic during a 9‐month postrandomization period, excluding the first postrandomization visit (index visit) BP reading if there was at least one additional clinic visit during this period. In the event that there was no additional clinic visit, the index visit BP provided a data point for intention‐to‐treat analysis.
Secondary outcomes included between‐group differences in measures of clinical inertia. Because both medication management and lifestyle counseling are appropriate strategies for controlling BP, 16 we operationally defined constitutive components of clinical inertia to include medication inertia and behavioral inertia. Following other study definitions of clinical inertia, 3 , 4 we defined medication inertia as PCP failure to initiate or intensify medications despite elevated BP. We defined behavioral inertia as PCP failure to document behavioral counseling despite elevated BP. Each type of clinical inertia was assessed as a dichotomous, true‐false variable for an individual primary care clinic visit. Combined clinical inertia was defined as the presence of both medication and behavioral inertia at every clinic visit during a defined period of time. For the prerandomization period, this was calculated on the basis of the last 2 clinic visits prior to randomization. For the postrandomization period, it was based on the first 1 or 2 clinic visits during the postrandomization period as these were most likely to correspond to the outreach‐facilitated visits in the intervention group.
Additional outcomes included between‐group differences in the number of primary care clinic visits during the 9‐month study period; the average time between randomization and the first postrandomization clinic visit; new hypertension diagnoses on the problem list for previously undiagnosed patients; and types of behavioral counseling. Behavioral counseling included advice documented in the medical record about antihypertensive medication adherence, sodium intake, weight loss, exercise or physical activity, diet, nonsteroidal anti‐inflammatory medications, and alcohol use.
Blinding
PCPs were accustomed to electronic reminders and patient outreach protocols used in unrelated clinical programs and were not aware that BP management was subject to evaluation in this specific quality‐improvement initiative. A research assistant blinded to study group assignment assessed behavioral inertia, medication inertia, hypertension diagnosis, and behavioral counseling through chart abstraction in which all OC‐created chart notes were filtered from view in the EHR. BP outcomes, number and frequency of PCP visits, and number of antihypertensive medications were determined through electronic queries of the EHR.
Statistical Analysis
Statistical procedures were carried out using SAS version 9.2 (SAS Institute, Inc, Cary, NC). For bivariate comparisons, we used chi‐square tests, as well as Mantel‐Haenszel and Fisher exact tests when appropriate for categoric variables. Because BP measurements, clinical inertia measurements, and frequency of postrandomization visits were not normally distributed, the nonparametric Wilcoxon test was used to compare prerandomization BP, prerandomization combined clinical, medication, and behavioral inertia, and postrandomization visits by study group.
To determine study group differences in ΔBP and Δcombined clinical inertia, we performed intention‐to‐treat analyses using restricted maximum likelihood (REML) for a repeated measures model with incomplete data (SAS PROC MIXED). As this analysis assumes that the occurrence of missing follow‐up data depends only on observed data (ie, prerandomization values), we also performed a sensitivity analysis using the method proposed by Little. 22 The estimates from the sensitivity analysis were virtually identical to the REML results, providing assurance that the missing data were missing at random (data not shown). Moderation analyses also assessed the effect of key demographic covariates (age, sex) and key clinical covariates (prerandomization BP medications [≥1 vs 0], prerandomization diagnosis of hypertension [yes/no], and prerandomization hypertension stage [2 vs 1, JNC 7 criteria]). Because many participants’ race and ethnicity were recorded as “unknown,” we could not perform moderation analyses using race.
Human Participants
This intervention was designed and carried out as a quality‐improvement program that relied on standard methods for creating patient registries and providing patient outreach. The Colorado Multiple institutional review board approved publication of results following the removal of protected health information. Because there were no patient exclusions based on informed consent requirements, the study population was representative of the clinic population.
Results
Sample
A sample of 591 individuals met eligibility criteria and the study group was randomly assigned. Figure 1 depicts the study flow per Consolidated Standards of Reporting Trials (CONSORT) recommendations for pragmatic RCTs. 15 The Table summarizes participant characteristics. At baseline, there were no group differences in BP or sociodemographic characteristics. Intervention group participants had more prerandomization clinic visits than usual care group participants (P=.05).
Figure 1.
