Abstract
AIM
To study the effects of geranylgeranylacetone (GGA) on the expression of heat shock protein70 (HSP70) on retinal ganglion cells (RGC) in rats with chronic intraocular pressure (IOP) elevation.
METHODS
Seventy Wistars were divided into blank control group (10 rats), chronic hypertension group (30 rats) and GGA group (30 rats). Chronic hypertension was created by cauterizing the superficial scleral veins. 800mg/kg/d GGA was given by oral daily after cauterization. Immunohistochemistry was used respectively to observe the changes of expression of HSP70 in the model rats and GGA interference rats at different time points during the course of chronic IOP elevation.
RESULTS
The successful model was identified as the IOP over 40% of normal rats. The retinal thickness was significantly reduced in model group and model+GGA group compared with normal rats from 21 days through 28 days after cauterization (P<0.05), and that of model rats was obviously decreased in comparison with model+GGA rats (P<0.05). The number of ganglion cells was significantly decreased in model rats and model+GGA rats compared with normal rats from 21 days and 28 days. The stronger expression intensity (IOD) value was seen for HSP70 in the model+GGA rats by immunochemistry (P<0.01).
CONCLUSION
Systemic administration of GGA protects retina from chronic IOP elevation by regulating the expression of HSP70.
Keywords: retina, chronic hypertention, geranylgeran- ylacetone, heat shock protein70, rentinal ganglion cell
INTRODUCTION
Glaucoma, one of the world's leading causes of blindness, is characterized by progressive optic nerve damage with selective loss of retinal ganglion cells (RGC). It has been postulated that apoptosis, a highly regulated process of cell death, is the final common pathway for RGCs in glaucoma which is not yet known. Therefore, it is very important to investigate how these apoptosis-related genes are involved in the apoptosis of RGCs at different levels and how they are regulated, so that we can provide better treatment for patients. The HSP70 family, classified according to molecular mass (70kDa), comprises several members, such as the inducible, the constitutive, the mitochondrial, and the endoplasmic reticular forms. Intracellular expression of HSP70 has been demonstrated to protect cerebral neurons against heat shock, oxidative stress, apoptotic stimuli, excitotoxic insults[1],[2], and ischemia-like conditions[3]. Neurons of transgenic mice expressing HSP70[4] or those of rats injected with the herpes virus containing HSP70 genes[5] also have been shown to be resistant to ischemia and seizures, which suggests that HSP70 is essential for neuroprotection. GGA, is a unique antiulcer drug that protects gastric mucosa without affecting gastric acid or pepsin secretion. Its cytoprotective effects have been found to correlate with the expression of HSPs in gastric mucosal cells induced by the systemic administration of GGA[6]. GGA induces HSPs in numerous tissues including rat small intestine, liver, lung, kidney, and heart[7]. We investigate in the current study whether HSP70 is induced in rat RGCs with systemic administration of GGA, whether the induction of HSP70 by GGA enhances RGC survival of a rat glaucoma model. We performed this experiment to reveal the protection of glaucoma to some degree, and we look forward to applying our findings to clinical treatment.
MATERIALS AND METHODS
Materials
Seventy male Wistar rats, weighing between 200-300g were supplied by experiment animal department of China Medical University. The animals and experimental conditions followed laboratory animal regulations of State Science and Technology Commission. The animals were randomly divided into 3 groups, which were 10 in blank control group (20 eyes), 30 in chronic IOP elevated group (60 eyes), and 30 in chronic IOP elevated with GGA application group (60 eyes). Mouse anti-rat HSP70 (Santa Cruz Biotechnology, INC.), and SP kit (Fuzhou Maixin Biotechnology) were pure.
Methods
Rat model of chronic IOP elevation
The rats were anesthetized by intraperitoneal injection of 100g/L chloral hydrate 3mL/kg bulbar conjunctiva was cut and two superficial venous tributaries were burnt (signs of successful burn showed episclera venous blood flow disappeared on the distal end of the burnt point, distension and darkness of the vessels near comeoscleral limbus); bulbar conjunctiva was reset with TobraDex drops and pasted as eyedrop application. IOP was also measured with Tonopen XL, and the measurement time points were before the operation, half an hour after the operation, the 1st day, the 3rd day, the 7th day, the 14th day, the 21st day and the 28th day. IOP that 40% beyond preoperative value (9-16mmHg) meant the modeling was successful. To group with GGA application GGA (Tokyo, Japan) was given after modeling with 800mg/kg/d. The modeling procedure was all the same.
