Abstract
AIM
All-trans retinoic acid (RA) is the only extrinsic biochemical candidate known to date that could act as a growth controller, the aim of this study was to investigate the expression cellular retinoic acid binding proteins I (CRABP-I) and retinoic acid receptor-β (RAR-β) in retina of the guinea pig eyes with experimental myopia.
METHODS
Ninety guinea pigs aged 14 days were equally and randomly divided into three groups: form deprivation (FD), -5D lens, and control. The diffusers for FD were white translucent hemispheres, and -5D lenses were used to introduce hyperopic defocus. Refraction was measured with streak retinoscopy after cycloplegia, and axial length was calculated with Cinescan A/B ultrasonography. Retina harvested at different time points were used to measure RA level with HPLC and expressions of cellular retinoic acid binding proteins I (CRABP-I) and RA receptor-β (RAR-β) were assayed with Western blot and Real-time PCR. SPSS13.0 software was used for statistical analysis.
RESULTS
Up-regulations of CRABP-I and RAR-β in ocular tissues correlated with changes in the refractive status and growth rate of the guinea pig eye (P<0.05). 14 days of monocular form-deprivation led to -5.14D myopia and a 0.281mm axial elongation; 14 days of monocular defocus produced -3.64D myopia and a 0.163 mm axial elongation. The level of retinal RA started to elevate in 7 days (P<0.05) after visual manipulation in both FD and -5D lens groups and became more prominent by 14 days (P<0.01) . The expressions of CRABP-I and RAR-β increased by 14 days after visual manipulation (P<0.05), the mRNA level of RAR-β, however, increased by 7 days after visual manipulation (P<0.05), which suggested that changes of expressions of CRABP-I and RAR-β might lag behind the change of RA.
CONCLUSION
The levels of CRABP-I and RAR-β were elevated in retina of the guinea pig eye with experimental myopia. During the progression of experimental myopia, the retinal RA level increased rapidly, and there might be a positive feedback between the increase of RA and up-regulation of RAR-β.
Keywords: retinoic acid, CRABP-I, RAR-β, experimental myopia, guinea pig
INTRODUCTION
Ocular growth is visually regulated by signals released from the retina. Whether the image is focused in front of or behind the retina can be sensed by the retina itself[1]. The retina can analyze the projected image, and then release unknown messengers to control the growth rates of the back of the eye so that the image remains at the photoreceptor plane during the development[2]. A growing eye becomes myopic after form deprivation (FD) or compensates for the power and sign of the spectacle lens imposed[3],[4]. Animal model studies have also shown that imposed spectacle lens can induce the eye to adjust its length to compensate for the power precisely and it is sensitive to both the amount of the imposed blur and the sign of the defocus[5],[6].
Although studies have been done in a variety of animal species, it is still unknown how the visual error signal is translated to affect the eye growth. One possible mediator is all-trans retinoic acid (RA). In 1984 Palestine first reported that a patient developed transient, acute myopia while on isotretinoin (Accutane) therapy for acne. There was a clear relationship between restarting the Accutane and recurrence of the transient myopia[7]. After that, many researchers investigated the role of RA in eye growth. Previous research indicated that visual conditions which enhanced or inhibited eye growth resulted in elevated or reduced levels of retinal RA[8]-[14]. It is known that the accessibility of retinoids and free retinoic acid is regulated by the amounts of cellular retinol-binding proteins (CRBP) and retinoic acid binding proteins (CRABP)[15],[16]. Retinoic acid exerts its effects on cells by binding to nuclear receptors which serve as transcription factors[17],[18]. By binding with nuclear receptors, RA can regulate the transcription of more than 300 different genes, such as b-FGF, TGF-β, which play important roles in the eye development[19]-[23].
Both CRABP-I and RAR-β are very important in RA system[15]-[20]. However, neither the change of RAR-β in guinea pigs nor the change of CRABP-I in experimental myopia has been reported. Therefore, we investigated the role of RA system in the guinea pig model of eye growth. In particular, we manipulated the refractive error (RE) and ocular elongation with diffusers and lenses and asked whether the temporal expression of CRABP-I and RAR-β in ocular tissues was correlated with the refractive state and growth rate of the guinea pigs' eye.
