Abstract
AIM
To investigate whether bis(7)-tacrine, a multifunctional drug, inhibits N-methyl-D-aspartate (NMDA) -activated current in retinal ganglion cells(RGC) and provides neuroprotection against retinal cell damage.
METHODS
Purified RGC cultures were obtained from retinas of 1-3 days old Sprague-Dawley(SD) rats, following a two-step immunopanning procedure. After 7 days of cultivation, the inhibition of NMDA-activated current by bis(7)-tacrine was measured by using patch-clamp recording techniques. In animal experiments, RGCs were damaged after intravitreal injection of NMDA (5µL, 40nmol) in adult rats. Bis(7)-tacrine(0.05, 0.1, 0.2mg/kg) or memantine(20mg/kg) was intraperitoneal administered to the rats fifteen minutes before intravitreally injection of NMDA. RGC damage was analyzed by histologic techniques, TUNEL and retrograde labeling techniques.
RESULTS
Whole-cell patch-clamp recordings demonstrated that NMDA (30µmol/L) resulted in approximately -50 pA inward currents that were blocked by bis(7)-tacrine(1µmol/L). Histological examination and retrograde labeling analysis revealed that bis(7)-tacrine induced a significant neuroprotective effect against NMDA-induced cell damage 7 days after NMDA injection. TUNEL staining showed that pretreatment with bis(7)-tacrine was effective in ameliorating NMDA-induced apoptotic cell loss in the retinal ganglion cell layer 18 hours after injection.
CONCLUSION
Bis(7)-tacrine possesses remarkable neuroprotective activities against retinal excitotoxicity through inhibition of NMDA receptors.
Keywords: bis(7)-tacrine, N-methyl-D-aspartate receptors, excitotoxicity, neuroprotection
INTRODUCTION
Retinal ganglion cells (RGC) are responsible for the transmission of visual signals to the brain via their axons that comprise the optic nerve. Progressive death of RGC occurs in glaucoma, and excitotoxic mechanisms have been extensively implicated in its pathogenic processes[1]. Glutamate is a major excitatory amino acid in the inner retina and high levels of glutamate in the vitreous humor are associated with glaucoma. Excessive activation of glutamate receptors, mainly the N-methyl-D-aspartate (NMDA) type, results in an increase in intracellular calcium and initiates a cascade of events that lead to necrosis and/or apoptosis. Retinal ganglion cells express NMDA receptors and are susceptible to NMDA-induced damage .
Multiple lines of evidence have showed that antagonists of the NMDA receptor are neuroprotective in animal models of diseases. Memantine is an uncompetitive NMDA receptor antagonist used for Alzheimer's disease[2]; however, two recent parallel clinical trials of oral memantine in patients with chronic progressive open-angle glaucoma were unsuccessful[3],[4]. A neuroprotectant that has a single mode of action, such as memantine, has a limited positive effect on reducing the ganglion cell death. Therefore, it is suggested that neuroprotectants with multiple modes of actions are likely to reveal clearer results than was found for memantine[3].
Bis(7)-tacrine (1,7-N-heptylene-bis-9,9′-amino-1,2,3,4-tetrahydroacridine) possesses multi-potencies including anti-acetylcholinesterase(AChE), anti- β Amyloid protein (Aβ)[5], anti-NMDA receptors, anti-nitric oxide synthase (NOS) signaling and the regulation of the downstream signal of NMDA receptors[6]. In view of its multiple neuroprotective activities, bis(7)-tacrine has been proposed as a promising agent for the treatment of Alzheimer's disease.
We have reported that bis(7)-tacrine prevents glutamate- induced hippocampal neuron apoptosis by noncompetitively antagonizing NMDA receptors[7],[8]. Furthermore, we found that bis(7)-tacrine had neuroprotective effects against glutamate-induced RGC damage in vitro and in vivo[9]. We hypothesized that bis(7)-tacrine attenuated glutamate- induced retinal ganglion cells damage through the blockade of NMDA receptors. Our research showed that bis(7)-tacrine inhibited NMDA-induced current in cultured RGC and protected RGC against NMDA-induced cell damge in vivo. Therefore, the results of this study suggest that bis(7)-tacrine is a neuroprotectant via the blockade of NMDA receptors.