Immunohistochemistry
After sampling fixation, dehydration and paraffin imbedding were performed according to the instruction of the kit. Positive cells were those with yellow or brownish-yellow granules deposited in cytoplasm or nuclei. We selected 5 discontinued high power fields from each section to assess the expression intensity with etaMorph/BX51 microgram analytical system through data analysis of the determination of integrated A of positive cells.
Statistical Analysis
The analysis was done with SPSS 13.0. The data were expressed as the mean±SEM. Mean data among groups were compared with one-way ANOVA, and data between groups were compared with the unpaired Student's t -test. Statistical significance was set at for P<0.05.
RESULTS
RGC Change and Thickness of Nerve Fiber Layer
On the 21st day after modeling the retina became thinner and the number of RGCs decreased, however, the change of medication group was relatively mild. Among three groups at least two of them had distinctive difference according to the analysis by Levene homogeneity test for variance. Inter-group multiple comparison test indicated that, in terms of thickness comparison, compared with control group the variances of other two groups both had statistical significance, thickness comparison between model group and model+GGA group had statistical significance (P<0.05, Table 1).
Table 1. Retinal thickness of rats with chronic IOP elevation.
| Group | Thickness RGCL and NFL in respect time |
|
| 21d | 28d | |
| Control | 27.5±9.7 | 25.7±6.4 |
| Model | 17.4±5.8a,c | 16.6±7.1a,c |
| Model+GGA | 22.3±7.3a | 20.4±5.5a |
a P<0.05 vs control; c P<0.05 vs model+GGA
(mean±SEM, µm, n=10)
HSP70 Expression
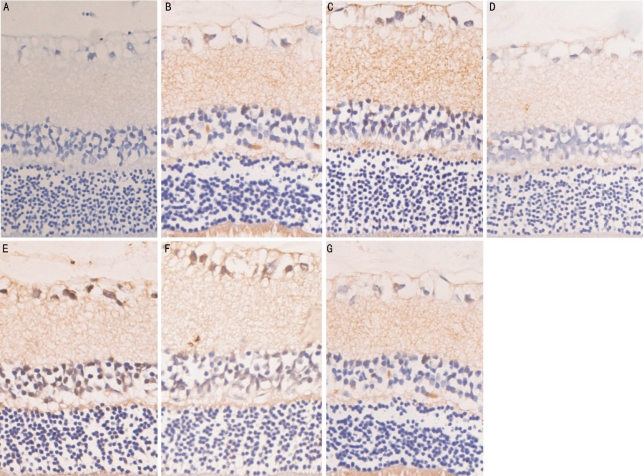
It is illustrated in Figure 1. Control group with only trace quantity of positive expression was merely detected in layer of ganglion cells in the retina of normal rats. HSP70 was expressed in the ganglion cell layer in ocular hypertension 3 days group. The positive immune cells for HSP70 seems to be reduced in retina of high intraocular pressure for 7 days group. In ocular hypertension for 21 days group, the expression of HSP70 in retina was significantly reduced. In GGA+3 days group of high IOP, the number of round syncytium big positive cells for HSP70 in ganglion cell layer began to increase. HSP70 was positively expressed in the ganglion cell layer in GGA+7 days group of high IOP. In GGA+ocular hypertension for 21 days, the positive ganglion cells for HSP70 reduce (Figure 1, Table 2).
Figure 1. HSP70 expression on retina of rats with chronic IOP elevation.
A: Control; B: 3 days after IOP elevation; C: 7 days after IOP elevation; D: 21 days after IOP elevation; E: GGA+3 days of high IOP; F: GGA+7 days of high IOP; G: GGA+ocular hypertension for 21 days
Table 2. HSP70 expression in rat retina.
| Group | IA |
|||||
| 1d | 3d | 7d | 14d | 21d | 28d | |
| Model | 31.7±4.7 | 26.5±3.7 | 16.8±1.8 | 16.0±2.8 | 14.5±1.0 | 15.0±0.5 |
| Model+GGA | 36.0±5.2 | 48.5±2.7b | 67.9±6.3b | 40.7±3.2b | 32.5±2.4b | 16.9±1.0 |
bP<0.01 vs 3 days
(mean±SEM, IA, n=10)
DISCUSSION
Beere et al [8] have proposed that HSP70 is an antiapoptotic chaperone protein that may interfere with multiple stages of the apoptotic pathway. These stages include suppression of c-Jun N-terminal kinase (JNK) activation,prevention of cytochrome c release, disruption of apoptosome formation, inhibition of apoptotic protease activating factor (Apaf)-1 oligomerization, and suppression of procaspase recruitment[8],[9]. More recently Ikeyama et al demonstrated that GGA induces a rapid accumulation of HSP70 mRNA and HAP70, suppresses hydrogen peroxide-and ethanol-induced phosphorylation of JNK, and interferes with caspase-9 and subsequent activation of caspase-3-like proteases in rat hepatocytes. It appears likely that HSP70 plays an important role in promoting the survival of RGC.
