Abstract
AIM
To investigate expression of factors related to angiogenesis: HIF-1α, iNOS, COX-2 and VEGF in choroidal melanoma and its clinical significance.
METHODS
Fifty samples of choroidal melanoma and 15 samples of melanocytic nevi of the eyelid identified by pathology were collected. Immunohistochemistry SP method was used to examine the expression of HIF-1α, iNOS, COX-2 and VEGF in these samples. The comparison among groups was done by SPSS 13.0 software.
RESULTS
The positive expression rates of HIF-1α, iNOS, COX-2 and VEGF in choroidal melanoma group were significantly higher than those in eyelid nevi group (χ2=6.5542, 7.7224, 8.5828, 15.1749). The positive expression rate of VEGF was associated with the tumor size (χ2=10.9194), but was not associated with pathological type (χ2=2.0712) and the situation of scleral invasion (χ2=5.4289). The positive expression rate of HIF-1α was associated with the tumor size (χ2=7.1216) and pathological type (χ2=9.0889), but was not associated with the situation of scleral invasion (χ2=3.3586). The positive expression rate of iNOS was associated with the tumor size (χ2=9.5503), but was not associated with pathological type (χ2=1.9450) and the situation of scleral invasion (χ2=2.3810). The positive expression rate of COX-2 was associated with the tumor size (χ2=7.2970), but was not associated with pathological type (χ2=1.8421) and the situation of scleral invasion (χ2=0.4018). The expression of HIF-1α, iNOS and COX-2 were significantly associated with the expression of VEGF (r=0.9429, 1, 0.9857). The expression of COX-2 was significantly associated with the expression of iNOS (r=0.9857). The expression of HIF-1α was significantly associated with the expression of COX-2 (r=0.9857). The expression of HIF-1α was significantly associated with the expression of iNOS (r=0. 9429).
CONCLUSION
The expression of HIF-1α, iNOS and COX-2 protein in choroidal melanoma were higher and may relate to angiogenesis and stimulate tumor growth. Determination of HIF-1α, iNOS and COX-2 may be helpful for the diagnosis and therapy of this tumor.
Keywords: choroidal melanoma, immunohistoehemistry, hypoxia inducible factor-1a, inducible nitric oxide synthase, cyclooxygenase-2
INTRODUCTION
Angiogenesis is important for the development and transitivity of tumors. There are lots of researches about factors of anoxia and angiogenesis like HIF-1α, iNOS and COX-2 in malignancy, but the relationship between these factors and choroidal melanoma are little. We tested the expressions of HIF-1α, iNOS, COX-2 and VEGF in choroidal melanoma by immunohistochemistry and studied the relationship between the expressions of them and that of VEGF and clinicopathological features of patients to get in-depth knowledge about choroidal melanoma.
MATERIALS AND METHODS
Materials
Paraffin-embedded sections of tumor samples, 50 choroidal melanoma and 15 melanocytic nevi of the eyelid, were collected from pathologic laboratory of ophthalmology, affiliated to the hospital of Qingdao University Medical Collage. All these samples were affirmed by hematoxylin-eosin staining. Fifty patients with melanoma, with mean age 45.8 years (ranged from 22 to 80 years old), were included in this study. Of them, 29 (58%) were male and 21 (42%) were female. There were three kinds of sorting methods: 1) according to the tumor size: 6 cases (accounting for 12%) had small tumors (height <3mm, basal diameter <10mm), 27 cases (54%) with medium-sized tumor (3mm ≤height ≤5mm, 10mm ≤=basal diameter ≤16mm), 17 cases (34%) with large tumors (height >5mm, basal diameter >16mm); 2) according to cell differentiation degree: spindle-shaped cell type of 15 cases (30%), large epithelioid type of 22 cases (44%) and mixed cell type of 13 patients (26%); 3) according to the situation of scleral invasion: not involving the sclera of 35 cases (70%), involving the sclera but not penetrating of 10 cases (20%), penetrating sclera of 5 cases (10%). None of patients was treated by anti-cancer drugs. Anti-COX-2 and anti-VEGF polyclonal antibody (Beijing Zhongshan Goldenbridge Co, China), anti-HIF-1α polyclonal antibody (Beijing Boaosen Co, China), anti-iNOS polyclonal antibody (Wuhan Boshide Co, China), PV6000 HistostainTM-Plus Kits (IgG /Bio, S-A/ HRP, DAB) (Beijing Zhongshan Goldenbridge Co, China) were purchased.
