Abstract
AIM
To examine the relationship between corneal lymphangiogenesis and hemangiogenesis after keratoplasty.
METHODS
Nineteen human corneas were obtained from 19 patients undergoing a second corneal transplantation in Zhongshan Ophthalmic Center in 2005. Blood and lymphatic vessels in human transplanted corneas were identified by lymphatic vessel endothelial receptor (LYVE-1) and platelet endothelial cell adhesion modecule-1 (PECAM-1) immunohistochemistry, and double enzyme-histochemistry; then the association of corneal blood vessel counting (BVC) with lymphatic vessel counting (LVC) was examined.
RESULTS
Corneal hemangiogenesis was present in 12 cases (63%), and lymphangiogenesis occurred in 5 cases (26%) human transplanted corneas. In addition, corneal lymphangiogenesis was only present in vascularized corneas. LVC was strongly and positively correlated with BVC (r=0.725, P<0.01).
CONCLUSION
Corneal lymphangiogenesis develops after keratoplasty and strongly associates with hemangiogenesis.
Keywords: cornea, keratoplasty, lymphangiogenesis, hemangiogenesis
INTRODUCTION
Lymphangiogenesis, the development of new lymphatic vessels, is a relatively new area of experimental and clinical investigations. In addition to fluid transportation, lymphatic vessels has an important role in immunological response by enhancing the speed and amount of antigenic material or antigen presenting cells (APCs) to reach regional lymph nodes[1]-[3]. There is no evidence both from animal studies and human corneas that lymphangiogenesis can occur in the cornea in several clinical and pathological state. However, in contrast to the studies with respect to processes of blood vessel proliferation (hemangiogenesis), the work on lymphangiogenesis is much scarce. Recently, we examined transplanted corneas and observed the presence of corneal hemangiogenesis and lymphangiogenesis with electron microscopy, which indicated that the graft had a chance of coming to contact with both the blood and lymphatic system in corneal transplantation[4]. Whereas the blood vessels provide a route of entry for immune effector cells (e.g., CD4+ alloreactive T lymphocytes, memory T lymphocytes), corneal lymphangiogenesis enables the exit of antigenic material, antigen-presenting cells (APCs), etc. from the graft to the regional lymph node. This can induce alloimmunization and subsequent graft rejection. However, to our knowledge, there is little literature available about the relationship between corneal lymphatic and blood vessels after keratoplasty. The aim of the present study is to examine the association of corneal lymphangiogenesis with hemangiogenesis in human transplanted corneas. Findings from the present study may potentially broaden our understanding of mechanisms that can be instrumental in corneal transplant rejection.
MATERIALS AND METHODS
Human Transplanted Cornea
