Abstract
Retinoblastoma (Rb) is the most common eye cancer in children and it can be inherited. Rb is quite rare and originators from the neural retina with a significant genetic component in etiology, which occurs in approximately 1 in every 20 0000 births. In children with the heritable genetic form of Rb, there is a mutation on chromosome 13, called the retinoblastoma 1 (Rb1) gene. Early diagnosis and intervention is critical to the successful treatment of the Rb. The Rb1 gene is the first cloned tumor suppressor gene. As a negative regulator of the cell cycle, Rb1 gene could maintain a balance between cell growth and development through binding to transcription factors and regulating the expression of genes involved in cell proliferation and differentiation. Thus, it is involved in cell cycle, cell senescence, growth arrest, apoptosis and differentiation. We summarized the recent advances on the epidemiology and Rb1 gene of Rb in this review.
Keywords: retinoblastoma, epidemiology, Rb1 gene, structure, expression, function
INTRODUCTION
Retinoblastoma (Rb) was reported firstly by Benedict[1] and it is a childhood cancer arising from immature retinal cells in one or both eyes and can strike from the time a child is in the womb up to 5 years of age, and accounts for about 3% of the cancers in children under the age of 15. This cancer is a rapidly developing cancer which develops in the cells of retina, the light detecting tissue of the eye. In the developed world, Rb has one of the best cure rates of all childhood cancers (95%-98%), with more than nine out of every ten sufferers surviving into adulthood[2],[3]. Rb can occur in two forms: 1) A heritable form where there are often tumors in both eyes (bilateral) or sometimes only in one eye, and 2) A non-heritable form where there is a tumor in only one eye (unilateral). Approximately 55% of children with Rb have the non-genetic form. If there is no history of the disease within the family, the disease is labeled “sporadic”, but this does not necessarily indicate that it is the non-genetic form. In about two thirds of cases, only one eye is affected (unilateral retinoblastoma); in the other third, the Rb develops in both eyes (bilateral retinoblastoma). The number and size of Rb on each eye may vary. In certain cases, the pineal gland is also affected (trilateral Rb). The position, size and quantity of tumors are considered when choosing the type of treatment for Rb[2]-[4].
The most common and obvious sign of Rb is an abnormal appearance of the pupil, leukocoria. Other less common and less specific signs and symptoms are: deterioration of vision, a red and irritated eye, faltering growth or delayed development. Some children with retinoblastoma can develop a squint[5],[6], commonly referred to as “cross-eyed” or “wall-eyed” (strabismus). Rb presents with advanced disease in developing countries and eye enlargement is a common finding. Depending on the position of the tumors, they may be visible during a simple eye exam using an ophthalmoscope to look through the pupil. A positive diagnosis is usually made only with an examination under anesthetic. A white eye reflection is not always a positive indication of Rb and can be caused by light being reflected badly or by other conditions such as Coats's Disease. In a photograph, the photographic fault red eye may be a sign of Rb, if in the photograph it is in one eye and not in the other eye[3],[7].
Early diagnosis and intervention is critical to the successful treatment of the Rb[4]. Rb is a very treatable cancer, and curable if caught early enough. However, 87% of the children stricken with this disease worldwide die, mostly in developing countries. In developed countries, 97% of those who do live have moderate to severe visual impairment or the child may loose one or both eyes. Treatment of Rb varies from country to country[9]. The first priority is to preserve the life of the child, then to preserve the vision and thirdly to minimize any complications or side effects of the treatment. The exact course of treatment will depend on the individual case and will be decided by the ophthalmologist in discussion with the paediatric oncologist[10],[11].
Options for Rb treatment include chemotherapy (which can be administered locally through a thin catheter that is threaded through the groin through the aorta and the neck into the optic vessels), cryotherapy, radioactive plaques, laser therapy, external beam radiotherapy and surgery[6]-[8]. Any combinations of these treatments may be adopted. In recent years, there has been an effort to find alternatives to enucleation and radiation therapy[10]. This retrospective review confirms a curable strategy based on local treatment and conventional plus high-dose chemotherapy. Patients with CNS involvement remain incurable[12].
EPIDEMIOLOGY AND ETIOLOGY
Epidemiology
Retinoblastoma (Rb) is the most common eye cancer in children and it can be inherited. Although the most common eye cancer in children, retinoblastoma is quite rare and occurs in approximately 1 in every 20 0000 births. In the UK, around 40 to 50 new cases are diagnosed each year. The therapeutic goal for retinoblastoma is early detection to maximize the visual outcome and the quality of life of the affected child. Fortunately, the survival rate for affected children is 96%. Rb can spread or metastasize from the eye to the brain, the central nervous system (brain and spinal cord), and the bones. In these cases, chemotherapy is prescribed by a pediatric oncologist and is administered through the peripheral blood vessels or into the brain for months to years after initial diagnosis of metastatic disease[7],[12],[13].
