Abstract
AIM
To investigate the expression of connexin 43 and epithelial cadherin (E-cadherin) in choroidal melanoma, to explore the clinical and pathological implications of expression of these proteins, and to determine their relations with malignant features.
METHODS
The expression of connexin 43 and E-cadherin in choroidal melanoma were detected by immunohistochemistry and correlated with clinicopathological features.
RESULTS
Positive rates of connexin 43 in choroidal melanomas and benign pigmented nevus tissues were 75% and 40% respectively with significant differences between the two groups (χ2=5.607, P=0.009). Positive rates of E-cadherin in choroidal melanomas and benign pigmented nevus tissues were 40% and 75% respectively with significant differences between the two groups (χ2=5.214, P=0.010). Significant overexpression of connexin 43 and reduction of E-cadherin expression was associated with the invasion to the sclera, and there were respectively significant differences between without and with scleral invasion groups (χ2=2.880, P=0.040; χ2=2.778, P=0.046). Overexpression of connexin 43 were correlated with tumor cell types and the expression of connexin 43 and E-cadherin may be correlated with each other.
CONCLUSION
The increased expression of connexin 43 and the decreased expression of E-cadherin may be involved in the process of invasion of choroidal melanoma. The overepression of connexin 43 and reduction of E-cadherin may contribute to the development of choroidal melanoma.
Keywords: choroidal neoplasms, melanoma, connexin 43, E-Cadherin, immunohistochemistry
INTRODUCTION
As the most common primary intraocular malignant tumor in adults, choroidal melanoma is prone to invasion and metastasis, and has high tumor-related mortality rate[1]. Until now there is no effective treatment for melanoma metastasis, and its biochemistry and molecular mechanisms are not clear yet. Therefore, studying on specific targets to understand the major pathophysiological process of invasion and metastasis is quite necessary, and may help us to find more effective treatment. Recent evidence suggests that gap junctions interact with cell adhesion-associated proteins, and both of them play critical roles in cell contact inhibition, differentiation and proliferation of various tumor cells[2]. Connexin 43, a common and important gap junction protein connexin[3], is the major connexin homolog expressed in choroidal melanoma. E-cadherin is a kind of cell adhesion-associated proteins. Loss and decline of expression of E-cadherin-mediated adhesion often exist in the process of the non-invasive tumors translating to invasive tumors[4]. Connexin 43 and E-cadherin participate in the same signaling events, and play important roles in carcinogenesis, malignant behavior and tumor metastasis[5]-[7]. However, in choroidal melanoma, it is still not quite clear that how connexin 43 and E-cadherin protein are expressed and whether they affect each other. In this study, we examined the expression levels of connexin 43 and E-cadherin in human primary choroidal melanoma to clarify the correlation between connexin 43 and E-cadherin, and explore the pathological and clinical implications of their expression.
MATERIALS AND METHODS
Materials
A total of 40 cases from the Department of Ophthalmic Pathology of Qingdao Affiliated Hospital and Department of Pathology of Tianjin Eye Hospital from 1990 through 2010 diagnosed as choroidal melanoma were enrolled. As the control group, 20 cases with benign pigmented nevus were collected from the Department of Ophthalmic Pathology of Qingdao Affiliated Hospital. In each case, routine hematoxylin and eosin (H& E)-stained slides were reviewed for classifying the tumors. According to the morphological features of the tumor cells, 40 cases of choroidal melanoma were identified as spindle cell type, epithelioid cell type and mixed cell type. According to the variation in size, 40 cases of choroidal melanoma were identified as small tumors (thickness<3mm, largest tumor diameter<10mm), medium tumors (thickness 3-5 mm, largest tumor diameter 10-16mm), and large tumors (thickness>5mm, largest tumor diameter>16mm). According to the sclera involvement, 40 cases of choroidal melanoma were identified as tumor without the scleral invasion, and tumor with the scleral invasion.
