Abstract
AIM
To assess the effectiveness of immunosuppressants in the prophylaxis of corneal allograft rejection after high-risk keratoplasty and normal-risk keratoplasty.
METHODS
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CNKI, VIP and reference lists of articles. Date of most recent search: 18 June, 2011. All randomised controlled trials (RCTs) assessing the use of immunosupressants in the prevention of graft rejection, irrespective of publication language. Two authors assessed trial quality and extracted data independently. Only dichotomous outcomes (clear graft survival, ratio of immune reactions and side effects) were available and were expressed as relative risk (RR) and 95% confidence intervals (CI).
RESULTS
Seven studies were included in this review. In the comparing of mycophenolate mofetil (MMF) with placebo, the results showed MMF could significantly reduce immune reactions compared with placebo (RR 1.08 95% Cl 0.95 to 1.21), but no effect on clear graft survival (RR 1.11 95% Cl 0.90 to 1.35). In clear graft survival and immune reactions, MMF and cyclosporine A (CsA) showed similar effect (RR 1.11 95% Cl 0.90 to 1.35, and RR 1.48, 95% Cl 0.56 to 3.93, respectively). Tacrolimus (FK506) and steroid showed similar effects on clear graft survival and immune reactions (RR 0.32, 95% CI 0.02 to 6.21, and RR 1.00, 95%CI 0.88 to 1.14, respectively). No drug relative side effect has been found.
CONCLUSION
MMF may reduce immune reactions in both normal-risk and high-risk rejection of penetrating keratoplasty. CsA and FK506 showed similar effects as MMF. However, due to the lack of large clinical trials, the evidence remain weak, the quality of evidences were rated as very low to moderate. Large, properly randomised, placebo-controlled, double masked trials are needed to evaluate the effect of immunosuppressants.
Keywords: immunosuppressants, penetrating keratoplasty, meta-analysis
INTRODUCTION
Penetrating keratoplasty is the corneal transplantation procedure in which a full-thickness cornea from the host is replaced by a graft from a donor. It has been performed in many eye diseases including pseudophakic corneal edema, keratoconus, aphakic corneal edema, and stromal corneal dystrophies[1]-[3]. Penetrating keratoplasty remains the most common tissue transplant procedure, the reported number of corneal transplants performed increased by 2.3% in 2009, from 41 652 to 42 606 cases in the United States[4]. Survival of first-time grafts is 90% at five years and 82% at 10 years with reported allograft rejection rates following penetrating keratoplasty ranging from 5% to 18%[5]. Initial regrafts have significantly lower five-year and 10-year survival rates, 53% and 41%, respectively[6]. Besides, the survival of transplants depends upon the condition of the recipient corneal bed. In high-risk corneal recipients, such as those with inflamed or vascularized recipient beds and large-diameter or eccentric transplants, the immune privileges of the corneas are broken. In these cases, the survival rates of transplants fall lower than normal people, even with immune-suppression therapy.
The eye has properties that permit the long-term survival of tissue grafts that are normally rejected at extraocular sites. This ocular immune privilege was originally attributed to a putative sequestration of antigens in the eye as a result of the conspicuous absence of intraocular lymphatic drainage channels[7]. However, a recent multivariate analysis suggests no difference between the long-term outcomes of corneal transplantation and other forms of transplantation[8]. The anterior segment of the eye is still regarded as an immune-privileged site because of the absence of vascular and lymphatic supply to the cornea. Cell-mediated immunity in corneal allograft rejection can result from the activation of limbal Langerhans cells and from T-cells activation by antigens released in the aqueous humor of the anterior chamber[9]. Nevertheless, the immunology of corneal transplantation is not fully understood[10]-[12]. Furthermore, corneal graft rejection remains the most common cause of graft failure in the late postoperative period and prophylaxis for allograft rejection is needed[13].
