Abstract
AIM
To investigate whether CD4+CD25+ regulatory T (Treg) cells play a role in the development of anterior chamber-associated immune deviation (ACAID).
METHODS
The dynamic changes in the frequency of CD4+CD25+ T cells, CD4+CD25+ FoxP3+ T cells and CD4+CD25+ PD-1+ T cells from spleens of mice with ACAID were analyzed by flow cytometry. Foxp3 mRNA expression in purified CD4+CD25+ T cells was analyzed using real-time PCR. The suppressive effect of purified CD4+CD25+ T cells on the proliferation of CD4+CD25− T cells was evaluated by [3H] thymidine incorporation. A blocking experiment was performed to further address the role of CD4+CD25+ T cells in ACAID. The expression of IL-10 in purified CD4+CD25+ T cells was evaluated by ELISA.
RESULTS
Increased frequencies of CD4+CD25+ T cells, CD4+CD25+ FoxP3+ T cells and CD4+CD25+ PD-1+ T cells were observed in ACAID. The CD4+CD25+ T cells from mice with ACAID showed enhanced suppressive effect on the proliferation of CD4+CD25− T cells. Treatment of BALB/c mice with anti-CD25 antibody after injection of OVA into the anterior chamber significantly inhibited the induction of ACAID. Furthermore, purified CD4+CD25+ T cells from ACAID mice secreted IL-10.
CONCLUSION
Our results demonstrate that Treg cells are induced in the mice undergoing ACAID. These Treg cells may play a role in the development of ACAID.
Keywords: CD4+CD25+ regulatory T cells, Foxp3, ACAID, IL-10, PD-1
INTRODUCTION
Intracameral injection of soluble protein antigens may elicit a deviant immune response, known as anterior chamber-associated immune deviation (ACAID) that is characterized by an impaired delayed-type hypersensitivity (DTH) response and suppressed complement-fixing antibodies. ACAID has been shown to be essential in the protection against sight-threatening immune responses. However, the exact mechanisms of ACAID are still not fully understood. An interesting study has revealed that thymus is directly involved in the induction of ACAID and immunoregulatory T cells are formed immediately after an intracameral injection of low doses of nonself antigen. Regulatory T (Treg) cells have been considered to be critical for the development of ACAID and at least two functionally distinct regulatory T cell populations have been identified in this immune deviation. The first regulatory T cell population consists of CD4+ T cells that inhibit the induction of a DTH response, but do not affect the expression of DTH if hosts have already been immunized. Thus, these CD4+ T cells block the induction or afferent component of the immune response. The second regulatory T cell population is comprised of CD8+ T cells, which inhibit the expression of DTH.
It has been established that CD4+CD25+ Treg cells, which constitutively express a high level of the IL-2 receptor alpha chain (CD25) and account for 5-10% of peripheral CD4+ T cells in normal rodents and human, are essential for the maintenance of self-tolerance by inhibiting the activation and expansion of self-reactive T cells[1]. Studies have demonstrated that the Treg cells are also involved in regulating T cell homeostasis, organ transplant tolerance and modulating immune responses to infection, allergy, and tumor immunity[2],[3]. However, the exact role of Treg cells in ACAID is not yet clear. A number of cell surface markers such as CD25, cytotoxic T-lymphocyte antigen 4 (CTLA4)[4]-[7] and glucocorticoid-induced tumor-necrosis factor (TNF)-receptor-related protein (GITR)[8]-[11] are found to be highly expressed in the Treg cells in naive mice and in humans. These molecules are also expressed by other types of activated T cells, and therefore cannot be used for the identification of Treg cells. Forkhead/winged helix transcription factor gene (Foxp3) was recently shown to be specifically expressed by the naturally occurring Treg cells and was virtually undetectable in resting and activated effector T cells. Foxp3 is also essential for Treg cell development and function. Foxp3 mutant scurfy and Foxp3-null mice developed a lethal autoimmune syndrome as the consequence of a deficiency in Treg cells. Adoptive transfer of CD4+CD25+ T cells into neonatal Foxp3-deficient mice prevented the development of disease[12],[13]. Recently, a novel negative regulatory molecule and a new member of the B7-CD28 superfamily has been identified as programmed death-1 (PD-1). This molecule on activated CD4+ and CD8+ T cells, regulates T cell responses and binds to two known ligands, PD-L1 (also termed B7-H1) and PD-L2 (also termed B7-DC), on APC as well as on diverse parenchymal cell types. It has been demonstrated that murine CD4+CD25+ T cells suppress effector T cell function in vitro through cell-cell contact[1]. However, in vivo, these cells can also function through induction of immunosuppressive cytokines, such as IL-10 and transforming growth factor β1 (TGF-β1)[14].