Methods
Immunohistochemistry was performed using the streptavidin-peroxidase method. Negative control slides were performed using PBS instead of primary antibodies (Figure 1 and Figure 2). Three-micron sections were cut from formalin-fixed, paraffin-embedded choroidal melanoma and benign pigmented nevus tissues. After de-paraffin in dimethylbenzene for 5 minutes twice, then rehydrated in gradient ethyl alcohol and distilled water, the sections were depigmented with 50g/L potassium permanganate for 5-15 minutes (according to the amount of melanin pigment), and then processed with 10g/L oxalic acid for 2-5 minutes. Epitope retrieval was performed by heating the slides in citrate buffer at pH 6.0 for 20 minutes. If necessary, epitope was retrieved twice. After getting rid of endogenous peroxidase with 30mL/L hydrogen peroxide for 10 minutes, sections reacted with confining liquid at room temperature for 15 minutes. After that, sections reacted with connexin 43 and E-cadherin antibody (mouse polyclonal antibody, Zhongshan Goldenbridge Biotechnology Corporation, China) separately at 4°C overnight, then with a biotin-conjugated anti-mouse secondary antibody for 15 minutes, and followed by a peroxidase-conjugated streptavidin for 15 minutes. Slides were developed with diaminobencidine. The developing time was controlled according to the scene under the standard light microscope. Then the sections were counterstained with hematoxylin, dehydrated, and finalized with neutral balsam.
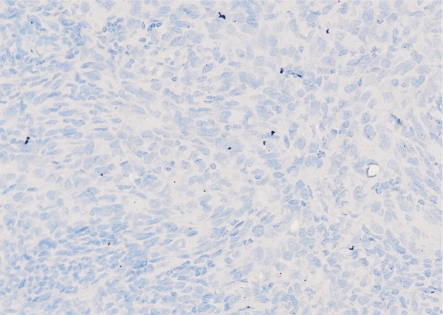
Figure 1. PBS instead of connexin 43 antibody in choroidal melanoma showed a negative staining (200×).
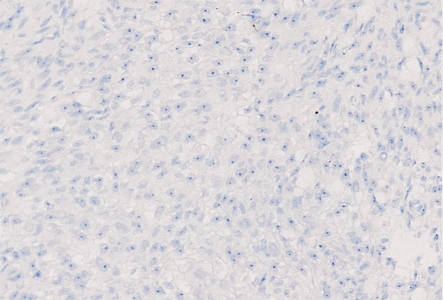
Figure 2. PBS instead of E-cadherin antibody in choroidal melanoma showed a negative staining (200×).
The immunostaining of connexin 43 and E-cadherin was graded for the percentage of the connexin 43 and E-cadherin positive tumor. That is, <25%, 25%-50%, 50%-75%, and >75% of tumor cells showed positive staining and were defined as (-), (+), (++), and (+++) respectively. Samples scoring (-) - (+) were considered negative and weak positive, and those that scored (++) - (+++) were considered strong positive[2].
Statistical Analysis
The χ2 test was used to compare the positive expression rate of connexin 43 and E-cadherin between choroidal melanoma and benign pigmented nevus, and the relationships were clarified among connexin 43, E-cadherin and clinicopathologic characteristics in choroidal melanoma. All statistical analysis was performed by using the SPSS software (version 11.5). P<0.05 was considered statistically significant.
RESULTS
The patients ranged in age from 24 to 80 (mean, 48 years). Twenty-three cases (57.5%) were men and 17 cases (42.5%) were women. Histologically, 13 cases (32.5%) were spindle cell type, 17 cases (42.5%) were epithelioid cell type and 10 cases (25%) were mixed cell type. Thirteen of the 40 cases (32.5%) were identified as small tumors, 14 cases (35%) were medium tumors, and 13 cases (32.5%) were large tumors. Twenty-five cases (62.5%) were identified without scleral invasion, while 15 cases (37.5%) with scleral invasion.