In this study, HSP70 expression was induced in rat RGCs by systemic administration of GGA, a heat shock protein inducer. We further demonstrated the protective effects of GGA on RGC survival in a rat glaucoma model. This is the first report to demonstrate that GGA induces HSP70 in retinal neurons in China, and provides evidence of a neuroprotective effect for RGCs in a rat glaucoma model. GGA is used clinically for the treatment of gastric ulcers and gastritis. There are several published reports that show GGA to be cytoprotective in other organs such as lung, heart, liver, and kidney. Our results indicate that the neuroprotective effects of GGA may be related to the expression of HSP70.
We administered GGA orally to the rats daily and demonstrated that there was an increased expression of HSP70 in RGCs after 3 days, with no observable side effects. And HSP70 is positively expressed in the ganglion cell layer after 7 days. The mechanism of HSP induction by GGA is not clearly understood, but it is likely that GGA activates HSF1, a transcription factor that stimulates synthesis of mRNA for HSP70[10]. In our study, with the application of GGA, the degree of retina thinning diminished compared with model group (P<0.05) was significantly inhibited; and the expression of HSP70 in retina of rat with chronic IOP elevation was positively induced. Immunohistochemical method showed that HSP70 IOD was induced and through statistical analysis the difference was significant (P<0.05). Our study confirms that the systemic administration of GGA positively induces the expression of HSP70 in RGCs and confers protection to RGCs in rats with chronic IOP elevation.
Footnotes
Foundation item: Scientific and Technological Research Founded Projects in Liaoning Province, China (No.2009225021)
REFERENCES
- 1.Caprioli J, Kitano S, Morgan JE. Hyperthermia and hypoxia increase tolerance of retinal ganglion cells to anoxia and excitotoxicity. Invest Ophthalmol Vis Sci. 1996;37(12):2376–2381. [PubMed] [Google Scholar]
- 2.Alavez S, Pedroza D, Moran J. Role of heat shock proteins in the effect of NMDA and KCI on cerebellar granule cells survival. Neurochem Res. 2000;25(3):341–347. doi: 10.1023/a:1007584802989. [DOI] [PubMed] [Google Scholar]
- 3.Lee JE, Yenari MA, Sun GH, Xu L, Emond MR, Cheng D, Steinberg GK, Giffard RG. Differential neuroprotection from human heat shock protein 70 overexpression in vitro and in vivo models of ischemia and ischemia-like conditions. Exp Neurol. 2001;170(1):129–139. doi: 10.1006/exnr.2000.7614. [DOI] [PubMed] [Google Scholar]
- 4.Plumier JC, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997;2(3):162–167. doi: 10.1379/1466-1268(1997)002<0162:tmethi>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yenari MA, Fink SL, Sun GH, Chang LK, Patel MK, Kunis DM, Onley D, Ho DY, Sapolsky RM, Steinberg GK. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy. Ann Neurol. 1998;44(4):584–591. doi: 10.1002/ana.410440403. [DOI] [PubMed] [Google Scholar]
- 6.Rokutan K, Teshima S, Kawai T, Kawahara T, Kusumoro K, Mizushima T, Kishi K. Geranylgeranylacetone stimulates mucin synthesis in cultured guinea pig gastric pit cells by inducing a neuronal nitric oxide synthase. J Gastroenterol. 2000;35(9):673–681. doi: 10.1007/s005350070046. [DOI] [PubMed] [Google Scholar]
- 7.Tsuruma T, Yagihashi A, Hirata K, Araya J, Katsuramaki T, Tarumi K, Yanai Y, Watanabe N. Induction of heat shock protein-70 (hsp-70) by intraarterial administration of geranylgeranylacetone. Transplant Proc. 2000;32(7):1631–1633. doi: 10.1016/s0041-1345(00)01441-x. [DOI] [PubMed] [Google Scholar]
- 8.Beere HM, Green DR. Stress management: heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001;11(1):6–10. doi: 10.1016/s0962-8924(00)01874-2. [DOI] [PubMed] [Google Scholar]
- 9.Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2(8):476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- 10.Hirakawa T, Rokutan K, Nikawa T, Kishi K. Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology. 1996;111(2):345–357. doi: 10.1053/gast.1996.v111.pm8690199. [DOI] [PubMed] [Google Scholar]