Methods
Paraffin-embedded sections of tumor samples were examined for melanoma tumor HIF-1α, iNOS, COX-2 and VEGF expression by immunohistochemistry following routine methods, but at first the pigments were removed by 5g/L potassium permanganate / 10g/L oxalic acid solution for 5-10 minutes (according to the tumor size and thick degree of pigment). Immunolabeling was scored separately for 2 variables: number of positive melanoma cells at high magnification (200×) vision and the staining intensity of the positive cells. Briefly, scoring for number of positive tumor cells was defined as follows: “0,” less than 5% positive cells; “1,” 5-25% positive cells; “2,” 26-50% positive cells; “3,” 51-75% positive cells; “4,” greater than 75% positive cells. According to the staining intensity: “1,” light brown; “2,” tan plan; “3,” deep brown. Add both above as a judge results: (-): 0-1; (+) : 2-3, (+ +): 4-5, (+ + +): 6-7.The slides were independently interpreted by 2 readers without knowledge of the clinical data.
Statistical Analysis
All data were analyzed using SPSS13.0. Ratio analysis were done with χ2 test and Spearman rank relationship analysis between HIF-1α, iNOS, COX-2 and VEGF. The differences of P<0.05 was considered to be significant.
RESULTS
VEGF Expression
The difference of positive expression rate of VEGF in melanocytic nevi and choroidal melanoma samples was statistically significant (χ2=6.5542, P<0.05). χ2 value according to the tumor size, pathologic types and the situation of scleral invasion were 10.9194, 2.0712 and 5.4289, indicated that VEGF expression was relevant with the tumor size, and was irrelevant with pathologic types and the situation of scleral invasion (Tables 1, 2; Figure 1).
Table 1. VEGF, HIF-1α, iNOS and COX-2 protein expressions.
| Degree | Choroidal melanoma |
Melanocytic nevi |
||||||
| VEGF | HIF-1α | iNOS | COX-2 | VEGF | HIF-1α | iNOS | COX-2 | |
| - | 15 | 23 | 11 | 18 | 10 | 13 | 10 | 14 |
| + | 14 | 10 | 18 | 12 | 4 | 1 | 1 | 0 |
| ++ | 13 | 13 | 12 | 12 | 1 | 0 | 2 | 1 |
| +++ | 8 | 4 | 9 | 8 | 0 | 1 | 2 | 0 |
Table 2. Relationship between the expressions of VEGF, HIF-1α, iNOS, COX-2 protein and clinical pathological features in choroidal melanoma.
| Clinical pathological feature | n | VEGF |
HIF-1α |
iNOS |
COX-2 |
||||
| (+) | (-) | (+) | (-) | (+) | (-) | (+) | (-) | ||
| Tumor size | |||||||||
| Small | 6 | 1 | 5 | 1 | 5 | 2 | 4 | 4 | 2 |
| Medium-sized | 27 | 19 | 8 | 13 | 14 | 21 | 6 | 13 | 14 |
| Large | 17 | 15 | 2 | 13 | 4 | 16 | 1 | 15 | 2 |
| Pathologic type | |||||||||
| Spindle-shaped cell | 15 | 9 | 6 | 12 | 3 | 12 | 3 | 10 | 5 |
| Large epithelioid | 22 | 15 | 7 | 12 | 10 | 16 | 6 | 12 | 10 |
| Mixed cell | 13 | 11 | 2 | 3 | 10 | 11 | 2 | 10 | 3 |
| Situation of scleral invasion | |||||||||
| Not | 35 | 21 | 14 | 16 | 19 | 29 | 6 | 22 | 13 |
| Yes | 10 | 9 | 1 | 7 | 3 | 6 | 4 | 6 | 4 |
| Penetrating sclera | 5 | 5 | 0 | 4 | 1 | 4 | 1 | 4 | 1 |
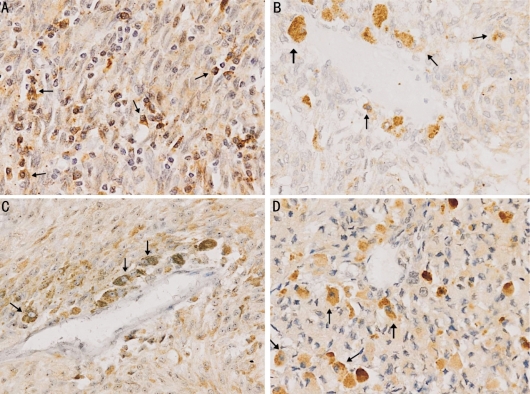
Figure 1. VEGF, HIF-1α, iNOS, COX-2 in choroidal melanoma cells (SABC×200).
