Abstract
AIM
To evaluate the dynamic ocular biometric changes of a modified form-deprivation myopia model in young guinea pigs.
METHODS
The animals were randomly assigned to two groups: the monocularly deprived facemask group (MDF, with all the right eyes covered, n=24) and the normal control group (free of facemask, n=24). Each group was then equally divided into four subgroups which were followed up for 2, 4, 6 and 8 weeks, respectively. Parameters measured from every eye included refraction, corneal curvature, axial length and the dry weight of sclera at the posterior pole.
RESULTS
All the facemasks remained in place during the follow-up. The covered eyes developed myopia with the vitreous chamber lengthening and the dry weight of posterior sclera reduced at each time point compared with the contralateral uncovered (P<0.05 at all time points). The changes had a linear correlation with the deprivation time (P<0.05). There were no significant differences in all the parameters between the uncovered eyes of MDF group and the normal control group (P>0.05 at all time points).
CONCLUSION
Monocular form deprivation with the facemask is highly effective and non-invasive in inducing axial myopia in guinea pigs. The axial myopia is mainly caused by the increased vitreous chamber length and the weakened posterior sclera rigidity. The form-deprivation eye didn't interfere with the natural development of the contralateral eye.
Keywords: dynamic change, form-deprivation, myopia model, guinea pig
INTRODUCTION
Visual form-deprivation can cause axial myopia which is a common refractive error in the early childhood of human. It can arise from neonatal eye closure, ptosis, corneal opacity, congenital cataract and hemangioma of the eyelid[1]-[3]. A variety of animals had been used to investigate the mechanisms of axial myopia, including monkeys, chickens, tree shrews, mice, cats and guinea pigs[4]-[6].
Guinea pigs is considered to be a suitable alternative to monkey in the study of myopia since it has an ocular structure and physiology similar to monkey and is relatively susceptible to myopic development after 1 or 2 weeks of form-deprivation[7]. Moreover, it is cooperative, easily breed, readily available and less expensive than most of the other small mammals.
To our knowledge, the development of changes in ocular parameters (such as refraction, corneal curvature, axial length and dry weight of sclera at the posterior pole) regarding a form-deprivation myopia model of young guinea pigs have not yet been reported. In this study, we designed a novel facemask to ensure the successfully monocular form-deprivation and continuously observed the effect of monocular cover on the development of eyeball in guinea pigs.
MATERIALS AND METHODS
Animals and assignment
Forty-eight male guinea pigs weighing 140-170g were used for this study. These animals were got form Experimental Animal Laboratory of Zhejiang Academy of Medical Sciences. All animal procedures were approved by local Animal Care Committee and in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Careful examination was performed to ensure that there was no abnormality in both anterior and posterior segment. The animals were randomly assigned to two groups: the MDF group (n=24) and the normal control (free of form-deprivation, n=24). Each group was then randomly divided into four subgroups (n=6 each) which were followed up for 2,4,6 and 8 weeks, respectively and baseline ocular parameters (refraction, corneal curvature and axial length) were recorded. At each of the four time points, aforementioned index and dry weight of sclera at the posterior pole were measured. All animals were raised on a shift of 12-hour illumination and 12-hour darkness each day. Water was supplemented with Vitamin C and food was freely available for the animals.
Form-deprivation
Monocular facemask
Opaque latex balloon (Suzhou, China) was modified into facemask which only covered the animal's right eye, leaving the left eye, nose, mouth and ears exposed (Figure 1). Two sizes of the facemasks were used according to the change of the animal's head size during the follow-up. Usually, the animals wore a small facemask (Size 6) at the beginning and a larger one (Size 8) in 6 weeks. The facemasks were checked three times weekly to ensure that they were in place and could be changed to another size in time.
Figure 1. A guinea pig (7 weeks old) well fitted with a MDF. The MDF only covered the right eye, leaving the left eye, nose, mouth and ears exposed.

Biometry
Retinoscopy
One hour before retinoscopy examination, 1% cyclopentolate hydrochloride (Alcon, Belgium) was topically administered every five minutes for four times to achieve a completely-dilated pupil. Retinoscopy was performed in a dark room with a streak retinoscope and trial lenses. The refraction was recorded as the sum of the spherical component and one-half of the cylindrical components.
