Abstract
AIM
To evaluate the peroxynitrite (ONOO−) of puerarin on retinal pigment epithelial (RPE) cells apoptosis induced partly by peroxynitrite via Fas/FasL.
METHODS
RPE cells from C57BL/6 mice eyes were cultured. Diabetes was induced in Sprague-Dawley (SD) rats by streptozotocin (STZ) intraperitoneal injection. Puerarin was administrated to cultured RPE cells and diabetic rats. Western blotting analysis, DNA ladder, RT-PCR, immunohistochemistry were used for determining the expression of nitrotyrosine (NT, the foot print of ONOO−), complement 3 (C3); apoptosis and inducible nitric oxide synthase (iNOS) mRNA as well as Fas/FasL signal transduction in RPE cells.
RESULTS
Both RPE cells in ONOO− and puerarin group developed apoptosis and expressed NT, C3, iNOS mRNA and Fas/FasL. But latter delayed the all changes in a time-dependent manner compared with control and STZ group (P<0.001). iNOS, C3 and Fas/FasL were up-regulated and associated with an increase of expression of ONOO− in vivo and in vitro.
CONCLUSION
Puerarin decreases RPE cells apoptosis partly induced by ONOO− for diabetic retinopathy.
Keywords: retinal pigment epithelial cells, oxidative, cell signal, complement, puerarin
INTRODUCTION
Retinal pigment epithelial (RPE) cells are multifunctional cells that are organized as a monolayer between the retina and choroid. RPE cells form a blood-retinal barrier that limits access of blood cells and serum proteins to the retina as well as transport nutrients from the vascular choroid to photoreceptors. This feature makes it especially sensitive to oxygen and/or nitrogen activated species. Several authors have postulated the importance of peroxynitrite (ONOO−) production in the development of diabetic complications[1]-[3]. Otherwise, the most important character of RPE cells is induction and regulation of immunity by complement components and complement receptors. However, the relations of RPE cells with complement, oxidation and cell signal transduction net work are still not clear. The purpose of this study was to evaluate that if ONOO− induced expression of inducible nitric oxide synthase (iNOS) and complement 3 (C3) via Fas/FasL pathway in RPE cells and in streptozotocin (STZ) -induced diabetic rats. We have used one of the antioxidants named puerarin that presented as a common feature with ONOO− scavenging capacity[4],[5] to ameliorate the oxidative stress that exists in the RPE cells in diabetic rats.
MATERIALS AND METHODS
Materials
Pathogen-free, aged 2-3 weeks, 40 C57BL/6 mice and 36 healthy, male, Sprague-Dawley (SD, weight ∼250g) rats were used in this study. The mouse eyes were used to culture RPE cells. All RPE cells were divided into control, ONOO− and puerarin groups. The latter two groups were treated with ONOO−. Puerarin group was added puerarin at the same time. Thirty-six SD rats were randomly divided into control, streptozotocin (STZ) and puerarin groups respectively. SD rats in STZ and puerarin groups were intraperitoneally injected with STZ (45mg/kg) to establish the animal model. Three days after STZ injection, rats with blood glucose levels >16mmol/L were considered diabetic. Three days later, the rats in puerarin group received puerarin 140mg/kg per day. The rats in the control group received the same amount of saline. The blood glucose and weight of the animals were monitored weekly by tail vein blood measurements and scale. The eyes of the animals were examined by slit-lamp and ophthalmoscope every other day. Passage 2 to 3 RPE cells and their supernatants were used and all these cells were routinely stained with antibodies against a broad range of epidermal keratins (AE1/AE3; RDI, Flanders, NJ07836) for determining their epithelial origins. The cell cultures were routinely ≥95% keratin+. The negative control incubated with isotype matched mouse IgG showed no positive staining. Specific binding was visualized and viewed under fluorescence optics (Olympus, Melville, NY). When examined by phase-contrast microscopy after 2-3 passages, the cells formed a monolayer and displayed an epithelial configuration, being predominately hexagonal in shape. Comparative studies had shown that the structure and function of RPE cells passed for 3-5 generations were indistinguishable from those of freshly prepared cells. Thus, in the proposed study, we only used RPE cells that had not been passed more than 3 times.
