Abstract
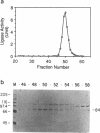
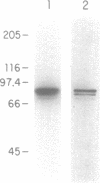
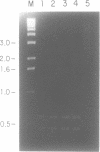
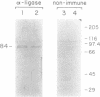
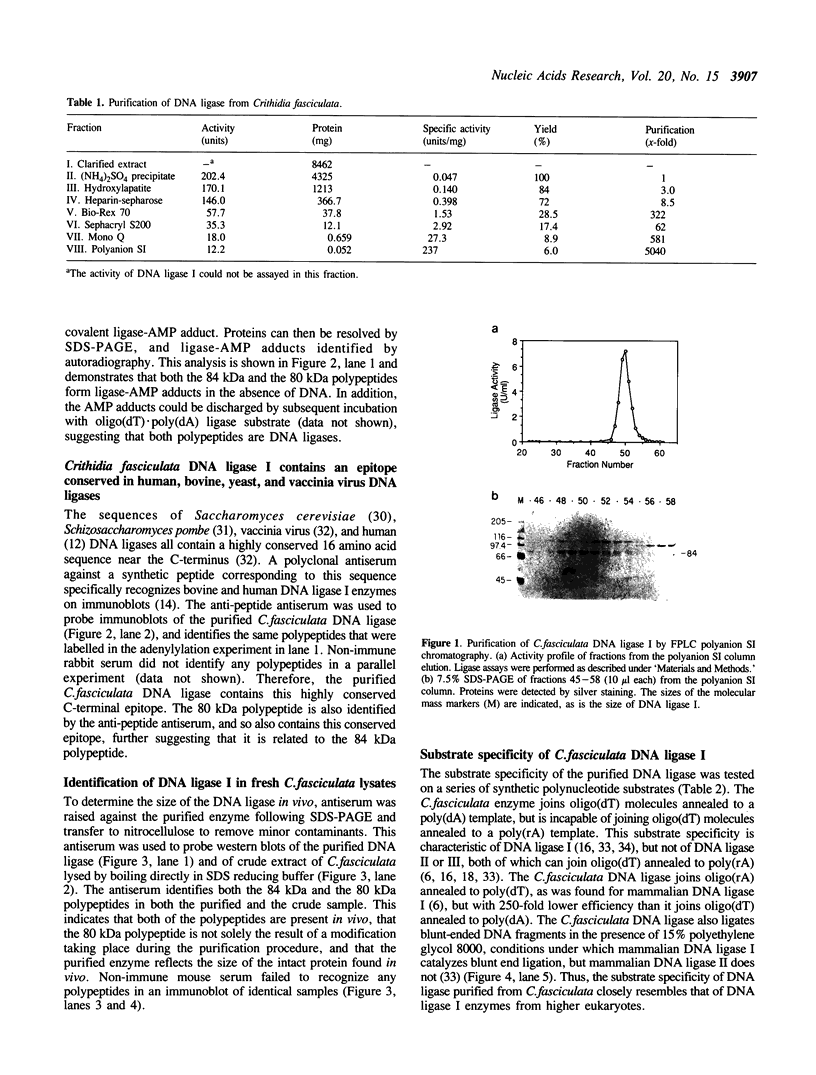
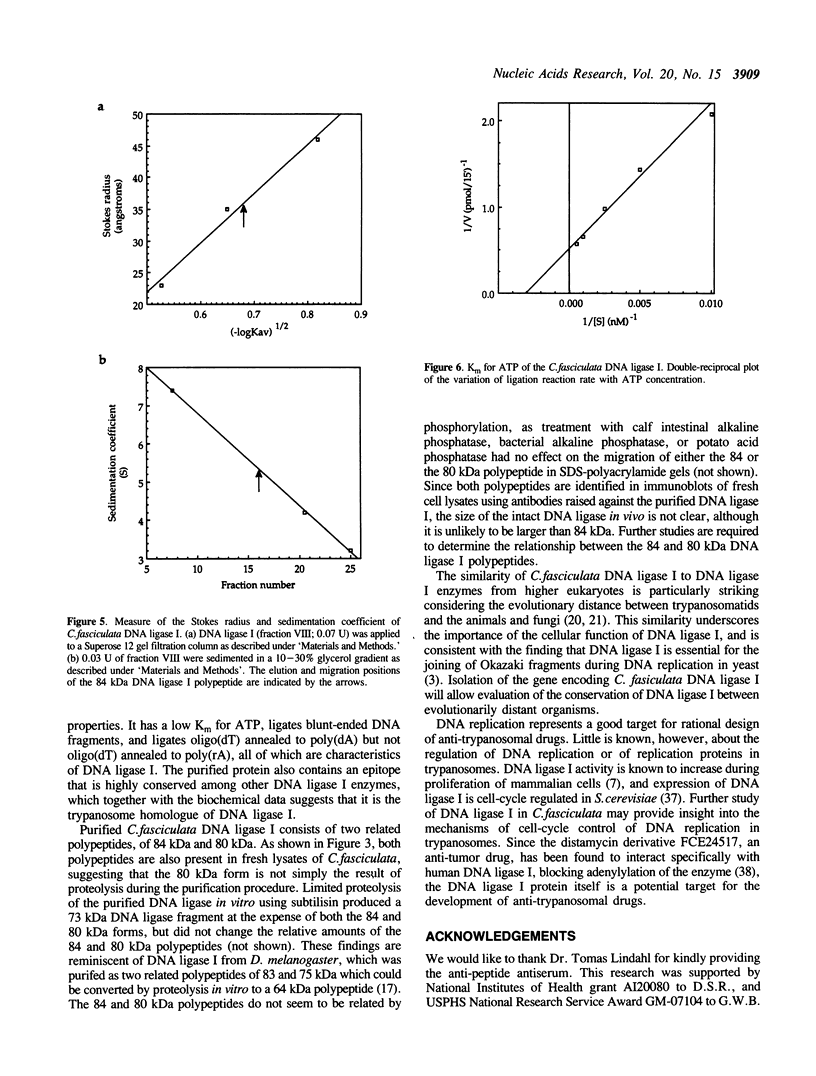
A DNA ligase has been purified approximately 5000-fold, to near homogeneity, from the trypanosomatid Crithidia fasciculata. The purified enzyme contains polypeptides with molecular masses of 84 and 80 kDa as estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Both polypeptides formed enzyme-adenylate complexes in the absence of DNA, contained an epitope that is highly conserved between human and bovine DNA ligase I and yeast and vaccinia virus DNA ligases, and were identified in fresh lysates of C. fasciculata by antibodies raised against the purified protein. Hydrodynamic measurements indicate that the enzyme is an asymmetric protein of approximately 80 kDa. The purified DNA ligase can join oligo(dT) annealed to poly(dA), but not oligo(dT) annealed to poly(rA), and can ligate blunt-ended DNA fragments. The enzyme has a low Km for ATP of 0.3 microM. The DNA ligase absolutely requires ATP and Mg2+, and is inhibited by N-ethylmaleimide and by KCI. Substrate specificity, Km for ATP, and the conserved epitope all suggest that the purified enzyme is the trypanosome homologue of DNA ligase I.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrand J. E., Willis A. E., Goldsmith I., Lindahl T. Different substrate specificities of the two DNA ligases of mammalian cells. J Biol Chem. 1986 Jul 15;261(20):9079–9082. [PubMed] [Google Scholar]
- Barker D. G., White J. H., Johnston L. H. Molecular characterisation of the DNA ligase gene, CDC17, from the fission yeast Schizosaccharomyces pombe. Eur J Biochem. 1987 Feb 2;162(3):659–667. doi: 10.1111/j.1432-1033.1987.tb10688.x. [DOI] [PubMed] [Google Scholar]
- Barker D. G., White J. H., Johnston L. H. The nucleotide sequence of the DNA ligase gene (CDC9) from Saccharomyces cerevisiae: a gene which is cell-cycle regulated and induced in response to DNA damage. Nucleic Acids Res. 1985 Dec 9;13(23):8323–8337. doi: 10.1093/nar/13.23.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. E., Johnston L. H., Kodama K., Tomkinson A. E., Lasko D. D., Lindahl T. Human DNA ligase I cDNA: cloning and functional expression in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6679–6683. doi: 10.1073/pnas.87.17.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Becker F. F. DNA ligase activities during hepatocarcinogenesis induced by N-2-acetylaminofluorene. Carcinogenesis. 1985 Sep;6(9):1275–1277. doi: 10.1093/carcin/6.9.1275. [DOI] [PubMed] [Google Scholar]
