Abstract
AIM
To investigate retinal vascular endothelial growth factor (VEGF) level and retinal cells apoptosis in the early stage of diabetic NOD mouse retina.
METHODS
Animals were divided into non-diabetes group, (control) (2-, 4-, 6-, 8- and 12-week sub-groups, n=30) and diabetes group (2-, 4-, 6-, 8- and 12-week sub-groups, n=30). Enzyme-linked immunosorbent assay (ELISA) was performed to detect VEGF level in both serum and retina. Transmission electron microscope method was used to examine retinal cell apoptosis.
RESULTS
Compared with the control group, VEGF levels in serum and retina were increased significantly in the NOD group (12 weeks: 4.9±0.4µg/g vs 0.19±0.1µg/g in serum sample, P<0.01; 165±9µg/g vs 17±5µg/g in retinal sample, P<0.01). There exists a positive correlation between serum VEGF and retinal VEGF levels in the early diabetic NOD mice (γ=0.9902, P=0.001). The number of the cells apoptosis in the ganglion cells and endothelium can also been found increased significantly in the NOD group (P<0.01).
CONCLUSION
The high VEGF expression may be contributed to increased retinal cells apoptosis. Many factors associated with retinal VEGF expression might involve in the early diabetes stage.
Keywords: retina, apoptosis, vascular endothelium growth factor, NOD mice
INTRODUCTION
Diabetic retinopathy is the most common diabetic eye disease and a leading cause of blindness. Diabetic retinopathy is characterized increased microvascular permeability, cell damage, abnormal blood flow autoregulation, disordered angiogenesis and increased adhesive properties of the endothelium. All these factors can cause capillary occlusion, microvascular degeneration and abnormal neovascularization, then eventually leading to irreversible blindness. Hyperglycemia is an independent risk factor for the development of cardiovascular disease[1]. However, the mechanisms of hyperglycemia and hyperglycemia's effect on tissue damage and clinical complications remain unclear. Vascular endothelial growth factor (VEGF) might play a particularly important role in diabetic retinopathy[2]. The expression changes and effect of VEGF on apoptosis in retinal cells induced by hyperglycemia are still elusive.
The nonobese diabetic (NOD) mouse is an useful and important model of autoimmune type 1 diabetes. The incidence of spontaneous diabetes in the NOD mouse varied from 60%-80% in females and 20%-30% in males. Diabetes onset typically occurs at 12-14 weeks of age in female mice and slightly later in male mice[3]. In this study, we aim to study the expression of VEGF on apoptosis in retinal cells in diabetic NOD mice.
MATERIALS AND METHODS
Materials
NOD mice (Jackson Laboratory, Bar Harbor, ME) were bred in the animal house of Central South University. Mice were housed by pairs in plastic cages under a pathogen-free environment, continuously access to food and water on a 12-hour light/dark cycle. All animal experiments were accordance with the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Mice were considered to be diabetic when non-fasting blood glucose levels were >16.67mmol/L (One-Touch Lifescan meter; Lifescan, Inc., Milpitas, CA) using whole blood collected from the tail vein. Only females were used in the studies because disease progression in males is slower and less consistent[2]. Then these diabetic NOD mice were divided into 5 groups according to duration of diabetes (2-, 4-, 6-, 8- and 12-week groups after onset of diabetes, n=30). The control groups were made up of sibling without diabetes. Body weight and blood glucose concentration were monitored every week. Diabetic mice were not given routine insulin injections. At week 2, 4, 6, 8 and 12 after onset of diabetes, the mice were anesthetized with sodium pentobarbital (100mg/kg, IP) and decapitated. The diabetic NOD mice had significantly elevated concentrations of blood glucose compared with those in control group. At death, the NOD mice also weighed significantly less than the control animals (Table 1).
Table 1. Body mass, blood glucose and VEGF levers in diabetes mice.