A variety of strategies to prevent corneal allograft rejection have been explored. Strategies include the use of several immunosuppressants through various delivery systems; human leukocyte antigens (HLA) matching and manipulation of antigen expression. Immunosuppressants include steroids, cyclosporine A (CsA), tacrolimus, mycophenolate mofetil (MMF), sirolimus and leflunomide. Topical and oral steroids are currently the gold standard for routine use in the prevention of graft rejection[5],[14],[15] and the use of topical cyclosporine for routine management of high-risk grafts is increasing[15]. CsA is a fungal protein that has a high degree of specificity for T-cell lymphocytes and as a calcineurin inhibitor prevents T-cell-mediated immune responses. Systemic CsA is believed to significantly increase the rate of graft survival in high-risk corneal transplantation when used prophylactically following transplantation. But this therapy also carries significant risks including hypertension, renal toxicity, hepatotoxicity, neurotoxicity[16],[17] and posttransplant lymphoproliferative disorders[18]. Although evidence is increasing on the effectiveness of topically administered CsA in the prevention of graft rejection[19], studies have yielded inconsistent results. For example, some investigators found that a combination use of topical CsA and steroids is better than steroids alone in preventing episodes of rejection[20]-[22]. However, other authors found topical CsA did not demonstrate any significant improvement in preventing corneal graft rejection[23],[24].
Tacrolimus (FK 506) has been shown to be effective for preventing corneal allograft rejection[25],[26] but uses a lower dose[27]. Systematic adverse effects such as hypertension and renal toxicity may be encountered with oral tacrolimus[26]. Mycophenolate mofetil (MMF) is thought to be a safe and effective immunosuppressive agent following renal transplantation due to less nephrotoxicity[28],[29]. Mycophenolate mofetil has been shown to be as effective as CsA in preventing acute rejection following high risk corneal transplantation[25],[30], but inferior to systemic tacrolimus in preventing graft rejection[27]. Rapamycin is a bacterial macrolide with both antifungal and immunosuppressive properties. It is commonly used in conjunction with CsA or tacrolimus after solid-organ transplantation[28].
Totally, immunosuppressants are widely used for the prophylaxis of corneal graft rejection after high-risk keratoplasty and normal-risk keratoplasty. However, the benefits and adverse reactions from their use have not yet been systematically reviewed. Our primary objective was to assess the effectiveness of immunosuppressants in the prophylaxis of corneal allograft rejection after high-risk and normal-risk keratoplasty.
MATERIALS AND METHODS
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library), MEDLINE, EMBASE, VIP and China National Knowledge Infrastructure (CNKI). We also searched the WHO International Clinical Trials Registry Platform (WHO ICTRP) Search Portal which included Austrlian New Zealand Clinical Trials Registry, ClinicalTrials.gov, Current Controlled Trials records. There were no date or language restrictions in the electronic search for trials. Non-English language papers will be translated so that they can be fully assessed for inclusion in the review. All databases are searched till 18 June, 2011.
We also searched the ISI Citation Index database, Science and Social Science Citation Index/Web of Science Services to find studies that had cited the identified trials. We were unable to contact the primary investigators of identified trials for details of additional trials and companies or pharmaceutical firms that produce immunosuppressants used for unpublished data they may possess.
Materials
We scanned the titles, abstracts, and keywords of every record retrieved to find any study that met our inclusion criteria. Full articles were retrieved for further assessment if the information given suggested that the studies:1) included participants after penetrating keratoplasty; 2) compared immunosuppressants such as CsA, tacrolimus and MMF with corticosteroids only; 3) assessed one or more relevant clinical outcome measures; 4) used random allocation for the comparison groups. Where randomisation was used, we were unable to contact the trial authors for confirmation of this. We analyzed the data using Review Manager 5.0. descriptively as different interventions were used in the included studies and used subgroup analyses based on different immunosuppressants and control interventions. We have used 95% confidence intervals throughout. The numbers of dropouts and lost-to-follow-up for each study were summarized, if available, using intention-to-treat analysis. We performed subgroup analyses in the descriptive form.
Methods
We included randomised controlled trials (RCT) only. We included patients undergoing high-risk and normal-risk keratoplasty and evaluated them as two separate groups. We included trials in which immunosuppressants such as CsA, tacrolimus, rapamycin and MMF were compared to placebo, corticosteroids or other immunosuppressants. Most outcomes were measured during a one-year, a two-year, a five-year and a 10-year follow-up if it was possible. For those studies where the aforementioned follow-up was not available even after correspondence with the principal investigator, we included the nearest point in time available in the general and subgroup analyses. The primary outcome if the proportion of graft survival at 12 months after penetrating keratoplasty; secondary outcomes are as follows: 1) incidence of graft rejection at 12 months (Rejection is defined as any immune reaction requiring a change in therapy, involves both epithelial rejection and endothelial rejection); 2) Best-correlated visual acuity; 3) Quality of life (QoL). The instrument of assessment for QoL should be evaluated by an international ‘minimum standard checklist’ and should be participant based[31]-[34]; 4) Cost-effect analysis. This includes the cost of the drugs and other palliative medications; the need for bedrest or hospitalisation versus outpatient care; the length of hospital stay.