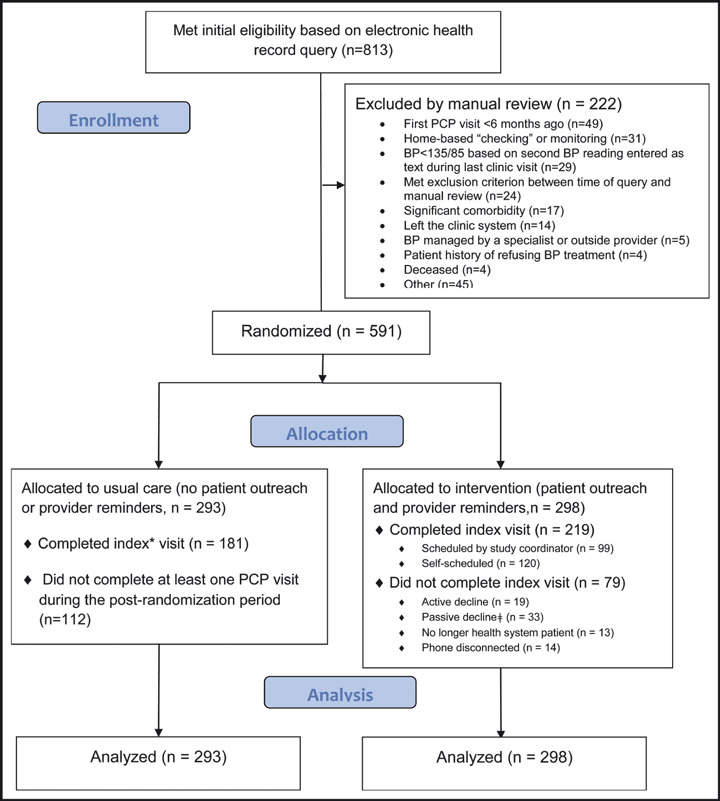
Study flow diagram. *The index is defined as the first postrandomization primary care provider (PCP) clinic visit; †passive decline refers to patients who did not respond to a full cycle of outreach (on letter and 3 phone calls) and to those who either cancelled or no‐showed to their appointments. BP indicates blood pressure.
Table I.
Participant Characteristics (n=591)
| Characteristic | Usual care N=293 (%) | Intervention N=298 (%) | P‐value |
|---|---|---|---|
| Demographic characteristics | |||
| Sex | |||
| Female | 52.2 | 57.1 | .24 |
| Age category | |||
| <45 | 23.9 | 24.8 | .75 |
| 45–54 | 23.6 | 18.8 | |
| 55–64 | 27.3 | 28.5 | |
| 65–79 | 25.3 | 27.9 | |
| Race | |||
| Non‐Latino White | 31.4 | 37.9 | .10 |
| Black | 23.9 | 22.5 | |
| Latino | 7.5 | 10.7 | |
| Other/Unknown | 37.3 | 28.9 | |
| Marital status | |||
| Single | 35.5 | 34.9 | .44 |
| Married/Partnered | 62.8 | 61.7 | |
| Unknown | 1.7 | 3.4 | |
| Insurance status | |||
| Commercial | 52.2 | 48.3 | .51 |
| Medicaid/Indigent | 9.6 | 10.4 | |
| Medicare | 27.7 | 26.9 | |
| Tricare | 10.6 | 14.4 | |
| Clinical characteristics | |||
| Hypertension diagnosed | |||
| No | 46.8 | 47.0 | .96 |
| Yes | 53.2 | 53.0 | |
| Hypertension stage | |||
| 1 | 70.7 | 68.8 | .62 |
| 2 | 29.4 | 31.2 | |
| Pre‐randomization systolic BP (mm Hg, median, interquartile range) | 149, 144–158 | 149, 144–160 | .88 |
| Pre‐randomization diastolic BP (mm Hg, median, interquartile range) | 91, 84–96 | 90, 83–95 | .23 |
| Pre‐randomization combined clinical inertia (mean, SD) | 0.59, 0.42 | 0.58, 0.44 | .76 |
| Pre‐randomization medication inertia (mean, SD) | 0.67, 0.40 | 0.67, 0.41 | .90 |
| Pre‐randomization behavioral inertia (mean, SD) | 0.77, 0.35 | 0.74, 0.38 | .41 |
| eGFR | |||
| ≤60 | 5.5 | 7.4 | .55 |
| >60 | 76.5 | 76.5 | |
| Missing | 18.1 | 16.1 | |
| Number of pre‐randomization anti‐hypertensive medications | |||
| None known | 55.6 | 52.7 | .61 |
| 1 | 19.1 | 21.5 | |
| ≥2 | 25.3 | 25.8 | |
| Number of PCP visits in 18 month pre‐randomization phase | |||
| 1–2 | 28.0 | 21.8 | .05 |
| 3–4 | 38.9 | 38.9 | |
| 5 or more | 33.1 | 39.3 | |
Abbreviations: BP, blood pressure; SD, standard deviation; GFR, glomerular filtration rate; PCP, primary care provider.