| Group(weeks) | Mass(g) | Glucose(mg/dL) | Retinal VEGF(µg/g) | Serum VEGF(µg/g) |
| Control 2 | 23.0±3.0 | 152.0±9.0 | 16.0±4.0 | 0.18±0.10 |
| 4 | 28.0±3.0 | 138.0±21.0 | 17.0±5.0 | 0.19±0.10 |
| 6 | 31.0±4.0 | 146.0±16.0 | 16.0±3.0 | 0.19±0.10 |
| 8 | 32.0±1.0 | 144.0±5.0 | 18.0±3.0 | 0.19±0.20 |
| 12 | 36.0±2.0 | 140.0±8.0 | 18.0±4.0 | 0.18±0.10 |
| Diabetes 2 | 23.8±1.2 | 479.0±33.0 | 23.0±5.0 | 0.53±0.10 |
| 4 | 24.0±2.3 | 477.0±23.0 | 33.0±7.0 | 0.76±0.10 |
| 6 | 22.0±3.2 | 488.0±27.0 | 64.0±6.0 | 1.24±0.20 |
| 8 | 20.0±4.3 | 558.0±44.0 | 92.0±7.0 | 2.37±0.20 |
| 12b | 18.0±3.5 | 576.0±27.0 | 165.0±9.0 | 4.9±0.40 |
bP<0.01 vs control group (unpaired t-test)
(mean±SD, n=30)
Methods
VEGF expression
The eyes of both groups were rapidly removed and retinas were dissected, immediately frozen in liquid nitrogen and stored at -70°C. ELISA method was used to determine VEGF protein concentration according to the manufacturer's protocol (Quantikine Mouse VEGF ELISA kit; R&D Systems, Minneapolis, MN). The measurement was performed on three samples for each experimental condition. Four retinas of each sample were placed into 200µL of buffer A and sonicated for 30 seconds at 50Hz. The lysate was centrifuged at 22 000g for 15 minutes at 4°C. VEGF concentration was determined spectrophotometrically (Microplate Reader 680 XR; Bio-Rad) at 450nm. In each experiment, all samples and standards were measured twice. Data were collected as picograms VEGF per milligrams of total protein and averaged in the same graph. Blood was centrifuged at 2000G for 20 minutes and then serum was stored at -20°C. Same protocol was applied to both serum VEGF and VEGF in retina.
Ultrastructural changes
The eyeballs from diabetic mice (diabetic 4 weeks and 12 weeks group) were prefixed by immersion in 22g/L glutaraldehyde in 0.1mol/L (pH 7.4) Sorensen's phosphate buffer for 4 hours, and then postfixed in 10g/L osmium tetroxide in 0.1mol/L (pH 7.2) sodium phosphate buffer. The samples were dehydrated in graded ethyl alcohol series and embedded in Araldite CY212. Ultrathin sections were contrasted with uranyl acetate and lead citrate for examination by transmission electron microscope (LEO 906E, Oberkochen, Germany).
Statistical Analysis
Two-way analysis of variance, t-test and correlation analysis via the SPSS statistical program were used to analyze the data. P<0.05 was considered statistically significant.
RESULTS
VEGF Expression
VEGF protein level in the diabetic NOD mice was increased compared with control mice. Retinal VEGF expression in diabetic mice of 2-, 4-, 6-, 8- and 12-week groups were (µg/g): 23.0±5.0, 33.0±7.0, 64.0±6.0, 92.0±7.0 and 165.0±9.0. There were no significant differences of retinal VEGF expression between 2, 4, 6, 8 and 12 weeks in non-diabetic groups(P<0.01).Serum VEGF expression in diabetic mice of 2-, 4-, 6-, 8- and 12-week groups were (µg/g): 0.53±0.10, 0.76±0.10, 1.24±0.20, 2.37±0.20, 4.96±0.40, and no significant differences in non-diabetic mice(P<0.01, Table 1). There was linear correlation between retinal VEGF and serum VEGF in diabetic NOD mice (γ=0.9902, P=0.001).
Ultrastructural Changes
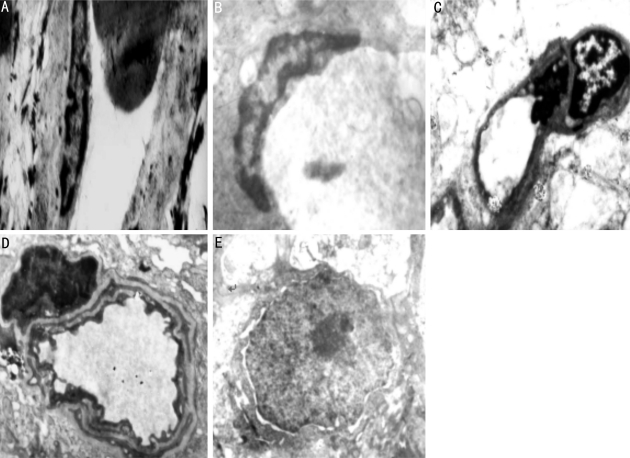
Retinal capillary basement membrane, endothelial cells and ganglion cells were normal in control group. Incresing membrane thickeness(Figure 1A), microvascular occlusion and perivascular edema can be observed in retinal capillary basement (Figures 1B, 1C); chromatin margination with nuclear deformation in the endothelial cells (Figure 1A) and pericyte (Figure 1B); an irregular aggregation of nuclear chromatin with vacuolar degeneration in the ganglion cells can also be observed in 4 weeks NOD group. Retinal pathological change became more obvious in 12 weeks NOD mice, which includes apparent thickening of capillary basement membrane (Figure 1D); nuclear membrane shrinkage and nuclear chromatin condensation in the endothelial cells(Figure 1D); cell shrinkage, pyknosis of nuclear, distribution of nuclear chromatin and loss of mitochondrial cristae in the ganglion cells (Figure 1E).
Figure 1. A: Endothelial cell apoptosis; B: Pericyte apoptosis; C: Perivascular edema, obstruction of microvascular; D: Capillary basement membrane thickening and microvascular wall change; E: Ganglion cells apoptosis.
