Abstract
AIM
To research the effect of erythropoietin (EPO) to the HIF-1\iNOS signal transduction path in retina in chronic ocular hypertension rat.
METHODS
One hundred and twenty Wistar rats were divided into 12 groups randomly. Two episcleral veins were coagulated unilaterally in rats with electric coagulator to establish the glaucoma model. PT-PCR and Western Blot analysis were used to examine the expression of Caspase-9 genes in retina. And the changes of ERG-b wave before and after were detected using EPO.
RESULTS
In EPO drug treatment group, the amplitude of ERG-b wave of retina restored remarkably. There was significant difference between two groups (P<0.05). The expressions of HIF-1\iNOS mRNA and protein in EPO drug treatment group were weakened remarkably. It was statistically different compared with the non-drug treatment group.
CONCLUSION
One of protect mechanisms of EPO to injured retina caused by chronic intraocular hypertension is through HIF-1\iNOS signal conduct path.
Keywords: EPO, HIF-1, iNOS, ocular hypertension, retina
INTRODUCTION
Erythropoietin (EPO) is a water-solubility sialic-contained glycoprotein. It has a 20-year-history as a safety treatment method to renal anemia. EPO is not only a new neurotransmitter in central nervous system, but also proved as a new perspective neuro-protective agent protecting neuron when hypoxia happened in central nerve system in recent researches[1],[2]. Wondering whether EPO has the same protection to hypoxia neuron in glaucoma, we inject rhEPO intraperitoneal to ocular hypertension rat, observe the effect of rhEPO to retina and the relationship between EPO and hypoxia accommodation factor HIF-1α, iNOS, then discuss the relation between the hypoxia signal conduct pathway and retina injury caused by glaucoma.
MATERIALS AND METHODS
Materials
A total of 120 Wistar male rats , age 40-50 days, weight 200-250g and without any eye disease, were provided by Experimental Animal Center of China Medical University. One hundred and twenty Wistar rats were divided into 12 groups randomly, included blank group, rats under ocular hypertension 3-, 7-, 14-, 21-, and 28-day groups, rats injected EPO group, rats injected EPO and under ocular hypertension 3-, 7-, 14-, 21-, and 28-day groups, each group had 10 rats 20 eyes. Wistar rat was intraperitoneal injected by 100g/L chloral hydrate 3mL/kg. Oxybuprocaine hydrochloride eye drop was dropped into Wistar rat's eye twice for topical anesthesia after it were totally sedated. And then the temporal and superior conjunctiva bulbi were opened for revealing the sclerotic superficial vein. There was a relative straight superficial vein in each of the follow sclera region, temporal sclera region, superior-temporal sclera region, inferior-temporal sclera region and nasal sclera region. Two or three of them were coagulated by heat cure haemostat in experiment group, while did nothing to the veins in blank group. The incision of the conjunctiva was not sutured. Dexamethasone eye drop and Erythromycin eye ointment were used after the surgery. Tono-Pen II tonometer was used to measure the intraocular tension of the Wister rats in the following times: before the surgery; half an hour after the surgery; 3, 7, 14, 21, and 28 days after the surgery. The ocular pressure after the surgery was 40% higher than that before surgery, and was considered as chronic ocular hypertension model successful.
Methods
The drug Epiao (rhEPO) were purchased by Shenyang Sansheng Medicine Limited Company. Wistar rats in drug group were intraperitoneally injected with rhEPO 1 day before the surgery, 2, 6, 13, 20 and 27 days after the surgery. Each time, rhEPO 5 000U/kg was injected. Bilateral eyeball were removed into 0°C normal sodium immediately after the animal was killed. Then the eyeball was incised in coronal plane along pars plana corporis ciliaris, threw away the anterior segment of the eye. The integrity retina was separated carefully, put into liquid nitrogen at once after blot the water by filter paper, and conserved in -80°C refrigerator after first 24 hours prepared for extracting RNA and protein.
Target gene mRNA measurement
We used RT-PCR for detecting the target gene mRNA. The total RNA in different group was detected according to the method of the Trizol kit produced by American Gibco Company. Endo-reference gene GAPDH, target gene HIF-1α and iNOS primer were synthesized according to references. Primer series was recorded in the Table 1 belowed, all the primer were synthesized by Dalian Bao Biotechnology Company. 95°C 5 minutes for reverse transcription, 94°C 40 seconds for degeneration, 60°C 40 seconds for anneal, 72°C 60 seconds for elongation, the RT-PCR procedure was totally 32 circulation of mentioned above. Production electrophoresis was amplified in 15g/L agarose gel, observed and photographed in UVI pro gel image analysis system. Flourchem V2.0 software was used for scanning analysis. The ratio of the target gene strap absorbance (A) and GAPDH trap absorbance (A) was used as the relative amount of the target gene mRNA.