Most children are diagnosed before the age of five years old. In the UK, bilateral cases usually present within the first year with the average age at diagnosis being 9 months. Diagnosis of unilateral cases peaks between 24 and 30 months[2],[3]. A systematic review of publications from least developed countries (LDCs) was performed by Canturk et al[13]. Articles were from multiple databases and written in seven languages. Results were correlated with socioeconomic indicators. Lower-income countries (LICs) and middle-income countries (MICs) were included in our analyses. An analysis of 164 publications including 14 800 patients from 48 LDCs was performed. Twenty-six per cent of the papers were written in languages other than English. Estimated survival in LICs was 40%; in lower MICs 77% and in upper MICs 79% (P=0.001). Significant differences were also found in the occurrence of metastasis: in LICs it was 32%; in lower MICs 12% and in upper MICs 9.5% (P=0.04). On multivariate analysis, physician density and human development index were significantly associated with the survival and metastasis. Maternal mortality rate and per capita health expenditure were significantly associated with treatment refusal. The results showed that it is not always available in English or in major databases. Indicators of socioeconomic development and maternal and infant health were related with the outcome.
The long-term cause-specific mortality among 998 Dutch retinoblastoma survivors, diagnosed from 1862 to 2005, according to follow-up time, treatment and heredity was examined. After a median follow-up of 30.8 years, retinoblastoma patients revealed an emerging excess risk of mortality in hereditary retinoblastoma survivors. This implies that lifelong follow-up is needed, whereas at the same time, patients and their physicians must be alerted to the increased second malignancy risks[14]. Although screening for familial retinoblastoma has been shown to be beneficial we suspected that such screening programs may be less than optimal in developing countries. The patients with familial Rb are less likely to be diagnosed by screening in developing countries and had higher morbidity and mortality caused by recurrent extraocular Rb[15]. The current survival rates for retinoblastoma exceed 90%; individual visual outcome and survival are dependent upon early detection and prompt referral. In addition in research of survivors and families, it is clear that advanced and practiced nurses play a key role in early detection and maintaining the current survival rate[4],[16].
Yang et al[17] described the survival outcomes and prognostic factors of patients with a retinoblastoma receiving primary treatment at their hospital over the last 20 years. A retrospective series study of 30 retinoblastoma cases treated from 1987 to 2006 was conducted from a review of medical records and histopathological sections. Variables, including age at onset, laterality, treatment modalities, treatment delay, and optic nerve invasion, were analyzed to elucidate the prognostic factors associated with cumulative survival. The results indicated that the tumor invasion of the optic nerve is the most significant prognostic factor for surviving of the retinoblastoma patients. Delayed treatment increases the risk of optic nerve invasion. Parental awareness of both the risk of this consequence and the significance of early treatment is vital to achieve improved survival rates. Survivors of hereditary retinoblastoma have an elevated risk of developing second malignancies, but data on the risk in middle-aged retinoblastoma survivors (i.e., those with more than 40 years of follow-up) are scarce. Life long follow-up studies are needed to evaluate the full spectrum of subsequent cancer risk in hereditary retinoblastoma survivors[18].
Global initiatives by nongovernmental organizations such as the International Network for Cancer Treatment and Research, Orbis International, and the International Agency for Prevention of Blindness were presented. Treatment of retinoblastoma in developing countries remains a challenge; however, it is possible to coordinate efforts at multiple levels, including public administrations and nonprofit organizations, to improve the diagnosis and treatment of Rb and to improve the outcome for these children[19]. The mean age-adjusted incidence rate of retinoblastoma of 11.8 cases per million children aged 0-4 years in the USA is similar to the rates reported from European countries. Over the last 30 years; there has been a gradual improvement in 5-year survival of children with retinoblastoma in the USA[20]-[22]. At least 9 out of every 10 children with retinoblastoma are cured. Following treatment, the eye specialist should frequently examine the child's eye under anaesthetic to check that the cancer has not come back. Follow-up is usually in a clinic for childhood cancers (a paediatric oncology clinic)[23],[24].