MATERIALS AND METHODS
Animals
Pigmented guinea pigs (n=90) aged 14 days were randomly divided into three groups: form deprived (FD) group (n=30), -5D lens group (n=30) and control group (n=30). Animals were reared in an environment with natural light and dark cycle. Vitamin C-supplemented water, hay and fresh vegetables were freely accessible to the animals. The room temperature was kept at 22°C. Experiments conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Committee for Animal Welfare of the EENT Hospital of Fudan University.
Visual Manipulations
FD group: The white translucent hemispheres were used to cover one randomly selected eye in each animal by the diffusers for two weeks[13]. -5D lens group: The -5D lenses were used to cover one eye in random for two weeks to defocus. The lenses were RGP lenses (power: -5.0D, diameter: 12mm, optical zone: 10.5mm-11.5mm, posterior surface radius: 8mm; Daiwa lens Co., Ltd, Japan). The RGP lenses were mounted onto a ring, then the ring were sutured onto the skin around the eye. This technique was similar to that routinely used on chicken and was already implemented on guinea pigs in previous studies. The control group received no intervention, one eye was picked up in random in each guinea pig.
Measurement of Refractive Parameters
0.25% tropicamide were used to dilate the pupil every 5 minutes for 3 times. RE measurements were obtained using streak retinoscopy 45 minutes after the first mydriasis. Axial length and vitreous depth were measured with Cinescan A/B ultrasonography (Optikon 2000 S.P.A., sensitivity 0.01mm). Limpid ultrasound traces included: the front of the cornea, the front and back of the lens, the retinal/vitreous interface and the back of sclera. Axial length was defined as the distance from the front of the cornea to the back of the sclera. RE data are presented as the mean spherical equivalent refractive error (MSE). Since the accommodation of guinea pigs' eyes were very powerful, retinoscopy was conducted in the central zone shortly after the ciliary muscle was completely paralyzed. The refraction parameters above were measured prior to visual manipulations, and on the 7th day and the 14th day after the manipulations were initiated. All refraction data were presented as mean derived from 5 repeated measures.
Collection of Retina Samples
The experimental and control eyes were removed and dissected along the ora serrata. Then a whole mount of the posterior layers of each eye was obtained. The retinal and choroidal plus scleral layers were isolated and the pigment epithelium was carefully removed. Procedures were performed on the surface of the ice and under subdued light. Samples were immediately stored in the -70°C liquid nitrogen. Two guinea pigs from each group were sacrificed prior to the visual manipulations. Twelve guinea pigs of each group were sacrificed on the 7th day after the manipulations, and the rest of the animals were sacrificed on the 14th day. Once sample collection was complete for all the time points, the collected samples went through assays together.
Measurement of Levels of RA, CRABP-I and RAR-β in Retina
Levels of retinal RA were obtained using HPLC. These methods have been previously described in detail by Mertz and Wallman[17], especially the chromatographic condition and the standard curve. Expressions of CRABP-I and RAR-β were obtained using Western Blot and Real-time PCR respectively at protein and mRNA level. More than 3 samples from each group were assayed by these methods. The samples were picked up randomly to take different measurements.
Measurement of CRABP-I and RAR-β by Western Blot
The samples were added into 100uL phenylmethyl sulfonylfluoride of required concentration. Bradford chromatometry was used to determine the levels of protein after the tissues were dispersed by ultrasound on ice. Thirty microliter of the electrophoresis sample was added into each slot in sequence. The SDS-PAGE electrophoresis was conducted under the voltage of 120V and the electric current of 60mA and stopped when bromphenol blue ran out of the separation gel. Western Bolt procedure: The gel was transferred into 100mL cathode buffer(125 mM Tris, pH 6.8, 10% SDS, 50% sucrose, 10mM EDTA, 1% bromophenol blue, and 100mM DTT; Bio-Rad, Hercules, CA, USA), balanced for 15 minutes; a piece of PVDF membrane(Millipore, Milford, MA, USA) was cut in the same size with the gel. The membrane was submerged into 10mL of 100% methanol for 15 seconds, then into distilled water for 2 minutes. The membrane was transferred into anode buffer II and balanced for 10 minutes. Semidrying process was applied to the transmembrane for 1 hour under 1.2mA/cm2. After transmembrane was completed the membrane was transferred into methanol for 10s, then put onto the filter paper until the methanol evaporated to dry in about 15 minutes. Ponceau red solution was used to detect the transmembrane efficiency then was washed-out by deionized water. We incubated the membrane in primary antibody(1:200) for 2 hours or to stay overnight under 4°C, the membrane was rinsed for 10 minutes in TTBS solution three times, and then incubated in secondary antibody (1:2 000) for 1 hour, rinsed for 10 minutes in TTBS solution three times. DBA solution was used to colorate the membrane. We washed the membrane to stop the reaction when coloration was enough. The membrane was shot by TANON GIS-2008 gel formatter. Date was analyzed by TANON gel-gram processing system. The density of the protein of interest on the film was measured using densitometric readings. Density measurements were then normalized to β-actin readings, which served as loading controls. All the reagents were produced by Santa Cruz. The antibody and the secondary reagent of CRABP-I was an affinity purified goat polyclonal antibody (sc-10062) and donkey anti-goat IgG-HRP(sc-2020). To RAR-β, the antibody and secondary reagent were rabbit polyclonal IgG (sc-552) and goat anti-rabbit IgG HRP (sc-2004).