Given the protective role of CD4+CD25+ T cells in autoimmunity, we hypothesized that these cells might also be important for the development of ACAID. Our results showed that there were increased frequencies of CD4+CD25+ T cells, CD4+CD25+ Foxp3+ T cells and CD4+CD25+ PD-1+ T cells in ACAID mice. Purified CD4+CD25+ T cells from mice with ACAID exhibited a much stronger suppressive effect on the proliferation of CD4+CD25− T cells in vitro. Treatment of BALB/c mice with anti-CD25 antibody after injection of OVA into the anterior chamber substantially inhibited the induction of ACAID; Furthermore, these T cells secreted a high amount of IL-10, but not TGF-β1, indicating that CD4+CD25+ T cells potently suppress Th1 responses and are actively involved in the development of ACAID.
MATERIALS AND METHODS
Materials
Balb/c mice (6-8 week-old) were purchased from the animal center of the Sun Yat-Sen University housed under specific pathogen-free conditions at the Zhongshan Ophthalmic Center, and maintained as outlined by the Association for Research in Vision and Ophthalmology (ARVO) resolution for use of animals in research. ACAID was induced as described previously using a microinjection of Ag into the anterior chamber of the eye of Balb/c mice. Briefly, mice were anesthetized with 0.66mg of ketamine hydrochloride by i.p. injection. A glass micropipette of approximately 70-80µm diameter was fitted onto a sterile infant feeding tube and mounted onto a 1mL syringe. Each mouse received a 100µg inoculation of OVA (Sigma) in 5µL phosphate-buffered saline (PBS) in the anterior chamber of the right eye. For subcutaneous(s.c.) sensitization, mice received a s.c. injection of OVA 200µg in 100µL PBS emulsified 1:1 in 100µL of complete Freund's adjuvant (CFA. Sigma) 1 to 4 weeks after intracameral injection. ACAID was successfully induced from 1 to 4 weeks after intracameral injection of OVA, and persisted for at least 4 weeks. Mice were challenged by intradermal injection of 200µg OVA in 20µL PBS into the right ear pinnae 7 days after s.c. immunization. The left ear pinnae received 20µL sterile PBS alone. Both ear pinnae were measured 24 hours later, and the difference in ear pinnae size was used as a measure of DTH. Results are expressed as: specific ear pinnae swelling= [(24-h measurement -0-h measurement) of right ear-(24-h measurement- 0-h measurement) of left ear]×10−3mm. Mice receiving only ear challenge were used as negative control[15]. Mice sensitized with OVA emulsified with CFA 7 days before ear challenge were used as positive controls.
Cell surface marker analysis
Single-cell suspensions were prepared from the spleens of mice 7 days after s.c. immunization. Tris-NH4Cl (0.83% in 0.01mol/L Tris-HCl, pH 7.2) was used for lysing the red blood cells (RBC). The RBC-free fresh single-cell suspensions were cell surface stained with PE-anti-CD3, FITC-anti-CD4, PE-Cy5-CD25 and isotype control antibodies (eBioscience, USA). After washing with PBS, the labeled cells were analyzed by FCM using the BD FACSDiVa software. Usually, a total number of 300000 cells were collected for each sample. Mice sensitized with OVA emulsified with CFA 7 days before ear challenge were used as positive controls. Mice receiving intraocular inoculation of OVA or systemic CFA alone (no OVA immunization) served as CFA controls and CFA controls respectively. To identify the expression of Foxp3 on CD4+CD25+ T cell, The RBC-free fresh single-cell suspensions were stained with FITC-anti-CD3, PE-anti-CD4, PE-Cy7-CD25, APC-Foxp3 or isotype control antibodies. To identify the expression of PD-1 on CD4+CD25+ T cells, the RBC-free fresh single-cell suspensions were stained with FITC-anti-CD3, APC-anti-CD4, PE-Cy7-CD25, PE-PD-1 or isotype control antibodies. The stained cells were washed and analyzed by FCM. Mouse CD4+CD25+ Treg cells were isolated using the mouse CD4+CD25+ regulatory T cells isolation kit by magnetic cells sorting (MACS) according to the manufacturer's instructions (Miltenyi Biotec, Germany) in a two-step procedure. Briefly, CD4+ T cells were enriched by depletion of unwanted cells and followed by a positive selection using anti-CD25-coupled Miltenyi microbeads. The CD4+CD25+ T cells and CD4+CD25− T cells were harvested and used for the following experiments. The purity of CD4+CD25+ T cells and CD4+CD25− T cells, determined by FCM, was >95% and 90%, respectively.