MATERIALS AND METHODS
Materials
Sprague-Dawley(SD) rats, including 1-3 days rats and adult male rats, were obtained from the Animal Center in Medical College, Yangtze University, and were housed in the animal facility under standard conditions of room temperature and a 12:12 h light-dark cycle with free access to food and water. All animal experiments followed the guidelines for the care and use of animals established by Medical College, Yangtze University and adhered to the tenets of the Declaration of Helsinki. Bis(7)-tacrine was purchased from Cayman Chemical Co.(USA). Fluorogold was purchased from Biotium (Hayward, USA). Unless noted, all other reagents were obtained from Sigma (St. Louis, MO, USA). Primary culture and purification of RGC from 1-3 days rats was prepared as previously described[9]. The retinal tissue was dissociated and incubated in a polypropylene tube coated with an anti-rat macrophage monoclonal IgG (Chemicon International, Inc, CA, USA) to exclude macrophages, and then incubated in a tube coated with an anti-rat Thy 1.1 monoclonal IgG (Chemicon International, Inc, CA, USA ). The tube was gently washed with PBS for five times, and adherent RGC were collected by centrifugation at 600g for 5 minutes. Next, RGC were grown in serum-free medium(Gibco,China), containing 1 mmol/L glutamine, 10µg/L gentamicin, B27 supplement (1:50), 40µg/L each of brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF), and 5µmol/L forskolin. Purified RGC were plated at a low density of approximately 500 cells/cm2 of growth substrate. This plating density provided cultures in which most RGC grew in physical isolation from other cells. RGC were cultured for 7 days and then exposed to NMDA and/or bis(7)-tacrine for patch-clamp recording.
Methods
Whole-cell electrophysiological recordings
Whole-cell patch-clamp recording was performed at room temperature using an Axopatch 200B (Molecular Devices, Sunnyvale, CA, USA) amplifier as described previously[8]. In brief, neurons were placed in Mg2+-free extracellular medium containing (in mmol/L): NaCl 150, KCl 5, CaCl2 0.2, HEPES 10, glucose 10, EDTA 10, and sucrose 10; pH was adjusted to 7.4 with NaOH and osmolality was adjusted to 340mOsmol/kg with sucrose. The low concentration of Ca2+ was used to minimize the calcium-dependent inactivation of NMDA-activated current. The patch-pipettes were filled with internal solution containing (mmol/L): KCl 140, MgCl2 2.5, HEPES 10, EGTA 11 and ATP 5; pH was adjusted to 7.2 with KOH and osmolality was adjusted to 310mOsmol/kg with sucrose. The membrane potential was held at -60mV, unless noted otherwise. Drug solutions were prepared in extracellular medium and applied to neurons by using one of two systems. Patches were exposed to 30µmol/L NMDA in the absence or the presence of 1µmol/L bis(7)-tacrine in external solution.
NMDA-induced neurotoxicity
Male rats (220-280g) were anesthetized by intraperitoneal injection of 100g/L (w/v) chloral hydrate (350mg/kg) and rectal body temperature was maintained at 37°C with a heating pad during the experiments. The pupils were dilated with tropicamide, and a single dose of 5µL of 8mmol/L NMDA (total amount 40nmol) in 0.01mol/L PBS (pH 7.4) was injected into the vitreous cavity using 32-gauge Hamilton needle and syringe. Fifteen minutes before intravitreal injection of NMDA, bis(7)-tacrine at the concentration of 0.05, 0.1, or 0.2mg/kg, 20mg/kg memantine or PBS was intraperitoneally administered to the rats in a volume of 1.5mL/kg. Animals intravitreally administrated with PBS alone were used as controls. Eighteen rats were evenly divided into six experiment groups as described. Six eyes per group were used. Rats were sacrificed with an overdose of chloral hydrate 7 days after intravitreally injection of NMDA and both eyes were enucleated. Eight paraffin-embedded sections cut through the optic disc of each eye were prepared and stained with hematoxylin and eosin. Three sections from each eye being used for the morphometric analysis. Light microscope images were photographed, and the cell counts in the ganglion cell layer (GCL) at a distance between 1mm and 2mm from the optic disc and the thickness of the inner plexiform layer (IPL) were measured on the photographs by a single observer. Data from three sections (selected randomly from the eight sections) were averaged for each eye and used to evaluate the cell count in the GCL and the thickness of the IPL.
