Abstract
AIM
To evaluate the therapeutic efficacy of intracameral amphotericin B (ICAMB) injection in the treatment of keratomycosis.
METHODS
The study design was a prospective controlled clinical trial. A total of 60 eyes of 60 patients were divided into two groups, 30 in the ICAMB injection group (group A) and 30 in the control group-topical application amphotericin B (group B). Serial measurements of the size of the keratomycosis-namely, two maximum linear dimensions perpendicular to each other, and the area and perimeter was done at start of therapy and follow up on day 3, 7, and 21. Rate of healing of the keratomycosis were measured as percentage decrease from the baseline parameter at each subsequent follow up. The data were analyzed by the non-parametric Wilcoxon rank sum test.
RESULTS
The mean time to disappearance of hypopyon was 9.6±9.2 (range:1-26) days in group A and 26.8±20.8 (range:14-62) days in group B (P=0.03). The median percentage decrease in the size of the keratomycosis was significantly greater than that in the cord serum group at day 21(P<0.05) when measured in terms of the area and perimeter. A greater number of patients showed complete re-epithelialization in group A (n=27) than in group B (n=14) (P<0.05). None of the patients reported any side effects or discomfort with either treatment.
CONCLUSION
ICAMB injection leads to faster healing of the keratomycosis refractory to all medical management and reducing time to disapperence of hypopyon compared to topical application amphotericin B.
Keywords: amphotericin B, management, intracameral injection, keratomycosis
INTRODUCTION
Fungal keratitis presents suppurative, usually ulcerative, lesions and results in significant ocular morbidity. It is caused by filamentous fungi, yeast, and dimorphic fungi. Fungal keratitis accounts for 5%-20% of all corneal infections. Unlike bacteria, fungi are capable of penetrating the intact Descemet's membrane and entering the anterior chamber, resulting in hypopyon, which is difficult to treat with antifungal agents, ocular penetration and bioavailability of many of the available topical antifungal preparations are poor[1]. Hypopyon and inflammation of the membranes of the anterior chamber may easily develop during the course of the disease, the selection of appropriate therapy for fungal infection remains an unsettled question. Therapeutic keratoplasty, especially if performed for larger ulcers, is not as effective as an optical keratoplasty done on a quiet eye after healing. While fungal ulcers are a major problem in developing countries, donor corneal tissue supply is erratic and limited. Although many antifungal agents are available, treatment with these agents is complicated by a narrow spectrum of activity, lack of effective penetration into the eye, and toxicity. Amphotericin B, a macrocyclic polyene, is effective against Candida and aspergillus species. It has been used by systemic, topical, and intravitreal routes in the trestment of fungal keratitis. Amphotericin B is also used intravitreally for endophthalmitis and is considered safe for intraocular injection if used in the appropriate dose.
To try to successfully treat those fungi that had penetrated into the anterior chamber, we tested the use of intracameral injection of amphotericin B as an alternative to conventional therapies. We hypothesised that amphotericin B can reach the fungi in cornea stroma by penetrating Descemet's membrane and this study aimed to evaluate beneficial role of the ICAMB injection in keratomycosis. To our knowledge, there has been no clinical comparison between the intracameral administration of amphotericin B and topical application amphotericin B treatment to date.
MATERIALS AND METHODS
Patients
We prospectively examined 30 eyes (18 right and 12 left) of 30 patients with keratomycosis by ICAMB injection (13 men and 17 women, group A), were compared with 30 eyes (16 right and 14 left) of 30 patients with keratomycosis by a topical application amphotericin B (15 men and 15 women, group B). The mean age of patients was 42.8 years (range, 28.6-58.9 years). All the subjects with the group B was established matching to patients (group A) as to age and gender. The diagnosis of fungal corneal ulcer was made on the basis of clinical features and confirmed by the presence of filamentous septate fungi using clinical confocal laser scanning microscopy. Patients of any age group, preferably cooperative for digital photography, were included. Pregnant and lactating women, patients unable to give consent for the therapy, those with active ocular or lid infections, patients with lid abnormalities contributory to the epithelial defect, impending perforations, elevated intraocular pressure, and with severe limbal ischaemia were excluded from the study. Written informed consent was given. The study followed the tenets of the Helsinki declaration and was approved by the institutional review board.