Besides, we evaluated the Side effects: The incidence of epithelial keratitis; The incidence of high intraocular pressure; Major calcineurin-inhibitor toxicity (for example new-onset diabetes or renal failure); Minor calcineurin-inhibitor toxicity (for example tremor, gingivitis or hirsutism); Dose reductions due to adverse events; Withdrawals and dropouts.
RESULTS
A total of 6249 hits are yield by the search strategy. After screening the title and abstracts, 40 trials were retrieved. Of them, only seven studies measure up to the inclusion criteria[35]-[41]. Four studies were found in the clinicalTrial.gov, all of them were completed for enrolment of participants, but the study report has not been searched out in the databases. The other studies were excluded due to they are not actually RCT.
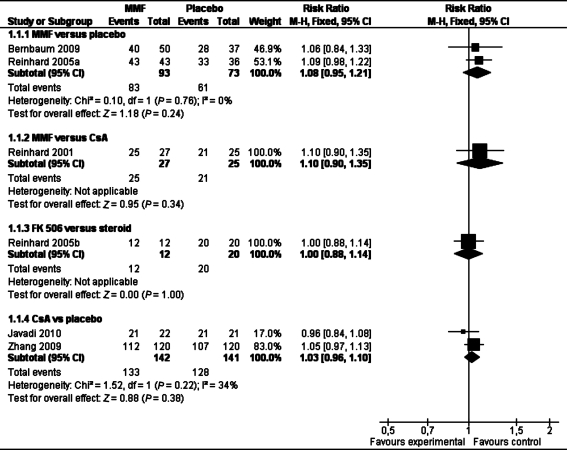
MMF vs placebo
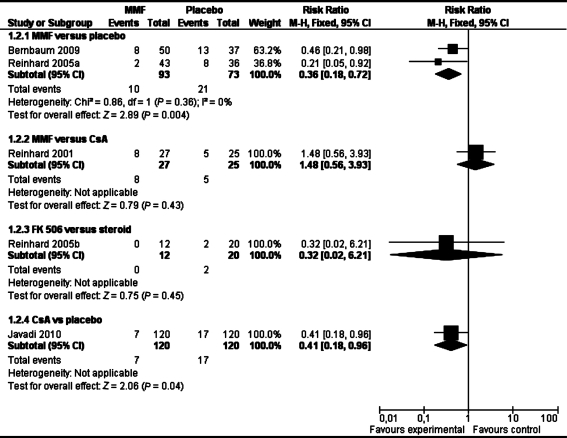
Although there was no significant difference in clear graft survival between the two groups in pooled analysis of two studies (RR 1.08 95% Cl 0.95 to 1.21) (see Table 1), MMF could significantly reduce immune reactions compared with placebo (RR 0.36 95% Cl 0.18 to 0.72, Table 2) in the combined analysis of these two studies in both normal-risk and high-risk of graft rejection patients.
Table 1. Clear graft survival.
Table 2. Immune reactions.
MMF vs CsA
There was no significant difference in clear graft survival (RR 1.11 95% Cl 0.90 to 1.35, Table 1) and immune reactions (RR 1.48 95% Cl 0.56 to 3.93)(Table 2) between the two groups in one study[37]. These results suggest that MMF and CsA have similar effect on clear graft survival and immune reactions in the high-risk of rejection patients.
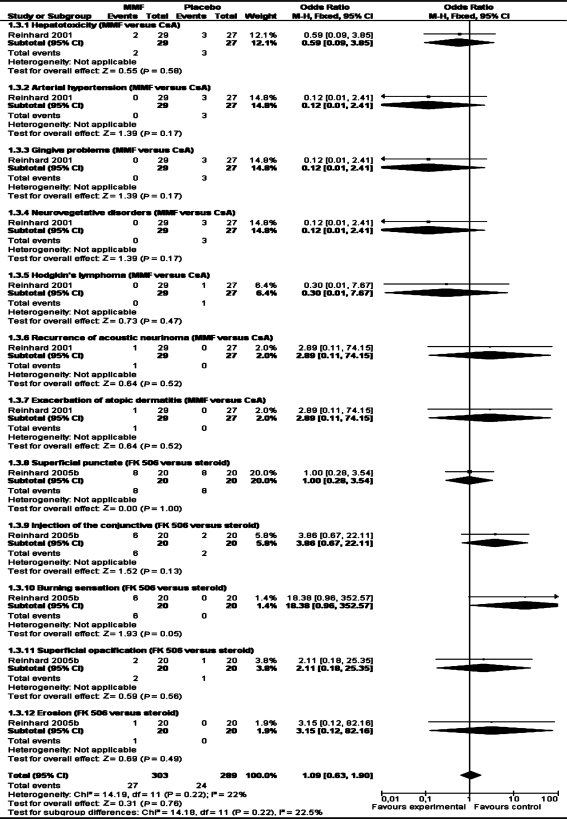
One study[38] reported adverse events such as hepatotoxicity, arterial hypertension, gingiva problems, neurovegetative disorders, Hodgkin's lymphoma, recurrence of acoustic neurinoma, exacerbation of atopic dermatitis and there were no significant differences between the two arms.