Measurement of CRABP-I and RAR-β mRNA using Real-time PCR
Extraction of total RNA: 1mL Trizol was added into the samples and then total RNA was extracted according to the operating instruction. RNA was dissolved into the DEPC solution. The relative value of OD260/ OD280 was measured by spectrophotometer after the total RNA was diluted by 400 times and then quantified using the following formula: RNA concentration (µg/mL)=OD260 value×40×dilution multiple (400 in current study). Reverse transcription: 2ug of total RNA in 20µL reaction system. Reverse transcripted RNA to cDNA according to the operating instruction. Then fluorescent quantitation PCR was used. The sequences for the primers were listed in Table 1.
Table 1. The sequences for the primers in fluorescent quantitation PCR.
| Upstream primers | Downstream primers | |
| CRABP-I | 5′—TGAACGCCATGCTGAGGAA—3′ | 5′—GGCGCCAAATGTCAGGATTA—3′ |
| RAR-β | 5′—TAAGATCGTGGAGTTCGCCA—3′ | 5′—TTTCCAAAGGCAGGAGCTG—3′ |
| GAPDH | 5′-ACC ACA GTC CAT GCC ATC AC-3′ | 5′-TCC ACC ACC CTG TTG CTG TA-3 |
Statistical Analysis
Data are presented as the mean±SEM. The differences of Refractive error, axial length, RA, mRNA and protein of CRIBP-I and RAR-β among FD group, -5D lens group and Control group were analyzed by ANOVA in SPSS V13.0. Fisher's LSD test was used as the post hoc test.
RESULTS
Effect of Visual Manipulations-Diffuser and Lens Wear
In this experiment, we found that both form deprivation and -5D lens wear were effective in inducing myopia in guinea pigs. There was no difference in refraction and axial length between three groups prior to the visual manipulation. After 7 and 14 days' visual manipulation, the differences among the three groups were both significant (P<0.01). Fisher's LSD test between FD group and control group suggested that FD for 7 days created a difference of -3.9D of myopia between the diffuser wearing eye and the control eye (-1.33±0.56D vs 2.57±1.02D, P<0.01) with a corresponding significant increase in axial length (7.923±0.066mm vs 7.694±0.067mm, P<0.05). This myopia increased -5.14D between the diffuser wearing eye and the control eye (-3.17±0.98D vs 1.97±0.39D, P<0.001) after 14 days of diffuser wear while there still existed a significant increase in axial length (8.135±0.051mm vs 7.844±0.035mm, P<0.05).
Fisher's LSD test between -5D lens group and control group showed: Hyperopic defocus for 7 days created a difference of -3.35D of myopia between the -5D lens wearing eye and the control eye (-0.78±0.98D vs 2.57±1.02D, P<0.01) with a corresponding significant increase in axial length (7.907±0.058mm vs 7.694±0.067mm, P<0.05). When an eye had been worn a -5D lens for 14 days, it became significantly more myopia (-3.64D on average) than the control eye (-1.67±0.59D vs 1.97±0.39D, P<0.01), while there was still a significant increase in axial length (8.017±0.077mm vs 7.844±0.035mm, P<0.05) (Table 2).