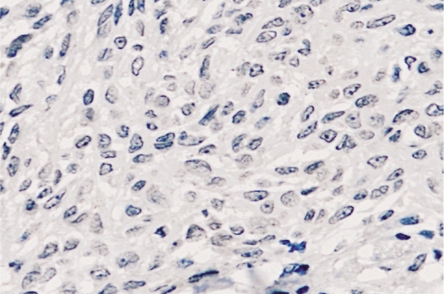
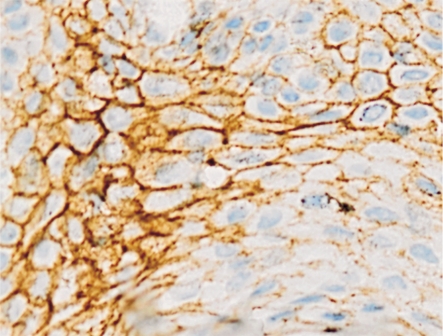
In choroidal melanoma, 30 cases (75%) showed connexin 43 immunostaining positive, and only 16 cases (40%) demonstrated E-cadherin positive. In benign pigmented nevus, 8 cases (40%) showed connexin 43 immunostaining positive with 12 cases (60%) negative (Figure 3), and 15 cases (75%) demonstrated E-cadherin positive (Figure 4). The expression of connexin 43 and E-cadherin proteins respectively had significant differences between the choroidal melanoma and benign pigmented nevus groups (χ2=5.607, P=0.009; χ2=5.214, P=0.010).
Figure 3. Connexin 43 expression in benign pigmented nevi showed a negative staining (400×).
Figure 4. E-cadherin expression in benign pigmented nevi showed a positive membranous and cytoplasmic staining (400×).
In the 25 cases (62.5%) of choroidal melanoma without scleral invasion, connexin 43 positive patterns were seen in 16 of 25 cases (64%), and 13 of these cases (52%) showed E-cadherin positive. In the 15 cases (37.5%) which had the scleral invasion, connexin 43 positive patterns were seen in 14 of 15 cases (93.9%), and only 3 of these cases (20%) showed E-cadherin positive. The expression of connexin 43 and E-cadherin proteins respectively had significant differences between without and with scleral invasion groups (χ2=2.880, P=0.040; χ2=2.778, P=0.046).
Seven of 13 spindle cell type tumors (53.8%) were observed connexin 43 immunostaining positive, 8 of 10 mixed cell type melanomas (80%) were connexin 43 reactive, while 16 of the 17 epithelioid cell type melanomas (94.1%) were connexin 43 positive. The expression of connexin 43 protein was distinct in different kinds of cell types. We observed that over-expression of connexin43 accompanied with low E-cadherin expression. Connexin 43 and E-cadherin expression may be correlated with each other. Several samples showed simultaneous mixed (membranous and cytoplasmic) expression of connexin 43 and E-cadherin, while some were cytoplasmic or membranous expression only.
DISCUSSION
Tumor cell migration and invasion play fundamental roles in cancer metastasis. For choroidal melanoma, tumor invasion can be an expansion in the vitreous cavity or infiltrating even breaking through the sclera. The eye lacks lymphatic vessels, and choroidal melanoma tends to spread by the hematogenous route, especially to the liver[1]. Choroidal melanoma invasion is a complex process involving a variety of mechanisms, and it is often related to tumor diameter, pathological cell type, advanced scleral invasion staging and so on. Meanwhile, the degree of violation to the sclera is an important prognostic indicator[8]. Sclera, almost entirely collagenous and relatively avascular, is a compact structure to form mechanical barrier against intraocular tumor spread. Advanced choroidal melanoma will infiltrate even break through sclera, and then followed by extraocular metastasis and ultimately life-threatening condition. A series of studies from Collaborative Ocular Melanoma Study (COMS, 1998) confirmed that scleral invasion was seen in 56% of the cases examined. In addition, some proteins secreted by tumor cells can also be used to identify patients at risk for metastasizing disease. Special high or low expression of biomarker in choroidal melanoma may be participated in tumor malignance[9]. Regulating their expressions facilitate possible adjuvant therapy and improve survival level. Therefore, it is becoming an urgent need to develop molecular biomarkers. There are already many molecules can be used as biomarkers guiding therapy selection and identifying prognosis[10].