FK 506 vs steroid
Reinhard[39] involving 32 patients reported data on clear graft survival and immune reactions. There was no significant difference in clear graft survival and immune reactions between the two groups (RR 0.32, 95% CI 0.02 to 6.21, and RR 1.00, 95%CI 0.88 to 1.14)(Table 1, 2). This study reported adverse events such as superficial punctate keratitis, injection of the conjunctiva, burning sensation, superficial opacification, erosion and there were no significant differences between the two arms (Table 3).
Table 3. Side effects.
None of studies reported best-corrected visual acuity, quality of life, major and minor calcineurin-inhibitor toxicity, cost-effect analysis and the incidence of high intraocular pressure.
CsA vs place
One study[40] reported there was no statistically significant difference in incidence of graft rejection between the study and the control group: seven participants experinced one graft rejection episodes in the study group; one episode of graft rejection and one participant had two episodes of graft rejection in the control group. The mean duration after which the participants developed graft rejection after keratophlasty (rejection-free period) was 7.92±1.45 months in study group and 6.50±2.72 months in control group (P=0.20). The mean duration of reversal of rejection episode in the study group was 12.33±2.94 days and 13.5±3.10 days in the control group (P=0.56). The rejection-free time period in the eyes that had rejection was more in the study group; however, the difference was not statistically significant. These results suggested that topical cyclosporine A 2% eye drops do not prevent occurrence of graft rejection in high-risk keratoplasty.
Six participants showed complete reversal of rejection in the study group, four in the control group (P=0.03). The reversal of rejection episode was seen in significantly greater number of eyes in the study group (P=0.03). These results suggest that the eyes receiving topical cyclosporine stand a better chance of reversal of the episode of graft rejection.
The mean keratometric astigmatism in the clear grafts at the end of 1 year was 3.69±1.37 diopters (D) in the study group and 4.53±1.88 D in the control group (P=0.06). The mean specular count was 1,365.33±471.63 in the study group and 1,151.18±293.31 in the control group at the end of year (P=0.06). Average corneal thickness was significantly higher in the control group than study group at the end of 1 year (P=0.008).
Visual acuity at 1 month after keratophlasty was 0.20±0.17 in the study group and 0.09±0.13 in the control group (P =0.003). At the end of 1 year 0.31±0.18 in study group and 0.24±0.17 in control group, P=0.14.
The authors of study Sinha 2010 recommended that polyvinyl alcohol is an ideal vehicle because there was no any adverse effects observed in the study.Study Javadi 2010 reported episodes of graft rejection 2.7(1.8, range 0-5) in group 1, 1.4(1.2, range 0-4) in group 2, P=0.03. Of these, 1.7(1.4, range 0-4) episodes occurred while the participants of group 1 were receiving topical CsA, and 1.0 (0.9, range 0-3) episodes recurred in group 2 during the corresponding period of receiving placebo, P=0.14.
Rejection-free graft survival rate 34.8% in group 1, 31.7 in group 2 at 20 months (P=0.89). The mean length of rejection-free graft survival was 10.5 (0.5) months in group 1 and 14.2 (2.7) months in group 2 with a median of 8 months in both groups. For the episode of graft rejection in which topical CsA or placebo was started, the participants in groups 1 and 2 received 0.1% betamethasone eyedrop for 50.6(10.6) (range 31-68) days and 60.3(21.1) (range 36-124) days, respectively (P=0.08). The episode took 25.6 (21.0) days in group 1 and 33.2(16.7) days in group 2 to completely resolve (P=0.22, Table 1, 2).