Primary Outcome: BP
There was no significant difference in ΔBP in intervention vs usual care groups (−10.1/−4.1 vs −9.1/−4.5 mm Hg, P=.50 and 0.71 for systolic and diastolic BP, respectively, Figure 2). There was also no difference in the percentage of patients achieving BP goal by the end of study (42.7% vs 37.4%, respectively; P=.27). Finally, there were no group differences in ΔBP in separate moderation analyses that adjusted for key demographic (age, sex) and clinical characteristics (hypertension diagnosis, hypertension stage, and prerandomization hypertensive medications), respectively (data not shown).
Figure 2.
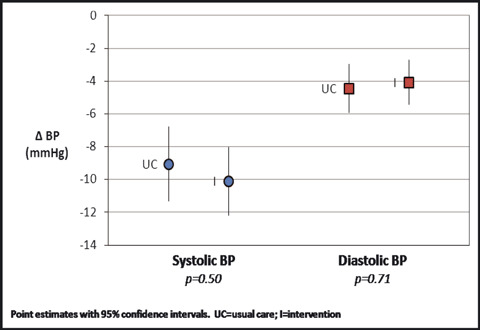
Blood pressure (BP) outcomes.
Secondary Outcomes
Compared with usual care, the intervention group’s combined clinical inertia improved by an additional 18.6% (95% confidence interval [CI], 7.4–29.7 percentage points; mean scores of −29.3% vs −10.7% in intervention and usual care, respectively, P=.001; Figure 3). In terms of constitutive components of inertia, the intervention group’s behavioral inertia improved by an additional 16.2% (95% CI, 4.5–27.9 percentage points; mean scores of −32.2% vs −16.0% in intervention and usual care, respectively; P=.007) and medication inertia improved by an additional 15.5% (95% CI, 3.5–27.4 percentage points; mean scores of −29.3% vs −13.8% in intervention and usual care, respectively; P=.01), as compared with usual care. The improvement in medication inertia was related primarily to uptitrating medications, however, as there was no significant difference in the number of end‐of‐study antihypertensive medications in intervention and usual care (63% vs 58% with ≥1 medication, P=.25).
Figure 3.
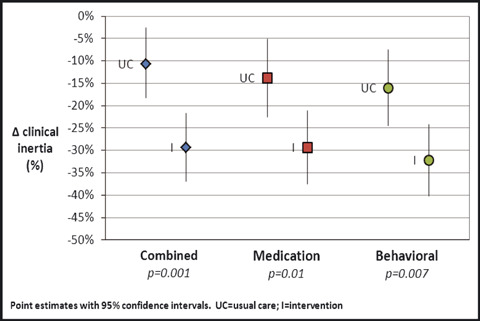
Clinical inertia outcomes.
The intervention was associated with improved primary care visit frequency and timeliness. In the 9‐month study period, there were 2.5 visits in the intervention group as compared with 1.8 visits in the usual care group (P<.0001). In addition, almost two thirds (65%) of intervention group participants completed their first postrandomization visit within 9 weeks, whereas just more than one third (39%) of the usual care group did so (P=.02). Among patients with undiagnosed hypertension at study entry, a new diagnosis of hypertension was added to the problem list for 26% of participants in the intervention group compared with 16% in the usual care group (P=.03).
The most commonly documented behavioral counseling elements were advice to exercise (16.1% intervention vs 7.2% usual care, P=.0007), restrict sodium intake (13.4% intervention vs 6.1% usual care, P=.003), modify diet (11.1% intervention vs 4.1% usual care, P=.001), lose weight (6.7% intervention vs 2.4% usual care, P=.01), and take antihypertensive medications as prescribed (4.4% intervention vs 2.4% usual care, P=.18).
