Abstract
AIM
To investigate 5-hydroxytryptamine (5-HT) function and 5-HT receptor 2A (5-HT2A) mRNA expression in the formation of lens-induced myopia (LIM).
METHODS
Lens-induced myopia construction method was applied to generate myopia on guinea pig right eye (LIM eye).
RESULTS
LIM eyes formed significant myopia with longer axial length. 5-HT level in retina, choroids and sclera from LIM eyes was significantly higher than that in control group. 5-HT2A mRNA expression was also significantly up-regulated.
CONCLUSION
Refraction lens could induce myopia in guinea pig and 5-HT may play an important role in the formation of myopia by binding with 5-HT2A receptor.
Keywords: guinea pig, high performance liquid chromatography/electrochemical detection, lens-induced myopia, 5-hydroxytryptamine, 5-HT receptor 2A
INTRODUCTION
Myopia is a common eye disease with high prevalence. The average incidence of disease rate is very high, around 25%-40% in the world. China is one of the countries with very high incidence (ID) rate. According to the cases investigation at Sunyi district in Beijing, myopia ID rate reached to 37.6% and 55% for male students and female students respectively at their 15 years old age[1]. ID rate of myopia keeps growing with human civilization advancement and currently it has become a social issue attracted enormous attention worldwide. Some serious myopia's syndromes could cause blindness. Now, myopia ranks around 4-6 among the causes leading to blindness. Although many hypothesizes have been proposed during 200 years research on myopia treatment, however, the mechanism of myopia etiology is still unclear. It is critical to treat myopia based on the etiology.
5-hydroxytryptamine (5-HT), a neurotransmitter in CNS and PNS, has extensive impact on eyes health. It plays roles in increasing intraocular pressure, contracting ocular blood vessels, improving mitosis and changing retinal amacrine cell processing. Some research results show that the level of metabolic substances yielded from 5-HT decreased significantly in the model of form deprivation myopia in chicken[2], which suggested that 5-HT engaged in generation of form deprivation myopia as a neurotransmitter. However, the role of 5-HT played in this model is still not clearly identified. 5-HT function varies upon binding with its different type receptors. Five receptor subtypes including 5-HT1A, 5-HT2A, 5-HT2C, 5-HT3 and 5-HT7 were found existing in rat retina. Among them, the content level of 5-HT7 and 5-HT2A are highest, 5-HT2C, 5-HT3 come second and 5-HT1A is lowest[3]. This report compared 5-HT level in retina, choroids and sclera, along with expression of mRNA of 5-HT2A in retina between LIM group and control group, explored relationship of 5-HT level and 5-HT2A receptors expression in the generation of lens-induced myopia(LIM).
MATERIALS AND METHODS
Materials
Four-week old healthy guinea pig were provided by Experimental Animal Center, China Medical University. All experiment procedures are under Hesinki declaration and carried out according to protocols approved by Experimental Animal Center and Ethnics Committee in China Medical University. The right eye of guinea pig was used to construct LIM. Briefly, we cut Velcro belt into a ring with 2cm in diameter, then, cut a hole with 0.8cm in diameter in the middle of the ring, and insert PMMA contact lens (Lens diameter 13.5mm, optical diameter 10.5mm, base curve 9.6mm, diopter -10D) into the hole with convex plane facing outwards. PMMA contact lens were placed in front of animal right eye by gluing Velcro belt on the animal with celloidin. The animals were specially cared by animal care personnel in the room with 12 hours: 12 hours light on/off cycle under 20-25°C. The animals were checked once every two hours in the daytime to clean the dirty lens with swab or refix lens in front of right eye if needed. Lens was demounted 14 days later and animals were processed for further experiment.
Four-week old healthy guinea pigs (n=48) were randomly divided into two groups, LIM (n=34, 68 eyes) groups including LIM refraction eyes (the right eyes wearing contact lens) and LIM refraction control eyes (the left eye without contact lens), and control (n=14, 28 eyes) group without intervention except eyes related examination.
Refraction examination and eye axial length measurement
Before and after LIM construction, all guinea pigs were administered with 10g/L tropicamide-phenylephrine ophthalmic solution on both eyes' conjunctival sac three times with 10 minutes interval, and then eyes were examined with streak retinoscopy. Meanwhile, before and after LIM construction, both eyes axial length (distance from apex of corneal to vitreoretinal interface at eye ball posterior polar) of all guinea pigs were also manually measured 10 times by A-scan ultrasonography (Compuscan, American) with accuracy at 0.01mm and then mean value was computed. All procedure was done after guinea pigs were anaesthetized with ketamine hydro-chloridum injection (i.m., 75-100mg/kg) and animals' conjunctival sacs were administered with 0.4% oxybuprocatine.