Keratometry
A +8.0D lens was attached onto the anterior surface of the keratometer (Topcon, OM-4, Japan) during the measurement to magnify the cornea of the guinea pigs. This made it possible to examine and record the readings on the steep cornea. A group of stainless balls with diameters from 5.5 to 11.0mm was measured by the modified keratometer (three measurements were averaged). The radius of corneal curvature of the guinea pig was then deduced from the results of the balls with known radii[8].
Ultrasonography
A-scan ultrasonography (Cinescan A/B scan at 11MHZ, France) was used to measure the axial length of the eye while animals were lightly anaesthetized with 1% halothane oxygen. The parameters included the depth of the anterior chamber, the thickness of the crystalline lens and the length of the vitreous chamber. The conducting velocity was set at 1540m/s to measure the anterior chamber and the vitreous body and at 1645m/s to measure the crystalline lens[8]. The result was the mean of 10 measurements.
Scleral dry weight
Bilateral eyes of MDF group and the normal control group were enucleated under deep anesthesia (pentobarbital sodium was injected peritoneally, 120mg/kg). Extraneous orbital tissue was dissected from the globe and the cornea was dissected out with a circumferential cut around the limbus. The iris, crystalline lens and vitreous were removed. A 6mm trephine was used to remove a nasal region of the posterior pole of the eye (1-2mm distance from optic nerve) and the retina and choroid tissues were also cleaned. Samples were dried at 105°C in an oven under an atmosphere of phosphorous pentoxide for 18 to 24 hours. Dry weights were recorded to the nearest 0.01mg. The final data was the mean of three readings obtained with an electric balance (BP221s, Germany)[9].
Statistical Analysis
The measurement and the data collection were performed by 2 researchers, both of whom were blinded to the study. In MDF group, the right covered eyes (RC) were compared with the left uncovered eyes (LUC). The LUC were also compared with the normal control. The ocular data in the normal control group were calculated as the mean of both eyes. SPSS 11.5 statistical software was used in the analysis. The paired-samples t-test and the independent-samples t-test were used for the comparison of the means. The P level of significance was set at 0.05.
RESULTS
Facemasks
All the facemasks were kept in place during the follow-up and the facemask was replaced by a larger one in all eyes between the 4th and 6th week.
Biometry
There was no significant difference among the RC, LUC and normal control group in refraction, corneal curvature and axial length at baseline (data not shown).
Right covered eyes (RC) vs left uncovered eyes (LUC)
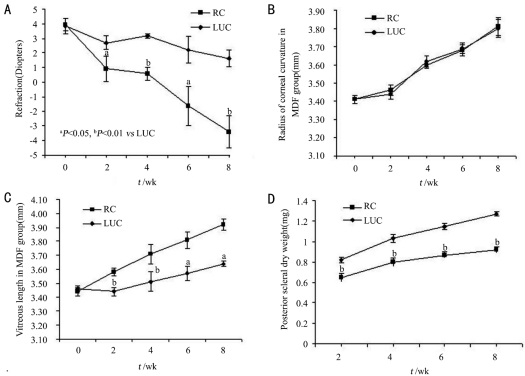
Significant differences were observed in refraction between the two groups at all time points (2, 4, 6 and 8 weeks, paired-samples t-test, P=0.028, 0.002, 0.045 and 0.004, respectively). As time passed, the difference in refraction gap between RC and LUC eyes had widened, and the changes were negatively correlated with the form-deprivation time (R=-0.476, Figure 2A).
Figure 2. The changes during the form-deprivation period in MDF group.
A: The changes in refraction; B: The changes in radius of corneal curvature; C: The changes in vitreous body length; D: The changes in posterior scleral dry weight
There were no significant differences in corneal curvature radius between RC and LUC eyes at all time points (2, 4, 6 and 8 weeks, paired-samples t-test P=0.248, 0.081, 0.930 and 0.773; Figure 2B). Similarly, no significant differences in depth of the anterior chamber and thickness of the crystalline lens could be found between the two groups during the follow-up. However, as shown in Figure 2C, significant differences could be found in the length of the vitreous chamber between the RC and LUC eyes at each of the time point (P=0.003, 0.000, 0.028 and 0.020, paired-samples t-test respectively).The difference had enlarged with the prolonged deprivation time, and the two were in a positive correlation (R=0.749).