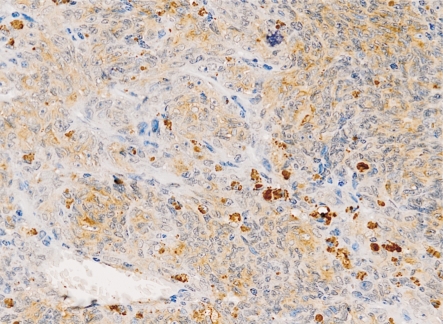
Connexin 43 belongs to a family of transmembrane proteins called connexins, and it is composed of six subunits forming Gap Junction Intercellular Communication (GJIC) between the neighboring cells. Connexins form channels between adjacent cells allowing the transport of small molecules (such as Ca2+, second messenger molecules, etc.) and many metabolites. Connexin 43 plays an important role in maintaining tissue homeostasis, and dysfunction of it may lead to cancer[11]. The role of connexin 43 in tumors is controversial. In many tumors, such as lung cancer[12] and ovarian cancer[13], connexin 43 acts as a tumor suppressor and the loss of it contributes to metastasis. Conversely, some other studies have found that the up-regulation of connexin 43 in breast cancer[14] as well as in gliomas[15] established GJIC between tumor cells and microenvironment, allowing for the passage of ions and second messengers, thus enhanced the metastatic process. Previous studies have showed that connexin 43 up-regulation was seen in tumor cells and endothelial cells in the contact area and played a role in tumor cell adherence, diapedesis and enhancing angiogenesis in vivo[16]. Villares et al[11] found in cutaneous melanoma connexin 43 does not act as a tumor suppressor but increased circulating melanoma cells to vascular endothelial cell adhesion and exudation through increased attachment and communication with the vascular endothelium. Our study found that the distribution of connexin 43 protein in choroidal melanoma showed clusters under low magnification microscope (Figure 5). There were intensive or high expression areas, and also sheets or cord-like no expression zones. The strongest expression zones of tumor cells were often located in peripheral tissue, non-developed region concentrated in the central part. The developing parts of tumor cells were also quite different, and the expression of connexin 43 protein in some sections was ectopic. In addition to distribution on the cell membrane, connexin 43 protein were presented with both cytoplasmic and membranous staining in most cases. Similar phenomena were also found in other cancers, and that might be an indirect evidence of lost of functional gap junction channels between cancerous cells[17],[18]. The local invasion to sclera may be facilitated by loss of GJIC which is also related to abnormal tumor cell survival and obtaining invasion ability, because the reduction in cell to cell communication could contribute to cellular dissociation. Our study also found that the expression of connexin 43 in the spindle cell type of choroidal melanoma was weaker than that in epithelial type and mixed type. And in some sections of choroidal melanoma, the expression of connexin 43 protein in spindle B cells is stronger than spindle A cells. This suggests that connexin 43 may be related to cell type transformation and may reflect a higher degree of differentiation of tumor cells in choroidal melanoma. Since histopathology type and tumor size can be used as indicators of choroidal melanoma prognosis[19], these findings may have clinical significance.
Figure 5. Connexin 43 expression in choroidal melanoma showed a positive membranous and cytoplasmic staining pattern with uneven dyeing (200×).
Invasion and diapedesis into blood vessels is necessary for tumor cell metastasis, and this potential mainly reflected in the adhesion and communication capabilities between tumor cells and endothelial cells. Our study found that in a case of choroidal melanoma specimen, connexin 43 protein was only presented with membranous staining around blood vessels, just as Pollmann et al[14] found in breast cancer. They reported that GJIC is necessary for emigration of metastatic breast cancer cells through the vascular endothelium. One specimen has been observed that some tumor cells with strong cytoplasmic staining had invaded into blood vessels. Maybe the meaning of this overexpression was to enhance its adhesion, and facilitate the transfer and capture by target vascular endothelial cells in organs. Some studies suggested that connexin 43 promotes cell adhesion and tumor cell diapedesis[20]. When diverting from the primary tissue, tumor cells adhered to the vascular endothelial cell under the help of connexin 43, established GJIC and then invaded through the vascular endothelium. Connexin 43 also played an important role when tumor cells were captured by vascular endothelium of metastatic organs. Villares et al[11] got similar findings in skin melanoma. Furthermore, connexin-positive metastases suggest that it is necessary for tumor cells to possess particular properties in order to acquire the metastatic capability. Possibly only connexin-positive clones of tumor cells possess metastatic potential[21]. In addition to cell communication, adhesion and promotion of exudation, in vivo studies have shown that connexin 43 could play a role in promoting angiogenesis[16]. It may also be meaningful to the metastasis role of connexin 43. Therefore, the exact role of connexin 43 in metastasis still needed more investigations in detail than ever.