Study[41] reported an obvious reduction of reject events in 6 months in CsA group than placebo group (7/120 versus 17/120, P=0.025), and the the incidence of adverse events was similar in the CsA and placebo groups.
Pooled analysis of two studies[40],[45] for clear graft survival showed there is no benefit by using CsA (112/120 in CsA group, 107/120 in placebo group, RR 1.03, 95%CI 0.96 to 1.10, P=0.25, Table 1).
DISCUSSION
Immunosuppressants have the function of preventing corneal graft rejection is based on inhibiting the immunity of the host. The targets of different drugs acted on are some different. The mechanism of CsA prophylaxis of corneal graft rejection is mainly by selectively inhibit cellular immunity, primarily inhibits the proliferation and action of T cells[42]. MMF prevents the replication of T- and B-lymphocytes by inhibiting the de novo pathway of purine synthesis[43]. FK 506, also a calcineurin inhibitor, is a macrolide antibiotic with potent immunosuppressive activity[44]. Steroids have anti-proliferative function[45].
Based on the seven small RCTs, MMF may have an effect on reducing immune reactions. MMF has a similar effect on clear graft survival as CsA. There was no difference between FK506 and steroid. However, the following factors may affect the results. The review included studies conducted in four countries include China, Germany, Iran and India. Four studies[36],[38],[40],[41] were well designed, conducted and reported. Although, there were some methodology shortcomings in other three studies[35],[37],[39], of them, ineligible method was used to allocated the participants in study[35],[37],[39] did not give information of randomisation procedure in detail, and totally, the sample size were not big enough, but the limited evidence from these studies could be used to estimate the effect size of rate of clear graft survival and immune reactions in normal risk and high risk of rejection patients who need to accept corneal penetrating keratoplasty in clinical practice.
Totally, the quality of evidence from included studies are rated from very low to moderate. The likely publication bias due to only one or two studies included in a outcome measure and limitations on study design resulted downgrade one level, respectively.
Four studies[36],[38],[40],[41] were of higher methodological quality. In these three studies, eligible randomisation procedure and allocation concealment were performed. In one study[41], intention-to-treat was used to calculate the effects. Other three studies were rated as poor methodological quality. Non of them provided information about randomisation procedure, allocation concealment and masking. None of these studies used ‘intention-to-treat analysis’ to test the robustness of the results. There were some participants who did not complete the study according to the protocol in all three studies and the duration of follow up for every participant was not the same. All the factors mentioned above could lead to selection bias, performance bias, or detection or incomplete data bias. This could result in false positive findings. Javadi et al[36] reported the calculation of the sample size, others did not, so whether the sample size was sufficient or not is unknown. Three trials[37]-[39] failed to report the information on interventions adequately. Dosage and duration of therapy should be considered when evaluating the effectiveness of immunosuppressants, but we cannot do this due to the limited number of included trials.
The outcomes observed in the included trials are few that we are unable to say whether immunosupressants have an effect on other indexes that evaluate penetrating keratoplasty, which contributes to the difficulty in analysing the effect of immunosuppressants thoroughly. There were so few trials identified and participants involved that we cannot analyse many confounding variables, such as indications for surgery, age, length of follow-up, route of administration, dosage etc. Our searches were limited to English and Chinese databases which could result in selection bias and language bias. It is impossible for us to investigate publication bias using a funnel plot in this review because of the insufficient number of trials. Topical MMF had been found is ineffective for prophylaxis of corneal graft rejection in an experimental keratoplasty model[46], but we have not find other review related to this topic.
The results suggest that MMF on the basis of fluocortolone may reduce immune reactions in penetrating high-risk keratoplasty, the effectiveness and safety of MMF, CsA, topical FK506 and steroid are the same. Due to the small number of RCTs and participants, there is insufficient evidence currently to ascertain which immunosuppressant is better for penetrating keratoplasty. Large,properly randomised, placebo-controlled and double masked trials are needed to evaluate the effectiveness of immunosuppressants. The following factors should be taken into account in future studies: sample size should be calculated before the start of the study; report the procedure of randomisation and the allocation concealment; apply masking and describe it in detail; describe the baseline of participants in detail; describe the manufacturer, composition, dosage and course of treatment of the drugs; describe the standard of outcomes and the time to measure them in detail; intention-to-treat analysis should be applied to analyse the outcomes when there are missing participants due to drop-out or loss of follow-up.