There were statistically significant differences in the posterior scleral dry weight between the two groups at each time point (P=0.001, 0.000, 0.000 and 0.000, paired-samples t-test respectively) and the gaps enlarged with the form-deprivation continuing (Figure 2D). The difference of dry weight was negatively correlated with the form-deprivation time (R=-0.738).
Left uncovered eyes vs normal control group
The ocular data of each guinea pig in the normal control group was calculated as the mean of both eyes. There were no significant differences in all the parameters (diopter, radius of corneal curvature, anterior chamber depth, thickness of the crystalline lens, vitreous body length and posterior scleral dry weight ) between the LUC and the normal control group at each of the follow-up time point (independent-samples t-test, Table 1).
Table 1. The ocular parameters of left uncovered eyes in MDF compared with those in the normal control group.
| Parameters | Follow-up time (weeks, n) | MDF (left eye) | Normal control | t value |
| Diopter | 0(24) | 3.84±2.64 | 4.60±1.37 | 0.217 |
| 2(6) | 3.71±1.16 | 3.63±1.34 | 0.910 | |
| 4(6) | 3.17±0.34 | 3.21±0.70 | 0.899 | |
| 6(6) | 2.21±2.27 | 2.88±0.65 | 0.516 | |
| 8(6) | 1.63±1.41 | 1.31±2.38 | 0.788 | |
| Radius of corneal curvature(mm) | 0(24) | 3.41±0.08 | 3.38±0.10 | 0.264 |
| 2(6) | 3.44±0.07 | 3.48±0.06 | 0.313 | |
| 4(6) | 3.62±0.08 | 3.59±0.06 | 0.413 | |
| 6(6) | 3.69±0.08 | 3.65±0.10 | 0.443 | |
| 8(6) | 3.80±0.12 | 3.76±0.07 | 0.467 | |
| Anterior chamber depth (mm) | 0(24) | 1.27±0.03 | 1.27±0.04 | 0.573 |
| 2(6) | 1.27±0.03 | 1.28±0.03 | 0.525 | |
| 4(6) | 1.29±0.01 | 1.29±0.01 | 0.666 | |
| 6(6) | 1.31±0.01 | 1.31±0.01 | 0.511 | |
| 8(6) | 1.30±0.02 | 1.31±0.01 | 0.436 | |
| Thickness of the crystalline lens (mm) | 0(24) | 3.14±0.14 | 3.09±0.17 | 0.266 |
| 2(6) | 3.33±0.10 | 3.39±0.12 | 0.316 | |
| 4(6) | 3.52±0.08 | 3.42±0.16 | 0.200 | |
| 6(6) | 3.50±0.10 | 3.46±0.12 | 0.586 | |
| 8(6) | 3.64±0.07 | 3.59±0.06 | 0.275 | |
| Vitreous body length (mm) | 0(24) | 3.46±0.11 | 3.47±0.13 | 0.718 |
| 2(6) | 3.44±0.07 | 3.41±0.12 | 0.704 | |
| 4(6) | 3.51±0.18 | 3.58±0.14 | 0.489 | |
| 6(6) | 3.57±0.13 | 3.66±0.16 | 0.298 | |
| 8(6) | 3.64±0.05 | 3.64±0.22 | 0.986 | |
| Posterior scleral dry weight (mg) | 2(6) | 0.82±0.08 | 0.83±0.11 | 0.880 |
| 4(6) | 1.03±0.10 | 1.10±0.08 | 0.247 | |
| 6(6) | 1.15±0.08 | 1.23±0.09 | 0.174 | |
| 8(6) | 1.27±0.05 | 1.26±0.16 | 0.907 |
All P>0.05; MDF: the monocularly deprived facemask group
Mean±SD
DISCUSSION
In this study, guinea pigs were used for the establishment of a form-deprived myopic model for the animals are cooperative and more susceptible to myopic development. At present, there are two common methods used for form-deprivation: eyelid suturing and use of an opaque goggle glued to the skin around the eye[7]-[9]. However, these methods have some defects. Suturing may cause injuries to the eyelid and the use of goggle affixation with glue may lead to erosion and infection of the eyelid[10]. In addition, the detachment of the sutures and removal of the goggle may decrease the number of samples and affect the study results[4],[8]. Therefore, in this study we design an effective and non-invasive device of the facemask, which differed from the traditional methods. No detachment or break of the masks happened during the study. This enhanced the reliability of the results and cut down on the number of samples. In addition, it seemed that the MDF would not flatten the cornea perhaps due to the flexibility of the latex material which had been proved by our data in corneal curvature. The modified facemask avoided mechanical pressure to the anterior segment and minimized the effect of the corneal curvature on the myopic development.