Etiology
Rb is an uncommon childhood tumor of the neural retina with a significant genetic component in its etiology. In children with the heritable genetic form of retinoblastoma there is a mutation on chromosome 13, called the Rb1 gene. The genetic codes found in chromosomes control the way in which cells grow and develop within the body[5]. If a portion of the code is missing or altered (mutation), a cancer may develop. The defective Rb1 gene can be inherited from either parent. In some children, however, the mutation occurs in the early stages of fetal development. It is unknown what causes the gene abnormality; and it is most likely to be a random mistake during the copy process which occurs when a cell divides. Inherited forms of Rb are more likely to be bilateral. In addition, they may be associated with pinealoblastoma (also known as trilateral retinoblastoma) with a dismal outcome. The genetic codes found in chromosomes control the way in which cells grow and develop within the body. Several methods have been developed to detect the Rb1 gene mutations and attempts have been made to correlate gene mutations to the stage at presentation[3],[5],[7].
A small proportion of patients have a deletion in chromosome 13 encompassing band 13q14, an observation which permitted the assignment of the Rb1 locus to this region. About 20% of Rb tumors exhibit microscopic deletions of band 13q14 or monosomy 13. Trisomy 1q and i(6p) have also been reported in a high percentage of tumors. The chromosome complements from direct preparations of 10 Rb tumors derived from seven patients were analyzed. Modal chromosome numbers ranged from 45 to 48, and occasional duplications of the genomes were noted. In general, the tumors were chromosomally stable, although karyotypic evolution and random chromosome loss were encountered. Consistent abnormalities included trisomy 1q, i(6p), 6q-, and del (13)(q12→14). It is reported that one patient with bilateral Rb had three tumor clones (two in one eye and one in the other) with chromosome abnormalities unrelated in origin. A second patient with unilateral Rb had two tumor clones with chromosome abnormalities again unrelated in origin. These two patients provide some of the first cytogenetic evidence for the multifocal origin of primary Rb. In the untreated tumor of a third patient, a homogeneously staining region (HSR) was detected in 1p32, indicating gene amplification in vivo; previously, an HSR at this site has been reported in the established Rb cell line Y79[25].
MacCarthy et al[22] examined the association between paternal occupational exposures and Rb using birth registration data for cases from the National Registry of Childhood Tumours (NRCT) and controls from the general population of Great Britain during 1962-1999, and 1318 controls matched on sex, date of birth and birth registration sub-district. The exposure to oil mists in metal work (a subset of metal workers) is not directly comparable to those for metal working previously reported in the literature. Overall, these findings do not support the hypothesis that paternal occupational exposure is an important aetiological factor for Rb; however, the study has low power and other methodological limitations. To identify the types of non-ocular tumor occurring in Rb survivors and to produce estimates of risk for these tumors, MacCarthy et al[23] carried out a cohort study that included 1927 cases of Rb diagnosed in Great Britain between 1951 and 2004. All cases were ascertained through the National Registry of Childhood Tumors and followed up for the occurrence of non-ocular tumors using the routine notification system based on the National Health Service Central Registers in Britain. There is a high risk of non-ocular tumors occurring in survivors of heritable Rb. These results have important implications for the clinical follow-up and counseling of survivors. Histopathologic risk factors are present in a significant proportion of patients enucleated for retinoblastoma and have identifiable clinical predictors[21]-[24].
RETINOBLASTOMA GENE
Retinoblastoma 1 (Rb1) gene is the first cloned tumor suppressor gene. The protein encoded by this gene is a negative regulator of the cell cycle. The encoded protein also stabilizes constitutive heterochromatin to maintain the overall chromatin structure. The active, hypophosphorylated form of the protein binds transcription factor E2F1. Defect in this gene is a cause of childhood cancer Rb, bladder cancer, and osteogenic sarcoma[2],[10]. Rb is a malignant tumor that originates from developing retina. The diagnosis should be based on clinical signs and symptoms and is usually made in children under the age of five years. Mutations in both alleles of the Rb1 gene are a prerequisite for this tumor to develop. In most patients with sporadic unilateral Rb, both Rb1 gene mutations occur in somatic cells and are not passed over to offspring (nonhereditary Rb)[7]-[9]. Almost all patients with sporadic bilateral and virtually all patients with familial Rb are heterozygous for Rb1 gene mutations that cause predisposition to Rb (hereditary Rb). In families, Rb predisposition is transmitted as an autosomal dominant trait (familial Rb). In addition to Rb, patients with hereditary disease also have an increased risk of tumors outside the eye (second cancer). This risk is enhanced in patients who have received external beam radiotherapy. Analysis of genotype-phenotype associations has shown that the mean number of tumor foci that develop in carriers of mutant Rb1 alleles is variable depending on which functions of the normal allele are retained and to what extent. Moreover, phenotypic expression of hereditary retinoblastoma is subject to genetic modification. Identification of the genetic factors that underlie these effects will not only help to arrive at a more precise prognosis, but may also point to mechanisms that can be used to reduce the risk of tumor development[26]-[30].