Synthesis of ONOO−
ONOO− was obtained by reacting ice-cold solutions of sodium nitrite (0.6mol/L) with H2O2 (0.7mol/L) in acidic medium (0.6mol/L HCl) and rapidly quenching the reaction in NaOH (1.5mol/L), as described previously. The reaction mixture solution was frozen at -20°C, and the ONOO− concentrated in the upper layer was collected. Its concentration was measured at 302nm using a molar extinction coefficient of 1670mol/L.cm.
NT and C3
RPE cells were prepared as described above and the protein content of the supernatants was determined by the Bradford method. After sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 120g/L linear slab gel, separated proteins were transferred to a polyvinylidene fluoride (PVDF) membrane using a semidry electrophoretic transfer cell (Trans-blot; Bio-Rad, Richmond, CA). Blot was stained at room temperature with 1:600 dilution of monoclonal mouse anti-NT and mouse anti-C3 antibody over night at 4°C, respectively. After washing and incubating with horseradish peroxidase-conjugated 1:1000 dilution secondary antibody, blot was developed using the enhanced chemiluminescence Western blot analysis detection system (ECL Plus; Amersham Pharmacia Biotech, USA).
DNA ladder for apoptosis
RPE cells and DNA ladder technique were prepared.
iNOS mRNA expression
RT-PCR was performed using 2µg of total RNA of RPE cells for the first-strand synthesis followed by amplification in the presence of specific primers for iNOS(5′-CGCCCTTCCGCAGTTCT-3′ and 5′-TCCAGG AGGACATGCAGCAC-3′) and β-actin (5′-GAGACCTTC AACACCCAGCC-3′ and 5′-GCGGGGCATCGGAACCGC TCA-3′). The amplification consisted of 29 cycles of denaturation for 1 minute at 94°C, annealing for 1 minute at 60°C, and extension for 1 minute at 72°C.
Fas/FasL transduction
Immunohistochemistry was performed as previously described. RPE cells and deparaffinized rats' retinal sections were incubated with hydrogen peroxide (peroxidase blocking reagent; Daco, Carpinteria, CA) to block endogenous peroxidase activity, then with 100mL/L goat serum for 30 minutes at room temperature to block non-specific antigen. After rinsed and washed in PBS, the block slides were incubated with Fas/FasL (1:200 dilution), then in goat biotinylated anti-rat Ig-G (LSAB2 System; Dako, as a secondary Ab). After washed with PBS, the slides were incubated in streptavidin conjugated with horseradish peroxidase. The color was developed with streptavidin and biotin chromogen (Liquid DAB+Substrate-Chromogen System; Dako).
Statistical Analysis
Statistical analysis of the data were performed on computer(SPSS 16.0).The results were expressed as mean±SD. Statistical significance was determined by an one-factor analysis of variance (ANOVA) followed by the Fisher post hoc test for multiple comparisons. P<0.05 was considered significant. All tests were repeated at least three times.
RESULTS
NT and C3 expression
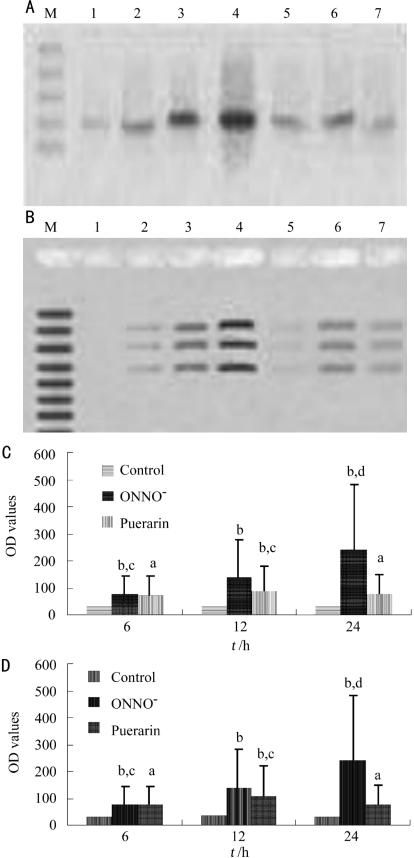
With Western blot analysis, a faint expression of NT and C3 could be seen in the control group. A gradually to strong expression of NT and C3 were observed at different stages of the experiment in ONOO− group. But expression of NT and C3 in puerarin group changed gradually from faint to strong during the period of 6 to 12 hours, then turn to weak at 24 hours (Figure 1). Computer photo-analysis indicated that there were significant differences among three groups (P<0.001).