TdT-dUTP TUNEL staining
TUNEL staining was performed according to the manufacturer's protocols (In Situ Cell Death Detection Kit; Roche China, Ltd.) to detect retinal cell apoptosis induced by NMDA. Twelve rats were evenly divided into four groups: three NMDA-intravitreally-injected groups with the pretreatment of bis(7)-tacrine-treated groups(0.2mg/kg), memantine-treated groups (20mg/kg), or PBS, and one control group. In this study, 18 h after the NMDA injection, rats were sacrificed and the eyes were immediately enucleated and fixed in 40g/L paraformaldehyde in PBS for the TUNEL studies. The specimens were then dehydrated and embedded in paraffin and 5µm sections were cut. These sections were stained by the TUNEL method. The yellow condensed TUNEL positive cells were counted in the retinal ganglion cell layer (GCL) manually at 1.0-2.0mm (both sides) from the center of the optic disc. No attempt was made to distinguish the cell types in the GCL, and displaced amacrine cells were not excluded from the counts. The average number of TUNEL positive cells/eye was obtained from three sections of each retina.
RGC counting
To examine the change in the number of RGC following NMDA injection, RGC were retrogradely labeled with a fluorogold. Eighteen rats were divided and treated as described in NMDA-induced neurotoxixity above. Four days after the NMDA injection, retrograde labeling of RGC was made. Briefly, rats were anesthetized by intraperitoneal injection with 100g/L (w/v) chloral hydrate (350mg/kg) and then the heads were fixed in a stereotaxic apparatus. Fluorogold was microinjected bilaterally into the superior colliculi (SC) and dorsal lateral geniculate nuclei (dLGN) of rats. Three days after the Fluorogold injection (seven days after the NMDA injection), the animals were sacrificed and the eyes were fixed with 40g/L paraformaldehyde for 1 hour. Retinas were removed from the sclera, divided into four radial cuts and mounted on slides. Analysis for the number of Fluorogold labeled RGC was carried out. Briefly, Tracer-labeled RGC counting was performed in 12 areas of 0.072 mm2 each (three areas per retinal quadrant) at 2/6, 3/6, and 5/6 of the retinal radius under a fluorescent microscope (Olympus IX71). Data from 12 areas from each eye were averaged.
Statistical Analysis
Data are expressed as mean±SEM, with a minimum n=6. One-way ANOVA and Tukey's test were used for statistical analysis for all measurements. P<0.05 was considered statistically significant.
RESULTS
NMDA-activated Current in Cultured RGC
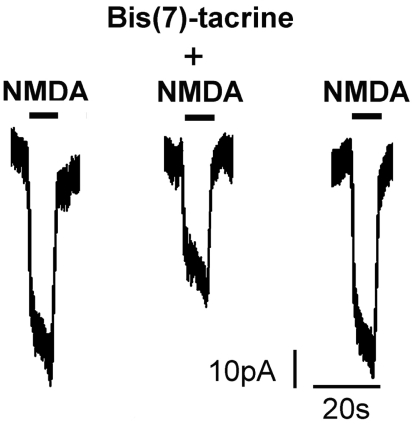
RGC in mixed retinal cultures have previously been shown to express NMDA currents[10]. To determine whether NMDA receptors in RGC could be inhibited by bis(7)-tacrine, we analyzed membrane currents activated by NMDA with or without bis(7)-tacrine. Currents were recorded in RGC cultured for 7 days. Thirty micromolars NMDA elicited small membrane currents, approximately -50 pA. One micromolar bis(7)-tacrine inhibited the NMDA current, and the inhibition was reversible upon washout of bis(7)-tacrine (Figure 1).
Figure 1. Inhibition of NMDA-activated current by bis(7)-tacrine in RGC in vitro.