Methods
Patients underwent a thorough examination, which involved a comprehensive ocular and medical history and examination including record of the best corrected visual acuity and slit lamp biomicroscopy. Tactile intraocular pressure was recorded for each patient. A photographic documentation of the epithelial defect was done with and without fluorescein staining. The size of the epithelial defect was measured at its greatest dimension, along two perpendicular axes with a slit lamp micrometer and later also with digital image analysis software Image Pro Plus. After examination the patients were first put on a one-day washout period with normal saline drops and preservative free chloramphenicol drops to clear the epithelium of all toxic preservatives. They were then started on therapy with either ICAMB injection or topical application amphotericin B after measuring the epithelial defect, the day of starting therapy being defined as day 0. Antifungal treatment include topical hourly application of 1.5% amphotericin B in group B. but in group A, we use ICAMB to treatment the patients with keratoinycosis.
For topical application, a preparation of amphotericin B (50-mg powder; Fungizone, Cedex, France) for intravenous administration was diluted to a 0.15% concentration with distilled water. For intracameral injection, the preparation was mixed with balanced salt solution (BSS; Alcon, Fort Worth, TX) under sterile conditions and was diluted to a concentration of 100µL/mL. ICAMB was administration under aseptic conditions by using an operating microscope. After instillation of topical proparacaine (Alcaine; Alcon), a speculum was inserted, A paracentesis was performed at the superior temporal limbus by using a sharp blade. After discharge of the aqueous humor, 10µg of amphotericin B in 0.1mL was injected, using a 27-gauge anterior-chamber cannula attached to a 1.0-mL syringe into the anterior chamber. The patients were followed up on day 3, 7, and 21. The variables recorded at each follow up visit were the best corrected visual acuity, the maximum size of the epithelial defect along two perpendicular axes measured with a slit lamp micrometer, and also a digital photograph of the same, a record of the side effects if any, and the digital intraocular pressure. After the 21st day the patients who did not heal were re-evaluated and were advised to have a surgery therapy.
Distribution of categorical variables in the two groups was compared using the χ2 test. For quantitative variables following normal distribution we used mean and standard deviation as the summary statistics. For variables following non-normal distribution, we computed median (range) as summary statistics. The various baseline characteristics in the two groups were statistically compared.
Statistical Analysis
Student's t-test was used to compare mean values in the two groups. Median values in the two treatment groups were compared using Wilcoxon rank sum test. SPASS 13.0 statistical software was used for data analysis. P<0.05 was considered as statistically significant.
RESULTS
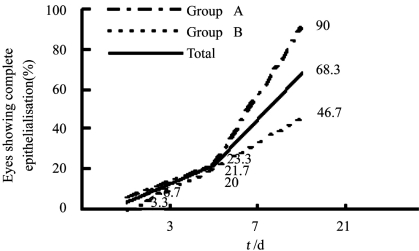
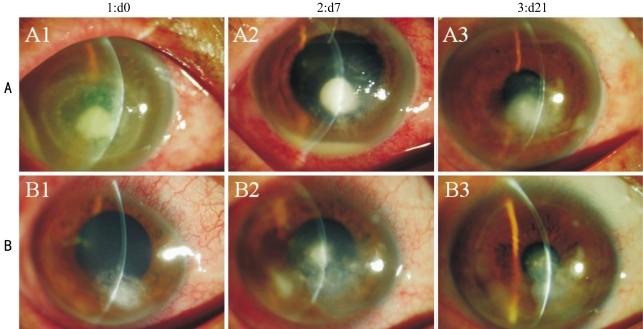