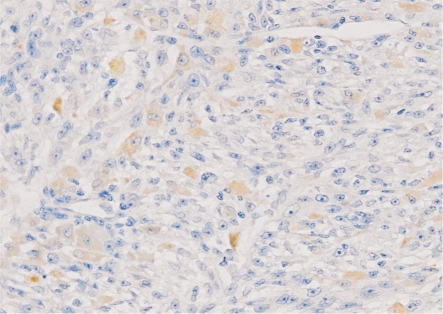
E-cadherin, which is localized in the adherent junction, is a kind of molecule transmembrane glycoprotein involved in cell-cell adhesion. It is considered to be a tumor suppressor and play a key role in the negative regulation of tumor invasion and metastasis. Decreased or loss expression of E-cadherin were reported in many tumors, including colorectal cancer, esophageal cancer, pancreatic cancer and so on[22]-[24]. In vitro studies found that transfecting tumor cells with increased the E-cadherin expression using cDNA technology will lead to a significant decline of their invasive capacity, and at the mean time, sealing off E-cadherin protein in use of inhibitory antibodies can enhance tumor cells' invasiveness[25]. In addition, results obtained from in vivo tumor models also confirmed that E-cadherin acts as a potent tumor suppressor[26],[27]. In skin malignant melanoma, low or loss of expression of E-cadherin was first found in vitro, and then confirmed in the tumor specimens by immunohistochemistry studies[28]. Our study found that E-cadherin showed a heterogeneous decrease immunostaining in choroidal melanoma compared with benign pigmented nevus. Both cytoplasmic and membranous staining of E-cadherin were expressed in choroidal melanoma specimens. These ectopic expressions of tumor cells may be relevant to the abnormal proliferation, because the tumor cells surrounding small blood vessels and capillaries with better metabolism and nutrient supply showed much more E-cadherin ectopic expression than others (Figure 6). At present, down-regulation mechanism for E-cadherin protein in malignant melanoma is not quite clear. It may be associated with promoter inactivation attributable to hypermethylation, which also have been found in the breast, stomach and prostate cancer[29]-[31]. Phenotypic changes distinguish tumor cells from normal cells. The most prominent of them are morphological changes, changes in adhesion and acquisition of an invasion and metastasis phenotype. As a critically important cell-cell adhesion molecule, E-cadherin was a potential molecular target involved in these alterations. Some studies showed that down-regulated of E-cadherin in melanoma leads to control deficiencies of reduced proliferation and invasive phenotype changes of tumor cells[32]. In many tumors[33] E-cadherin gene mutations may exist in the process of protein inactivation, but in melanoma, the expression decrease of E-cadherin protein is not associated with gene change[34]. Its expression may be regulated by the tumor microenvironment[35] and could be reversible in most cases. It provides a theoretical basis for regulation of the E-cadherin becoming the target to treatment.
Figure 6. E-cadherin expression in choroidal melanoma showed obvious reduction, and most positive expression located in tumor cells surrounding the small vessels or capillaries (200×).
Accumulating evidences indicated that there was close relationship between connexins and adhesion molecules. E-cadherin has been implicated in connexins docking. Its activation played an important role on and may determine the type of GJIC. Connexins and cadherins may be regulated by common signaling pathways. Promoter regulation of connexin 43 has been involved in many recent studies[36]. Connexin 43 and E-cadherin are expressed in many tumors. Hernandez-Blazquez et al found that E-cadherin was implicated in the regulation of connexin 43 expression and function in lung cancer cells[37]. In choroidal melanoma, their relationship and connections with clinical or pathological features of tumor cells are still unclear. According to our observation, we found that, in the same specimen, the higher connexin 43 protein was expressed, the lower E-cadherin protein was expressed in choroidal melanoma, and this phenomenon was correlated with the degree of tumor invasion to the sclera. The correlation between E-cadherin and connexin 43 may be meaningful for studying the occurrence and development of choroidal melanoma. The expression change of connexin 43 and E-cadherin occurs in a particular stage of the transfer process, especially in tumor cell transendothelial migration process. That could increase the adhesion and communication between tumor cell and target cell and then increase the chance of metastasis.