Foxp3 expression detection
Total RNA was extracted from 106 purified CD4+CD25+ T cells or CD4+CD25− T cells using TRIzol reagent. The resulting RNA was transcribed into cDNA using oligo-dT and Superscript First Strand synthesis system (Qiagen, German) according to the manufacturer's instructions. The reaction mixture was incubated for 60 minutes at 37°C and the reverse transcriptase was inactivated by incubating the cDNA samples for 3 minutes at 95°C. The cDNA samples were then subjected to PE 7000 automatic quantitative PCR apparatus (Perkin Elmer, USA) for quantitative real-time PCR assay. The sequences of the forward (-FW) and reverse (-RV) primers and probes (-TP) for GAPDH and Foxp3 were as follows: GAPDH-FW:5′-CGTGTTCCTACCCCCAATGT-3′.GAPDH-RV: 5′-TGTCATCATACTTGGCAGGTTTCT-3′.GAPDH-TP:5′-FAM-TCGTGGATCTGACGTGCCGCC-TAMRA-3′.FoxP3-FW:5′-AGGCCCTTCTCCAGGACAGA-3′. FoxP3-RV: 5′-AAGGTGGTGGGAGGCTGATC-3′. FoxP3-TP:5′-FAM-ACTTCATGCATCAGCTCT CCACTGTGGA-TAMRA-3′, using a ABI 3900 Detector. All primers and probes were designed with the assistance of the computer program Primer Express 2.0. All results were normalized to GAPDH to compensate for differences in the amount of cDNA in all samples.
Cell proliferation assay
T-depleted spleen cells were used as APC and were prepared by first lysing the erythrocytes with Tris-NH4Cl, followed by treatment with 50mg/L of mitomycin C (Sigma, St. Louis, MO) for 20 minutes at 37°C and wash them three times with DMEM plus 100mL/L FBS before using them in proliferation assays. Purified CD4+CD25− T cells (5×104cells/well) from naive mice were co-cultured with different concentrations of purified CD4+CD25+ T cells from mice with ACAID, naive or positive mice in the presence of anti-CD3 mAb (145-2C11, 3mg/L, Pharmargin) and soluble anti-CD28 mAb (1mg/L, Biolegend, USA) for 72 hours as described previously by Cai et al[16]. The ratio of CD4+CD25− T cells to CD4+CD25+ T cells was 1: 0, 0: 1, 1:1, 2: 1 and 5: 1 respectively. In an attempt to evaluate the influence of non-specific stimulator, unrelated antigen and specific antigen on the role of CD4+CD25+ T cells, CD4+CD25− T cells (5×104) from naive mice were co-cultured with CD4+CD25+ T cells from mice with ACAID stimulated with equal numbers of mitomycin C-treated APC (T cell–depleted splenocytes) and anti-CD3/anti-CD28 mAb, BSA(5µg/mL) and OVA(5µg/mL) respectively, and assayed for the inhibition of CD4+CD25+ T cells on the CD4+CD25− T cells (the ratio of CD4+CD25+ T cells to CD4+CD25− T cells was 1:1). Cells were cultured in complete media (RPMI 1640 supplemented with 100mL/L heat-inactivated FCS, 50µmol/L 2-ME, 2mmol/L L-glutamine, 0.1mmol/L nonessential amino acids, 1mmol/L sodium pyruvate and 10g/L streptomycin/penicillin) (Gibco, USA) at 37°C in 50mL/L CO2. To assess cell proliferation, half of the culture supernatant (100µL) was removed from each well before 18.5kBg [3H] thymidine was added to each well for the last 6 hours of culture. Cells were subsequently harvested and [3H] thymidine incorporation was measured by scintillation counter (Beckman, LS6500). All data shown represent an average of at least three independent experiments.