Nineteen human corneas were obtained from 19 patients undergoing a second corneal transplantation in Zhongshan Ophthalmic Center in 2005. Only cases whose cornea prior to the first transplant was devoid of blood vessels were selected. Among them, 1 had a transplantation history (TH) within 1 year, 6 had a history of transplantation between 1 and 2 years, 8 cases had a history of 2 to 5 years, and the remaining 4 had a history of more than 5 years. After transplantation, the excised corneas were divided into half, one was fixed for immunohistochemistry examination, the other was for 5′-nase-alkaline phosphatase (5′-NA-ALP) double enzyme-histochemistry. All patients were informed of the experimental nature of this procedure and signed consent was obtained beforehand. All procedures were conducted according to the principles expressed in the declaration of Helsinki
Double Enzyme-histochemistry
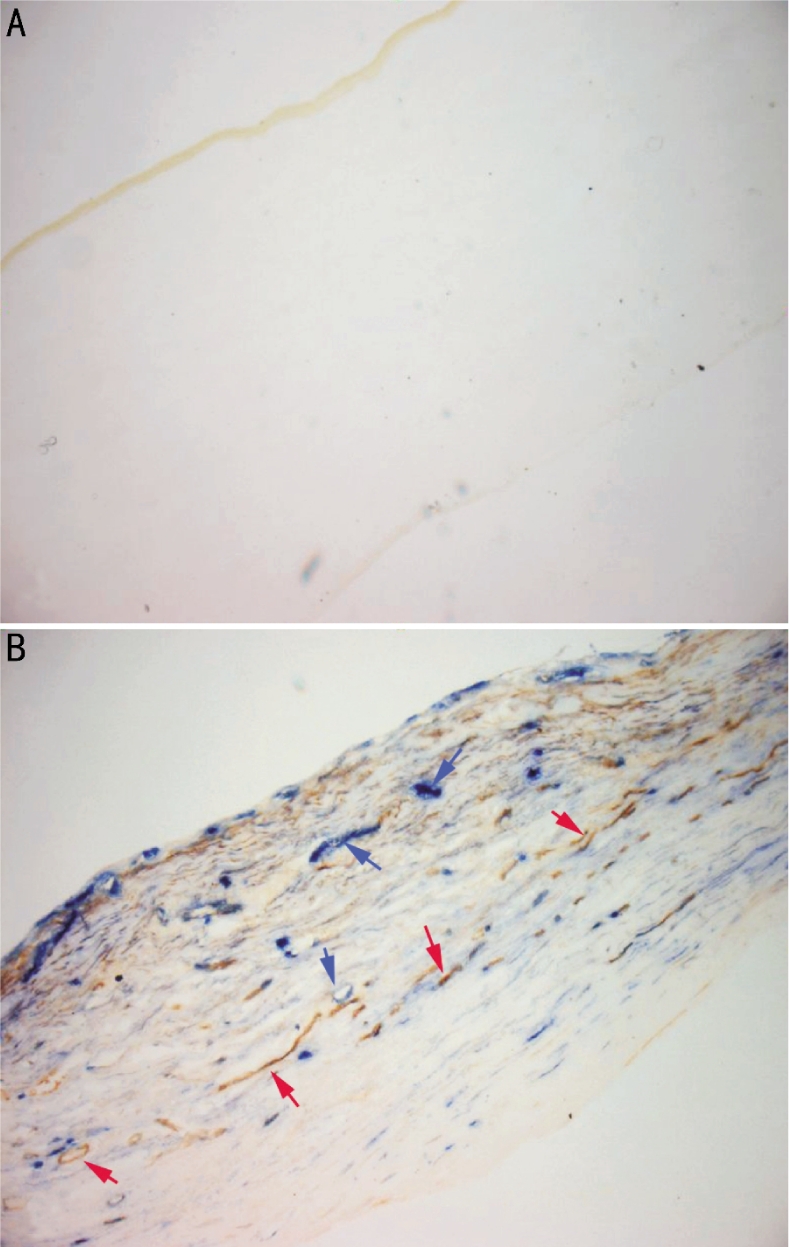
After corneas were fixed with 99% acetone for 10 minutes, 10µm thick serial cryostatic cross sections were prepared with 20 sections per sample, washed and treated with the enzyme reaction for 60 minutes at 37°C in a substance mixture [20mL of 0.2mol/L Tris-maleate buffer, pH7.2, 25mg adenocine-5-monophospate (Sigma), 20mg L-tetramisole, 3g sucrose, 5mL of 25g/L magnesium sulphate, 3mL of 20g/L Pb(NO3)2, 22mL diluted water]. The samples followed to react with 10g/L ammonium sulphide solution for 2 minutes at room temperature. This was followed by a final rinse in distilled water for 1-2 minutes. Finally, the sections were incubated in the reaction medium for ALP activity for 60 minutes at room temperature with 40mL of 0.1mol/L Tris-HCl buffer, pH9.0, 20mg naphthol AS-B1 phosphate disodium salt (Sigma), 40mg fast blue BB (Sigma), and 0.5mL of N, N-dimethylformamide. The (5-NA)−ALP+ vessels (the blue vessels) were blood vessels, and the (5-NA)+ALP− vessels (the brown vessels) were identified as lymphatic vessels..
Immunohistochemistry
After being fixed in 40g/L neutral formaldehyde for 24 hours, embedded in paraffin, serially sectioned for 4µm in thickness, and rehydrated with graded ethanol-water mixtures, human and rat corneas were washed with distilled water. Endogeneous peroxidase activity was blocked after incubatipn with 30mL/L hydrogen peroxidase for 20 minutes. Tissue sections were then autoclaved at 121°C in 10 mmol/L citrate buffer (pH6.0) for 10 minutes for antigen retrieval and cooled at room temperature for 30 minutes. After that, human cornea sections were incubated for 3 hours with mouse anti human LYVE-1 molyclonal antibody (R&D systems, MN), mouse anti human CD31 (R&D systems, MN) respectively, and biotin marked rabbit anti mouse immunoglobulin as the secondary antibody. The slides were visualized for peroxidase activity with 3,3′-diaminobenzidine (DAB) and counterstained with hematoxylin.