Discussion
Clinical inertia arises from factors such as a lack of provider awareness of in‐clinic BP readings; a failure to formally diagnose hypertension; postponement of BP treatment intensification when BP is close to but nonetheless above goal levels; patient and provider reticence to add more medication; an overreliance on lifestyle strategies rather than pharmacotherapy; an assumption that in‐clinic BP readings may represent a “white coat” phenomenon; and medical concerns that compete with BP for attention during time‐limited clinic visits. 4 , 5 , 6 , 23 , 24 , 25 , 26 , 27 , 28 In this study, we evaluated a population‐based BP management program designed to mitigate patient, provider, and office system influences on clinical inertia. Specifically, we attempted to raise patient and provider awareness of unmet BP goals, recalled patients for BP‐focused clinic visits, and furnished medical providers with EHR prompts prior to BP‐focused clinic visits that included a Web address for treatment decision support. Compared with usual care, the intervention resulted in almost one additional primary care visit (2.5 vs 1.8 visits) and in reduced measures of clinical inertia (−29% vs −11%). Nonetheless, although BP appeared to improve in both study groups, most likely reflecting regression toward the mean, the intervention did not result in superior BP control compared with usual care. Regression toward the mean is common when a population characteristic, such as BP, is selected on the basis of extreme values and then re‐measured over time.
Although improved clinical inertia has been associated with improved BP in other trials, 11 , 13 , 29 , 30 there are several possible reasons we did not observe this result here. First, a majority of hypertensive patients require ≥2 antihypertensive agents to achieve BP control. 16 In this study, however, there was relatively little initiation of new medication. Also, while dose intensification took place, and was the major reason for improved medication inertia measures, the intensification may not have been sufficiently aggressive. It is quite possible that these weaknesses could have been mitigated if we had provided PCPs with specific medication treatment protocols such as those employed in nurse‐ and pharmacist‐based hypertension programs. 10 , 11 , 13 An EHR‐based point‐of‐care decision support tool might have been particularly helpful for this purpose.
Because medication adjustments and behavioral counseling require follow‐up clinic visits to assess BP responses, and because there were only 0.7 greater visits in the intervention group, patient follow‐up may have been insufficient to achieve a beneficial effect on BP. By helping providers to focus on inadequate BP management during at least 2 clinic visits, we envisioned that providers would proactively and autonomously maintain this focus during a longer period until BPs were controlled. Unfortunately, this did not happen. Other BP interventions have incorporated more persistent and prolonged follow‐up between providers and patients. 11 , 12 , 13 Although it is unclear how intense and sustained this activity needs to be, treatment goals are typically reached more quickly as a result of multiple, frequent encounters. 31 However, more persistent and prolonged follow‐up also incurs additional costs to both the practice and patient, so further study is warranted to identify the optimal frequency and duration of patient outreach and follow‐up clinic visits.
We also suspect that poor medication adherence might have undercut any potential intervention benefit. US rates of nonadherence to antihypertensive medications are 20% to 50%, 32 , 33 , 34 and adherence tends to be poorer in populations with poorly controlled BP. 34 Our intervention did not assess adherence nor incorporate strategies to help patients and providers minimize the widespread problem of poor adherence. In other studies, promising strategies have included simplifying medication regimens and motivational strategies ranging from medication reminder charts to culturally appropriate storytelling. 14 , 21 , 33
Finally, “best practices” can increase the accuracy of BP measurements including, for example, ensuring that proper cuff sizes are employed and that patients are seated for ≥5 minutes before BPs are assessed. 16 Although medical assistants in our clinics have been trained to follow proper BP measurement techniques, 35 fidelity to these techniques is likely variable. This was not an efficacy trial that incorporated strict protocols for assessing outcomes in an ideal or controlled setting, but was instead an evaluation of a real‐world clinical practice. It is more likely that BPs are erroneously high than low when proper measurement techniques are not followed, and repeat measurement produces regression towards the mean. In this study, the magnitude of such a phenomenon might have blunted a possible intervention effect. Greater attention to standardizing BP measurement would be important for future interventions.
Beyond identifying possible ways of improving hypertension treatment where PCPs maintain exclusive responsibility for medication management, our results suggest that a more fine‐grained approach to measuring clinical inertia might be useful in future studies. For example, the reduction in behavioral inertia that we observed (an outcome rarely assessed in other studies) might actually reflect clinicians’ sometimes misplaced emphasis on lifestyle counseling over medication management. Alternatively, lifestyle counseling in lieu of medication intensification may reflect patient or provider resistance to starting additional medications. Thus, we propose that medication inertia should be subdivided into two types: inertia to adding medications and inertia to escalating medication doses. In a population‐based program, both are important but the former is probably more important for reaching BP goals.