Acknowledgments
We thank Anupa Shah, Richard Wormald, and Iris Gordon from the Cochrane Eyes and Vision Group, for help on writing the protocol. We also thank Jod Mehta for his advice on this protocol and Catey Bunce, Keryn Williams and Doug Coster for their comments on the protocol.
REFERENCES
- 1.Dobbins KR, Price FW, Jr, Whitson WE. Trends in the indications for penetrating keratoplasty in the midwestern United States. Cornea. 2000;19(6):813–816. doi: 10.1097/00003226-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Liu E, Slomovic AR. Indications for penetrating keratoplasty in Canada 1986-1995. Cornea. 1997;16(4):414–419. [PubMed] [Google Scholar]
- 3.Ramsay AS, Lee WR, Mohammed A. Changing indications for penetrating keratoplasty in the west of Scotland from 1970 to 1995. Eye. 1997;11(Pt 3):357–360. doi: 10.1038/eye.1997.75. [DOI] [PubMed] [Google Scholar]
- 4.Eye Bank Association of America. EBAA Eye Banking Statistical Report. 2011;7(6):133–137. [Google Scholar]
- 5.Tabbara KF. Pharmacologic strategies in the prevention and treatment of corneal transplant rejection. International Ophthalmology. 2007;28(3):223–232. doi: 10.1007/s10792-007-9100-7. [DOI] [PubMed] [Google Scholar]
- 6.Thompson RW, Price MO, Bowers PJ, Price FW. Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110(7):1396–1402. doi: 10.1016/S0161-6420(03)00463-9. [DOI] [PubMed] [Google Scholar]
- 7.Niederkorn JY. Mechanisms of immune privilege in the eye and hair follicle. Journal of Investigative Dermatology. Symposium Proceedings. 2003;8(2):168–172. doi: 10.1046/j.1087-0024.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 8.Williams KA, Esterman AJ, Bartlett C, Holland H, Hornsby NB, Coster DJ. How effectiveis penetrating corneal transplantation? Factors influencing long-term outcome in multivariate analysis. Transplantation. 2006;81(6):896–901. doi: 10.1097/01.tp.0000185197.37824.35. [DOI] [PubMed] [Google Scholar]
- 9.Yamagami S, Hamrah P, Zhang Q, Liu Y, Huq S, Dana MR. Early ocular chemokine gene expression and leukocyte infiltration after high-risk corneal transplantation. Mol Vis. 2005;11:632–640. [PubMed] [Google Scholar]
- 10.Arentsen JJ. Corneal transplant allograft reaction: possible predisposing factors. Transactions of the American Ophthalmological Society. 1983;81:361–402. [PMC free article] [PubMed] [Google Scholar]
- 11.Chandler JW, Kaufman HE. Graft reactions after keratoplasty for keratoconus. Am J Ophthalmol. 1974;77(4):543–547. doi: 10.1016/0002-9394(74)90469-3. [DOI] [PubMed] [Google Scholar]
- 12.Hughes WF. The treatment of corneal dystrophies by keratoplasty. Am J Ophthalmol. 1960;50(6):1100–1114. doi: 10.1016/0002-9394(60)90997-1. [DOI] [PubMed] [Google Scholar]
- 13.Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998;105(10):1855–1865. doi: 10.1016/S0161-6420(98)91030-2. [DOI] [PubMed] [Google Scholar]
- 14.Hill JC, Maske R, Watson P. Corticosteroids in corneal graft rejection. Oral versus single pulse therapy. Ophthalmology. 1991;98(3):329–333. doi: 10.1016/s0161-6420(91)32291-7. [DOI] [PubMed] [Google Scholar]
- 15.Randleman JB, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns (2004) Cornea. 2006;25(3):286–290. doi: 10.1097/01.ico.0000178731.42187.46. [DOI] [PubMed] [Google Scholar]
- 16.Hill JC. The use of cyclosporine in high-risk keratoplasty. Am J Ophthalmol. 1989;107(5):506–510. doi: 10.1016/0002-9394(89)90494-7. [DOI] [PubMed] [Google Scholar]
- 17.Hill JC. Systemic cyclosporine in high-risk keratoplasty. Short-versus long-term therapy. Ophthalmology. 1994;101(1):128–133. doi: 10.1016/s0161-6420(13)31253-6. [DOI] [PubMed] [Google Scholar]