Rb1 Gene Structure
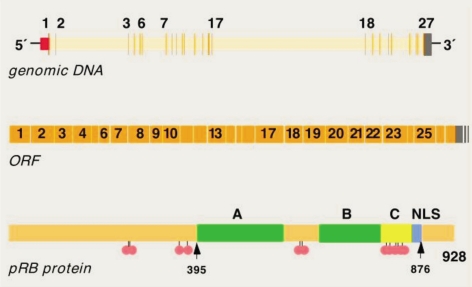
Rb1 gene composition contains 27 exons and 26 introns. Human Rb1 gene (GenBank accession number: NM_000321) located in the long arm of chromosome 13 (13q14.2), DNA length of 178 143 bp, mRNA length of 4 772 bp, CDS length of 2 787 bp, encoding 928 amino acids. Rb1 gene in mice (GenBank accession number: NM_009029) located in chromosome 14 (14D3), DNA length of 130 238 bp, mRNA length 4 591 bp, CDS length of 2 766 bp, encoding 921 amino acids. Chicken Rb1 gene (GenBank accession number: NM_204419) located in chromosome 1, DNA sequence of length 78 217 bp, mRNA length of 4 464 bp, CDS length of 2 766 bp, encoding 921 amino acids. Rb1 gene encoding a protein pRb is a nuclear phosphoprotein in the molecular mass of about 104-110 kDa. pRb contains three domains: N-terminal domain, A/B pocket domain (A/B pocket domain) and C-terminal domain. N-terminal domains of cellular proteins and viral proteins exists binding sites[2],[31]-[34]. Graphical presentations (Figure 1) show Rb1 mutation database for structure of Rb1 gene.
Figure 1. Rb1 gene structure.
Rb1 Gene Function
Rb1 can inhibit a variety of tumors and play an indispensable role in cancers, such as retinoblastoma, small cell lung cancer, osteosarcoma, pancreatic cancer and breast cancer. Numerous studies show that, Rb1 tumor suppressor and its role in cell cycle, cell differentiation, cell aging, apoptosis and growth suppression is closely related to the regulation[35]-[37]. Rb1 is a negative regulator of cell cycle. The regulation of cell is cycle and mainly by E2F combining, thereby preventing the cells through the G1-S checkpoint, the cell cycle arrest. Rb1 has been considered as the key cell proliferation and differentiation regulatory factors, and pRb with tissue-specific transcription factors regulate differentiation specific gene expression in different tissues of various key players in the process of cell differentiation[2],[3],[7].
Key regulator of Rb1 in cell division acts as a tumor suppressor, and also acts as a transcription repressor of E2F1 target genes. The underphosphorylated, active form of Rb1 interacts with E2F1 and represses its transcription activity, leading to cell cycle arrest. Rb1 directly involved in heterochromatin formation by maintaining overall chromatin structure and, in particular, that of constitutive heterochromatin by stabilizing histone methylation. There are recruits and targets histone methyltransferases SUV39H1, SUV420H1 and SUV420H2, leading to epigenetic transcriptional repression, which control histone H4 “Lys-20” trimethylation, or inhibits the intrinsic kinase activity of TAF1[4]-[6]. Transcriptional repression of SMARCA4/BRG1 by recruiting a histone deacetylase (HDAC) is complex to the c-fos promoter. In resting neurons, transcription of the c-fos promoter is inhibited by BRG1-dependent recruitment of a phospho-Rb1-HDAC1 repressor complex. Upon calcium influx, Rb1 is dephosphorylated by calcineurin, which leads to release the repressor complex (by similarity). In case of viral infections, interactions with SV40 large T antigen, HPV E7 protein or adenovirus E1A protein induce the disassembly of Rb1-E2F1 complex thereby disrupting activity of Rb1[2],[7],[38]-[40].