IL-10 measurement
To deplete CD4+D25+ T cells in vivo, each mouse was intravenously injected with 1mg anti-CD25 mAb (PC61) (eBioscience, USA) twice times a week for 2 weeks, and followed by injection of OVA into the anterior chamber one week after the last injection of the mAb. Rat IgG1 (1mg/mouse) (eBioscience, USA) was used as isotype control. The in vivo depletion of CD4+CD25+ T cells in peripheral blood was monitored by FCM on day 7 and day 14 after intravenous injection. A total number of 1 ×105 CD4+CD25+ or CD4+CD25− T cells in 200µL total volume in each well of 96-well round-bottom plates was cultured for 72 hours at 37°C and 50mL/L CO2 in the presence or absence of plate-bound anti-CD3 antibody(145-2C11, 3mg/L) and soluble anti-CD28 antibody (1mg/L). The supernatants were harvested, and the levels of IL-10 were assessed using an ELISA kit (Biosource, USA) according to the manufacturer's instructions. The minimal levels of detection of IL-10 were 13pg/mL. The supernatants collected before addition of [3H] thymidine incorporation for proliferation assays were also tested for IL-10 production according to the manufacturer's instructions.
Statistical Analysis
Data were analyzed using the SPSS11. Analysis of variance (ANOVA)-Dunnett-t test. Data are presented as means ± SEM. P <0.05 were considered as a significant difference.
RESULTS
Increased T cell Subset Frequencies in ACAID Mice
To address the role of the Treg cells in the induction and maintenance of ACAID, we first examined the frequencies of CD4+CD25+ T cells from the spleen at several time points after intracameral injection of OVA. The percentage of CD4+CD25+ T cells in the CD4+ T cells was identified by FCM after cells were labeled with fluorescence-conjugated anti-CD3, anti-CD4 and anti-CD25 antibody. The frequencies of CD4+CD25+ T cells in the spleen of ACAID mice were significantly increased from 9.9% to 8.4% from 1 to 4 weeks as compared with those in the positive controls (6.7 %, P<0.001). However there was no difference among different time points of ACAID concerning the proportions of CD4+CD25+ T cells in the spleens (P>0.05). Furthermore, the frequency of CD4+CD25+ T cells were also increased in positive controls compared with those naive controls.
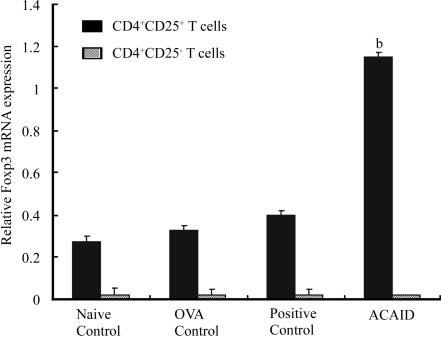
In order to exclude the influence of M.tb in the adjuvant on the frequencies of CD4+ T cells and CD4+CD25+ T cell, we used two additional controls. Mice receiving only intracameral injection of OVA not receiving the subcutaneous challenge subsequently were used as OVA control, and mice receiving systemic CFA alone (no OVA immunization) were used as CFA control. These frequencies were assayed by FCM. The results showed that intracameral injection of OVA could greatly enhance the frequency of CD4+ T cell (48.1%), which equal to that elicited by s.c. injection of OVA and CFA (positive control, 48.8%, P=0.526 for OVA control vs positive control), as compared with that in naive control (45.7%, P<0.05 for OVA control vs naive control). This response seemed higher than that elicited by sc injection of CFA (47.0%), but there was no difference between them (P=0.347 for OVA control vs CFA control). It is also noted that the frequency of CD4+CD25+ T cells in OVA control was significantly higher than that in naive control but lower than that in ACAID mice, (P<0.01 for ACAID groups vs positive controls, OVA control