Table 2. The refraction, axial length and retinal-RA level in FD group, -5D lens group and control group with/without visual manipulation.
| Time/Groups | Sample | Refraction (D) | Axial length (mm) | Sample (eye) | Retinal-RA (µg/g wet wt) |
| prior to the visual manipulation: | |||||
| FD Group | 30 | 3.06±0.98 | 7.813±0.084 | 2 | 1.303/1.469 |
| -5D lens Group | 30 | 3.14±0.69 | 7.787±0.059 | 2 | 1.236/1.362 |
| Control Group | 30 | 2.96±0.78 | 7.799±0.056 | 2 | 1.32/1.364 |
| 7 days later after visual manipulation: | |||||
| FD Group | 28 | –1.33±0.56b | 7.923±0.066a | 4 | 1.732±0.036b |
| -5D lens Group | 27 | –0.78±0.98b | 7.907±0.058a | 4 | 1.648±0.075b |
| Control Group | 28 | 2.57±1.02 | 7.694±0.067 | 4 | 1.198±0.076 |
| 14 days later after visual manipulation: | |||||
| FD Group | 14 | –3.17±0.98b | 8.135±0.051b | 4 | 2.360±0.098b |
| -5D lens Group | 13 | –1.67±0.59b | 8.017±0.077a | 3 | 2.273±0.032b |
| Control Group | 15 | 1.97±0.39 | 7.844±0.035 | 4 | 1.386±0.083 |
aP<0.05, bP<0.01
Retinal RA Levels after Visual Manipulations
No difference in retinal RA level prior to the visual manipulation between form deprivation, -5D lens wear and control group was showed in our results. After visual manipulation, the difference between the three groups became statistically significant (P<0.05, 7 days; P<0.01, 14 days). Fisher's LSD test shows: Guinea pig eye which had experienced FD for 7 days had significantly higher levels of retinal-RA than the control eye (1.732±0.036µg/g wet wt vs 1.198±0.076µg/g wet wt, P<0.001). After 14 days of FD, the diffuser wearing eyes developed nearly double the level of retinal-RA relative to their control eyes (2.360±0.098µg/g wet wt vs 1.386±0.083µg/g wet wt, P<0.01).
Those eyes which had experienced -5D lens wear for 7 days had significantly higher level of retinal-RA than the control eye(1.648±0.075µg/g wet wt vs 1.198±0.076µg/g wet wt, P<0.01). After defocus for 14 days, the level of retinal-RA in -5D lens wear eyes were significantly higher than the control eye(2.273±0.032µg/g wet wt vs 1.386±0.083µg/g wet wt, P<0.001).
Measurements of Levels of CRABP-I and RAR-β Using Western Blot and Real-time PCR
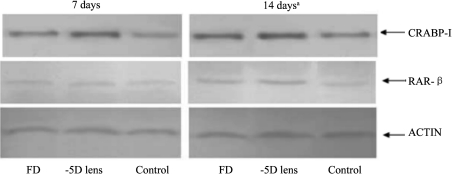
CRABP-I and RAR-β protein levels in visually manipulated retina were obtained using Western Blot. No difference of CRABP-I protein level was found between three groups after visual manipulation for 7 days. By the 14th day, the difference was significant (P<0.05). Fisher's LSD test showed that level of CRABP-I protein in FD eyes was nearly 1.55 times the level in control eyes (P<0.05). In -5D lens group, this ratio was 2.06 (P<0.01).
There was also no significant difference of RAR-β protein expression between three groups at the 7th day. On the 14th day, however, the difference was significant (P<0.05). The levels of RAR-β protein in FD and lens wearing group were 1.38 (P<0.05) and 1.88 (P<0.05) times the level in control eyes, respectively (Figure 1).
Figure 1. Western blot showing the expression of retinal CRABP-I and RAR-β in the diffuser wearing eye, -5D lens wearing eye and the control eye 7 days and 14 days after visual manipulation respectively (aP<0.05).
