Abstract
Correspondence to
Fang-Tian Dong. Department of Ophthalmology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing 100730, China. d_fangtian@sina.com
AIM
To access the differentiation of rat mesenchymal stem cell (MSC) in the microenvironment of retinal degeneration induced by the administration of sodium iodate.
METHODS
In-vitro cultured Lewis rat MSC were injected into the sub-retinal space of NaIO3 induced retinal degeneration rat eyes (30g/L NaIO3 100mg/kg). To observe the trace and differentiation of MSC by immuno-fluorescent method successively in 5 weeks after the surgery.
RESULTS
The majority of the transplanted cells stay in retinal pigment epithelium layer and cones & rods layer. From the 2nd week after transplantation, the engrafted MSC express PCK and rhodopsin under fluorescent microscope.
CONCLUSION
MSC can survive mainly in the outer layer of retina in the microenvironment of retinal degeneration and differentiate forward the RPE cell and photoreceptor.
Keywords: mesenchymal stem cell, NaIO3, transplantation in subretinal space, differentiation, retinal degeneration
INTRODUCTION
In the ongoing discussion of using stem cells for cell replacement strategies, the most promising group of stem cells are those isolated from the adult organism. Among these adult stem cells are the mesenchymal stem cells from the bone marrow stroma, which have recently experienced a high level of attention as they can be easily isolated and can be obtained in almost unlimited numbers. Bone marrow stromal cell (BMSC) can be expanded through as many as 20 to 50 population doublings in approximately 10 weeks[1]. Recently, it has been reported that bone marrow cells differentiate into various cell types, including hepatocytes, endothelial cells of the blood vessels, cardiac muscle, and skeletal muscle. It has also been reported that MSC differentiate into neural cells and astrocytes in vitro. Furthermore, they differentiate into astrocyte-like cells in vivo when transplanted into the normal or ischemic brain. In addition, in the case of the rat eye, it has been published that MSC can differentiate into photoreceptor-like cells[2]. This study is to observe the growth and differentiation of MSC that are transplanted into the sub-retinal space of rats with the destruction of RPE cells.
MATERIALS AND METHODS
Materials
DMEM/F12(1:1) culture medium and fetal calf serum (Hyclone), NaIO3, Dulbecco fluid (Gibco), Bromodeoxyuridine (BrdU, Sigma), mouse monoclonal anti-BrdU antibody (Boster Biotechnology Co. Ltd.), PE conjugated anti-rat CD45 monoclonal antibody and FITC conjugated anti-rat CD90 monoclonal antibody (Caltag Laboratory), anti-rat rhodopsin (Opsin) monoclonal antibody (NeoMarkers), anti-rat PCK monoclonal antibody (NeoMarkers), Cy3 conjugated anti-mouse secondary antibody, Sheep blood serum (Boster Biotechnology Co. Ltd.). Lewis rats, depuratory, 4-5weeks old, weigh from 100-120g.