Specimen processing
Eyeballs were extracted under aseptic condition after guinea pigs in two groups were killed. Front part of eye tissue and vitreous body were removed by splitting eyeball along ora serrata under surgery microscope (MOLLER-2102, Germany). Retina, choroids and sclera were rapidly dissected out with iris restitutor and stored into sterilized Eppendorf tube in the liquid nitrogen after properly labeled. If needed, tissues were moved to -70°C refrigerator for further processing.
HPLC chromatogram parameter
BDS C18 chromatogram column (Dalian Elit Analytical instrument Co, China) 250mm×4.6mm, voltage 0.7V, buffer: 3mmol/L 1-heptanesulfonic acid, sodium salt, 100mmol/L sodium acetate, 85mmol/L sodium citriate, 0.2mmol /L EDTA, PH=3.7, moving phase: buffer/methanol (volume, 92:8), speed: 1.0mL/min.
Eye tissue specimen wet weight
Eye tissue specimen wet weight was obtained by the following procedures: first, we weighed empty eppendorf tube with electrical scale (Sartorois, DP211D, Germany), then weighed eppendorf tube with eye tissue specimen inside, eye tissue specimen wet weight was weight of eppendorf tube containing specimen minus empty eppendorf tube. Wet weight of frozen retina, choloid and sclera specimens from 18 LIM guinea pigs (18 LIM eyes and 18 control eyes) and 6 control guinea pigs (12 eyes) were obtained by this method.
5-HT measurement
Weighed specimen was ultrasonically homogenized (Dr, hhielscher UP2000H, Germany) on ice with 95µL 0.1mol/L perchloric acid and 5µL inner standard solution (3,4-Dihydroxybenzylamine (DHBA), 4µg/mL, Sigma),then centrifuged 15 minutes at 2 000r/min, supernatant 20µL were kept for analysis. 5-HT level of specimen was obtained by measuring supernatant with HPLC and calculating the value from standard curve.
RT-PCR to test mRNA expression of 5-HT receptor in retina, choroids and sclera from LIM and control guinea pig
Whole RNA extraction: whole RNA of frozen retina, choroids and sclera from 16 LIM guinea pigs (16 LIM eyes, 16 control eyes) and 8 control guinea pigs (16 eyes) were extracted with Trizol, quantified with ultraviolet spectrophotometer (UV-310, UK) and then reverse transcribed into cDNA. 5-HT2A and β-actin primers were designed according to their cDNA sequence in GenBank. Sequence of primers are: for 5-HT2A, 5′-CTGCAGGATG ATTCCAAGGT-3′ and 5′- TCTGCTCATTGCTGATGGA C-3′, amplified fragment length was 316bp; for β-actin, 5′-GACGAAGCCCAGAGCCA-3′ and 5′- CAGAGGCATA CAGGGACAG-3′, amplified fragment length was 271bp. RT-PCR was performed with RT-PCR kit (Biometra, Germany). After RT-PCR, we loaded 5µL PCR product with 2µL loading buffer onto 20g/L agar gel for electrophoresis (120V, 45 minutes), then analyzed electrophoresis result with gel image analysis system, calculated aim gene mRNA value with Metamorph software by dividing aim gene mRNA mass Value with β-actin mRNA mass value.
Statistical Analysis
All statistical analysis was done by Student's t-test with software SPSS 11.4.
RESULTS
Refraction degree and eye axial length
Prior to LIM construction, no statistical significance on refraction was found between both eyes of 4-week guinea pig (P>0.05), both eyes were under hypermetropia status (+2.71±0.25D). Fourteen days later after LIM construction, LIM eyes generate significant higher degree myopia (-8.69±0.12D) in comparison to contralaterl control eyes (-0.20±0.25D, P<0.01). On the other hand, there was no significant difference for refraction degree between LIM control eyes with normal control eyes (P>0.05). Similar result was found for eye axial length during LIM construction. There was no significant difference between two eyes before LIM construction (P>0.05). If the eye was myopia induced, its axial length increased significantly (P<0.05). Meanwhile, LIM control eye was not seen to have significant difference compared with control eye (P>0.05, Table 1).