Retinoblastoma-associated Protein
Rb1 can express in a variety of normal cells, without the cell cycle change significantly, which may regulate the expression of a variety of cell proliferation and differentiation. Rb1 expressions in different tissues and different developmental stages have some differences[2]-[5],[7]. 1) Size: 928 amino acids, 106 159 Da; and 2) Subunit interacts with ATAD5 (by similarity)[41]-[43]. The hypophosphorylated form interacts with and sequesters the E2F1 transcription factor which interacts with heterodimeric E2F/DP transcription factor complexes containing TFDP1 and either E2F1, E2F3, E2F4 or E2F5, or TFDP2 and E2F4. The unphosphorylated form interacts with EID1, ARID3B, KDM5A, SUV39H1, MJD2A/JHDM3A and THOC1. They interact with the N-terminal domain of TAF1, and with AATF, DNMT1, LIN9, LMNA, SUV420H1, SUV420H2, PELP1 and TMPO-alpha. It may interact with NDC80, and with GRIP1 and UBR4. Concurrently, they interact with ARID4A and KDM5B, and with E4F1 and LIMD1, with SMARCA4/BRG1 and HDAC1 (by similarity), with adenovirus E1A protein, HPV E7 protein and SV40 large T antigen, with PSMA3 and USP4. 3) Interacting proteins for Rb1 (Table 1)[44]-[47]. The retinoblastoma susceptibility protein, Rb, has a key role in regulating cell-cycle progression via interactions involving the central “pocket” and C-terminal regions. While the N-terminal domain of Rb is dispensable for this function, it is nonetheless strongly conserved, and harbors missense mutations are found in hereditary retinoblastoma, indicating that disruption of its function is ontogeny. The crystal structure of the Rb N-terminal domain (RbN) reveals a globular entity formed by two rigidly connected cyclin-like folds. The similarity of RbN to the A and B boxes of the Rb pocket domain suggests that Rb evolved through domain duplication. Structural and functional analysis provides insight into oncogenicity of mutations in RbN and identifies a unique phosphorylation-regulated site of protein interaction. Additionally, this analysis suggests a coherent conformation for the Rb holoprotein in which RbN and pocket domains directly interact, and which can be modulated through ligand binding and possibly Rb phosphorylation[41]-[43].
Table 1. Interacting proteins for Rb1[44]-[47].
| Genecard | Interaction details |
| E2F1 | EBI-491274, EBI-448924, MINT-1777462, MINT-4793606, MINT-1777305, MINT-4793665, MINT-73329, MINT-4793592, STRING: ENSP00000345571 |
| HDAC1 | EBI-491274,EBI-301834,MINT-73395,MINT-6628404, MINT-77956, STRING: ENSP00000362649 |
| E2F2 | MINT-4793621, MINT-4793646, STRING: ENSP00000355249 |
| MDM2 | MINT-1776635, STRING: ENSP00000258149 |
| TAF1 | EBI-491274,EBI-491289, STRING: ENSP00000276072 |
SUMMARY
Retinoblastoma (Rb) is a malignant tumor that originates from developing retina. Rb can occur in one eye (unilateral) or in both eyes (bilateral). Rb is usually confined to the eye and has not spread to other tissues. The present challenge for those who treat Rb is to prevent blindness and other serious effects of treatment that reduce the life span or the quality of life after treatment. In about 40% of the cases, Rb is hereditary, or germline[2],[42]-[45]. The genetic locus responsible for a predisposition to Rb is located within the q14 band of chromosome 13. Patients with Rb, particularly the hereditary type, have an increased frequency of second malignancies. Diagnosis should be based on clinical signs and symptoms and is usually made in children under the age of five years. Mutations in both alleles of the Rb1 gene are a prerequisite for this tumor to develop. Table 2 summarizes the epidemiology and Rb1 gene of Rb[2],[3],[7],[46]-[48].
Table 2. Epidemiology and Rb1 gene of retinoblastoma[2],[3],[7],[46]-[48].
| Incidence, per 100,000 | 0.5 (up to 5 years of age) |
| Prevalence, per 100,000 | 1.5 (under the age of 15) |
| Etiology | Significant genetic component |
| Genetic locus | q14 band of chromosome 13 |
| Age of onset | The womb up to 15 years of age |
| Sex | No differences |
| Race | No differences |
| The best cure rates | 95%-98% in the developed world |
| Rb1 gene composition | 27 exons and 26 introns |
| GeneCard | E2F1, HDAC1, E2F2, MDM2, TAF1 |
In most patients with sporadic unilateral Rb, both Rb1 gene mutations occur in somatic cells and are not passed over to offspring (nonhereditary Rb). Almost all patients with sporadic Rb are bilateral and virtually all patients with familial Rb are heterozygous for Rb1 gene mutations that cause predisposition to Rb (hereditary Rb). In families, Rb predisposition is transmitted as an autonomic dominant trait (familial Rb)[2],[7],[14]. In addition to Rb, patients with hereditary disease also have an increased risk of tumors outside the eye (second cancer). This risk is enhanced in patients who have received external beam radiotherapy. Analysis of genotype-phenotype associations has shown that the mean number of tumor foci that develop in carriers of mutant Rb1 alleles is variable depending on which functions of the normal allele are retained and to what extent. Moreover, phenotypic expression of hereditary retinoblastoma is subject to genetic modification. Identification of the genetic factors that underlie these effects will not only help to arrive at a more precise prognosis but may also point to mechanisms that can be used to reduce the risk of tumor development.