Our study revealed the dynamic changes of major ocular parameters in juvenile guinea pigs form-deprivation myopia model. The statistical analysis demonstrated that during the course of form-deprivation, the refraction, vitreous chamber length and posterior scleral dry weight of the covered eyes had significantly changed compared with the contralateral uncovered eyes and the differences enlarged as the time passed. The differences in refraction and the posterior scleral dry weight were in negative correlation with the form-deprivation time (R=-0.476, R=-0.738, respectively), while the vitreous body length was positively correlated (R=0.749). No significant differences were observed in the corneal curvature, anterior chamber depth and crystalline lens thickness between the two groups. These results indicated that in juvenile guinea pigs the myopia development in the covered eyes was mainly caused by the lengthening of the vitreous chamber and was rarely correlated with the corneal curvature, anterior chamber depth or the thickness of the crystalline lens. These findings agreed with those experiments using chickens and tree shrews[9],[11]-[13].
In mammals, the collagen and protein account for 90% of the dry weight of the scleral tissue[14]. The sclera itself can accommodate the metabolism of extracellular matrix (ECM) accurately according to the changes of the visual environment. In the normal eye, the synthesis and degradation of ECM is in a state of dynamic equilibrium, and the key to maintaining this coordinative state is a secretory balance of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs)[15],[16]. Norton et al[17] discovered that in tree shrews, sulfated glycosaminoglycans level was significantly lower in the deprived eyes, as compared to the control eyes, and the hydroxyproline level was significantly lower only at the posterior pole. These results suggested form deprivation slowed or reversed the normal process of extracellular matrix accumulation in sclera. Zorn et al[18] found that in tree shrew deprived eyes, the hydroxyproline contents in the posterior sclera were notably reduced. These findings supported that in MDF model the dynamic balance between MMPs and TIMPs was altered, resulting in the degradation of scleral matrix, the thinning of the sclera and the reduction of resistance which might explain why the eyeball prolongated in the axial myopia. In our study, the posterior scleral dry weight of the covered eyes was significantly reduced as compared with the contralateral uncovered eyes. The differences were -0.17±0.02mg, -0.23±0.02mg, -0.28±0.02mg and -035±0.04mg at 2, 4, 6 and 8 weeks. It seemed that this pathological change developed early in the form-deprived eyes and became more severe with the deprivation time prolonged. These results also conformed to the previous study made by Curtin et al[19].
In order to find out whether the monocular cover would affect the normal development of the contralateral eye, we added the normal control group in which neither eye of the guinea pig was covered. The major ocular parameters including diopter, radius of corneal curvature, anterior chamber depth, thickness of the crystalline lens, vitreous body length and posterior scleral dry weight were compared between the contralateral uncovered eyes and the control group. However, no significant differences were observed in any of these parameters at each follow-up time point, and these ocular data were also consistent with those from emmetropization of the normal guinea pigs[20]. The result revealed the form-deprivation covered eye would not interfere with the natural development of the contralateral eye and in other words the cover really could cause severe pathological change, this result was also found in the study of tree shrew[8]. This also is consistent with some clinical findings in human being. We can find the other eye well developed and with normal visual function in children with early developed monocular severe congenital cataract or corneal disease.The small size of samples and the mid-term observation are the limitation of this study. A large size, randomized and long-term follow up study is being planned to make general exploration of form-deprivation myopia. The molecular biological changes concerning the myopic development in the form-deprived eyes would also be further studied.
Taken together, with the modified MDF model, we successfully induced axial myopia which was mainly caused by the lengthening of the vitreous chamber. The increase of vitreous chamber length and the decrease of posterior scleral dry weight in the covered eyes were in linear correlation with the deprivation time. Moreover, the form-deprivation covered eye would not interfere with the natural development of the contralateral eye. Further study is still needed to make a general exploration of form-deprivation myopia.