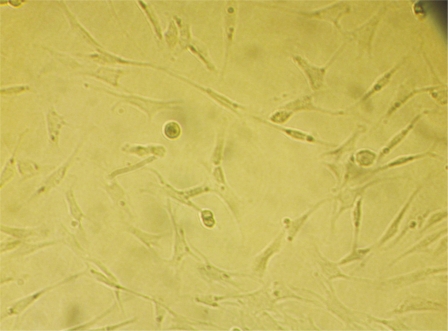
Anesthetize the Lewis rat in overdose, take the tibia and fibula and immerse them in 750mL/L alcohol for 10 minutes. Wash the medullary cavity of bones with the PBS fluid repeatedly. Centrifugate the washed PBS fluid at the speed of 1 000r/min for 5 minutes. Collect the cells and put them in the culture of DMEM/F12 including 100mL/L fetal calf serum. Finally put them in the petri dish and culture them at the condition of 37°C, 50mL/L CO2 and saturated humidity. Change the culture media on alternate days. 12-14 days later, transfer the passage when the cells confluence mostly. Digest the MSC that confluence mostly with the 2.5g/L trypsin. Put PE-conjugated CD45 and FITC-conjugated CD90 monoclonal antibody after centrifugation. Place them in the dark at 4°C for 30 minutes and then wash them by PBS fluid, suspend them again and check them by flow cytometry. 2-3 days later, there are some sporadic spindle cells adherent to the culture dish. 5-7 days later, the sporadic cells change into colonies. 9-10 days later, the colonies increase and coalesce. 12-14 days later, there are whirlpools in some place. We can transfer the passage when cells confluence. 6-7days later after transferring, the cells can confluence again. The result of flow cytometry indicates that more than 70% of the cells are CD90(+) and CD45(-) which are from passage 1 to 2. And more than 93% of the cells are CD90(+) and CD45(-) which are from passage 3 to passage 6. MSC cells labeled by Brdu monoclonal antibody can be stained into brownish red. The cytoplasm is not stained. Un-labelled cells are not stained at all (Figure 1). We can see the damage of RPE cells and photoreceptor cells and the inner nuclear layer being in direct contact with Bruch's membrane.Put DMEM culture with 10mg/L Brdu, 100mL/L fetal cow blood serum in the MSC of passage 3, then culture at the condition of 37°C, 50mL/L CO2 and saturated humidity, change the culture media on alternate days. Observe the label effect by immunohist- ochemistry. When MSC of passage 3 labelled by BrdU confluence mostly, digest with 2.5g/L trypsin and centrifugate them. Dilute with PBS fluid to the concentration of 3×1010/L and put in the sterile canoula for use.
Figure 1. BrdU labeled, MSCs red brown nucleus.
Twenty-five Lewis rats, 4 weeks old, weigh from 100-120g, intra-peritoneally administered 100mg/kg NaIO3 dissolved just before use for one week. We observed the pathological sections of RPE cells and photoreceptor cells.
Methods
We anesthetized the Lewis rat, dilated the pupil, stabbed the sclera and pars plana of ciliary body under the operating microscope. Then we put the slender glass needle made by ourselves and the micro-amount syringe in the vitreous through the pars plana of ciliary body stabbed just now, and injected 10µL fluid into the sub-retinal space. We repeated the procedures and completed the surgeries to 25 rats. The 25 right eyes were considered as experimental eyes and 25 left eyes as controls. The fluid injected in the right eye was 10µL MSC suspension while in the left eye was PBS fluid in equal amount. All the fundus of the rat eyes were observed at day 1, 7, 14, 21 and 28 after operation. Twenty-five Lewis rats were divided into 5 groups randomly. One group contained 5 rats. We anesthetized the Lewis rat in overdose, enucleated the eyes of the rats at day 7, 14, 21 and 28 after operation. The eyes were frozen immediately and cut in sections labelled by mouse monoclonal Anti-BrdU antibody, FITC-conjugated anti-mouse secondary antibody; Anti-rat rhodopsin monoclonal antibody, Cy3-conjugated anti-mouse secondary antibody; Anti-rat PCK monoclonal antibody, Cy3-conjugated anti-mouse secondary antibody; Fluorescence microscope was used to observe the result.