and CFA control. P=0.019 for OVA control vs naive control, P=0.023 for CFA control vs naive control. P=0.974 for OVA control vs CFA control), All these results suggest that intracameral injection of OVA could induce the increased frequency of CD4+ T cells as well as CD4+CD25+ T cells and the M.tb in the adjuvant may enhance this response elicited by intracameral injection of antigen. All purified CD4+CD25+ cells, regardless of their origins, showed a higher expression of Foxp3, whereas CD4+CD25− T cells in all the groups showed a uniformly much lower expression of Foxp3. Approximately 3-5 fold higher level of Foxp3 mRNA was observed in CD4+CD25+ T cells from mice with ACAID as compared with positive control and OVA control or naive controls (P<0.001, Figure 1). The frequency of CD4+CD25+Foxp3+ T cells from mice with ACAID was significantly higher than that of positive controls (P=0.042 ), OVA control(P=0.021) and naive controls (P=0.0068).These results indicated that purified CD4+CD25+ T cells from mice with ACAID included more regulatory T cells than those from naive or positive controls and OVA control. Intraocular inoculation OVA or s.c. immunization (OVA+CFA) could also increase the frequency of CD4+CD25+ T cells. CD4+CD25+ T cells from ACAID mice expressed higher level of PD-1 in frequency as compared with those from positive controls, OVA controls and naive controls
Figure 1. Foxp3 mRNA expression.
bP<0.01 vs three controls
CD4+CD25+ T cells Suppression
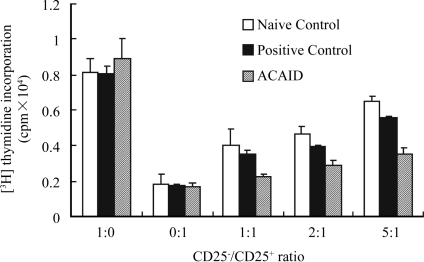
CD4+CD25− T cells from naive mice, positive mice and ACAID mice proliferated robustly in response to anti-CD3/anti-CD28 mAb stimulation. Such proliferation was inhibited by CD4+CD25+ T cells in a dose-dependent manner, which was consistent with the property of Treg cells. CD4+CD25+ T cells from the spleen of mice with ACAID proliferated poorly, exhibiting an anergic profile similar to that of Treg cells from naive and positive controls. In contrast, CD4+CD25− T cells proliferated vigorously. The proliferation of CD4+CD25− T cells was inhibited by all the CD4+CD25+ T cells in a dose-dependent manner. Importantly, this suppression was much stronger when co-cultured with CD4+CD25+ T cells from mice with ACAID as compared with those from naive or positive control mice. CD4+CD25+ T cells inhibited the proliferation of CD4+CD25− T cells only when stimulated with polyclonal stimulatory or specific antigen, id anti-CD3/anti-CD28 mAb, or OVA (Figure 2). CD4+CD25+ T cells did not show any inhibitation on proliferation of CD4+CD25− T cells when stimulated with non-specific antigen, id, BSA. The suppression of CD4+CD25+ T cells on the proliferation of CD4+CD25− T cells was antigen-specific.
Figure 2. [3H] thymidine incorporation.
Effect of CD25+ cells Depletion
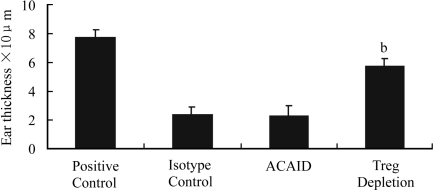
A significantly decreased percentage of CD4+CD25+ T cells from PBL was detected on day 14 after injection of anti-CD25 antibody into mice. ACAID was not detected in these anti-CD25 antibody-treated mice, as an overt DTH response developed in these mice (Figure 3). Furthermore, there was a significantly lower percentage of CD4+CD25+ T cells (0.8%) in the spleen of anti-CD25 antibody-treated mice than those with ACAID (10.4%). The injection of isotype controls did not result in a decreased percentage of CD4+CD25+ T cells in the spleen and had no influence on the development of ACAID.