Table 1. PCR primer and product length of target gene HIF-1α and iNOS GAPDH.
| Gene | Primer series | PCR product length |
| Endo-reference | ||
| GAPDH | Upstream: 5'- ACC ACA GTC CAT GCC ATC AC-3' | 452bp |
| Downstream: 5'- TCC ACC ACC CCT GTT GCT GTA-3' | ||
| Target gene | ||
| HIF-1α | Upstream: 5'- CTG ATT GCA TCT CCA CCT TCT ACC -3' | 343bp |
| Downstream: 5'- TTC CAA GAA AGC GAC ATA GTA GGG -3' | ||
| iNOS | Upstream: 5'- TCC AGG AGG ACA TGC AGC AC -3' | 260bp |
| Downstream: 5'- TCT TGA CGC CTT CCC GC -3' | ||
Protein measurement
We used Western blot for detecting protein: the retina tissue was put hypersound homogenate, 12 000r/min, centrifuged for 30 minutes,then took the supernatant, detected the protein concentration by phenol reagent method, regulated the protein concentration of different groups into the same level. 30μL protein was taken into sample buffer and boiled for 5 minutes, electrophoresis in 150g/L SDS-PAGE, then electro-trarsmembrane to nitrocellulose filter, sealed off in room temperature with 80g/L dried skim milk prepared by TBS (20mmol/L Tris-HCl,150mmol/L NaCl, pH7.4). Rabbit-anti-rat HIF-1α, iNOS (Wuhan Boshide Company, work concentration 1:200) was incubated overnight at 4°C. TTBS (TBS add 10g/L Tween220) and washed membranes. Second antibodies (horseradish peroxidase labeling Caprine-anti-rabbit IgG, 1:2 000), incubated 2 hours at room temperature and terminated after added coloration fluid 15-30 minutes. Then computer scanned after coloration. The result scanned and analyzed by Floorchem V2.0 software, using the ratio of the absorbance (A) of interest protein strap and absorbance (A) of β-actin strap as the relative amount of the interest protein.
Electroretinography
We measured the change of ERG-b by VETS-3 electrophsiolography, the amplitude of ERG-b by the method of cornea-contacted electrode after Wistar rat was totally anesthetized, mydriasis and dark adaptationed. Floor-electrode was pricked into homonymy subcutaneous of ear edge, reference-electrode pricked into subcutaneous of frontal, corneal working electrode connected with corneal contact lens. With full-field-vision flashing stimulator (distance of corneal plane to white stimulation light was 15cm, intensity of light was 3.93cd·m−2, frequency of stimulation was 1.9Hz, band width was 1-100Hz), we recorded the average value of the measurement of three times continuous ERG-b wave amplitude, the interval time of two stimulate was 5 seconds. ERG-b wave amplitude means the vertical dimension of wave trough of a wave to wave crest of b wave, the unit is μV.
Statistical Analysis
The result was expressed in mean±standard deviation. The comparison among multi-groups was performed with one way-ANOVA using SPSS12.0 software.
RESULTS
ERG-b wave amplitude in retina
ERG-b wave of the medication group was recovered obviously on 7th intraocular hypertention. On 28th intraocular hypertention the b wave of the control group was only 61% of the normal, while the medication group was 80%. The difference was significant (Table 2).
Table 2. ERG-b wave amplitude in retina after EPO injection.
| Operation | Control | EPO |
| before | 113.05±9.53 | 119.45±11.43 |
| 3 days after | 92.71±7.38 | 97.34±8.45 |
| 7 days after | 72.37±8.15 | 98.21±10.23a |
| 14 days after | 65.51±0.042 | 95.57±9.29b |
| 21 days after | 68.65±0.059 | 93.32±6.42a |
| 28 days after | 69.91±0.037 | 95.32±9.12a |
aP<0.05, bP<0.01 vs control group
(mean±SD, μV, n=10)
HIF-1α, iNOS mRNA expression in retina
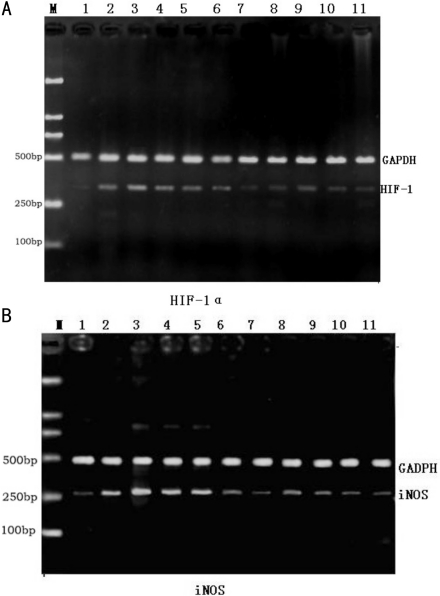
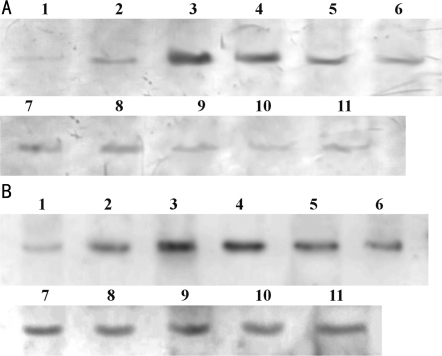
iNOSmRNA and HIF-1αmRNA in control group were compared with those in medication group (P>0.05); iNOSmRNA increased gradually accompanied with intraocular condition lasted, and increased to the maximum on 7th day. While in the medication group, the increasing tendency of iNOSmRNA and HIF-1αmRNA was obviously weaken, except under ocular hypertension 3-day group, other medication group compared with control group (P<0.05), difference was statistical significance (Figure 1, Table 3). Protein expression of HIF-1α and iNOS in retina after EPO injection was shown in Figure 2 and Table 4.