- 18.Algros MP, Angonin R, Delbosc B, Cahn JY, Kantelip B. Danger of systemic cyclosporine for corneal graft. Cornea. 2002;21(6):613–614. doi: 10.1097/00003226-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Belin MW, Bouchard CS, Phillips TM. Update on topical cyclosporin A. Background, immunology, and pharmacology. Cornea. 1990;9(3):184–195. [PubMed] [Google Scholar]
- 20.Cosar CB, Laibson PR, Cohen EJ, Rapuano CJ. Topical cyclosporine in pediatric keratoplasty. Eye & Contact Lens. 2003;29(2):103–107. doi: 10.1097/01.ICL.0000062460.03555.32. [DOI] [PubMed] [Google Scholar]
- 21.Inoue K, Amano S, Kimura C, Sato T, Fujita N, Kagaya F, Kaji Y, Oshika T, Tsuru T, Araie M. Long-term effects of topical cyclosporine A treatment after penetrating keratoplasty. Jpn J Ophthalmol. 2000;44(3):302–305. doi: 10.1016/s0021-5155(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 22.Xi XH, Qin B, Jiang DY. Cyclosporin A combined with dexamethasone in preventing and treating immune rejection after penetrating keratoplasty. Bulletin of Hunan Medical University. 2003;28(6):627–630. [PubMed] [Google Scholar]
- 23.Price MO, Price FW. Efficacy of topical cyclosporine 0.05% for prevention of cornea transplant rejection episodes. Ophthalmology. 2006;113(10):1785–1790. doi: 10.1016/j.ophtha.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Shepherd WF, Coster DJ, Fook TC, Rice NS, Jones BR. Effect of cyclosporin A on the survival of corneal grafts in rabbits. Brit J Ophthalmol. 1980;64(3):148–153. doi: 10.1136/bjo.64.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinhard T, Mayweg S, Sokolovska Y, Seitz B, Mittelviefhaus H, Engelmann K, Voiculescu A, Grodehardt E, Sundmacher R. Systemic mycophenolate mofetil avoids immune reactions in penetrating high-risk keratoplasty: preliminary results of an ongoing prospectively randomized multicentre study. Transplant International. 2005;18(6):703–708. doi: 10.1111/j.1432-2277.2005.00126.x. [DOI] [PubMed] [Google Scholar]
- 26.Sloper CM, Powell RJ, Dua HS. Tacrolimus (FK506) in the management of high-risk corneal and limbal grafts. Ophthalmology. 2001;108(10):1838–1844. doi: 10.1016/s0161-6420(01)00759-x. [DOI] [PubMed] [Google Scholar]
- 27.Reis A, Reinhard T, Sundmacher R, Braunstein S, Godehardt E. Comparative investigation of FK506 and cyclosporin A in murine corneal transplantation. Graefes Archive for Clinical & Experimental Ophthalmology. 1998;236(10):785–789. doi: 10.1007/s004170050159. [DOI] [PubMed] [Google Scholar]
- 28.Guerra G, Srinivas TR, Meier-Kriesche HU. Calcineurin inhibitor-free immunosuppression in kidney transplantation. Transplant International. 2007;20(10):813–827. doi: 10.1111/j.1432-2277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 29.Land W, Vincenti F. Toxicity-sparing protocols using mycophenolate mofetil in renal transplantation. Transplantation. 2005;80(2 Suppl):S221–234. doi: 10.1097/01.tp.0000186386.13597.cb. [DOI] [PubMed] [Google Scholar]
- 30.Reis A, Reinhard T, Voiculescu A, Kutkuhn B, Godehardt E, Spelsberg H, Althaus C, Sundmacher R. Mycophenolate mofetil versus cyclosporin A in high risk keratoplasty patients: a prospectively randomised clinical trial. Brit J Ophthalmol. 1999;83(11):1268–1271. doi: 10.1136/bjo.83.11.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efficace F, Bottomley A, Osoba D, Gotay C, Flechtner H, D'haese S, Zurlo A. Beyond the development of health-related quality-of-life (HRQOL) measures: a checklist for evaluating HRQOL outcomes in cancer clinical trials-does HRQOL evaluation in prostate cancer research inform clinical decision making? Journal of Clinical Oncology. 2003;21(18):3502–3511. doi: 10.1200/JCO.2003.12.121. [DOI] [PubMed] [Google Scholar]