At initial presentation most of the patients (80%) had a visual acuity of <3/60. The distribution of visual acuities between the two groups was not significantly different. Among the patients in the group A, 24 received one injection, 6 received two injections at one-week intervals. Of the 60 patients, 68.3% (n=41) healed on either therapy; 90% (n=27/30) in the group A and 46.7% (n=14/30) in the group B (Pearson χ2=0.07).The mean time to disappearance of hypopyon was 9.6±9.2 (range,1-26) days in group A and 26.8±20.8 (range,14-62) days in group B (P=0.03). The total number of patients showing re-epithelialisation in the different groups with different Etiology on day 21 is shown in Table 1. There was no significant gain in visual acuity in both the groups in cases showing complete re-epithelialisation. The trend lines for the percentage of eyes that showed complete healing of the epithelial defect in the two groups at each of the follow up days are shown in Figure 1. These were significantly different in complete healing of the epithelial defect at day 21. The median time for closure of epithelial defects was 20.2±10.6 days in group A and 31.2±21.4 days in group B (P=0.16). However, the percentage decrease in size of the epithelial defect as measured in terms of area and perimeter was different in the two groups, being significantly more in the group A (Table 2). On the follow up of 21 day of the 41 patients who had healed, 36 patients remained on follow up and none of them showed a recurrence of the epithelial defect. Among the 19 patients who did not show complete healing at day 21 underwent a temporary lateral tarsorrhaphy, with a subsequent healing of the epithelial defect(Figure 2). Thirteen patients underwent an amniotic membrane transplantation (AMT) and at last follow up ten of those had a complete re-epithelialisation of the corneal surface. One patient who underwent AMT was lost to follow up. The remaining three patients in the failure group developed a perforation. Both these patients underwent therapeutic penetrating keratoplasty. None of our patients from either group reported any adverse event during the therapy.
Table 1. Complete re-epithelialization in patients with different etiology on day 21.
| Etiology | Group A |
Group B |
||||
| n(%) | Healed(n=27) | Not healed(n=3) | n(%) | Healed (n=14) | Not healed (n=16) | |
| Cause not identified | 15(50) | 15 | 0 | 15(50) | 7 | 8 |
| Not vegetality trauma | 6(20) | 6 | 0 | 7(23.3) | 4 | 3 |
| Vegetality trauma | 6(20) | 5 | 1 | 5(16.7) | 2 | 3 |
| Herpes simplex keratitis | 1(3.33) | 1 | 0 | 1(3.33) | 1 | 0 |
| Exposure keratitis | 1(3.33) | 0 | 1 | 1 (3.33) | 0 | 1 |
| Sjogren syndrome | 1(3.33) | 0 | 1 | 1(3.33) | 0 | 1 |
Figure 1. Eyes showing complete re-epithelialization.
Table 2. Healing rate in the two groups.
| Outcome variables | Percentage decrease |
|
| group A(range) | group B (range) | |
| Horizontal diameter | ||
| Day 3 | 4.2 (–226.0–59.2) | 9.6 (–136.6–68.4) |
| Day 7 | 26.7 (–14.9–100.0) | 10.8 (–100.0–100.0) |
| Day 21 | 34.6 (–8.9–100.0) | 14.3 (–55.0–50.0) |
| Vertical diameter | ||
| Day 3 | 3.43 (–972.0–35.23) | 9.66 (–220.0–85.6) |
| Day 7 | 21.3 (–100.0–100.0) | 9.67 (–76.4–100.0) |
| Day 21 | 22.4 (–100.0–100.0) | 10.6(–84.6–54.3) |
| Area | ||
| Day 3 | 12.1 (–1269.6–68.9) | 0.82 (–646.4–86.2) |
| Day 7 | 54.0 (–49.8–100.0) | 2.89 (–67.4–16.4 )a |
| Day 21 | 69.4 (0.0–78.8) | 6.8 (–42.5–34.2)a |
| Perimeter | ||
| Day 3 | 13.9 (–366.85–66.34) | 1.4 (–589.7–73.1) |
| Day 7 | 33.8 (–47.3–100.0) | 3.8 (–37.2–16.9)a |
| Day 21 | 49.4 (0.0–76.5) | 2.9 (–17.6–32.4)a |
aP<0.05 vs group A (Wilcoxon rank sum test)
Median (n=30)
Figure 2. Healing of epithelial defect.