As a malignant/aggressive cancer, improvement of the prognosis and new treatments in choroidal melanoma are important. Adding specific drugs to affect connexin 43 expression at the transcriptional level and using genetic engineering technique to increase E-cadherin expression might reduce the growth, invasion and survival of melanoma cells[38]. As targets to control the process of invasion and metastasis, connexin 43 and E-cadherin proteins may provide new clinical treatment strategy for choroidal melanoma. It is hoped that these findings will contribute to the personalization of choroidal melanoma therapy using novel molecularly targeted agents.
REFERENCES
- 1.Sato T, Han F, Yamamoto A. The biology and management of uveal melanoma. Curr Oncol Rep. 2008;10(5):431–438. doi: 10.1007/s11912-008-0066-z. [DOI] [PubMed] [Google Scholar]
- 2.Xu HT, Li QC, Zhang YX, Zhao Y, Liu Y, Yang ZQ, Wang EH. Connexin 43 recruits E-cadherin expression and inhibits the malignant behaviour of lung cancer cells. Folia Histochem Cytobiol. 2008;46(3):315–321. doi: 10.2478/v10042-008-0057-9. [DOI] [PubMed] [Google Scholar]
- 3.Pahujaa M, Anikin M, Goldberg GS. Phosphorylation of connexin43 induced by Src: regulation of gap junctional communication between transformed cells. Exp Cell Res. 2007;313(20):4083–4090. doi: 10.1016/j.yexcr.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Haass NK, Smalley KS, Herlyn M. The role of altered cell-cell communication in melanoma progression. J Mol Histol. 2004;35(3):309–318. doi: 10.1023/b:hijo.0000032362.35354.bb. [DOI] [PubMed] [Google Scholar]
- 5.Kato Y, Hirano T, Yoshida K, Yashima K, Akimoto S, Tsuji K, Ohira T, Tsuboi M, Ikeda N, Ebihara Y, Kato H. Frequent loss of E-cadherin and/or catenins in intrabronchial lesions during carcinogenesis of the bronchial epithelium. Lung Cancer. 2005;48:323–330. doi: 10.1016/j.lungcan.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Chen JT, Cheng YW, Chou MC, Sen-Lin T, Lai WW, Ho WL, Lee H. The correlation between aberrant connexin 43 mRNA expression induced by promoter methylation and nodal micrometastasis in non-small cell lung cancer. Clin Cancer Res. 2003;9:4200–4204. [PubMed] [Google Scholar]
- 7.Brehm R, Rüttinger C, Fischer P, Gashaw I, Winterhager E, Kliesch S, Bohle RM, Steger K, Bergmann M. Transition from preinvasive carcinoma in situ to seminoma is accompanied by a reduction of connexin 43 expression in Sertoli cells and germ cells. Neoplasia. 2006;8:499–509. doi: 10.1593/neo.05847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alyahya GA. Melanoma associated spongiform scleropathy: characterization, biochemical and immunohistochemical studies. Acta Ophthalmol. 2008;86:1–21. doi: 10.1111/j.1755-3768.2008.1436.x. [DOI] [PubMed] [Google Scholar]
- 9.Pardo M, Dwek RA, Zitzmann N. Proteomics in uveal melanoma research: opportunities and challenges in biomarker discovery. Expert Rev Proteomics. 2007;4(2):273–286. doi: 10.1586/14789450.4.2.273. [DOI] [PubMed] [Google Scholar]
- 10.Rubin H. Contact interactions between cells that suppress neoplastic development: can they also explain metastatic dormancy? Adv Cancer Res. 2008;100(2):159–202. doi: 10.1016/S0065-230X(08)00006-7. [DOI] [PubMed] [Google Scholar]