- Elder R. H., Rossignol J. M. DNA ligases from rat liver. Purification and partial characterization of two molecular forms. Biochemistry. 1990 Jun 26;29(25):6009–6017. doi: 10.1021/bi00477a019. [DOI] [PubMed] [Google Scholar]
- Fabre F., Roman H. Evidence that a single DNA ligase is involved in replication and recombination in yeast. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4586–4588. doi: 10.1073/pnas.76.9.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Game J. C., Johnston L. H., von Borstel R. C. Enhanced mitotic recombination in a ligase-defective mutant of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4589–4592. doi: 10.1073/pnas.76.9.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S., Aoufouchi S., Thiebaud P., Prigent C. DNA ligase I from Xenopus laevis eggs. Nucleic Acids Res. 1991 Feb 25;19(4):701–705. doi: 10.1093/nar/19.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. H., Nasmyth K. A. Saccharomyces cerevisiae cell cycle mutant cdc9 is defective in DNA ligase. Nature. 1978 Aug 31;274(5674):891–893. doi: 10.1038/274891a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasko D. D., Tomkinson A. E., Lindahl T. Mammalian DNA ligases. Biosynthesis and intracellular localization of DNA ligase I. J Biol Chem. 1990 Jul 25;265(21):12618–12622. [PubMed] [Google Scholar]
- Lindahl T., Edelman G. M. Polynucleotide ligase from myeloid and lymphoid tissues. Proc Natl Acad Sci U S A. 1968 Oct;61(2):680–687. doi: 10.1073/pnas.61.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Melendy T., Ray D. S. Novobiocin affinity purification of a mitochondrial type II topoisomerase from the trypanosomatid Crithidia fasciculata. J Biol Chem. 1989 Jan 25;264(3):1870–1876. [PubMed] [Google Scholar]
- Melendy T., Ray D. S. Purification and nuclear localization of a type I topoisomerase from Crithidia fasciculata. Mol Biochem Parasitol. 1987 Jun;24(2):215–225. doi: 10.1016/0166-6851(87)90108-3. [DOI] [PubMed] [Google Scholar]
- Merril C. R. Gel-staining techniques. Methods Enzymol. 1990;182:477–488. doi: 10.1016/0076-6879(90)82038-4. [DOI] [PubMed] [Google Scholar]
- Montecucco A., Fontana M., Focher F., Lestingi M., Spadari S., Ciarrocchi G. Specific inhibition of human DNA ligase adenylation by a distamycin derivative possessing antitumor activity. Nucleic Acids Res. 1991 Mar 11;19(5):1067–1072. doi: 10.1093/nar/19.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin B. A., Chase J. W. DNA ligase from Drosophila melanogaster embryos. Substrate specificity and mechanism of action. J Biol Chem. 1987 Oct 15;262(29):14105–14111. [PubMed] [Google Scholar]