Bis(7)-tacrine: 30µmol/L; NMDA: 1µmol/L
Neuroprotective Effects of Bis(7)-tacrine
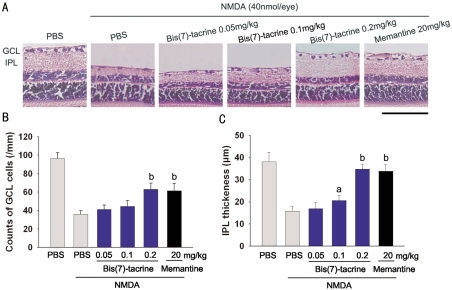
Typical photomicrographs of the retina taken 7 days after intravitreal injection of NMDA at 40 nmol/eye are shown in Figure 2A. No attempt was made to distinguish the cell types in the GCL, and displaced amacrine cells were not excluded from the counts. NMDA reduced both the cell count in GCL [(36.0±3.9)cells/mm] and the thickness of IPL[(15.8±2.0)µm] in the retina versus those of the vehicle-treated normal retina [(96.50±6.21)cells/mm for GCL and (38.1±4.3)µm for IPL] (Figure 2B, 2C). The cell number of GCL in pretreatment with bis(7)-tacrine (0.05, 0.1, 0.2mg/kg) or memantine(20mg/kg) were (41.4±4.4, 44.5±6.3, 63.0±6.8,61.4±8.0)cells/mm, respectively. The results demonstrate that pretreatments with 0.2mg/kg bis(7)-tacrine or 20mg/kg memantine significantly suppressed the decreases in the cell count in GCL induced by NMDA(P<0.01). Bis(7)-tacrine at the dose of 0.2mg/kg also inhibited thinning of the IPL in comparison with NMDA-injected eyes[(34.8±2.3)µm vs (15.8±2.0)µm, P<0.01].
Figure 2. Protective effects of bis(7)-tacrine on retinal damage induced by intravitreal injection of NMDA(40nmol).
aP<0.05, bP<0.01 vs NMDA; Scale bar, 100µm
TUNEL-positive Cell Counts
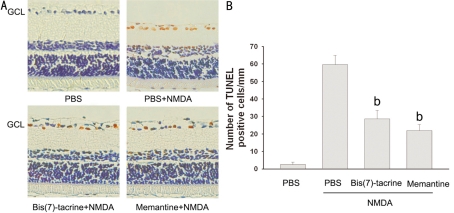
To examine whether bis(7)-tacrine reduces apoptotic cell death resulting from NMDA excitotoxicity, we conducted TUNEL staining of the retina 18 h after intravitreal injection of NMDA. Morphometric analysis showed that little TUNEL positivity was detected in the control (PBS-injected) eyes. However, at 18 h after NMDA was injected into the eyes, prominent TUNEL-positive cells were found in the ganglion cell layer (GCL) (Figure 3A). The number of TUNEL-positive cells was (59.6±5.3)cells/mm in the NMDA group whereas it was (28.6±4.9)cells/mm in the animals receiving bis(7)-tacrine at 0.2mg/kg and (22.0±3.4)cells/mm receiving memantine at 20mg/kg (Figure 3B). Both bis(7)-tacrine and memantine significantly reduced NMDA-induced TUNEL-positive cells in the GCL(P<0.01).
Figure 3. Bis(7)-tacrine reduces NMDA-induced apoptosis of ganglion cell layer(GCL) cells in vivo.
A: Representative photographs of retina in the vehicle group, the NMDA group and the NMDA group with peritoneal injection of bis(7)-tacrine(0.2mg/kg) or memantine(20mg/kg); B: The number of TUNEL-positive cells in GCL was counted. NMDA treatment plus NMDA receptor blocker was tested vs NMDA treatment alone bP<0.01 vs NMDA; Scale bar, 100µm
Retrograde Labeling RGC
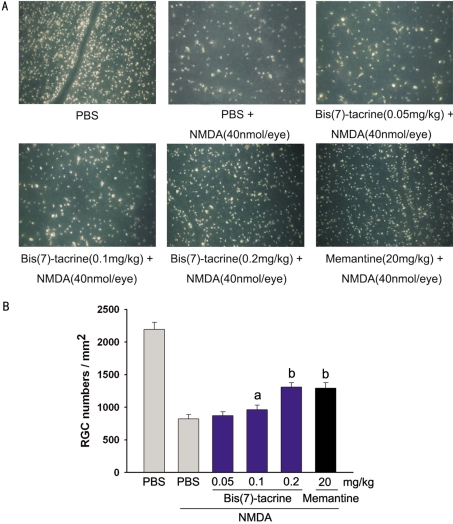
The RGC layer is composed of about equal numbers of RGC and displaced amacrines. Therefore, to ensure accurate identification of RGC, we retrogradely labeled the RGC in rats with fluorogold allowing us to selectively label greater than 99% of the RGC. In PBS-injected eyes, the mean density of RGC was (2194±108)cells/mm2 (n=6) (Figure 4A). Fifteen minutes after intraperitoneal injection of bis(7)-tacrine (0.05, 0.1, 0.2mg/kg), memantine (20mg/kg) or PBS, 40nmol NMDA was intravitreally injected into the same animals. At 7 days after NMDA was injected into the eyes, the mean densities for the RGC for positive control (PBS-injected) and experimental (0.05, 0.1, 0.2mg/kg bis(7)-tacrine and 20mg/kg memantine) eyes were (822±66, 871±60, 962±71, 1311±65, 1293±84)cells/mm2 (n=6)(Figure 4B), respectively, and the differences were statistically significant (PBS vs 0.1mg/kg bis(7)-tacrine, P=0.011; PBS vs 0.2mg/kg bis(7)-tacrine, P<0.01; PBS vs 20mg/kg memantine, P<0.01). These data showed that both of bis(7)-tacrine and memantine possessed neuroprotective effects against NMDA-induced RGC death, and bis(7)-tacrine (0.2mg/kg) had the same effects as did memantine (20mg/kg).