- 32.Efficace F, Horneber M, Lejeune S, Van Dam F, Leering S, Rottmann M, Aaronson NK. Methodological quality of patient-reported outcome research was low in complementary and alternative medicine in oncology. Journal of Clinical Epidemiology. 2006;59(12):1257–1265. doi: 10.1016/j.jclinepi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Efficace F, Osoba D, Gotay C, Sprangers M, Coens C, Bottomley A. Has the quality of health-related quality of life reporting in cancer clinical trials improved over time? Towards bridging the gap with clinical decision making. Annals of Oncology. 2007;18(4):775–781. doi: 10.1093/annonc/mdl494. [DOI] [PubMed] [Google Scholar]
- 34.Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, editors; Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. (updated September 2008) [Google Scholar]
- 35.Bernbaum F, Mayweg S, Reis A, Bohringer D, Seitz B, Engelmann K, Messmer EM, Reinhard T. Mycophenolate mofetil (MMF) following penetrating highrisk keratoplasty: long-term results of a prospective, randomised, multicentre study. Eye. 2009;(23):2063–2070. doi: 10.1038/eye.2008.402. [DOI] [PubMed] [Google Scholar]
- 36.Javadi MA, Feizi S, Karbasian A, Rastegarpour A. Efficacy of topical ciclosporin A for treatment and prevention of graft rejection in corneal grafts with previous rejection episodes. Brit J Ophthalmol. 2010;94(11):1464–1467. doi: 10.1136/bjo.2009.172577. [DOI] [PubMed] [Google Scholar]
- 37.Reinhard T, Reis A, Bohringer D, Malinowski M, Voiculescu A, Heering P, Godehardt E, Sunmacher R. Systemic mycophenolate mofetil in comparison with systemic cyclosporin A in high-risk keratoplasty patients: 3 years' results of a randomised prospective clinical trial. Graefes Archive for Clinical & Experimental Ophthalmology. 2001;239(5):367–372. doi: 10.1007/s004170100285. [DOI] [PubMed] [Google Scholar]
- 38.Reinhard T, Mayweg S, Sokolovska Y, Seitz B, Mittelviefhaus H, Engelmann K, Voiculeseu A, Godehardt E, Sundmacher R. Systemic mycophenolate mofetil avoids immune reactions in penetrating high-risk keratoplasty: preliminary results of an ongoing prospectively randomised multicentre study. Transplant International. 2005;18(6):703–708. doi: 10.1111/j.1432-2277.2005.00126.x. [DOI] [PubMed] [Google Scholar]
- 39.Reinhard T, Mayweg S, Reis A, Sundmacher R. Topical FK506 as immunoprophylaxis after allogeneic penetrating normal-risk keratoplasty:a randomised clinical pilot study. Transplant International. 2005;18(2):193–197. doi: 10.1111/j.1432-2277.2004.00006.x. [DOI] [PubMed] [Google Scholar]
- 40.Sinha R, Jhanji V, Verma K, Sharma N, Biswas NR, Vajpayee RB. Efficacy of topical cyclosporine A in prevention of graft rejection in high-risk keratoplasty: a randomised controlled trial. Graef Arch Clin Exp. 2010;248(8):1167–1172. doi: 10.1007/s00417-010-1388-8. [DOI] [PubMed] [Google Scholar]
- 41.Zhang YQ, Chen JQ, Zhou LH, Shi WY, Wang LY, Wang YQ. The randomised double blindness, multi-centre clinical trial for CsA on rejection following penet rating keraloplasty (PRK) Chinese Phythalogy Research. 2009;27(5):407–411. [Google Scholar]
- 42.Utine CA, Stern M, Akpek EK. Clinical review: topical ophthalmic use of cyclosporin A. Ocular Immunology and Inflammation. 2010;18(5):352–361. doi: 10.3109/09273948.2010.498657. [DOI] [PubMed] [Google Scholar]
- 43.Siconolfi L. Mycophenolate mofetil (CellCept): immunosuppression on the cutting edge. AACN Clinical Issues. 1996;7(3):390–402. doi: 10.1097/00044067-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Pillans P. Immunosuppressants-mechanisms of action and monitoring. Australian Prescriber. 2006;(29):99–101. [Google Scholar]
- 45.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Critical Reviews in Oncology/Hematology. 2005;56(1):23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Bertelmann E, de Ruijter M, Gong N, Knapp S, Pleyer U. Survival of corneal allografts following topical treatment with the immunomodulator mycophenolate mofetil. Ophthalmologica. 2010;224(1):38–41. doi: 10.1159/000233237. [DOI] [PubMed] [Google Scholar]