Figure 3. Effect of CD 25+ cell depletion.
bP<0.01 vs ACAID, Isotype control and Positive control
IL-10 production
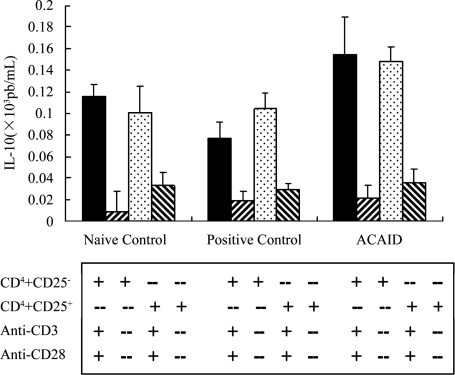
CD4+CD25+ T cells from mice with ACAID secreted a significantly higher level of IL-10 than did the CD4+CD25+ T cells from naive and positive controls. Interestingly, the CD4+CD25− T cells from mice with ACAID also produced a higher level of IL-10 (Figure 4).
Figure 4. IL-10 production “+” meaning in the presence of , “-” meaning in the absence of.
DISCUSSION
In the present study, we successfully induced ACAID using OVA as a soluble antigen, which was demonstrated by an impaired DTH response. These results are well in accordance with those in previous reports. The frequencies of CD4+CD25+ T cells, CD4+CD25+Foxp3+ T cells and CD4+CD25+PD-1+ T cells from ACAID mice were significantly higher than those of positive controls, OVA control and naive controls, The CD4+CD25+ T cells from mice with ACAID were more effective than those from naive and positive controls on the suppression of CD4+CD25− T cells. and inhibited the proliferation of CD4+CD25− T cells in a dose-dependent manner. Depleting the CD25+ T cells in vivo using specific mAb resulted in an abortion of ACAID. Furthermore, the CD4+CD25+ T cells of the spleen from mice with ACAID were found to produce a higher amount of IL-10 than did CD4+CD25+ T cells of the spleen from naive or positive controls. All these results indicate that Treg cells may play an important role in the development of ACAID.ACAID has been intensively studied mainly because it protects the eye from antigen-specific, immune- mediated injury inflicted by DTH that can lead to blindness. OVA is a commonly used antigen, among many others, to induce ACAID. In our ACAID model, the DTH response was significantly impaired during the first to 4 weeks after OVA injection into the anterior chamber of the eye without a dramatic difference among different time points. This was accompanied by a down-regulation of the Th1 and an up-regulation of the Th2 cytokine response.
The mechanisms of ACAID have not been well understood. Recent studies suggest that down-regulation of DTH is mediated by antigen-specific regulatory T cells. Three types of regulatory T cells, i.e. Th3, Tr1 and Treg cells have been described. Their functions are partially overlapped. The Treg cells are the best characterized ones to date in immune tolerance and autoimmune response. However, their role in ACAID has not been addressed. We, for the first time, demonstrated that the frequency of CD4+CD25+ T cells in the spleen was significantly increased during ACAID. Such an increase of cell frequency seemed to coincide with the suppression of the ear swelling response during the development of ACAID. Furthermore, we noticed that intracameral injection of OVA could induce the increased frequency of CD4+ T cells as well as CD4+CD25+ T cells and the M.tb in the adjuvant may enhance this response elicited by intracameral injection of antigen. The frequencies of CD4+CD25+ T cells were all increased in positive controls mice, OVA control and CFA control as compared with naive control mice. All these results suggest that activated effector T cells may be one part of this population. More importantly, the expression of the Treg cell marker Foxp3 was significantly elevated during ACAID at the mRNA level, and the frequency of CD4+CD25+Foxp3+ T cells from ACAID mice was significantly higher than that of positive controls. Moreover increased expression of PD-1 was detected on these CD4+CD25+ T cells, consistent with the previous study[37]. In contrast, Foxp3 message was essentially undetectable in the CD4+CD25− T cells. There results suggest that a larger proportion of Treg cells are present in the CD4+CD25+ T cells of ACAID mice than in the naive and positive control mice[19]. Since there was no difference among different time points of ACAID (1 to 4 weeks) concerning the DTH response and the cell frequency in the spleen, the day 7 of ACAID after injection of OVA into the anterior chamber was selected in the subsequent experiments.