Figure 4. Protective effects of bis(7)-tacrine on retinal ganglion cells(RGC) following intravitreal injection of NMDA (40nmol).
aP<0.05, bP<0.01 vs NMDA; Scale bar, 100µm
DISCUSSION
In this study, it was shown that bis(7)-tacrine inhibited NMDA-activated current in cultured retinal ganglion cells and possessed remarkable neuroprotective effects in a rat NMDA-induced excitotoxicity model. This is the first report to demonstrate the blockade of NMDA receptors by bis(7)-tacrine in RGC, and provide evidence of a neuroprotective effect of bis(7)-tacrine on RGC in a rat excitotoxicity model. Bis(7)-tacrine is a multifunctional drug that has been proposed as a promising agent to treat Alzheimer's disease[6]. This unique compound acts through a multitude of mechanisms, including concurrent inhibition of AChE, the NMDA receptor, nitric oxide synthase, and amyloid precursor protein/β-amyloid cascade[6],[11]. Because there are multiple factors closely indicated in the pathogenesis of glaucoma, multiple drug therapy will be required to address the varied pathological aspects of this disease. It has been demonstrated that there is a strong link between basic cellular processes in glaucoma and Alzheimer's disease[12]. Therefore, we attempted to study the neuroprotective effects of bis(7)-tacrine against excitotoxin-induced RGC damage. In our previous study, we found that bis(7)-tacrine attenuated glutamate-induced RGC injury and speculated that the actions of the drug might be related with the inhibition of NMDA receptors[9]. In this study, the electrophysiological properties of NMDA responses on retinal ganglion cells were examined with the use of the whole-cell voltage-clamp technique. We found that the whole-cell current amplitude was smaller in RGC than in hippocampal neurons in our previous study[8]. These findings in the present study were in agreement with previous reports. Furthermore, our results showed that 1µmol/L bis(7)-tacrine reduced NMDA-activated current in retinal ganglion cells, indicating that bis(7)-tacrine is a NMDA receptor antagonist. At this point, however, further studies will be required to verify the blocking kinetics of bis(7)-tacrine on NMDA receptor and determine the role of calcium permeation through NMDA receptor.
Intraocular injection of NMDA in the adult rats' eye provides a useful model of excitotoxicity to explore the pathogenesis of various degenerative diseases inclusive of glaucoma. In this study, histological examination showed that intravitreal injection of NMDA at 40 nmol/eye induced NMDA receptor-mediated loss of nuclei from the retinal ganglion cell layer (GCL) and thinning of the thickness of the inner plexiform layer (IPL). These results are consistent with some previous reports on NMDA-induced retinal damage models[13]. Cell apoptosis in ganglion cell layer was evaluated by TUNEL method. Our data show that bis(7)-tacrine prevented NMDA-induced cell apoptosis in GCL. Retrograde labeling of RGC also documented that pretreatment with bis(7)-tacrine protects RGC against NMDA-induced neuronal damage. Memantine, a moderate NMDA receptor antagonist with a similar affinity and potency to bis(7)-tacrine in blocking NMDA receptors, was used as the positive control in the experiment. In three evaluations as above, bis(7)-tacrine(0.2 mg/kg) showed the same protective effects as did memantine (20mg/kg). In summary, present study documents that pretreatment with bis(7)-tacrine, as well as memantine, induces a neuroprotective effect against NMDA-induced retinal damage. The results of patch clamp suggests that the neuroprotection of bis(7)-tacrine is related to inhibition of NMDA receptors. These findings suggest that bis(7)-tacrine may be a potential therapeutic neuroprotectant in glaucoma.