REFERENCES
- 1.Benedict WL. Homologous retinoblastoma in identical twins. Trans Am Ophthalmol Soc. 1929;27:173–176. [PMC free article] [PubMed] [Google Scholar]
- 2.Lohmann D. Retinoblastoma. Adv Exp Med Biol. 2010;685:220–227. doi: 10.1007/978-1-4419-6448-9_21. [DOI] [PubMed] [Google Scholar]
- 3.Dimaras H, Dimba EA, Gallie BL. Challenging the global retinoblastoma survival disparity through a collaborative research effort. Br J Ophthalmol. 2010;94(11):1415–1416. doi: 10.1136/bjo.2009.174136. [DOI] [PubMed] [Google Scholar]
- 4.Canty CA. Retinoblastoma: an overview for advanced practice nurses. J Am Acad Nurse Pract. 2009;21(3):149–155. doi: 10.1111/j.1745-7599.2008.00378.x. [DOI] [PubMed] [Google Scholar]
- 5.Du W, Pogoriler J. Retinoblastoma family genes. Oncogene. 2006;25(38):5190–5200. doi: 10.1038/sj.onc.1209651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields CL, Shields JA. Diagnosis and management of retinoblastoma. Cancer Control. 2004;11(5):317–327. doi: 10.1177/107327480401100506. [DOI] [PubMed] [Google Scholar]
- 7.Chintagumpala M, Chevez-Barrios P, Paysse EA, Plon SE, Hurwitz R. Retinoblastoma: review of current management. Oncologist. 2007;12(10):1237–1246. doi: 10.1634/theoncologist.12-10-1237. [DOI] [PubMed] [Google Scholar]
- 8.Melamud A, Palekar R, Singh A. Retinoblastoma. Am Fam Physician. 2006;73(6):1039–1044. [PubMed] [Google Scholar]
- 9.Sovinz P, Urban C, Lackner H, Benesch M, Langmann G. Retinoblastoma: a proposal for a multimodal treatment concept for intraocular retinoblastoma in Austria. Wien Klin Wochenschr. 2006;118(1-2):22–30. doi: 10.1007/s00508-005-0503-z. [DOI] [PubMed] [Google Scholar]
- 10.Parsam VL, Kannabiran C, Honavar S, Vemuganti GK, Ali MJ. A comprehensive, sensitive and economical approach for the detection of mutations in the Rb1 gene in retinoblastoma. J Genet. 2009;88(4):517–527. doi: 10.1007/s12041-009-0069-z. [DOI] [PubMed] [Google Scholar]
- 11.Arora RS, Eden TO, Kapoor G. Epidemiology of childhood cancer in India. Indian J Cancer. 2009;46(4):264–273. doi: 10.4103/0019-509X.55546. [DOI] [PubMed] [Google Scholar]
- 12.Cozza R, De Ioris MA, Ilari I, Devito R, Fidani P, De Sio L, Demelas F, Romanzo A, Donfrancesco A. Metastatic retinoblastoma: single institution experience over two decades. Br J Ophthalmol. 2009;93(9):1163–1166. doi: 10.1136/bjo.2008.148932. [DOI] [PubMed] [Google Scholar]
- 13.Canturk S, Qaddoumi I, Khetan V, Ma Z, Furmanchuk A, Antoneli CB, Sultan I, Kebudi R, Sharma T, Rodriguez-Galindo C, Abramson DH, Chantada GL. Survival of retinoblastoma in less-developed countries impact of socioeconomic and health-related indicators. Br J Ophthalmol. 2010;94(11):1432–1436. doi: 10.1136/bjo.2009.168062. [DOI] [PubMed] [Google Scholar]
- 14.Marees T, van Leeuwen FE, de Boer MR, Imhof SM, Ringens PJ, Moll AC. Cancer mortality in long-term survivors of retinoblastoma. Eur J Cancer. 2009;45(18):3245–3253. doi: 10.1016/j.ejca.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Chantada GL, Dunkel IJ, Qaddoumi I, Antoneli CB, Totah A, Canturk S, Nawaiseh I, Fandiño A, Pífano I, Peksayar G, Ribeiro KB, Abramson DH. Familial retinoblastoma in developing countries. Pediatr Blood Cancer. 2009;53(3):338–342. doi: 10.1002/pbc.21970. [DOI] [PubMed] [Google Scholar]
- 16.Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8(9):671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang IH, Kuo HK, Chen YJ, Lee JJ, Lin SA. Review of 20 years' clinical experience with retinoblastomas in southern Taiwan. Changgung Med J. 2008;31(5):484–491. [PubMed] [Google Scholar]