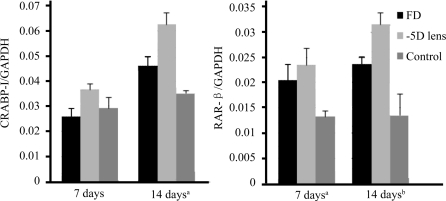
To investigate the transcriptions of CRABP-I and RAR-β genes in the retina, the mRNA level of CRABP-I and RAR-β were also measured using Real-time PCR. No difference of CRABP-I mRNA was found between three groups on the 7th day. By the 14th day, it developed nearly 1.31 times level of CRABP-I/GAPDH mRNA (P<0.05) in FD group and 1.78 times level of CRABP-I/GAPDH mRNA (P<0.05) in -5D lens group compared to the control group.
Difference in RAR-β mRNA levels was showed between three groups on the 7th day (P<0.05) and became more significant another 7 days later (P<0.01). Fisher's LSD test showed: FD group developed nearly 1.54 and 1.77 times level of RAR-β/GAPDH mRNA in control eye on the 7th day and the 14th day respectively (P<0.05, P<0.05). -5D lens eye showed 1.75 times level of RAR-β/GAPDH mRNA(P<0.05) in control eye by the 7th day and developed to 2.33 times after another 7 days. The results of Real time-PCR are similar to those of Western Blot (Figure 2).
Figure 2. Real-time PCR showing the transcriptions of the CRABP-I gene(A) and RAR-β gene(B) in the diffuser wearing eye, -5D lens wearing eye and the control eye 7 days and 14 days after visual manipulation respectively (aP<0.05, bP<0.01).
DISCUSSION
In this study, we found that both visual manipulations were effective in inducing myopic RE and axial elongation of the eye within 7 days, which became more prominent by day 14. The level of retinal RA in the experimental eyes was higher than that in control eyes. The increase of retinal RA started within one week from initial visual manipulations and continued through day 14 days. The time frame of retinal RA elevation mirrored the progression of experimental myopia closely. To investigate the role of RA in the mechanism of the development of myopia in the guinea pig, the expressions of CRABP-I and RAR-β in myopia and control retina were tested. We found that the expressions of CRABP-I and RAR-β increased by 14 days after visual manipulation, the mRNA level of RAR-β, however, increased by 7 days after visual manipulation, which suggested that changes of expressions of CRABP-I and RAR-β might lag behind the change of RA.
The increase of RA might be induced by visual error signal on the retina directly. It is well known that all-trans retinaldehyde is released by photoreceptor cells after photoisomerization, and then enters RPE cells. In RPE, all-trans retinaldehyde is isomerized back to all-trans RA and re-activated. The step from retinaldehyde to RA is irreversible. The CRABP proteins mediate the downstream effects of RA in distinct ways. In the retina, we can only check the CRABP-I[24]. CRABP-I negatively regulates the activity of RA by enhancing the production of RA metabolizing enzymes and increasing the rate of RA degradation. The abundance of CRABP-I may reflect the amount of RA the cell needs.
We found that the expression of CRABP-I significantly increased 14 days later after visual manipulations for FD and -5D lens wearing groups. Although 7 days after visual manipulations the expression of CRABP-I did not change in either myopic group, the level of RA increased significantly in both groups. Change of CRABP-I lagged behind the change of RA. As we know the abundance of CRABP may reflect the amount of RA the cell needs, an increase in CRABP-I level may indicate that the RA level in the cell is too high and the cell is trying to degrade more RA. Thus we hypothesize that during myopia development, the increase of retinal RA isn't induced by the more requirement of RA in retinal cells, it might be directly caused by visual error signals on the retina by making the photoreceptor cells release more retinaldehyde or the higher ratio of oxidation from retinaldehyde to RA. It is found recently that amacrine cells in the inner retina are involved in retinoid metabolism. It is also assumed that retinal amacrine cells are at the output level of the retinal image processing that is involved in the visual control of eye growth[2]. Although in the early stage of myopia development, the CRABP-I didn't raise, we suspect that retina cells need time to react for the increasing of RA and if we can elevate the CRABP-I more quickly, it might delay the myopic progression.
There is a positive feedback between the increase of RA, expression of RAR-β in cell nucleus and myopic progression. Retinoic acid exerts its effects on cells by binding to nuclear receptors which serve as transcription factors[25]. When the nuclear receptors bind to RA, two receptors will polymerize to form a dimeride, and the dimeride will bind to the cis-acting element upstream to the target gene, which is called Retinoid acid response element (RARE). RARE not only is present in many functional genes, but also participates in transcription and expression of these genes (e.g. MMP-2, b-FGF and TGF-β), which play important roles in the development of the eye. Besides, RARE is even present in RAR-β gene itself.