Lymphatic vessels counting (LVC) and blood vessels counting (BVC) of human corneas were evaluated independently by two observers without prior knowledge of the experimental details and were repeated once. The CD31+LYVE-1− vessels of stained human cornea sections were identified as blood vessels, whereas the CD31+LYVE-1+ vessels were identified as lymphatic vessels. Each cornea sample was excised into 40 slices. The number of BVC was calculated by summing up all blood vessels in the 40 slices divided by 40. Similarly, the number of LVC was calculated by summing up all lymphatic vessels in the 40 slices divided by 40.
RESULTS
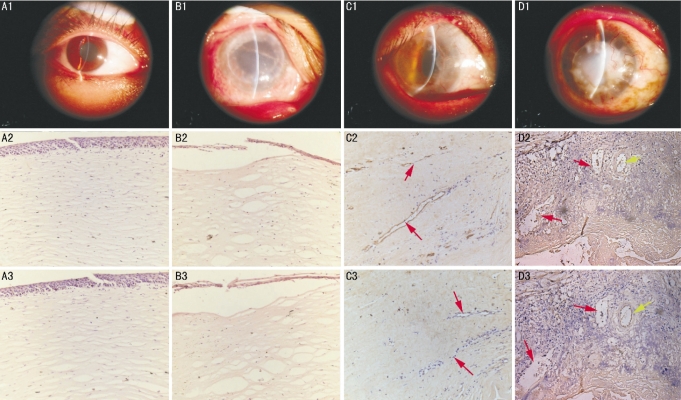
The activity of 5-Nase was high in lymphatic endothelial cells, and low or absent in blood capillary endothelial cells, while ALP was high only in blood vessels in transplanted corneas (5′-NA-ALP double enzyme-histochemistry, Figure 1). CD31 stains both blood and lymphatic vessels, and LYVE-1 specially stains on lymphatic endothelium (Figure 2). Corneal hemangiogenesis was present in 12 of 19 cases (63%), whereas lymphangiogenesis occurred in 5 of 19 (26%) human transplanted corneas including 1 cornea (100%) with a TH within 1 year, 2 cases (33%) with a TH between 1 and 2 years, and 2 cases (25%) with a TH between 2 and 5 years. In addition, corneal lymphang- iogenesis was only present in vascularized corne as.
Figure 1. Lymphatic (red arrows) and blood vessels (blue arrow) of human cornea (Enzyme-histochemistry×100).
A: Normal; B: Transplanted
Figure 2. LYVE-1 and CD31 expression in human corneas.
A: Normal; B: Keratoconus patient with a TH of 1.5 years; C: Viral keratitis patient with a TH of 1.3 years; D: Viral keratitis patient with a TH of 1.6 years (Yellow arrows: lymphatic vessels; Red arrows: blood vessels; 1: Human cornea; 2: CD31; 3:LYVE-1 ×200)
BVC was significantly increased in corneas with lymphatic vessels detectable by immunohistochemistry as compared with those without detectable lymphatic vessels (8.49±1.84 vs 1.95±2.41, P<0.01). LVC was strongly and positively correlated with both BVC (y=0.141x, r=0.725; P<0.01). Although the changes of BVC and LVC were parallel in general, corneal lymphangiogenesis disappeared followed by the corneal blood vessels.
DISCUSSION
The cornea is normally devoid of blood vessels and lymphatic vessels, implicating that cornea is a relative immune privileged site that is likely to have more favorable prognosis after corneal transplantation as compared with other solid organ transplantation[5]. However, cornea can be vascularized, by a number of inflammatory stimuli such as surgical trauma, infection, and contact lens wear, through penetration of new vessels from the neighboring conjunctiva which has a rich supply of both blood and lymphatic vessels. It is widely recognized that new blood vessels and lymphatic vessels in cornea are high-risk factors for graft failure. The current study focuses on the relationship between corneal hemangiogenesis and lymphangiogenesis and elucidates corneal lymphatic vessels develop closely with blood vessels after keratoplasty. Compared with hemangiogenesis, lymphangiogenesis is poorly understood, partly because of the lack of specific lymphatic endothelium markers. This situation has been improved since the identification of LYVE-1[6] LYVE-1, a hyaluronan receptor related to CD44 expressed on lymph vessel endothelial cells of both normal and neoplastic tissues and on both the luminal and abluminal surfaces of the lymphatic endothelial cells, is a powerful maker of lymphatic structure and function[7]. By using double enzyme-histochemistry in combination with LYVE-1 immunohistochemistry, we had investigated new corneal lymphatic and blood vessels and elucidated the development of corneal lymphangiogenesis and hemangiogenesis after corneal transplantation.