- 11.Villares GJ, Dobroff AS, Wang H, Zigler M, Melnikova VO, Huang L, Bar-Eli M. Overexpression of protease-activated receptor-1 contributes to melanoma metastasis via regulation of connexin 43. Cancer Res. 2009;69(16):6730–6737. doi: 10.1158/0008-5472.CAN-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elzarrad MK, Haroon A, Willecke K, Dobrowolski R, Gillespie MN, Al-Mehdi AB. Connexin-43 upregulation in micrometastases and tumor vasculature and its role in tumor cell attachment to pulmonary endothelium. BMC Med. 2008;6:20. doi: 10.1186/1741-7015-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershon E, Plaks V, Dekel N. Gap junctions in the ovary: expression, localization and function. Mol Cell Endocrinol. 2008;282:18–25. doi: 10.1016/j.mce.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Pollmann MA, Shao Q, Laird DW, Sandig M. Connexin 43 mediated gap junctional communication enhances breast tumor cell diapedesis in culture. Breast Cancer Res. 2005;7(4):522–534. doi: 10.1186/bcr1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JH, Takano T, Cotrina ML, Arcuino G, Kang J, Liu S, Gao Q, Jiang L, Li F, Lichtenberg-Frate H, Haubrich S, Willecke K, Goldman SA, Nedergaard M. Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. J Neurosci. 2002;22:4302–4311. doi: 10.1523/JNEUROSCI.22-11-04302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellafiore M, Sivverini G, Palumbo D, Macaluso F, Bianco A, Palma A, Farina F. Increased connexin 43 and angiogenesis in exercised mouse hearts. Int J Sports Med. 2007;28:749–755. doi: 10.1055/s-2007-964899. [DOI] [PubMed] [Google Scholar]
- 17.Kanczuga-Koda L, Sulkowski S, Koda M, Sobaniec-Lotowska M, Sulkowska M. Expression of connexins 26, 32 and 43 in the human colon-an immunohistochemical study. Folia Histochem Cytobiol. 2004;42(4):203–207. [PubMed] [Google Scholar]
- 18.Krutovskikh VA, Troyanovsky SM, Piccoli C, Tsuda H, Asamoto M, Yamasaki H. Differential effect of subcellular localization of communication impairing gap junction protein connexin43 on tumor cell growth in vivo. Oncogene. 2000;19(4):505–513. doi: 10.1038/sj.onc.1203340. [DOI] [PubMed] [Google Scholar]
- 19.Guo LJ, Wu ZY, Zhang S, Chen JQ, Ai SM, Zheng HL. Research on the relationship between pathological features of the uveal melanoma and prognosis. Chin J Pathol. 2002;31(6):518–521. [PubMed] [Google Scholar]
- 20.Li G, Satyamoorthy K, Herlyn M. Dynamics of cell interactions and communications during melanoma development. Crit Rev Oral Biol Med. 2002;13(1):62–70. doi: 10.1177/154411130201300107. [DOI] [PubMed] [Google Scholar]
- 21.Tang B, Peng ZH, Yu PW, Yu G, Qian F. Expression and significance of Cx43 and E-cadherin in gastric cancer and metastatic lymph nodes. Med Oncol. 2010 Apr 6; doi: 10.1007/s12032-010-9492-5. [DOI] [PubMed] [Google Scholar]
- 22.Tsanou E, Peschos D, Batistatou A, Charalabopoulos A, Charalabopoulos K. The E-cadherin adhesion molecule and colorectal cancer. A global literature approach. Anticancer Res. 2008;28(6A):3815–3826. [PubMed] [Google Scholar]
- 23.Nair KS, Naidoo R, Chetty R. Expression of cell adhesion molecules in esophageal carcinoma and its prognostic value. J Clin Pathol. 2005;58(4):343–351. doi: 10.1136/jcp.2004.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Natalwala A, Spychal R, Tselepis C. Epithelial-mesenchymaltransition mediated tumourigenesis in the gastrointestinal tract. World J Gastroenterol. 2008;14(24):3792–3797. doi: 10.3748/wjg.14.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spangler B, Vardimon L, Bosserhoff AK, Kuphal S. Post-transcriptional regulation controlled by E-cadherin is important for c-Jun activity in melanoma. Pigment Cell Melanoma Res. 2011;24(1):148–164. doi: 10.1111/j.1755-148X.2010.00787.x. [DOI] [PubMed] [Google Scholar]