SekoY et al[26] measured the expression of RAR-β in the sclera of myopic chick with RT-PCR. They demonstrated that t-RA in the myopic retina increased 5 days after visual deprivation, but expression of RAR-β in the myopic sclera increased 14 days later after visual deprivation and did not increase 7 days later after visual deprivation. Based on their in vitro data, expression of RAR-β in the sclera was thought to be induced by RA, which indicated that RA influenced proliferation and differentiation of scleral cells. They concluded that retinal RA induced the expression of RAR-β in the sclera of FDM eyes.Xie et al[20] found that TGF-beta2 mRNA level decreased in FDM eyes. Disulfiram, which inhibits the synthesis of retinoid acid, can up-regulate the level of TGF-beta2 mRNA.
Our results in the retina of guinea pig are consistent with the previous studies. With both manipulations, we found that the level of RA raised 7 days later after visual manipulation, and the expression of RAR-β in the myopic sclera increased 14 days but not 7 days after visual deprivation. The change of RAR-β followed the change of RA in retina, we hypothesize that the increase in RA level can make more RA-RAR-β combination and create more dimerides that can bind with RARE. Since RARE is present in RAR-β gene itself, the activated RARE can up-regulate expression of RAR-β directly.
Along with this biochemical change, the axial length of experimental eye increased significantly. From the previous researches we know that in sclera of a myopic eye the activities of MMP-2 and TGF-β increase and the activity of b-FGF decreases, although the mechanism of these factors in the development of myopia is still not clear. Considering the presence of RARE in genes of these factors, it is possible that the RA system is likely to initiate a biochemical cascade effect, in which the increase in RA is the first step, which is followed by RAR-β up-regulation and myopic progression. In other words, there is a positive feedback between the increase of RA, expression of RAR-β in cell nucleus and progression of myopia.
In conclusion, during the progression of experimental myopia, retinal RA level elevates rapidly and the expression of RAR-β is promoted by combing more RA to itself. Although the vision system reacts to this change by increasing the CRABP-I level to down-regulate the RA level, this effect is slow and relatively small. The unbalanced activation of RAR-β may directly induce the progression of myopia. In order to investigate more about the role of RA signal, in further research, antagonists of the RA-signaling pathway will be used to test whether the progression of experimental myopia would be influenced.
Footnotes
Foundation items: Key Program of National Natural Science Foundation of China (No.30530770);
Scientific Research Program of Shanghai Municipal Health Bureau, China (No.054072)
REFERENCES
- 1.Schaeffel F, Diether S. The growing eye: an autofocus system that works on very poor images. Vision Res. 1999;39:1585–1589. doi: 10.1016/s0042-6989(98)00304-6. [DOI] [PubMed] [Google Scholar]
- 2.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 4.Venkataraman S, Nguyen L, McBrien NA. Compensatory ocular growth responses to positive lens defocus in the tree shrew. Ophthalmol Vis Sci. 2005;46:E-Abstract 1973. [Google Scholar]
- 5.Howlett MC, McFadden SA. A fast and effective mammalian model to study the visual regulation of growth. Invest Ophthalmol Vis Sci. 2002;43:2928. [Google Scholar]
- 6.Whatham AR, Judge SJ. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001;41:267–273. doi: 10.1016/s0042-6989(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 7.Palestine AG. Transient acute myopia resulting from isotretinoin (accutane) therapy. Ann Ophthalmol. 1984;16:660–662. [PubMed] [Google Scholar]