Study Limitations and Strengths
This study has several potential limitations. The academic setting, patient population, and use of a specific type of EHR might limit generalizability. A spillover effect of increased provider vigilance with regard to BP in the control group was possible because randomization took place at the patient rather than clinical practice level; however, if present, it is unlikely to have been significant because providers were unaware of a formal quality‐improvement evaluation plan, the total number of enrolled patients per provider was small, the duration of patient outreach was only a few months, and the frequency of BP‐focused clinic visits in the intervention group was low. Adherence measures were not included, limiting our ability to determine whether poor medication adherence affected intervention effectiveness. We included patients with elevated BPs who met criteria for a hypertension diagnosis, but half had not yet been formally diagnosed. However, patients with and without a formal hypertension diagnosis might require different management strategies as patients in the latter group require more education and time to accept their diagnosis.
Despite these limitations, the study had a number of unique strengths. It employed a pragmatic trial approach in which a large and diverse cohort of patients was enrolled in a real‐world setting without exclusions based on informed consent requirements. Also, the intervention incorporated multiple strategies to address the types of patient, provider, and office system factors identified as important in systematic reviews. 4 , 10
Conclusions
There is still little evidence about how the care of hypertensive patients can be better organized and delivered by PCPs. 10 Our multifaceted intervention emphasized appointment scheduling for a specific purpose and the timely provision of information and advice to both PCPs and patients. While these efforts clearly affected the behavior of both parties and reduced clinical inertia as traditionally measured, they did not result in improved BP control. This is counterintuitive and is therefore worth noting for future interventions. Despite the negative result, and viewed from an orientation of clinical effectiveness (rather than efficacy), a useful lesson is that measurements of clinical inertia should reflect aspects of treatment intensification that are most likely to be associated with improvements in BP control. One possibility is to differentiate between inertia to adding new medications, on the one hand, and inertia to titrating doses of existing medications, on the other. Another lesson to be drawn is that future success will likely require alteration or augmentation of some elements of our intervention. Compelling options might include the use of specific medication protocols for PCPs, more sustained patient outreach to address unmet BP goals, greater attention to measuring and overcoming poor medication adherence, and the implementation of “best practices” related to measuring BP.
Acknowledgments and Disclosures: The study funder, Novartis Pharmaceuticals Corporation, had no role in the data analysis or manuscript preparation; however, as part of the study agreement, Novartis approved the investigators’ study design and final manuscript. Dr Huebschmann is supported by NIH/NCRR Colorado CTSI Grant Number KL2 RR025779. The authors acknowledge the efforts of Julie A. Porter, PharmD, Regional Scientific Associate Director for Novartis Pharmaceuticals Corporation, who gave input regarding the pragmatic study design elements and approved the final manuscript.
References
- 1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 2. Cherry DK, Hing E, Woodwell DA, et al. National ambulatory medical care survey: 2006 summary. Natl Health Stat Report. 2008;3:1–39. [PubMed] [Google Scholar]
- 3. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825–834. [DOI] [PubMed] [Google Scholar]
- 4. O’Connor PJ, Sperl‐Hillen JM, Johnson PE, et al. Clinical inertia and outpatient medical errors. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation ( Volume 2: Concepts and Methodology ). Rockville, MD: Agency for Healthcare Research and Quality (US); 2005:293–308. [PubMed] [Google Scholar]
- 5. Phillips LS, Twombly JG. It’s time to overcome clinical inertia. Ann Intern Med. 2008;148:783–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Viera AJ, Schmid D, Bostrom S, et al. Level of blood pressure above goal and clinical inertia in a Medicaid population. J Am Soc Hypertens. 2010;4:244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alonso‐Moreno FJ, Llisterri Caro JL, Rodriguez‐Roca GC, et al. Primary care physicians behaviour on hypertensive patients with poor blood pressure control. The PRESCAP 2006 study. Rev Clin Esp. 2008;208:393–399. [DOI] [PubMed] [Google Scholar]
- 8. Marquez CE, Martel CN, Gil GV, et al. Control of therapeutic inertia in the treatment of arterial hypertension by using different strategies. Aten Primaria. 2009;41:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okonofua EC, Simpson KN, Jesri A, et al. Therapeutic inertia is an impediment to achieving the Healthy People 2010 blood pressure control goals. Hypertension. 2006;47:345–351. [DOI] [PubMed] [Google Scholar]