REFERENCES
- 1.Robb RM. Refractive errors associated with hemangiomas of the eyelids and orbit in infancy. Am J Ophthalmol. 1977;83(1):52–58. doi: 10.1016/0002-9394(77)90191-x. [DOI] [PubMed] [Google Scholar]
- 2.O'Leary DL, Millodot M. Eyelid closure causes myopia in humans. Experientia. 1979;35(11):1478–1479. doi: 10.1007/BF01962795. [DOI] [PubMed] [Google Scholar]
- 3.Hoyt CS, Stone RD, Fromer C, Billson FA. Monocular axial myopia associated with neonatal eyelid closure in human infants. Am J Ophthalmol. 1981;91(2):197–200. doi: 10.1016/0002-9394(81)90173-2. [DOI] [PubMed] [Google Scholar]
- 4.Goss DA, Criswell MH. Myopia development in experimental animals: a literature review. Am J Optom Physiol Opt. 1981;58(10):859–869. doi: 10.1097/00006324-198110000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Edwards MH. Animal models of myopia: a literature review. Acta Ophthalmologica Scandinavica. 1996;74(3):213–219. doi: 10.1111/j.1600-0420.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 6.Phillips JR, Khalaj M, Mcbrien NA. Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest Ophthal Vis Sci. 2000;41(8):2028–2034. [PubMed] [Google Scholar]
- 7.Mcfadden S, Howlett MHC, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004;44(7):643–653. doi: 10.1016/j.visres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Marsh-Tootle W, Norton TT. Refractive and structural measures of lid-suture myopia in tree shrew. Invest Ophthalmol Vis Sci. 1989;30(10):2245–2257. [PubMed] [Google Scholar]
- 9.McBrien NA, Cornell LM, Gentle A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci. 2001;42(10):2179–2187. [PubMed] [Google Scholar]
- 10.Tejedor J, Villa P. Refractive changes induced by form deprivation in the mouse eye. Invest Ophthalmol Vis Sci. 2003;44(1):32–36. doi: 10.1167/iovs.01-1171. [DOI] [PubMed] [Google Scholar]
- 11.Siegwart JT, Jr, Norton TT. The time course of changes in mRNA levels in tree shrew sclera during induced myopia and recovery. Invest Ophthalmol Vis Sci. 2002;43(7):2067–2075. [PMC free article] [PubMed] [Google Scholar]
- 12.McBrien NA, Lawlor P, Gentle A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Invest Ophthalmol Vis Sci. 2000;41(12):3713–3719. [PubMed] [Google Scholar]
- 13.Troilo D, Li T, Glasser A, Howland HC. Differences in eye growth and the response. to visual deprivation in different strains of chicken. Vision Res. 1995;35(9):1211–1216. doi: 10.1016/0042-6989(94)00230-j. [DOI] [PubMed] [Google Scholar]
- 14.Norton TT, Miller EJ. Collagen and protein levels in sclera during normal development, induced myopia, and recovery in tree shrews. Invest Ophthalmol Vis Sci. 1995;36(Suppl):S760. [Google Scholar]
- 15.Siegwart JT, Jr, Norton TT. Steady state mRNA levels in tree shrew sclera with form-deprivation myopia and during recovery. Invest Ophthalmol Vis Sci. 2001;42(6):1153–1159. [PubMed] [Google Scholar]
- 16.McBrien NA, Gentle A. Anastasopoulos FA. TIMP-2 regulation of MMP-2 activity during visually guided remodeling of the tree shrew sclera in lens-induced myopia. Invest Ophthalmol Vis Sci. 2001;42(suppl):56. [Google Scholar]
- 17.Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995;35(9):1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- 18.Zorn M, Hernandez MR, Norton TT, Yang J, Ye HO. Collagen gene expression in the developing tree shrew sclera. Invest Ophthalmol Vis Sci. 1992;33(suppl):1053. [Google Scholar]
- 19.Curtin BJ, Iwamoto T, Renaldo DP. Normal and staphylomatous sclera of high myopia: an electron microscopic study. Arch Ophthalmol. 1979;97(5):912–915. doi: 10.1001/archopht.1979.01020010470017. [DOI] [PubMed] [Google Scholar]
- 20.Zhou X, Qu J, Xie R, Wang R, Jiang L, Zhao H, Wen J, Lu F. Normal development of refractive state and ocular dimensions in guinea pigs. Vision Res. 2006;46(18):2815–2823. doi: 10.1016/j.visres.2006.01.027. [DOI] [PubMed] [Google Scholar]