Figure 1. HIF-1α and iNOS mRNA expression in retina.
A: HIF-1α; B: iNOS. M: DNA ladder; 1: control group; 2-, 3-, 4-, 5-, 6-day groups; 3-, 7-, 14-, 21-, 28-day groups; 7, 8, 9, 10, 11: EPO injection in 3-, 7-, 14-, 21-, 28-day groups
Table 3. HIF-1α and iNOS mRNA expression in retina after EPO injection.
| Operation | HIF-1α mRNA |
iNOS mRNA |
||
| Control | EPO | Control | EPO | |
| Before | 0.248±0.087 | 0.234±0.072 | 0.259±0.053 | 0.284±0.037 |
| 3 days after | 0.408±0.029 | 0.240±0.027a | 0.423±0.085 | 0.358±0.026 |
| 7 days after | 0.512±0.028 | 0.288±0.031b | 0.676±0.065 | 0.448±0.0441b |
| 14 days after | 0.406±0.032 | 0.312±0.026a | 0.526±0.042 | 0.376±0.029b |
| 21 days after | 0.342±0.044 | 0.226±0.027a | 0.414±0.059 | 0.336±0.032a |
| 28 days after | 0.338±0.026 | 0.240±0.020a | 0.394±0.037 | 0.328±0.026a |
aP<0.05, bP<0.01 vs control group
(mean±SD, A, n=5)
Figure 2. Protein expression of HIF-1α and iNOS in retina.
A: HIF-1α; B: iNOS. 1: control; 2-, 3-, 4-, 5-, 6-day groups; 3-, 7-, 14-, 21-, 28-day groups; 7, 8, 9, 10, 11: EPO injection 3-, 7-, 14-, 21-, 28-day groups
Table 4. HIF-1α and iNOS protein expression in retina after EPO injection.
| Operation | HIF-1α protein |
iNOS protein |
||
| Control | EPO | Control | EPO | |
| Before | 0.084±0.021 | 0.118±0.019 | 0.264±0.037 | 0.228±0.029 |
| 3 days after | 0.210±0.026 | 0.164±0.011 a | 0.436±0.034 | 0.404±0.019 |
| 7 days after | 0.624±0.029 | 0.262±0.019 b | 0.860±0.050 | 0.472±0.038 b |
| 14 days after | 0.456±0.032 | 0.216±0.019 b | 0.792±0.063 | 0.414±0.029 b |
| 21 days after | 0.274±0.027 | 0.214±0.027 b | 0.528±0.032 | 0.370±0.027 a |
| 28 days after | 0.242±0.019 | 0.176±0.017 b | 0.378±0.026 | 0.320±0.026 a |
aP<0.05, bP<0.01 vs control group
(mean±SD, A, n=5)
DISCUSSION
EPO a kind of sialic-contained acidoglycoprotein, composed of 165 amino acids, comes from kidney and brepho-liver. EPO has α, β type in natural[3]. r-HuEPO is a kind of glycoprotein, which produced by recombinant DNA technology and has been used in clinic generally, has the same amino acids series and the same biologic activity with the natural separated EPO. The protection to the central nerve system of EPO has become a research hot spot in recent years. Many research proved that EPO can inhibit neurocyte apoptosis while promote neurocyte alive[4]-[6]. Bocker-Meffert et al[7] added EPO when cultured the retina tissue of newborn rats, found that VEGF presented dose-dependent promoted the growth of retina ganglia cells' axonal. When EPO-R antibody was added into medium to block the action of EPO, the action of promoted growth of axonal was inhibited. By detecting the expression of EPO and EPOR in retina by RT-PCR method and immunohistochemistry method, the results indicate that EPO not only has the neuroprotective effect, but also promotes nerve regeneration effect in ischemic retina. Our experiment compared non-medication group with medication group by intraperitoneal injected rHuEPO. From the amplitude of b wave in electroretinogram, we can see that the amplitude of b wave decreased 39% in 28-day non-medication group while the amplitude only decreased 20%, the difference was significant (P<0.05). The results indicate injected ectogenic EPO can protect the ischemic retina, coincidence with other researches in the world.