- 10. Glynn LG, Murphy AW, Smith SM, et al. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010;3:CD005182. [DOI] [PubMed] [Google Scholar]
- 11. Five‐year findings of the hypertension detection and follow‐up program. III. Reduction in stroke incidence among persons with high blood pressure. Hypertension Detection and Follow‐up Program Cooperative Group. JAMA. 1982;247:633–638. [PubMed] [Google Scholar]
- 12. Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008;299:2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simpson SH, Majumdar SR, Tsuyuki RT, et al. Effect of adding pharmacists to primary care teams on blood pressure control in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2011;34:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morgado MP, Morgado SR, Mendes LC, et al. Pharmacist interventions to enhance blood pressure control and adherence to antihypertensive therapy: review and meta‐analysis. Am J Health Syst Pharm. 2011;68:241–253. [DOI] [PubMed] [Google Scholar]
- 15. Zwarenstein M, Treweek S. What kind of randomised trials do patients and clinicians need? Evid Based Med Aug. 2009;14:101–103. [DOI] [PubMed] [Google Scholar]
- 16. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 17. Denberg TD, Myers BA, Eckel RH, et al. A patient outreach program between visits improves diabetes care: a pilot study. Int J Qual Health Care. 2009;21:130–136. [DOI] [PubMed] [Google Scholar]
- 18. Denberg TD, Myers BA, Lin CT, et al. An outreach intervention increases bone densitometry testing in older women. J Am Geriatr Soc. 2009;57:341–347. [DOI] [PubMed] [Google Scholar]
- 19. Fahey T, Schroeder K, Ebrahim S. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2006;4:CD005182. [DOI] [PubMed] [Google Scholar]
- 20. Weber CA, Ernst ME, Sezate GS, et al. Pharmacist‐physician comanagement of hypertension and reduction in 24‐hour ambulatory blood pressures. Arch Intern Med. 2010;170:1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Houston TK, Allison JJ, Sussman M, et al. Culturally appropriate storytelling to improve blood pressure: a randomized trial. Ann Intern Med. 2011;154:77–84. [DOI] [PubMed] [Google Scholar]
- 22. Haynes RB, Gibson ES, Taylor DW, et al. Process versus outcome in hypertension: a positive result. Circulation. 1982;65:28–33. [DOI] [PubMed] [Google Scholar]
- 23. Howes F, Hansen E, Williams D, et al. Barriers to diagnosing and managing hypertension – a qualitative study in Australian general practice. Aust Fam Physician. 2010;39:511–516. [PubMed] [Google Scholar]
- 24. Ogedegbe G. Barriers to optimal hypertension control. J Clin Hypertens. 2008;10:644–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Redon J, Coca A, Lazaro P, et al. Factors associated with therapeutic inertia in hypertension: validation of a predictive model. J Hypertens. 2010;28:1770–1777. [DOI] [PubMed] [Google Scholar]
- 26. Powers BJ, Olsen MK, Smith VA, et al. Measuring blood pressure for decision making and quality reporting: where and how many measures? Ann Intern Med. 2011;154:781–788. [DOI] [PubMed] [Google Scholar]
- 27. Safford MM, Shewchuk R, Qu H, et al. Reasons for not intensifying medications: differentiating “clinical inertia” from appropriate care. J Gen Intern Med. 2007;22:1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kerr EA, Zikmund‐Fisher BJ, Klamerus ML, et al. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148:717–727. [DOI] [PubMed] [Google Scholar]
- 29. Rinfret S, Lussier MT, Peirce A, et al. The impact of a multidisciplinary information technology‐supported program on blood pressure control in primary care. Circ Cardiovasc Qual Outcomes. 2009;2:170–177. [DOI] [PubMed] [Google Scholar]
- 30. Luders S, Schrader J, Schmieder RE, et al. Improvement of hypertension management by structured physician education and feedback system: cluster randomized trial. Eur J Cardiovasc Prev Rehabil. 2010;17:271–279. [DOI] [PubMed] [Google Scholar]
- 31. Morrison F, Shubina M, Turchin A. Encounter frequency and serum glucose level, blood pressure, and cholesterol level control in patients with diabetes mellitus. Arch Intern Med. 2011;171:1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caro JJ, Salas M, Speckman JL, et al. Persistence with treatment for hypertension in actual practice. CMAJ. 1999;160:31–37. [PMC free article] [PubMed] [Google Scholar]
- 33. Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure‐lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med. 2004;164:722–732. [DOI] [PubMed] [Google Scholar]
- 34. Hill MN, Miller NH, Degeest S, et al. Adherence and persistence with taking medication to control high blood pressure. J Am Soc Hypertens. 2011;5:56–63. [DOI] [PubMed] [Google Scholar]
- 35. Bonewitt‐West K HS, Applegate EJ. Today’s Medical Assistant: Clinical and Administrative Procedures, 1st edn. St. Louis, MO: Saunders; 2009. [Google Scholar]