- Rabin B. A., Hawley R. S., Chase J. W. DNA ligase from Drosophila melanogaster embryos. Purification and physical characterization. J Biol Chem. 1986 Aug 15;261(23):10637–10645. [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Chan Y. S., Kerr S. M. Transcriptional mapping and nucleotide sequence of a vaccinia virus gene encoding a polypeptide with extensive homology to DNA ligases. Nucleic Acids Res. 1989 Nov 25;17(22):9051–9062. doi: 10.1093/nar/17.22.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L. Early evolution and the origin of eukaryotes. Curr Opin Genet Dev. 1991 Dec;1(4):457–463. doi: 10.1016/s0959-437x(05)80192-3. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- Söderhäll S. DNA ligases during rat liver regeneration. Nature. 1976 Apr 15;260(5552):640–642. doi: 10.1038/260640a0. [DOI] [PubMed] [Google Scholar]
- Söderhäll S., Lindahl T. DNA ligases of eukaryotes. FEBS Lett. 1976 Aug 1;67(1):1–8. doi: 10.1016/0014-5793(76)80858-7. [DOI] [PubMed] [Google Scholar]
- Söderhäll S., Lindahl T. Mammalian DNA ligases. Serological evidence for two separate enzymes. J Biol Chem. 1975 Nov 10;250(21):8438–8444. [PubMed] [Google Scholar]
- Takahashi M., Senshu M. Two distinct DNA ligases from Drosophila melanogaster embryos. FEBS Lett. 1987 Mar 23;213(2):345–352. doi: 10.1016/0014-5793(87)81520-x. [DOI] [PubMed] [Google Scholar]
- Teraoka H., Tsukada K. Eukaryotic DNA ligase. Purification and properties of the enzyme from bovine thymus, and immunochemical studies of the enzyme from animal tissues. J Biol Chem. 1982 May 10;257(9):4758–4763. [PubMed] [Google Scholar]
- Tomkinson A. E., Lasko D. D., Daly G., Lindahl T. Mammalian DNA ligases. Catalytic domain and size of DNA ligase I. J Biol Chem. 1990 Jul 25;265(21):12611–12617. [PubMed] [Google Scholar]
- Tomkinson A. E., Roberts E., Daly G., Totty N. F., Lindahl T. Three distinct DNA ligases in mammalian cells. J Biol Chem. 1991 Nov 15;266(32):21728–21735. [PubMed] [Google Scholar]
- White J. H., Barker D. G., Nurse P., Johnston L. H. Periodic transcription as a means of regulating gene expression during the cell cycle: contrasting modes of expression of DNA ligase genes in budding and fission yeast. EMBO J. 1986 Jul;5(7):1705–1709. doi: 10.1002/j.1460-2075.1986.tb04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock D., Lane D. P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature. 1991 Jan 31;349(6308):429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- Yang S. W., Becker F. F., Chan J. Y. Fingerprinting of near-homogeneous DNA ligase I and II from human cells. Similarity of their AMP-binding domains. J Biol Chem. 1990 Oct 25;265(30):18130–18134. [PubMed] [Google Scholar]