CD4+CD25+ Treg cells have been shown to inhibit the proliferation of CD4+CD25− T cells in vitro. The increased frequency of CD4+CD25+ T cells containing Foxp3+ T cells in the ACAID mice indicated the presence of a higher percentage of Treg cells in these mice. Similar to Treg cells, these CD4+CD25+ T cells did not proliferate in response to the stimulation of anti-CD3/anti-CD28 mAb. They could inhibit the proliferative response of CD4+CD25− T cells, thus exhibiting Treg cell-like properties. In the mice with ACAID induced by OVA, CD4+CD25+T cells exerted stronger inhibition on the proliferation of CD4+CD25− T cells when exposed to this antigen or anti-CD3/anti-CD28 mAb as compared with that in the naive mice and positive control, and the suppression of CD4+CD25+ T cells from mice with ACAID on the proliferation of CD4+CD25− T cells was antigen-specific. These results, together, demonstrate that increased Treg-like cells and function correlated with the development of ACAID.
Anti-CD25 mAb administration in vivo has been reported to mainly lead to depletion of Treg cells in naive mice and activated lymphocytes after immunization[20],[21]. The use of this antibody before ACAID and DTH induction allows more likelihood of Treg cell deficient. Our results showed that there were only about 1.4±0.2% of CD4+CD25+ T cells left in the PBL after anti-CD25 antibody treatment, which was significantly lower than the percentage of CD4+CD25+ T cells in naive mice (4.85±0.2%). Mice treated with anti-CD25 antibody, but not the isotype control antibody, failed to develop ACAID, as evidenced by an apparent DTH response that was similar to the positive control. Our results are consistent with previous report[22],[23], in which in vitro depletion of Treg cells using anti-CD25 antibody resulted in a preferential Th1 response and an impaired Th2 response[22], and in vivo treatment with the same antibody mainly led to an increased Th1 response[23]. Reconstitution of CD4 knockout mice with CD4+CD25− T cells does not generate ACAID suppressor cells. These results, together with ours, suggest that the Th1 response dominates after the depletion of CD25+ cells that are necessary for the induction and/or maintenance of ACAID.
IL-10 is an immune inhibitory cytokine that inhibits the synthesis of Th1 cytokines. It was found to be crucial in the development of ACAID. A higher production of IL-10 was detected from CD4+CD25+ T cells in mice with ACAID than in the positive or naive control groups of mice. Previous studies showed that increased expression of Foxp3, a specific marker of Treg cells, was accompanied by the increased expression of IL-10. Moreover, Treg cells over-express a subset of Th2 gene transcripts including IL-10. Therefore, the regulatory cells in the CD4+CD25+ population of ACAID mice are likely to function through the production of IL-10. The regulatory T cell-derived IL-10 may skew Th0 cell differentiation toward Th2 cells. A high level of IL-10 that was comparable to that of CD4+CD25+ T cells was also seen in supernatant of the CD4+CD25− T cells from ACAID mice. This might be due to the induction of a strong Th2 response, in addition to the Treg up-regulation, during ACAID. Th2 and Treg cells may cooperate to play a role in ACAID.
In conclusion, our study showed an increased frequency of CD4+CD25+ T cells in association with an enhanced expression of Foxp3 and PD-1 during ACAID. These CD4+CD25+ T cells produced a higher level of IL-10 and markedly inhibited the proliferation of CD4+CD25− T cells. Treatment with anti-CD25 antibody inhibited the induction of ACAID. These results suggest these CD4+CD25+ T cells are involved in the development of ACAID. However, our study did not distinguish the effect of naturally occurring CD4+CD25+ Tregs from that of induced CD4+CD25+ Tregs. Recent study by keino et al [15] has demonstrated that induction of ACAID in Doll.10 mice is not dependent on the presence of natural CD4+CD25+ Tregs. More studies are needed to clarify whether the natural CD4+CD25+ Tregs is are involved in the induction of ACAID in these BALB/C mice and what is the relationship between induced Tregs and natural Tregs in this immune tolerance.