Figure 1. NT and C3 protein expression in RPE cells with Western blotting.
M: Marker; 1: Control; 2-4: ONOO- at 6, 12, 24 hours; 5-7: Puerarin at 6, 12, 24 hours. A:NT; B:C3; C:Gray values of NT; D:Gray values of C3. aP<0.05, bP<0.01 vs control; cP<0.05, dP<0.01 vs puerarin
DNA ladder for apoptosis
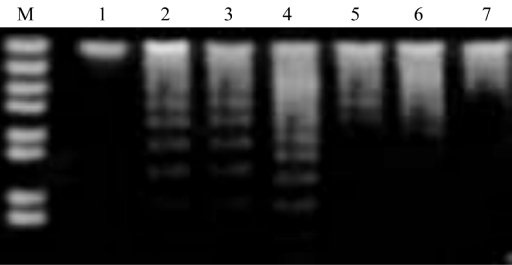
There was no appearance of DNA ladder band in the control group, but there was distinctly typical DNA ladder band in the ONOO− group as time passed. The expression of DNA ladder band in the puerarin group appeared from faint to strong gradually during the period of 6 to 12 hours, then became weak at 24 hours (Figure 2).
Figure 2. DNA ladder for apoptosis of RPE cells.
M: Marker; 1: Control; 2-4: ONOO− at 6, 12, 24 hours; 5-7: Puerarin at 6, 12, 24 hours
iNOS mRNA expression
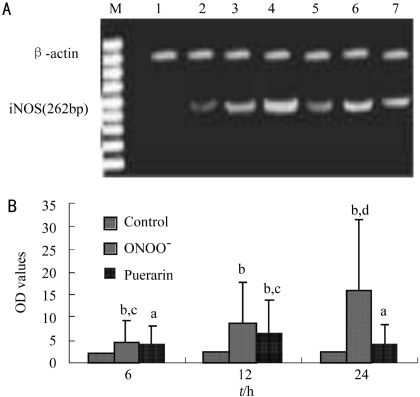
There was no expression of iNOS mRNA in the control group, but there was distinct up-regulation of iNOS mRNA in the ONOO− group as time passed by. The expression of iNOS mRNA in the puerarin group strengthened gradually with up-regulation of iNOS mRNA during the period of 6 to 12 hours of the experiment, then appeared down-regulated at 24 hours (Figure 3).With computer photo-analysis, there were significant differences among the three groups (P<0.01).
Figure 3. iNOS expression in RPE cell RT-PCR.
M: Marker; 1: Control; 2-4: ONOO− at 6, 12, 24 hours; 5-7: Puerarin at 6, 12, 24 hours. aP<0.05, bP<0.001 vs control; cP<0.05, dP<0.001 vs puerarin
Fas/FasL transduction
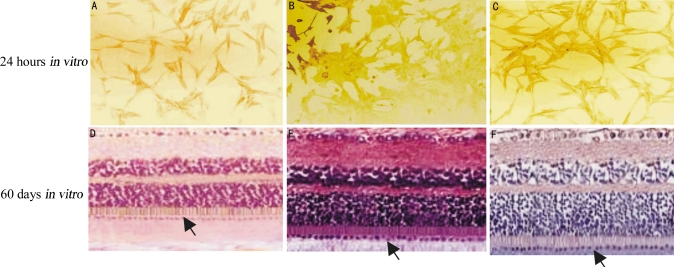
Immunohistochemistry staining revealed that the specific expression of Fas/FasL was yellow, brown-yellow or brown staining in the cell nucleus and cytoplasm. In the control group, a very faint yellow color could be observed. At different times in the ONOO− group, staining ranged from yellow to brown-yellow, then to brown in the cell nucleus and cytoplasm. Gradually decreasing staining expression could be observed in 12 to 24 hours and 40 to 60 days of the puerarin groups, respectively (Figure 4).
Figure 4. Fas/FasL transduction in RPE cells ( ).
).