- 8.Seko Y, Shimizu M, Tokoro T. Retinoic acid increases in the retina of the chick with form deprivation myopia. Ophthalmic Res. 1998;30:361–367. doi: 10.1159/000055496. [DOI] [PubMed] [Google Scholar]
- 9.Mertz JR, Wallman J. Choroidal retinoic acid synthesis: A possible mediator between refractive error and compensatory eye growth. Exp Eye Res. 2000;70:519–527. doi: 10.1006/exer.1999.0813. [DOI] [PubMed] [Google Scholar]
- 10.Mertz JR, Howlett MC, McFadden S, Wallman J. Retinoic acid from both the retina and choroid influences eye growth. Invest Ophthalmol Vis Sci( 1999;40(ARVO Suppl):S849. [Google Scholar]
- 11.Bitzer M, Feldkaemper M, Schaeffel F. Visually induced changes in components of the retinoic acid system in fundal layers of the chick. Exp Eye Res. 2000;70:97–106. doi: 10.1006/exer.1999.0762. [DOI] [PubMed] [Google Scholar]
- 12.McFadden SA, Howlett MC, Mertz JR. Retinoic acid signals the direction of the ocular elongation in the guinea pig eye. Vision Res. 2004;44:643–653. doi: 10.1016/j.visres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 13.McFadden SA, Howlett MC, Mertz JR, Wallman J. Acute effects of dietary retinoic acid on ocular components in the growing chick. Exp Eye Res. 2006;83(4):949–961. doi: 10.1016/j.exer.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Qu XM, Chu RY. The role of all-trans retinoic acid in the development of myopia in the guinea pig. Chin J Optometry Ophthalmol. 2006;8:69–72. [Google Scholar]
- 15.Napoli JL, Boerman MH, Chai X, Zhai Y, Fiorella PD. Enzymes and binding proteins affecting retinoic acid concentrations. Steroid Biochem Mol Bio. 1995;53:497–502. doi: 10.1016/0960-0760(95)00096-i. [DOI] [PubMed] [Google Scholar]
- 16.Propping C, Mönig B, Luksch H, Mey J. Distribution of the cellular retinoic acid binding protein CRABP-I in the developing chick optic tectum. Brain Res. 2007;1168:21–31. doi: 10.1016/j.brainres.2007.06.089. [DOI] [PubMed] [Google Scholar]
- 17.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe A, Richman JM, Brickell PM. Development of the spatial pattern of retinoic acid receptor-β transcripts in embryonic chick facial primordia. Development. 1992;114:805–813. doi: 10.1242/dev.114.3.805. [DOI] [PubMed] [Google Scholar]
- 19.Yan DS, Zhou XT, Chen XY, Lu F, Wang J, Hu DN, Qu J. Expression of retinoid acid receptors in human scleral fibroblasts and regulation of growth of fibroblasts by retinoic acid. Chin J Ophthalmol. 2007;43(8):750–753. [PubMed] [Google Scholar]
- 20.Xie F, Chen YG. Influence of disulfiram on the expression of transforming growth factor-beta2 in form-deprived eyes in chicks. Beijing Da Xue Xue Bao. 2008;40(6):610–615. [PubMed] [Google Scholar]
- 21.Chen JT, Liang JB, Chou CL, Shyu RC, Lu DW. Retinoic acid induces VEGF gene expression in human retinal pigment epithelial cells (ARPE-19) J Ocul Pharmacol Ther. 2005;21(6):413–419. doi: 10.1089/jop.2005.21.413. [DOI] [PubMed] [Google Scholar]
- 22.Fujieda H, Bremner R, Mears AJ, Sasaki H. Retinoic acid receptor-related orphan receptor alpha regulates a subset of cone genes during mouse retinal development. J Neurochem. 2009;108(1):91–101. doi: 10.1111/j.1471-4159.2008.05739.x. [DOI] [PubMed] [Google Scholar]
- 23.Roberts MR, Hendrickson A, McGuire CR. Retinoid X receptor (gamma) is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest Ophthalmol Vis Sci. 2005;46(8):2897–2904. doi: 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- 24.Dong D, Ruuska SE, Levinthal DJ, Noy N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J Biol Chem. 1999;274:23695–23698. doi: 10.1074/jbc.274.34.23695. [DOI] [PubMed] [Google Scholar]
- 25.Drager UC, McCaffery P. Retinoic acid and development of the retina. Prog Ret Res. 1997;16:323–351. [Google Scholar]
- 26.Seko Y, Shimizu M, Tokoro T. In vivo and in vitro association of retinoic acid with form deprivation myopia in the chick. Exp Eye Res. 1996;63:443–452. doi: 10.1006/exer.1996.0134. [DOI] [PubMed] [Google Scholar]