DISCUSSION
Increased glucose levels and fluctuation in blood glucose are two leading causes of retinal vascular injury in diabetic patients[4]. However, effect of VEGF on the pathogenesis of DR has not clear. Therefore, the study of the effect of VEGF on apoptosis in NOD retinal cells can help us to understand diabetic retinopathy. Increased glucose level can be observed in diabetic NOD mice at age of 12 weeks. After 4 weeks of onset of diabetes, retinal capillary basement membrane became thickening. Perivascular edema, pericytes, endothelial cells and ganglion cells apoptosis can also be found in NOD group. The pathological process became more obvious in the 12 weeks of diabetes. Capillary basement membrane became thicker; Under the transmission electron microscope, more apoptosis of endothelial cells and ganglion cells can be observed to. The vascular endothelial cell is a dynamic cell layer where they communicate chemical signals with other cells in the vessel wall. These signals contribute to monolayer integrity and vascular function[5]. The balance is seriously disrupted in the diabetic retinal microvasculature because of accelerated apoptosis of pericytes and endothelial cells resulting in progressive vasodegeneration[6]. Elevated glucose levels in diabees can interrupt normal cell substrate communication and vascular function, then induce the endothelial cell loss[7]. Damage of the endothelium is accompanied by breakdown of the inner blood-retinal barrier. the process can lead the local release of cytokines and chemokines such as VEGF, IGF-1, SDF-1 by the adjacent endothelium[8],[9]. Further more, increased VEGF expression can be found in the retina and serum of 4 weeks and 12 weeks NOD.
The potential relationship between endothelial cell apoptosis and VEGF expression is still ambiguous. Hyperglycemia induces cells hypoxia and vascular inflammation in retina. Then these retinopathy will stimulate retinal cells apoptosis and vascular changes. In the other side, these apoptotic bodies can make uninjured endothelial monolayers to upregulate VEGF expression. VEGF is responsible for the growth of new blood vessels via the stimulation of endothelial cell proliferation. VEGF also stimulates endothelial cell proliferation, migration, and survival. When retinal pigment epithelial cells begin to apoptosis because of lack of nutrition (ischemia), VEGF takes over to create neovascularization and acts as a restorative function in other parts of the body. VEGF can be massively upregulated by hypoxia. Its levels are increased in the retina and vitreous of patients and in animal models of ischemic retinopathy. A recent report had showed that 14 weeks diabetic mice from streptozotocin injection exhibited neuronal cell death in the retinal ganglion cell layer[10]. In this study, we demonstrate that the retinas of the NOD mice showed retinal vascular pathlogical changes, apoptosis of both retinal vascular cells and retinal neuronal cells. These hyperglycemia-induced retinopathy were attenuated with time, which indicating that hyperglycemia plays an important role in the pathogenesis of this disease. These changes become worse with the increasing VEGF expression in the retina and blood. Retinopathy in NOD mice, such as retinal vascular cells and neuronal degeneration were all deteriorated by hyperglycemia and VEGF, which indicate that VEGF and hyperglycemia plays an important role in the pathology of this disease. There are many factors related with retinal VEGF expression in the early diabetes, such as hyperglycemia, increased retinal cells apoptosis, inflammation and ischemia. All these risk factors have an effect on the diabetic retinopathy.
REFERENCES
- 1.Dabir P, Marinic TE, Krukovets I, Stenina OI. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ Res. 2008;102(12):1558–1565. doi: 10.1161/CIRCRESAHA.108.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6(4):511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Tang M, Wasserfall C, Campbell-Thompson M, Gardemann T, Crawford J, Atkinson M, Song S. Alpha1-antitrypsin gene therapy modulates cellular immunity and efficiently prevents type 1 diabetes in nonobese diabetic mice. Hum Gene Ther. 2006;17(6):625–634. doi: 10.1089/hum.2006.17.625. [DOI] [PubMed] [Google Scholar]
- 4.Santos KG, Tschiedel B, Schneider JR, Souto KEP, Roisenberg I. Prevalence of retinopathy in Caucasian type 2 diabetic patients from the South of Brazil and relationship with clinical and metabolic factors. Braz J Med Biol Res. 2005;38(2):221–225. doi: 10.1590/s0100-879x2005000200010. [DOI] [PubMed] [Google Scholar]
- 5.Heissig B, Hattori K, Friedrich M, Rafii S, Werb Z. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol. 2003;10(2):136–141. doi: 10.1097/00062752-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, vom Hagen F, Lin J, Hammes HP. Incipient diabetic retinopathy: insights from an experimental model. Ophthalmologica. 2007;221(4):269–274. doi: 10.1159/000101930. [DOI] [PubMed] [Google Scholar]
- 7.Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005;11(18):2279–2299. doi: 10.2174/1381612054367300. [DOI] [PubMed] [Google Scholar]
- 8.Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB, Scott EW. l.SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115(1):86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 10.Martin PM, Roon P, van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45(9):3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]