- 26.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392(6672):190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Gu D, Wang M, Zhang Z, Tang J, Chen J. The E-cadherin (CDH1)-160C>A Polymorphism associated with gastric cancer among Asians but not Europeans. DNA Cell Biol. 2011 Jan 8; doi: 10.1089/dna.2010.1091. [DOI] [PubMed] [Google Scholar]
- 28.George E, Polissar NL, Wick M. Immunohistochemical evaluation of p16INK4A, E-cadherin, and cyclin D1 expression in melanoma and Spitz tumors. Am J Clin Pathol. 2010;133(3):370–379. doi: 10.1309/AJCP52YVYCTLUOPI. [DOI] [PubMed] [Google Scholar]
- 29.Junxia W, Ping G, Yuan H, Lijun Z, Jihong R, Fang L, Min L, Xi W, Ting H, Ke D, Huizhong Z. Double strand RNA-guided endogeneous E-cadherin up-regulation induces the apoptosis and inhibits proliferation of breast carcinoma cells in vitro and in vivo. Cancer Sci. 2010;101(8):1790–1796. doi: 10.1111/j.1349-7006.2010.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borges Bdo N, Santos Eda S, Bastos CE, Pinto LC, Anselmo NP, Quaresma JA, Calcagno DQ, Burbano RM, Harada ML. Promoter polymorphisms and methylation of E-cadherin (CDH1) and KIT in gastric cancer patients from northern Brazil. Anticancer Res. 2010;30(6):2225–2233. [PubMed] [Google Scholar]
- 31.Kwon O, Jeong SJ, Kim SO, He L, Lee HG, Jang KL, Osada H, Jung M, Kim BY, Ahn JS. Modulation of E-cadherin expression by K-Ras; involvement of DNA methyltransferase-3b. Carcinogenesis. 2010;31(7):1194–1201. doi: 10.1093/carcin/bgq071. [DOI] [PubMed] [Google Scholar]
- 32.Herlyn M, Berking C, Li G, Satyamoorthy K. Lessons from melanocyte development for understanding the biological events in naevus and melanoma formation. Melanoma Res. 2000;10(4):303–312. doi: 10.1097/00008390-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Wen YG, Wang Q, Zhou CZ, Qiu GQ, Peng ZH, Tang HM. Mutation analysis of tumor suppressor gene PTEN in patients with gastric carcinomas and its impact on PI3K/AKT pathway. Oncol Rep. 2010;24(1):89–95. doi: 10.3892/or_00000832. [DOI] [PubMed] [Google Scholar]
- 34.Poser I, Domìnguez D, de Herreros AG, Varnai A, Buettner R, Bosserhoff AK. Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J Biol Chem. 2001;276(27):24661–24666. doi: 10.1074/jbc.M011224200. [DOI] [PubMed] [Google Scholar]
- 35.Ch'ng S, Tan ST. Genetics, cellular biology and tumor microenvironment of melanoma. Front Biosci. 2009;14:918–928. doi: 10.2741/3286. [DOI] [PubMed] [Google Scholar]
- 36.Huang T, Wan Y, Zhu Y, Fang X, Hiramatsu N, Hayakawa K, Paton AW, Paton JC, Kitamura M, Yao J. Downregulation of gap junction expression and function by endoplasmic reticulum stress. J Cell Biochem. 2009;107(5):973–983. doi: 10.1002/jcb.22202. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez-Blazquez FJ, Joazeiro PP, Omori Y, Yamasaki H. Control of intracellular movement of connexins by E-cadherin in murine skin papilloma cells. Exp Cell Res. 2001;270:235–247. doi: 10.1006/excr.2001.5342. [DOI] [PubMed] [Google Scholar]
- 38.Hsu MY, Meier FE, Nesbit M, Hsu JY, Van Belle P, Elder DE, Herlyn M. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am J Pathol. 2000;156(5):1515–1525. doi: 10.1016/S0002-9440(10)65023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]