RESULTS
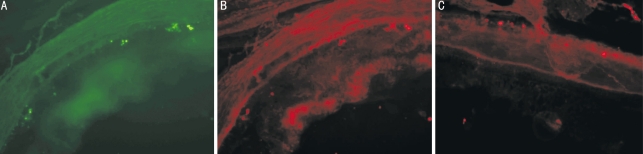
Day 1 after operation, the conjunctivas were congestive in all eyes. All the corneas were transparent. Seven lenses became slightly opaque including 4 right eyes and 3 left eyes. No hemorrhage was seen in vitreous and retina of all eyes checked by indirect ophthalmoscope. Local retinal detachment could be seen in all eyes. Day 7 after operation, the congestion in conjunctiva regressed. Seven lenses became opaque including 4 right eyes and 3 left eyes. Their fundus could not be seen accordingly. The detached retina became reattachment. Week 2-4 after operation, seven eyes including 4 right eyes and 3 left eyes changed into cataract. The retinas remained no change. One week later after injection of MSC into sub-retinal space, the slices of the retina of Lewis rats indicate that MSC labelled by Brdu show green conjugated with FITC. The cells that express PCK or Rhodopsin showed red conjugated with Cy3. If one scene is overlapped to another, we can see that some cells express PCK and the other express Rhodopsin. This indicates the MSC can differentiate into the way of RPE and photoreceptor cells (Figure 2).
Figure 2. After MSC sub-retinal transplantation.
A: Brdu(+); B: Rhodopsin(+); C: PCK(+)
DISCUSSION
There are a number of animal models of retinal degeneration. They contain the inherited animal models and chemical induced retinotoxicity models. In the inherited animal models, arrested outer segment development followed by photoreceptor cells loss is the primary event in the rd3 and rds mouse. By contrast, defects of the phagocytic activity in the RPE cells is the primary site of the genetic mutation in the RCS rat, with the rdy mutation in which photoreceptor cells are affected occurring as a secondary event. Among the chemicals, N-methyl-N-nitrosourea (MNU) and urethane cause photoreceptor cell degeneration without initial damage to the RPE cells. NaIO3 possesses selective toxicity for the RPE cells. The damage of photoreceptor is secondary to the damage of RPE cells. In the literature, the RPE cell nuclei were hyperchromatic at 6 hours after the NaIO3 treatment, and the RPE cell number decreased at 12 hours. At this time point, photoreceptor cell nuclei as well as photoreceptor segments remained mormal. At 24 hours, the number of RPE cells was decreased and the arrangement of the photoreceptor segments was disorganized. But by day 3, destruction of the photoreceptor segments was more advanced, macrophage migration was seen, and the number of photoreceptor cell nuclei was decreased in some areas. Finally, at day 7 and day 28, an abrupt transition from a relatively normal retina to an area with loss of RPE cells and photoreceptor cells resulting in the inner nuclear layer being in direct contact with Bruch's membrane was seen. In this study, we select NaIO3 to induce our animal model. As a result, we can see in the image 5 the damage of RPE cells and photoreceptor cells and the inner nuclear layer being in direct contact with Bruch's membrane.
MSC are the the precursor of the bone marrow stroma cells. There are several sight-threatening retinal disorders for which treatment is not yet available or produces poor results. In some of these diseases, retinal degeneration occurs early in life and might be quite rapid, whereas in other disorders, retinal degeneration begins later and progresses very slowly. There are some possible sources for the repair of retinal degeneration. A current therapeutic approach to neovascular eye diseases involves the application of angiostatic or antiproliferative agents to the eye. A cell-based therapy, however, should be explored, because, in contrast to inhibiting angiogenesis with small molecules or recombinant factors, a cell-based approach might enable the cell to adapt and respond to a changing environment. Cell-based therapy likely involves numerous factors produced by the cell that can be appropriately modulated in response to changing conditions[3]. It was demonstrated that MSC are able to secrete neurotrophic factors that promote neural cell survival[4]. The multipotentiality of BM-MSC as well as their easy isolation and culture properties and their high expansion potential makes these cells an ideal source for autologous transplantation aimed at a cell-based therapy for retinal degeneration repair[5]. We select the marker of CD90 and CD45 to identificate the MSC by flow cytometry. We have achieved a lot in the transplantation of NSC. NSC could incorporate into the retina and express the Cytokeratin and CD68 which are the markers of RPE cells when transplanted into sub-retinal space[6],[7]. MSC can incorporate into the retina and express the GFAP which is the marker of astrocyte and rhodopsin which is the marker of photoreceptor cells. Anthony et al.[8] transplanted the MSC into the sub-retianl space of RCS rat and discovered that most of the MSC distributed in the layer of cones & rods and express rodopsin which is the marker of photoreceptor cells. Pan et al.[9] discovered there are about 0.174%±0.082% MSC can survive and incorporate into all the layers of the retina 2 weeks after transplantation. Inoue et al.[5] discovered transplanted MSC can delay the retina degeneration of RCS rat. Also, a recent study compared MSC and retinal progenitor cells to evaluate their potential as a source for retinal transplantation. Both cell types expressed neuronal markers in vitro but some MSC differentiated into cells that resembled microglia rather than neural cells. These findings suggested that retinal progenitor cells are the best choice for retinal transplantation studies, but MSC remain an attractive candidate as a therapeutic tool for retinal repair in autologous transplantation.