DISCUSSION
Fungal keratitis is a significant cause of ocular morbidity in rural areas where the climate is warm and humid. The two most common forms of fungi that are of interest to ophthalmologists are filamentous fungi and yeast. Medical treatment, if possible, is still the best primary mode of therapy for keratomycosis. Amphotericin B was the first polyene effective in treating systemic mycosis[2]. This is the treatment of choice against yeasts and natamycin-resistant filamentous fungi, notably Aspergillus. It has a wider spectrum of activity and, in addition, has immunoadjuvant properties and an immunopotentiating effect. Improvement of the efficacy of amphotericin B may lie in its formulation and mode of application. In an attempt to achieve optimal drug levels in the cornea, topical use of amphotericin B has been tried in the form of drops, collagen shields, and ointment[3]. The efficacy of topical amphotericin B drops has been studied clinically and experimentally using concentrations ranging from 0.003% to 0.3%. Amphotericin B diluted to a concentration of 0.15% is efficacious in vivo and produces far fewer toxic effects than the higher concentrations previously used. Subconjunctival injection can cause longstanding periocular inflammation, epithelial ulcerations, and marked tissue necrosis at the injection site. Topical amphotericin B as a 0.15% solution or ointment is well tolerated and effective in the treatment of fungal keratitis[4]. Recently, intra-stromal corneal injection combined with intravitreal injection of amphotericin B has been tried to treat recurrent fungal keratitis and endophthalmitis[5]. The role of intracameral injections of amphotericin B for keratomycosis is ill defined. Animal studies have shown that up to 50µg of intracameral amphotericin B is well tolerated by rabbit eyes. At this dose, it causes only reversible iritis and clouding of lens. Amphotericin B has been shown to have some toxic effect on a porcine corneal endothelial cell culture line. In a rabbit model, it is found that anterior chamber injection of as much as 50µg amphotericin B failed to cause corneal or lenticular toxicity. The clinical does recommended is 10 to 30µg in 0.1 to 0.2mL. Clinical reports of intracameral amphotericin B for keratomycosis are few. In a case of colletotrichum graminicola corneal ulcer[6], intracameral amphotericin B was repeated twice in addition to repeated karatoplasties, and the infection was controlled. As dose between 5 and 10µg have been shown to be safe and effective for intravitreal injections, we decided to use this dose for intraocular injections. Surgery in the form of a therapeutic penetrating keratoplasty has been advocated for deep keratitis with retroconeal or anterior chamber involvement that is unresponsive to medical therapy. Because the patients were not randomized for treatment assignment, there was a possibility of bias from this assignment method. Further studies are needed with a prospective randomized design and a larger sample size. In conclusion, although there is no difference in treatment success rate between ICAMB and conventional treatment,
In conclusion, we recommend that ICAMB injection can reduce time to disappearance of hypopyon and time to final improvement in the treatment of fungal keratitis. It is an effective means of promoting epithelialisation and healing of the keratomycosis refractory, and can be safely used.
REFERENCES
- 1.Pleyer U, Grammer J, Pleyer JH, Kosmidis P, Friess D, Schmidt KH, Thiel HJ. Amphotericin B-bioavailability in the cornea. Studies with local administration of liposome incorporated amphotericin B. Ophthalmologe. 1995;92(4):469–475. [PubMed] [Google Scholar]
- 2.Abad JC, Foster CS. Fungal keratitis. Int Ophthalmol Clin. 1996;36(1):1–15. doi: 10.1097/00004397-199603630-00003. [DOI] [PubMed] [Google Scholar]
- 3.Hirose H, Terasaki H, Awaya S, Yasuma T. Treatment of fungal corneal ulcers with amphotericin B ointment. Am J Ophthalmol. 1997;124(6):836–838. doi: 10.1016/s0002-9394(14)71701-5. [DOI] [PubMed] [Google Scholar]
- 4.Isipradit S. Efficacy of fluconazole subconjunctival injection as adjunctive therapy for severe recalcitrant fungal corneal ulcer. J Med Assoc Thai. 2008;91(3):309–315. [PubMed] [Google Scholar]
- 5.Garcia-Valenzuela E, Song CD. Intracorneal injection of amphothericin B for recurrent fungal keratitis and endophthalmitis. Arch Ophthalmol. 2005;123(12):1721–1723. doi: 10.1001/archopht.123.12.1721. [DOI] [PubMed] [Google Scholar]
- 6.Ritterband DC, Shah M, Seedor JA. Colletotrichum graminicola: a new corneal pathogen. Cornea. 1997;16(3):362–364. [PubMed] [Google Scholar]