A:VEGF(+); B:HIF-1α(+); C: iNOS (+); D: COX-2(+)
HIF-1α Expression
The difference of positive expression rate of HIF-1α in melanocytic nevi and choroidal melanoma samples was statistically significant (χ2=7.7224, P<0.05). χ2 value according to the tumor size, pathologic types and the situation of scleral invasion were 7.1216, 9.0889 and 3.3586, indicated that HIF-1α expression was relevant with the tumor size and pathologic types, and was irrelevant with the situation of scleral invasion (Tables 1, n3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-l and -2 and HIF-1α induction pathway 2; Figure 1).
INOS Expression
The difference of positive expression rate of iNOS in melanocytic nevi and choroidal melanoma samples was statistically significant (χ2=8.5828, P<0.05). χ2 value according to the tumor size, pathologic types and the situation of the scleral invasion were 9.5503, 1.9450 and 2.3810, indicated that iNOS expression was relevant with the tumor size, and was irrelevant with pathologic types and the situation of scleral invasion (Tables 1, 2; Figure 1).
COX-2 Expression
The difference of positive expression rate of COX-2 in melanocytic nevi and choroidal melanoma samples was statistically significant (χ2=15.1749, P<0.05). χ2 value according to the tumor size, pathologic types and the situation of scleral invasion were 7.2970, 1.8421 and 0.4018, indicated that COX-2 expression was relevant with the tumor size, and was irrelevant with pathologic types and the situation of scleral invasion (Tables 1, 2; Figure 1).
Relationship Analysis
Spearman rank relationship analysis showed that the expression levels of HIF-1α (r=0.9429>0.886, P<0.05=, iNOS (r=1, P<0.05= and COX-2 (r=0.9857, P<0.05= had positive correlation with that of VEGF. The expression levels of COX-2 and iNOS (r=0.9857>0.886, P<0.05=, n3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-l and -2 and HIF-1α induction pathway HIF-1α and iNOS (r=0.9429, P<0.05=, HIF-1α and COX-2 (r=0.9857, P<0.05= all had positive correlation (Tables 1, 22; Figure 1).
DISCUSSION
Choroidal melanoma is the most common primary intraocular malignant tumor in adults, mainly originated from the uveal pigment cells. Bloodborne metastases occur mostly, with the liver being the most common distant site of involvement. Index used previously to clinical estimate prognosis included age, tumor diameter, growth situation outside sclera boundary, location of anterior tumor margin, etc. The choroid is rich in blood vessels, and tumor cells are mainly hematogenous metastases, so angiogenesis may play an important part in growth of choroidal melanoma. Many studies show that in other malignant tumors angiogenesis plays a key role. Tumor growth is dependent on blood vessels and blood vessels as important component of tumor interstitium can interact with tumor cells and constitute a complete micro ecology system. Newborn blood vessels provide nutrition necessary for tumor growth and through these tumor cells obtain the ability of division, invasion and metastasis. There is only a layer of endothelial cells within new blood vessels without smooth muscle. Basement membrane is very thin and even missing, so its structure is relatively incomplete, which makes them more likely to be penetrated by tumor cells than normal blood vessels. Therefore, newborn of microvascular in tumor is the first step in tumor invasion and metastasis, and to study the role it plays can help us better understand growth and transfer of tumors. The mechanism of angiogenesis is very complex, which consists of a series of stimulating and restraining factors. There are little studies on angiogenesis of intraocular malignant tumors, so we focused on the relationship between factors related to angiogenesis and clinicopathological features to provide a new angle on the diagnosis and treatment of choroidal melanoma.