In this study, we examined the relationship between BVC and LVC in transplanted human corneas. In addition, to reduce the heterogeneity of ocular disease phenotype, we restricted to the original ocular diseases only without corneal hemangiogenesis. There was also no significant heterogeneity of TH and mean age among groups. Our results showed that there was a significantly positive correlation of BVC with LVC, suggesting that after keratoplasy, there would be a greater opportunity of corneal lymphatic occurrence in strongly vascularized corneas. It also suggested that the survival rate of regrafting in such a recipient bed would be low and that antilymphangiogenic therapies might be necessary.
We also observed that although corneal lymphangiogenesis paralleled with corneal hemangiogenesis after keratoplasty, the duration of lymphatic vessels was shorter than that of blood vessels, which was consistent with the notions that lymphatic vessels occurred later but regressed earlier than blood vessels in vascularized corneas. Therefore, provided that the time point of regrafting when corneal hemangiogenesis regressed, lymphatic vessels had already disappeared and the success rate of the graft might be much promoted by suppressing both “arms” (hemangiogenesis and lymphangiogenesis) of a potential “immune reflex” which could lead to transplant rejection after keratoplasty. However, sometimes corneal hemangiogenesis is not easy to regress, especially among the elders, whose established blood vessels do not depend any longer on angiogenic growth factors and might wait for a long time to disappear[8]. For these cases, we may assess corneal lymphangiogenesis and decide the time point of regrafting according to it. Further studies of large samples are warranted.
In summary, our study revealed the presence of corneal lymphangiogenesis and eluciates a close relationship beween corneal hemangiogenesis and lymphangiogenesis in human corneal transplantation. These findings are particular valuable in view of the key role of corneal lymphangiogenesis in enhancing the traffic of graft-derived antigen to regional lymph nodes and promoting immune rejection.
Footnotes
Foundation item: National Natural Science Foundation of China (No.30700927); Guangdong Technology Program Foundation, China (No.93068)
REFERENCES
- 1.Mouta C, Heroult M. Inflammatory triggers of lymphangiogenesis (Review) Lymphat Res Biol. 2003;1(3):201–218. doi: 10.1089/153968503768330247. [DOI] [PubMed] [Google Scholar]
- 2.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5(1):74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 3.Lohela M, Saaristo A, Veikkola T, Alitalo K. Lymphangiogenic growth factors, receptors and therapies. Thromb Haemost. 2003;90(2):167–84. doi: 10.1160/TH03-04-0200. [DOI] [PubMed] [Google Scholar]
- 4.Ling S, Xiao Q, Hu Y. lymphangiogenesis occurring in transplanted corneas. J Huanzhong Univ Sci Technolog Med Sci. 2006;26(4):241–244. doi: 10.1007/BF02895827. [DOI] [PubMed] [Google Scholar]
- 5.Lam H, Dana MR. Corneal graft rejection. Int Ophthalmol Clin. 2009;49(1):31–41. doi: 10.1097/IIO.0b013e3181924e23. [DOI] [PubMed] [Google Scholar]
- 6.Bono P, Wasenius VM, Heikkila P, Lundin J, Jackson DG, Joensuu H. High LYVE-1-positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clin Cancer Res. 2004;10(21):7144–7149. doi: 10.1158/1078-0432.CCR-03-0826. [DOI] [PubMed] [Google Scholar]
- 7.Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112(7-8):526–38. doi: 10.1111/j.1600-0463.2004.apm11207-0811.x. [DOI] [PubMed] [Google Scholar]
- 8.Cursiefen C, Hofmann-Rummelt C, Kuchle M, Schlötzer-Schrehardt U. Pericyte recruitment in human corneal angiogenesis: an ultrastructural study with clinicopathological correlation. Br J Ophthalmol. 2003;87(1):101–106. doi: 10.1136/bjo.87.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]