A, D: Control; B,E: ONOO-; C,F: Puerarin
DISCUSSION
Cell apoptosis or programmed cell death is the term used to describe active cell death to maintain stability under physiological and pathological conditions. Interaction of the death receptor and death ligand is one of the main ways to induce apoptosis, of which, the Fas/FasL system is considered as the major signal transduction pathway to mediate apoptosis[6]-[8]. Loss of RPE cells via apoptosis plays a prominent role in several retinal degenerative diseases, such as age-related macular degeneration. That means the occurrence and development of many eye diseases are related to the regulation imbalance of RPE cell's apoptosis[9]-[11]. Strategies for preservation of vision that would interrupt the apoptotic signal require understanding the molecular events associated with apoptosis. This study investigated the susceptibility of RPE to Fas/FasL-dependent apoptotic pathways when challenged with different stimuli, including oxidants ONOO−, anti-Fas/FasL antibody, activated iNOS and C3 and antagnism of puerarin. We found, intensity of DNA ladder band continued to increase in ONOO− group. In puerarin group, it was increased from 6 to 12 hours, but decreased from 12 to 24 hours. This may indicate a protective role of puerarin on RPE cells. These results are consistent with our previous work.
The traditional oxygen-free radical stress mechanism pays more attention to the role of hydrogen-peroxide (H2O2), nitric-oxide (NO) and superoxide-anion (O2−.), while the new theory includes ONOO−, a product from rapid reaction of NO and O2−, which may be an important mediator of cytotoxicity in oxidation[12]. It is also highly reactive and interacts with cellular constituents inflicting damage on cells. Our study supports the new theory. We observed that the ONOO−-mediated protein nitration product, NT, was located in RPE cells and decreased under the intervention of puerarin. We found that NT greatly increased in ONOO− group. The expression of a small amount of NT in the control group provided physiological evidence for the existence of ONOO−. Purerarin could inhibit the expression of iNOS, therefore decreased the formation of ONOO−[5]. It is likely that iNOS may contribute to oxidation stress by helping develop more powerful oxidative agents such as ONOO− under pathological conditions, up-regulation of iNOS mRNA in RPE cells to over production of NO, accompanied by activation of the oxidant enzyme as well as increasing the O2−. extra NO and O2−. Produce extra ONOO− which acts as a strong oxidant[13].
Cell apoptosis is the result of cascade gene expression. Up to date, more genes contribute to production and regulation of cell apoptosis. It is believed that genes in the inner layer of the cell directly regulate the production and development of the apoptosis, while related elements in the outer layer of the cell affect the expression of the genes through signal transduction way[14]. Our results also suggest that RPE cells play the important roles in regulating complement activation that attributed to the apoptotic events. Increased complement activation in the RPE cells may be important for retinal homeostasis in the context of accumulating photoreceptor waste products. Complement activation is involved in the pathogenesis of age-related macular degeneration[15]. Some studies reported, complement factor H (CFH) is constitutively expressed by retinal pigment epithelial (RPE) cells and the production of CFH is negatively regulated by inflammatory cytokines and oxidative insults[16]. Increased CFB expression in RPE cells in vivo is accompanied by the accumulation of C3 and C3a deposition at the Bruch's membrane and the basal layer of RPE cells[17].
To summarize, apoptosis of RPE cells partly induced by ONOO− (C3 joined events) may be the new way of oxidative damage to the RPE cells. Puerarin decreased RPE cells apoptosis and it is a potential drug for therapy of diabetic retinopathy. Fas/FasL cell signal transduction route, C3 activation and many other apoptotic factors may affect and strengthen the apoptosis process mediated by ONOO−. The mechanism of puerarin dealing with RPE cells might be related to its direct inhibition of apoptosis of RPE cells and antagnism of damage of ONOO− to RPE cells.