- 18.Marees T, Moll AC, Imhof SM, de Boer MR, Ringens PJ, van Leeuwen FE. Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer Inst. 2008;100(24):1771–1779. doi: 10.1093/jnci/djn394. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Galindo C, Wilson MW, Chantada G, Fu L, Qaddoumi I, Antoneli C, Leal-Leal C, Sharma T, Barnoya M, Epelman S, Pizzarello L, Kane JR, Barfield R, Merchant TE, Robison LL, Murphree AL, Chevez-Barrios P, Dyer MA, O'Brien J, Ribeiro RC, Hungerford J, Helveston EM, Haik BG, Wilimas J. Retinoblastoma: one world, one vision. Pediatrics. 2008;122(3):e763–e770. doi: 10.1542/peds.2008-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiss S, Leiderman YI, Mukai S. Diagnosis, classification, and treatment of retinoblastoma. Int Ophthalmol Clin. 2008;48(2):135–147. doi: 10.1097/IIO.0b013e3181693670. [DOI] [PubMed] [Google Scholar]
- 21.Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA:1975-2004. Br J Ophthalmol. 2009;93(1):21–23. doi: 10.1136/bjo.2008.138750. [DOI] [PubMed] [Google Scholar]
- 22.MacCarthy A, Bunch KJ, Fear NT, King JC, Vincent TJ, Murphy MF. Paternal occupation and retinoblastoma: a case-control study based on data for Great Britain 1962-1999. Occup Environ Med. 2009;66(10):644–649. doi: 10.1136/oem.2007.037218. [DOI] [PubMed] [Google Scholar]
- 23.MacCarthy A, Bayne AM, Draper GJ, Eatock EM, Kroll ME, Stiller CA, Vincent TJ, Hawkins MM, Jenkinson HC, Kingston JE, Neale R, Murphy MF. Non-ocular tumours following retinoblastoma in Great Britain 1951 to 2004. Br J Ophthalmol. 2009;93(9):1159–1162. doi: 10.1136/bjo.2008.146035. [DOI] [PubMed] [Google Scholar]
- 24.Gupta R, Vemuganti GK, Reddy VA, Honavar SG. Histopathologic risk factors in retinoblastoma in India. Arch Pathol Lab Med. 2009;133(8):1210–1214. doi: 10.5858/133.8.1210. [DOI] [PubMed] [Google Scholar]
- 25.Chaum E, Ellsworth RM, Abramson DH, Haik BG, Kitchin FD, Chaganti RS. Cytogenetic analysis of retinoblastoma: evidence for multifocal origin and in vivo gene amplification. Cytogenet Cell Genet. 1984;38(2):82–91. doi: 10.1159/000132037. [DOI] [PubMed] [Google Scholar]
- 26.van Harn T, Foijer F, van Vugt M, Banerjee R, Yang F, Oostra A, Joenje H, te Riele H. Loss of Rb proteins causes genomic instability in the absence of mitogenic signaling. Genes Dev. 2010;24(13):1377–1388. doi: 10.1101/gad.580710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buiting K, Kanber D, Horsthemke B, Lohmann D. Imprinting of Rb1 (the new kid on the block) Brief Funct Genomics. 2010;9(4):347–353. doi: 10.1093/bfgp/elq014. [DOI] [PubMed] [Google Scholar]
- 28.Vance KW, Shaw HM, Rodriguez M, Ott S, Goding CR. The retinoblastoma protein modulates Tbx2 functional specificity. Mol Biol Cell. 2010;21(15):2770–2779. doi: 10.1091/mbc.E09-12-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berge EO, Knappskog S, Geisler S, Staalesen V, Pacal M, Børresen-Dale AL, Puntervoll P, Lillehaug JR, Lønning PE. Identification and characterization of retinoblastoma gene mutations disturbing apoptosis in human breast cancers. Mol Cancer. 2010;9:173. doi: 10.1186/1476-4598-9-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajasekaran R, Sethumadhavan R. Exploring the structural and functional effect of pRB by significant nsSNP in the coding region of Rb1 gene causing retinoblastoma. Sci China Life Sci. 2010;53(2):234–240. doi: 10.1007/s11427-010-0039-y. [DOI] [PubMed] [Google Scholar]
- 31.Dimaras H, Rushlow D, Halliday W, Doyle JJ, Babyn P, Abella EM, Williams J, Héon E, Gallie BL, Chan HS. Using Rb1 mutations to assess minimal residual disease in metastatic retinoblastoma. Transl Res. 2010;156(2):91–97. doi: 10.1016/j.trsl.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Sábado Alvarez C. Molecular biology of retinoblastoma. Clin Transl Oncol. 2008;10(7):389–394. doi: 10.1007/s12094-008-0220-y. [DOI] [PubMed] [Google Scholar]
- 33.Ganguly A, Shields CL. Differential gene expression profile of retinoblastoma compared to normal retina. Mol Vis. 2010;16:1292–1303. [PMC free article] [PubMed] [Google Scholar]