A lot of studies have shown that endothelial cell specific mitosis agent VEGF which is the key factor to stimulate angiogenesis in tumors and is highly correlated with tumor microvessel density (MVD) could stimulate differentiation of vascular endothelial cells and bring about angiogenesis to promote the growth and metastasis of tumors. In our experiment, VEGF was positive in cytoplasm and membrane of choroidal melanoma cells, and the surrounding area near vessels was heavier than the peripheral tissues, which was coincident to report of. Statistical analysis showed that the expression rate of VEGF in choroidal melanoma was much higher than that in control group, and was relevant with tumor size difference, which suggested that VEGF played a certain role in occurrence of choroidal melanoma by stimulating angiogenesis to promote growth of tumor. Expression of VEGF in solid tumors is affected by many factors, among which hypoxia is the main reason that up-regulate level of VEGF, and HIF-1α is the key factor to induce expression of VEGF following hypoxia. The level of HIF-1α is low during normoxia, but under hypoxic condition, transcription and translation level of HIF-1α increases greatly, with the increase of expression of its downstream target genes such as VEGF, erythropoietin, glucose transporter factor and glycolysis to solve blood oxygen supplement and energy metabolism problems of tumor tissue under anoxic condition and lift survival rate of tumor cells. Many scholars believe that HIF-1α is closely related with tumor prognosis and could be an independent index for prognosis. This study showed that expression of HIF-1α was positive in cytoplasm and membrane of tumor cells, and the surrounding area near vessels was heavier than other parts, and its rate was statistically significant in correlation with that of VEGF, which illustrated its close relationship with angiogenesis. In addition, statistical analysis also showed that expression of HIF-1α was relevant with tumor size and pathologic types, and with increase of tumor size and malignant degree, the expression was adding, which could conclude that HIF-1α can promote tumor growth by stimulating angiogenesis. Tumor tissues grow rapidly and could lead to relative lack of blood supply and hypoxia, so HIF-1α can activate and overexpress and combine to hypoxia response components in VEGF promoter, which is HIF-1α binding site and form pre-initiation complex (PIC) to start transcription of VEGF target genes. Under hypoxic condition, HIF-1α significantly increases stability of VEGFmRNA, thus up-regulates expression of corresponding protein, which could combine with VEGF R1 and VEGF R2 receptors and stimulate angiogenesis to relieve hypoxia of tumor organization. At present, there are little research on expression of HIF-1α in choroidal melanoma, but in other tissues or cells reports have shown that interaction between VEGF and HIF-1α was regulated by MAPK, PI3K/Akt, CaMKII (Ca2+/Cal modulin-dependent kinase II) pathways, and could further regulate Matrix metalloproteinases-9 (MMP-9) by these means. Also some researches have proved that lack of HIF-1α gene could lead to decline of VEGF to inhibit tumor angiogenesis and growth of solid tumor.
Some reports have suggested that high concentration of NO is toxic to tumor cells, and in contrary, low concentration of endogenous NO can promote growth and metastasis of tumor through some antiapoptotic effect[1], and its mechanism may be mediating DNA damage, preventing the repairment of DNA damage, promoting invasiveness, metastasis and angiogenesis, influencing corresponding proteins, etc. NO is important for expression of VEGF, though relevant mechanism is still unclear. It is generally accepted that NO can raise expression level of VEGF through PI3K-Akt pathway, and NO can increase permeability of vascular to play an important role in stimulating proliferation and migration of vascular endothelial cell. In addition, NO can also regulate formation of MMP-9 and MMP-2, and MMP-9 and MMP-2 can degrade vascular basement membrane and extracellular matrix (ECM) in order to increase proliferation and transitions of vascular endothelial cell, and then MMP-2 can also raise VEGF expression in the process of degradation of ECM, so as to promote tumor angiogenesis. Also, someone have found that in tumor cells NO/cGMP way may supply assistance on angiogenesis caused by VEGF, which are through CaM-Akt and PKC pathways and could also play a role of negative feedback regulation on NOS. iNOS is the rate-limiting enzyme of NO, mainly produced by the tumor cells and macrophages. And on its expression, L-Arginine is continuously catalyzed to generate NO. NO is extremely unstable with its half-life only half an hour or so, so we often detect level of iNOS to indirectly indicate that of NO. Our results showed that expression of iNOS was positive in cytoplasm and membrane, and area near vessels was heavier, and was statistically significant in correlation with tumor size and expression rate of VEGF , which illustrated that iNOS may be involved in angiogenesis of choroidal melanoma. Johansson et al[2] further studied expression of iNOS in primary cancer and metastatic hepatic tumor of uveal melanoma patients with and without liver metastases and found that expression of iNOS suggest worse prognosis regardless of appearing in metastatic hepatic tumor or just in primary uveal lesions.