Acknowledgments
The authors have great gratitude to Prof. Chao-Ying Li for helpful discussions and technical assistance.
Footnotes
Foundation items: National Natural Science Foundation of China (No.81000380H1204); Natural Science Foundation of Hubei Province, China (No.2008CDA053); Scientific Research Fund of the Ministry of Health, Hubei Province, China (No.QJX2010-53)
REFERENCES
- 1.Casson RJ. Possible role of excitotoxicity in the pathogenesis of glaucoma. Clin Experiment Ophthalmol. 2006;34(1):54–63. doi: 10.1111/j.1442-9071.2006.01146.x. [DOI] [PubMed] [Google Scholar]
- 2.Gilling KE, Jatzke C, Hechenberger M, Parsons CG. Potency, voltage-dependency, agonist concentration-dependency, blocking kinetics and partial untrapping of the uncompetitive N-methyl-d-aspartate (NMDA) channel blocker memantine at human NMDA (GluN1/GluN2A) receptors. Neuropharmacology. 2009;56(5):866–875. doi: 10.1016/j.neuropharm.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Osborne NN. Recent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandoned. Acta Ophthalmol. 2009;87(4):450–454. doi: 10.1111/j.1755-3768.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- 4.Danesh-Meyer HV, Levin LA. Neuroprotection: extrapolating from neurologic diseases to the eye. Am J Ophthalmol. 2009;148(2):186–191. doi: 10.1016/j.ajo.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Fu H, Li W, Luo J, Lee NT, Li M, Tsim KW, Pang Y, Youdim MB, Han Y. Promising anti-Alzheimer's dimer bis(7)-tacrine reduces beta-amyloid generation by directly inhibiting BACE-1 activity. Biochem Biophys Res Commun. 2008;366(3):631–636. doi: 10.1016/j.bbrc.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Mak M, Jiang H, Wang Q, Pang Y, Chen K, Han Y. Novel Anti-Alzheimer's Dimer Bis(7)-Cognitin: Cellular and Molecular Mechanisms of Neuroprotection Through Multiple Targets. Neurotherapeutics. 2009;6(1):187–201. doi: 10.1016/j.nurt.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YW, Li CY, Luo JL, Li WM, Fu HJ, Lao YZ, et al. et al. Bis(7)-tacrine prevents glutamate-induced excitotoxicity more potently than memantine by selectively inhibiting NMDA receptors. Biochem Biophys Res Commun. 2008;369(4):1007–1011. doi: 10.1016/j.bbrc.2008.02.133. [DOI] [PubMed] [Google Scholar]
- 8.Liu YW, Luo JL, Ren H, Peoples RW, Ai YX, Liu LJ, Pang YP, Li ZW, Han YF, Li CY. Inhibition of NMDA-gated ion channels by bis(7)-tacrine: whole-cell and single-channel studies. Neuropharmacology. 2008;54(7):1086–1094. doi: 10.1016/j.neuropharm.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Fang JH, Wang XH, Xu ZR, Jiang FG. Neuroprotective effects of bis(7)-tacrine against glutamate-induced retinal ganglion cells damage. BMC Neurosci. 2010;11(1):31. doi: 10.1186/1471-2202-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aizenman E, Frosch MP, Lipton SA. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J Physiol. 1988;396:75–91. doi: 10.1113/jphysiol.1988.sp016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van der Schyf CJ, Youdim MB. Multifunctional drugs as neurotherapeutics. Neurotherapeutics. 2009;6(1):1–3. doi: 10.1016/j.nurt.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung W, Guo L, Cordeiro MF. Neuroprotection in glaucoma: drug-based approaches. Optom Vis Sci. 2008;85(6):406–416. doi: 10.1097/OPX.0b013e31817841e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimazawa M, Yamashima T, Agarwal N, Hara H. Neuroprotective effects of minocycline against in vitro and in vivo retinal ganglion cell damage. Brain Res. 2005;1053(1-2):185–194. doi: 10.1016/j.brainres.2005.06.053. [DOI] [PubMed] [Google Scholar]