- 34.Macleod KF. The RB tumor suppressor: a gatekeeper to hormone independence in prostate cancer? J Clin Invest. 2010;120(12):4179–4182. doi: 10.1172/JCI45406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim Z, Quah BL. Unilateral retinoblastoma in an eye with Peters anomaly. J AAPOS. 2010;14(2):184–186. doi: 10.1016/j.jaapos.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Kanber D, Berulava T, Ammerpohl O, Mitter D, Richter J, Siebert R, Horsthemke B, Lohmann D, Buiting K. The human retinoblastoma gene is imprinted. PLoS Genet. 2009;5(12):e1000790. doi: 10.1371/journal.pgen.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson PF, Nagasawa H, Fitzek MM, Little JB, Bedford JS. G2-phase chromosomal radiosensitivity of primary fibroblasts from hereditary retinoblastoma family members and some apparently normal controls. Radiat Res. 2010;173(1):62–70. doi: 10.1667/RR1943.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CY, Xu CM, Du ZF, Chen XL, Ren GL, Zhang XN. A c.1363C>T (p.R455X) nonsense mutation of Rb1 gene in a southern Chinese retinoblastoma pedigree. Genet Test Mol Biomarkers. 2010;14(2):193–196. doi: 10.1089/gtmb.2009.0162. [DOI] [PubMed] [Google Scholar]
- 39.Parsam VL, Kannabiran C, Honavar S, Vemuganti GK, Ali MJ. A comprehensive, sensitive and economical approach for the detection of mutations in the Rb1 gene in retinoblastoma. J Genet. 2009;88(4):517–527. doi: 10.1007/s12041-009-0069-z. [DOI] [PubMed] [Google Scholar]
- 40.Xiao H, Zhang X, Goodrich DW. Construction of a dual affinity tagged allele of the Rb1 tumor suppressor gene in the mouse. Genesis. 2010;48(2):121–126. doi: 10.1002/dvg.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassler M, Singh S, Yue WW, Luczynski M, Lakbir R, Sanchez-Sanchez F, Bader T, Pearl LH, Mittnacht S. Crystal structure of the retinoblastoma protein N domain provides insight into tumor suppression, ligand interaction, and holoprotein architecture. Mol Cell. 2007;28(3):371–385. doi: 10.1016/j.molcel.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schüler A, Weber S, Neuhäuser M, Jurklies C, Lehnert T, Heimann H, Rudolph G, Jöckel KH, Bornfeld N, Lohmann DR. Age at diagnosis of isolated unilateral retinoblastoma does not distinguish patients with and without a constitutional Rb1 gene mutation but is influenced by a parent-of-origin effect. Eur J Cancer. 2005;41(5):735–740. doi: 10.1016/j.ejca.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Marmorstein R. Structure of the retinoblastoma protein bound to adenovirus E1A reveals the molecular basis for viral oncoprotein inactivation of a tumor suppressor. Genes Dev. 2007;21(21):2711–2716. doi: 10.1101/gad.1590607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nichols KE, Walther S, Chao E, Shields C, Ganguly A. Recent advances in retinoblastoma genetic research. Curr Opin Ophthalmol. 2009;20(5):351–355. doi: 10.1097/ICU.0b013e32832f7f25. [DOI] [PubMed] [Google Scholar]
- 45.Swiss VA, Casaccia P. Cell-context specific role of the E2F/Rb pathway in development and disease. Glia. 2010;58(4):377–390. doi: 10.1002/glia.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leiderman YI, Kiss S, Mukai S. Molecular genetics of Rb1--the retinoblastoma gene. Semin Ophthalmol. 2007;22(4):247–254. doi: 10.1080/08820530701745165. [DOI] [PubMed] [Google Scholar]
- 47.Krishna SM, Yu GP, Finger PT. The effect of race on the incidence of retinoblastoma. J Pediatr Ophthalmol Strabismus. 2009;46(5):288–293. doi: 10.3928/01913913-20090903-06. [DOI] [PubMed] [Google Scholar]
- 48.Lumbroso-Le Rouic L, Aerts I, Lévy-Gabriel C, Dendale R, Sastre X, Esteve M, Asselain B, Bours D, Doz F, Desjardins L. Conservative treatments of intraocular retinoblastoma. Ophthalmology. 2008;115(8):1405–1410. doi: 10.1016/j.ophtha.2007.11.009. [DOI] [PubMed] [Google Scholar]