Studies have found that COX-2 and PGE2 could promote expression of VEGF and induce angiogenesis, and this promoting role can be blocked by selectively COX-2 inhibitors PUFA[3]. Cyclooxygenase(COX) is a key enzyme to catalyse prostaglandin, and revellent COX-2 is a kind of fast reaction gene and could start producing after corresponding incentives (e.g. growth factor, mitogen and cytokine, etc), and then would catalyze production of prostaglandin (PGE2, PGF2, PGI2, during which PGE2 is the main catalyst), TXA2 and apoptosis factors such as Bcl-2, during which PGE2 and PGI2 can directly stimulate endothelial cell migration and accelerate growth factors to induce angiogenesis, and Bcl-2 and Akt can inhibit endothelial cell apoptosis. All these mechanisms are to induce expression of VEGF, and conversely, plenty of VEGF can rise up the expression of COX-2, thus form a positive feedback network to stimulate tumor angiogenesis. Currently, there are more and more reports about expression of COX-2 in melanoma, and we found that expression of COX-2 was positive in cytoplasm and membrane in choroidal melanoma, and area near vessels was heavier than other parts, and statistical analysis showed that expression of COX-2 was relevant with tumor size. In addition, its positive rate was statistically significant in correlation with that of VEGF, which illustrated its close relationship with angiogenesis. This was consistent with research of Lorna et al[4], and they also confirmed that in uveal melanoma excessive expression of COX-2 was relevant with adverse outcomes and shortened survival time through integrated follow-up data. But also some scholars pointed out that in malignant melanoma expression of COX-2 were relevant with histologic subtypes and pathologic stage[5],[6], and the reason might be caused by sample difference, inconsistent pathologically classification and antibodies difference. Despite of the difference between domestic and foreign researches, the role of COX-2 in melanoma has been confirmed by most of scholars who all think that COX-2 have adequate sensitivity and specificity to be a good immunohistochemical index to identify early malignant melanoma and related benign lesions[7] .
In recent years, there are many researches about correlation between expression of COX-2 and iNOS in various malignant tumors, and in endometrial carcinoma, brain star glioma, colorectal cancer, patients with breast cancer all have found high expression rate of COX-2 and iNOS. And they assume different degree of positive correlation and also have certain relationship with high MVD and expression of VEGF, indicating that COX-2 and iNOS may influence tumor invasion and metastasis by angiogenesis together, but the specific mechanism of interaction between these two factors remain unclear. iNOS and COX-2 are two important signal protein molecules in NF-κB signaling pathway, and “cross-talk” exists between them. COX-2 can combine NF-κB specifically, and iNOS co-ordinate with COX-2 to directly damage cellular DNA and protein to activate NF-κB. NO can activate COX-2 through two mechanisms, at first, NO couple super oxide anion to form ONOO- to activate COX-2, and secondly, production of NO and cGMP can improve activity of COX-2 and promote release of PGE2. NO can also promote activity and expression of COX through a way without cGMP, and with existence of both NOS and COX, NO will sharpen inflammation by promoting level of prostaglandin. However, there are also different opinions about the relationship between these two factors, e.g. in the intestinal epithelium cells of mice their role are mutually opposite, in which COX-2 inhibit iNOS through stabling protein level of Ik-Ba in order to decrease NF-κB. And similar results have also been found in nasopharyngeal carcinoma, in which the author analyzed that this could be caused by difference between measurement methods and organizational expression[8]. In melanoma, COX-2 and iNOS comparative studies also draw different conclusions, focused on the argument that which factor can be a better index for prognosis. Our study showed that expression of iNOS and COX-2 has certain correlation, but because of lack of specific follow-up data, we were unable to determine which could be a better prognostic indicator and further studies are needed to take.
It is unclear about the mutual inducing relationship and specific mechanisms among HIF-1α, iNOS and COX-2. This experiment proved that expression of HIF-1α, iNOS and COX-2 in choroidal melanoma were positive correlated, and VEGF was common target gene of HIF-1α, iNOS and COX-2, through which these three factors could regulate tumor growth and formation of new blood vessels. Regulation of HIF-1α generation is mainly carried out by hydroxylase induced by oxygen, and under common oxygen condition, proline hydroxyl of enzyme (PHD) make the proline dipeptide in oxygen dependent degradation structure area of HIF-1α hydroxylated,and then the hydroxylated proline dipeptide combine a tumor suppressor protein pVHL and lead to ubiquitination and degradation. And NO is inhibitor of hydroxylase which could competitively combine iron activating locus in PHD with oxygen, and prevent the combination of oxygen and PHD to inhibit the activity of PHD and reduce degradation of HIF-1α. On the other hand HIF-1α can regulate formation of iNOS. Under anoxic condition, HIF-1α can combine 5'flanking region 5'-TACGTGCT-3'of iNOS gene encoding area and start coding process to induce synthesis of NO and further iNOS, which produce vasodilatation and increased blood flow to improve tissue oxygen supplement. Research has shown that COX-2 can catalyze HIF-1α through its product PGE2 by COX - 2 / PGE2 / ERKp/ HIF-1α /VEGF way and promote angiogenesis[9], and HIF-1α is the main adjusting factor for PGE2 to induce VEGF expression. HIF-1α can combine anoxic reaction component of COX-2 promoter to start transcription of COX-2, and COX-2 could activate MAPK and/or PI3K signal transduction pathway through its production PGE2 counter-product on HIF-1α to regulate its expression, thus all these form a positive feedback network to commonly induce tumor angiogenesis and promote the growth of tumors.