Acknowledgments
We thank Jun Huang, Zhan Peng, Li-TaoYang and Rui Ma for excellent technical assistance
Footnotes
Foundation item: National Natural Science Foundation of China (No. 30400487); International Cooperation Project of Guangdong Province, China (No. 2004B50301002); “1135” Talent Doctor Foundation of Daping Hospital (No. 2008-2010)
REFERENCES
- 1.Schwartz RH. Natural regulatory T cells and self-tolerance. Nat Immunol. 2005;6(4):327–330. doi: 10.1038/ni1184. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 3.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5(4):271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 4.Birebent B, Lorho R, Lechartier H, de Guibert S, Alizadeh M, Vu N, Beauplet A, Robillard N, Semana G. Suppressive properties of human CD4+CD25+ regulatory T cells are dependent on CTLA-4 expression. Eur J Immunol. 2004;34(12):3485–3496. doi: 10.1002/eji.200324632. [DOI] [PubMed] [Google Scholar]
- 5.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34(11):2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 6.Kataoka H, Takahashi S, Takase K, Yamasaki S, Yokosuka T, Koike T, Saito T. CD25(+)CD4(+) regulatory T cells exert in vitro suppressive activity independent of CTLA-4. Int Immunol. 2005;17(4):421–427. doi: 10.1093/intimm/dxh221. [DOI] [PubMed] [Google Scholar]
- 7.Vasu C, Prabhakar BS, Holterman MJ. Targeted CTLA-4 engagement induces CD4+CD25+CTLA-4high T regulatory cells with target (allo)antigen specificity. J Immunol. 2004;173(4):2866–2876. doi: 10.4049/jimmunol.173.4.2866. [DOI] [PubMed] [Google Scholar]
- 8.Nocentini G, Riccardi C. GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur J Immunol. 2005;35(4):1016–1022. doi: 10.1002/eji.200425818. [DOI] [PubMed] [Google Scholar]
- 9.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173(8):5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 10.Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I, Azuma M. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172(12):7306–7314. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 11.Ji HB, Liao G, Faubion WA, Abadía-Molina AC, Cozzo C, Laroux FS, Caton A, Terhorst C. Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J Immunol. 2004;172(10):5823–5827. doi: 10.4049/jimmunol.172.10.5823. [DOI] [PubMed] [Google Scholar]
- 12.Feuerer M, Benoist C, Mathis D. Green T(R) cells. Immunity. 2005;22(3):271–272. doi: 10.1016/j.immuni.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 2004;6(8):745–751. doi: 10.1016/j.micinf.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-β1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172(2):834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 15.Keino H, Takeuchi M, Kezuka T, Hattori T, Usui M, Taguchi O, Streilein JW, Stein-Streilein J. Induction of eye-derived tolerance does not depend on naturally occurring CD4+CD25+ T regulatory cells. Invest Ophthalmol Vis Sci. 2006;47(3):1047–1055. doi: 10.1167/iovs.05-0110. [DOI] [PubMed] [Google Scholar]
- 16.Cai G, Karni A, Oliveira EM, Weiner HL, Hafler DA, Freeman GJ. PD-1 lignads, negative regulators for activation of naive, memory, and recently activated human CD4+ T cells. Cellul Immunol. 2004;230(2):89–98. doi: 10.1016/j.cellimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O'Garra A. mparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172(10):5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 18.Meng Q, Yang P, Li B, Zhou H, Huang X, Zhu L, Ren Y, Kijlstra A. CD4+PD-1+ T cells acting as regulatory cells during the induction of anterior chamber-associated immune deviation. Invest Ophthalmol Vis Sci. 2006;47(10):4444–4452. doi: 10.1167/iovs.06-0201. [DOI] [PubMed] [Google Scholar]
- 19.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16(11):1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 20.Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol. 2005;174(1):90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi M, Keino H, Kezuka T, Usui M, Taguchi O. Immune responses to retinal self-antigens in CD25+CD4+ regulatory T-cell-depleted mice. Invest Ophthalmol Vis Sci. 2004;45(6):1879–1886. doi: 10.1167/iovs.02-1030. [DOI] [PubMed] [Google Scholar]
- 22.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development1. J Immunol. 2004;173(2):1224–1231. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 23.Cosmi L, Liotta F, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, Lasagni L, Vanini V, Romagnani P, Maggi E, Annunziato F, Romagnani S. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood. 2004;103(8):3117–3121. doi: 10.1182/blood-2003-09-3302. [DOI] [PubMed] [Google Scholar]