Footnotes
Foundation items: This work was supported by the Hebei Province Science Foundation, China (No.07276101D-3); Clinical Science Project Fund of the Ministry of Health in Hebei Province, China (No.03078)
REFERENCES
- 1.Pacher P, Szabó C. Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr Opin Pharmacol. 2006;6(2):136–141. doi: 10.1016/j.coph.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanneken A, Lin FF, Johnson J, Maher P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Invest Ophthalmol Vis Sci. 2006;47(7):3164–3177. doi: 10.1167/iovs.04-1369. [DOI] [PubMed] [Google Scholar]
- 3.Bucolo C, Ward KW, Mazzon E, Cuzzocrea S, Drago F. Protective effects of a coumarin derivative in diabetic rats. Invest Ophthalmol Vis Sci. 2009;50(8):3846–3852. doi: 10.1167/iovs.08-3328. [DOI] [PubMed] [Google Scholar]
- 4.Hao LN, He SZ, Luo XM, Ma QM, Mao QY, Ling YL. Effect of puerarin on prevention of peroxynitrite-induced damage to the LEC and Fas/FasL apoptosis signal in culture. Chin J Ophthalmol. 2008;44(2):163–168. [PubMed] [Google Scholar]
- 5.Hao LN, He SZ, Luo XM, Mao QY, Ling YL. Puerarin decreases lens epithelium cell apoptosis induced partly by peroxynitrite in diabetic rats. Acta Physiologic Sinica. 2006;58(6):584–592. [PubMed] [Google Scholar]
- 6.Korkolopoulou P, Saetta AA, Levidou G, Gigelou F, Lazaris A, Thymara I, Scliri M, Bousboukea K, Michalopoulos NV, Apostolikas N, Konstantinidou A, Tzivras M, Patsouris E. c-FLIP expression in colorectal carcinomas: association with Fas/FasL expression and prognostic implications. Histopathol. 2007;51(2):150–156. doi: 10.1111/j.1365-2559.2007.02723.x. [DOI] [PubMed] [Google Scholar]
- 7.Zacks DN, Boehlke C, Richards AL, Zheng QD. Role of the Fas-signaling pathway in photoreceptor neuroprotection. Arch Ophthalmol. 2007;125(10):1389–1395. doi: 10.1001/archopht.125.10.1389. [DOI] [PubMed] [Google Scholar]
- 8.Semkova I, Fauser S, Lappas A, Smyth N, Kociok N, Kirchhof B, Paulsson M, Poulaki V, Joussen AM. Overexpression of FasL in retinal pigment epithelial cells reduces choroidal neovascularization. FASEB. 2006;20(10):1689–1691. doi: 10.1096/fj.05-5653fje. [DOI] [PubMed] [Google Scholar]
- 9.Lukinova N, Iacovelli J, Dentchev T, Wolkow N, Hunter A, Amado D, Ying GS, Sparrow JR, Dunaief JL. Iron chelation protects the retinal pigment epithelial cell line ARPE-19 against cell death triggered by diverse stimuli. Invest Ophthalmol Vis Sci. 2009;50(3):1440–1447. doi: 10.1167/iovs.08-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrington DA, Tran TN, Lew KL, Van Remmen H, Gregerson DS. Different death stimuli evoke apoptosis via multiple pathways in retinal pigment epithelial cells. Exp Eye Res. 2006;83(3):638–650. doi: 10.1016/j.exer.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 11.McKechnie NM, King BC, Fletcher E, Braun G. Fas-ligand is stored in secretory lysosomes of ocular barrier epithelia and released with microvesicles. Exp Eye Res. 2006;83(2):304–314. doi: 10.1016/j.exer.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 12.Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;20:436–443. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang IM, Yang CH, Yang CM, Chen MS. Comparative effects of fatty acids on proinflammatory gene cyclooxygenase 2 and inducible nitric oxide synthase expression in retinal pigment epithelial cells. Mol Nutr Food Res. 2009;53(6):739–750. doi: 10.1002/mnfr.200800220. [DOI] [PubMed] [Google Scholar]
- 14.Kociok N, Joussen AM. Varied expression of functionally important genes of RPE and choroid in the macula and in the periphery of normal human eyes. Graefes Arch Clin Exp Ophthalmol. 2007;245(1):101–113. doi: 10.1007/s00417-006-0266-x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Kim SR, Westlund BS, Sparrow JR. Complement activation by bisretinoid constituents of RPE lipofuscin. Invest Ophthalmol Vis Sci. 2009;50(3):1392–1399. doi: 10.1167/iovs.08-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogt SD, Barnum SR, Curcio CA, Read RW. Distribution of complement anaphylatoxin receptors and membrane-bound regulators in normal human retina. Exp Eye Res. 2006;83(4):834–840. doi: 10.1016/j.exer.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Muckersie E, Robertson M, Forrester JV, Xu H. Up-regulation of complement factor B in retinal pigment epithelial cells is accompanied by complement activation in the aged retina. Exp Eye Res. 2008;87(6):543–550. doi: 10.1016/j.exer.2008.09.005. [DOI] [PubMed] [Google Scholar]