To sum up, this experiment was about relationship between the expressions of HIF-1α, iNOS and COX-2 with that of VEGF in choroidal melanoma to test role of the above three kinds of protein in cancer development and metastasis process in angiogenesis aspect. Because this experiment was only done in the level of protein, in order to give the complete description of role of HIF-1α, iNOS and COX-2 in choroidal melanoma development, we have to do related research in gene level, which will be a follow-up experiment. In addition, due to lack of complete follow-up data, we had another shortage that did not make clear of the above three proteins in clinical prognostic evaluation. And we will carry out further studies to know significance of HIF-1α, iNOS and COX-2 in the tumor prevention, diagnosis, treatment and prognostic judgment.
REFERENCES
- 1.Tang CH, Grimm EA. Depletion of endogenous nitric oxide enhances cisplatininduced apoptosis in a p53-dependent manner in melanoma cell lines. J Biol Chem. 2004;279(1):288–298. doi: 10.1074/jbc.M310821200. [DOI] [PubMed] [Google Scholar]
- 2.Johansson CC, Mougiakakos D, Trocme E, All-Ericsson C, Economou MA, Larsson O, Seregard S, Kiessling R. Expression and prognostic significance of iNOS in uveal Melanoma. Int J Cancer. 2009;126(11):2682–2689. doi: 10.1002/ijc.24984. [DOI] [PubMed] [Google Scholar]
- 3.Calviello G, Di Nicuolo F, Gragnoli S. n3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-l and -2 and HIF-1α induction pathway. Carcinogenesis. 2004;25:2303–2310. doi: 10.1093/carcin/bgh265. [DOI] [PubMed] [Google Scholar]
- 4.Cryan LM, Paraoan L, Hiscott P, Damato BE, Grierson I, Gray D, Farrell M, Doherty GA, Fitzgerald DJ, O'Brien C. Expression of COX-2 and prognostic outcome in uveal melanoma. Curr Eye Res. 2008;33(2):177–184. doi: 10.1080/02713680701867908. [DOI] [PubMed] [Google Scholar]
- 5.Kuzbicki L, Sarnecka A, Chwirot BW. Expression of cyclooxygenase-2 in benign naevi and during human cutaneous melanoma progression. Melanoma Res. 2006;16(1):29–36. doi: 10.1097/01.cmr.0000194430.77643.a0. [DOI] [PubMed] [Google Scholar]
- 6.Becker MR, Siegelin MD, Rompel R, Enk AH, Gaiser T. COX-2 expression in malignant melanoma: a novel prognostic marker? Melanoma Res. 2009;19(1):8–16. doi: 10.1097/CMR.0b013e32831d7f52. [DOI] [PubMed] [Google Scholar]
- 7.Chwirot BW, Kuzbicki Ł. Cyclooxygenase-2 (COX-2): first immunohistochemical marker distinguishing early cutaneous melanomas from benign melanocytic skin tumours. Melanoma Res. 2007;17(3):139–145. doi: 10.1097/CMR.0b013e3280dec6ac. [DOI] [PubMed] [Google Scholar]
- 8.Soo R, Putti T, Tao Q, Goh BC, Lee KH, Kwok-Seng L, Tan L, Hsieh WS. Overexpression of cyclooxygenase-2 in nasopharyngeal carcinoma and association with epidermal growth factor receptor expression. Arch Otolaryngol Head Neck Surg. 2005;31(2):147–152. doi: 10.1001/archotol.131.2.147. [DOI] [PubMed] [Google Scholar]
- 9.Huang SP, Wu MS, Shun CT, Wang HP, Hsieh CY, Kuo ML, Lin JT. Cyclooxygenase-2 increases hypoxia-inducible factor-1 and vascular endothelial growth factor to promote angiogenesis in gastric carcinoma. J Biomed Sci. 2005;12(1):229–241. doi: 10.1007/s11373-004-8177-5. [DOI] [PubMed] [Google Scholar]