Many research also proved that the hypoxia signal pathway mediated by HIF-1α and iNOS participates in signal transduction of retinal neurons apoptosis during retinal injury on chronic high ocular pressure[8]-[10]. We also wondering whether EPO can influence the hypoxia signal pathway mediated by HIF-1α. Therefore, we approached the retina protect effect of EPO by observing the changes in transcriptional level and protein synthesis of hypoxia-adaption gene iNOS in hypoxia signal conduct path. On transcriptional level of mRNA, we can see that the EPO inhibits the mRNA high-expression to HIF-1α in every time point under intraocular hypertension after inject exogenous EPO (P<0.05). The transcription level of mRNA to iNOS also has statistical significance in every time point. On protein synthesis level, the EPO inhibits the protein synthesis high-expression to HIF-1α in every time point under intraocular hypertension after inject exogenous EPO (P<0.05). Except under ocular hypertension 3-day group, other group (under ocular hypertension 7-, 14-, 21-, and 28-day group) has statistical significance on protein synthesis to iNOS. The results above first indicate that EPO inhibition effect to hypoxia-adaption gene in intraocular hypertension retina, especially to HIF-1α. And then the inhibition effect of EPO to protein synthesis is delayed than to mRNA transcription. On one hand, it just proved that the inhibition effect is through a hypoxia signal conduct path. As another target gene of HIF-1α, EPO first inhibits the activation of HIF-1α by negative feedback, then inhibits the transcription and protein synthesis of downstream target gene through HIF-1α. On the other hand, it also illustrates that the protection effect of EPO to injured retina has a concentration accumulation procedure, needs repeat and multiple application. In a word, the protection mechanism of EPO to injured retina caused by chronic intraocular hypertension is through HIF-1α mediated hypoxia signal conduct path. EPO inhibits the activation of HIF-1α through negative feedback, then inhibits the transcription of iNOS to avoid neurotoxicity caused by over-synthesis of iNOS. Besides, EPO protects injured retina caused by intraocular hypertension through regulate the hypoxia adaption gene, it also proves that the hypoxia signal conduct path mediated by HIF-1α is the major pathomechanism of retina injury caused by glaucoma.
REFERENCES
- 1.Juul S. Erythropoietin in the central nervous system, and its use to prevent hypoxic ischemic brain damage. Acta Paediatr Suppl. 2002;91(438):36–42. doi: 10.1111/j.1651-2227.2002.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Xiong Y, Mahmood A, Meng Y, Qu C, Schallert T, Chopp M. Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit. Brain Res. 2009;1294:153–164. doi: 10.1016/j.brainres.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buemi M, Cavallaro E, Floccari F, Sturiale A, Aloisi C, Trimarchi M, Grasso G, Corica F, Frisina N. Erythropoietin and the brain: from neurodevelopment to neuroprotection. Clin Sci (Lond) 2002;103(3):275–282. doi: 10.1042/cs1030275. [DOI] [PubMed] [Google Scholar]
- 4.Morishida E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76(1):105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 5.Weishaupt JH, Rohde G, Pölking E, Siren AL, Ehrenreich H, Bähr M. Effect of Erythropoietin axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45(5):1514–1522. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- 6.Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, Rosenbaum PS, Cerami A, Brines M, Rosenbaum DM. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2002;99(16):10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Böcker-Meffert S, Rosenstiel P, Röhl C, Warneke N, Held-Feindt J, Sievers J, Lucius R. Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest Ophthalmol Vis Sci. 2002;43(6):2021–2026. [PubMed] [Google Scholar]
- 8.Pang IH, Johnson EC, Jia L, Cepurna WO, Shepard AR, Hellberg MR, Clark AF, Morrison JC. Evaluation of inducible nitric oxide synthase in glaucomatous optic neuropathy and pressure-induced optic nerve damage. Invest Ophthalmol Vis Sci. 2005;46(4):1313–1321. doi: 10.1167/iovs.04-0829. [DOI] [PubMed] [Google Scholar]
- 9.Tezel G, Wax MB. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol. 2004;122(9):1348–1356. doi: 10.1001/archopht.122.9.1348. [DOI] [PubMed] [Google Scholar]
- 10.Whitlock NA, Agarwal N, Ma JX, Crosson CE. Hsp27 upregulation by HIF-1 signaling offers protection against retinal ischemia in rats. Invest Ophthalmol Vis Sci. 2005;46(3):1092–1098. doi: 10.1167/iovs.04-0043. [DOI] [PubMed] [Google Scholar]