The purpose of the study is to observe if the MSC can differentiate into RPE cells which are damaged. RPE cells are damaged by celiac injection of NaIO3. In this case, the MSC can differentiate into the way of RPE cells. Because the photoreceptor cells are damaged at the same time, we can also observe that the MSC differentiate into the way of photoreceptor cells. As for the differentiated cells, we don't know if they have the function of the RPE and photoreceptor cells and need further study. But it is difficult to detect the retinal function of the animals because it needs to keep the most retina intact to examine the electrophysiology or action. At least, we can deduce two principles from this study: 1) the transplanted MSC can survive in the sub-retinal space, without exclusion and proliferation; 2) the MSC can differentiate into the way of RPE and photoreceptor cells.
REFERENCES
- 1.Lee HS, Huang GT, Chiang H, Chiou LL, Chen MH, Hsieh CH, Jiang CC. Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells. 2003;21(2):190–199. doi: 10.1634/stemcells.21-2-190. [DOI] [PubMed] [Google Scholar]
- 2.Kicic A, Shen WY, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci. 2003;23(21):7742–7749. doi: 10.1523/JNEUROSCI.23-21-07742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedlander M. Fibrosis and diseases of the eye. J Clin Invest. 2007;117(3):576–586. doi: 10.1172/JCI31030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Q, Long Y, Yuan X, Zou L, Sun J, Chen S, Perez-Polo JR, Yang K. Protective effects of bone marrow stromal cell transplantation in injured rodent brain: synthesis of neurotrophic factors. J Neurosci Res. 2005;80(5):611–619. doi: 10.1002/jnr.20494. [DOI] [PubMed] [Google Scholar]
- 5.Inoue Y, Iriyama A, Ueno S, Takahashi H, Kondo M, Tamaki Y, Araie M, Yanagi Y. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res. 2007;85(2):234–241. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Guo Y, Saloupis P, Shaw SJ, Rickman DW. Engraftment of adult neural progenitor cells transplanted to rat retina injured by transient ischemia. Invest Ophthalmol Vis Sci. 2003;44(7):3194–3201. doi: 10.1167/iovs.02-0875. [DOI] [PubMed] [Google Scholar]
- 7.Enzmann V, Howard RM, Yamauchi Y, Whittemore SR, Kaplan HJ. Enhanced induction of RPE lineage markers in pluripotent neural stem cells engrafed into the adult rat subretinal space. Invest Ophthalmol Vis Sci. 2003;44(12):5417–5422. doi: 10.1167/iovs.03-0468. [DOI] [PubMed] [Google Scholar]
- 8.Kicic A, Shen WY, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci. 2003;23(21):7742–7749. doi: 10.1523/JNEUROSCI.23-21-07742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan H, Liu X, Wu J, Tian Y, Zhang S, Lin Z, Huang Q. Fate and protective effect of marrow stromal cells after subretinal transplantation. Acta Biochim Biophys Sin (Shanghai) 2008;40(3):202–208. doi: 10.1111/j.1745-7270.2008.00398.x. [DOI] [PubMed] [Google Scholar]