Table 1. Comparison of diopter and eye axis length of two groups.
| Group Eye | n | Diopter (D) |
Eye axis length (mm) |
||
| Pre-experiment | Post-experiment | Pre-experiment | Post-experiment | ||
| Experiment | |||||
| Right eye | 34 | +2.71±0.25 | -8.69±0.12 | 6.91±0.12 | 7.60±0.04 |
| Left eye | 34 | +2.75±0.21 | -0.20±0.25 | 6.95±0.11 | 7.07 ±0.05 |
| Control | 28 | +2.74±0.25 | -0.10±0.16 | 6.94±0.12 | 7.06±0.08 |
(mean±SD)
5-HT level in retina, choroids and sclera
In the anatomical structure of guinea pig eye, all retina, choroids and sclera have 5-HT existing. Among them, choroids contains highest level of HT, then sclera comes the second, retina the third. 5-HT level was found significantly increased in the retina, choroids and sclera of LIM eye (P<0.01). However, 5-HT level was not found significantly different between LIM control eyes and normal control eyes (Table 2).
Table 2. Comparison of 5-HT in retina, choroids and sclera of two groups.
| Group Eye | n | Retina | Choroids | Sclera |
| Experiment | ||||
| Right eye | 18 | 48.74±4.52 | 451.56±32.82 | 269.16±41.49 |
| Left eye | 18 | 39.09±8.67 | 374.45±17.72 | 147.38±20.18 |
| Control | 12 | 37.28±9.95 | 354.32±20.15 | 142.95±12.87 |
(mean±SD, ng/g)
RT-PCR result
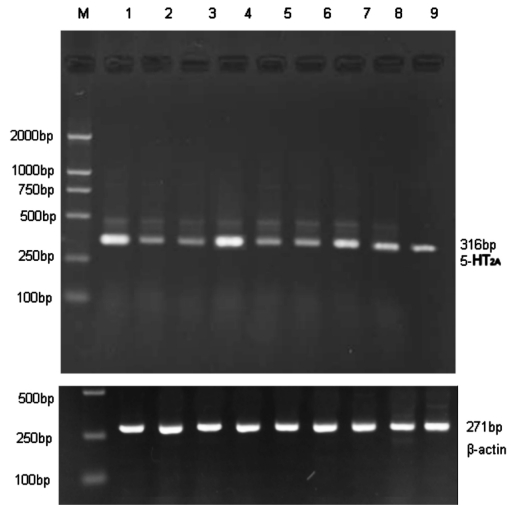
RNA extracted from retina, choroids and sclera in LIM group and normal control group was not degenerated and contaminated, which was identified by OD260/280 ratio at 1.8-2.0 obtained by ultraviolet spectrophotometer (UV-310, UK). Electrophoresis result also showed sharp and clear band (Figure 1).
Figure 1. Electrophoresis result of 5-HT2A mRNA expression in retina, choroids and sclera of two groups.
M: DNA maker (100bp-1000bp); 1-3 Retina, 1: LIM eye; 2: LIM control eye; 3: Normal control eye. 4-6 Choroids, 4: LIM eye; 5: LIM control eye; 6: Normal control eye. 7-9 Sclera, 7: LIM eye; 8: LIM control eye; 9: Normal control eye
5-HT2A receptor mRNA was found in guinea pig retina, choroids and sclera, and formed a 316bp length band on the gel after RT-PCR. At the same time, inner control β-actin formed a 194bp band. Compared with normal control and LIM control, 5-HT2A mRNA levels in retina, choroids and sclera from LIM eyes were significantly up-regulated (P<0.01, Table 3).
Table 3. Comparison of 5-HT2A mRNA expression in retina, choroids and sclera of two groups.
| Group Eye | n | Retina | Choroids | Sclera |
| Experiment | ||||
| Right eye | 16 | 1.239±0.021 | 1.311±0.015 | 0.888±0.020 |
| Left eye | 16 | 0.543±0.012 | 0.613±0.018 | 0.618±0.014 |
| Control | 16 | 0.565±0.037 | 0.553±0.013 | 0.593±0.021 |
(mean±SD)
DISCUSSION
LIM construction
The mechanism of myopia formation is still unknown and currently there is no effective method to treat myopia based on its etiology. Although experimental myopia is one type of myopia only induced in the lab, it is now commonly used to study human myopia because of the similarity of its anatomical structure and characteristics of refraction to human spontaneous myopia and its easy manipulation in the lab. Both form deprivation and lens induction could elongate eye axial length and cause myopic refraction on chicken, squirrel, guinea pig and young monkey[4]-[7]. In the past, chicken is usually chosen to make myopia model on basis of its easy construction in short time and cheap price. However, chicken's sclera comprises of two layers, layer cartilage and layer fiber, which is different with mammal's only one fiber layer sclera. Guinea pig belongs to mammal and has similar anatomical structure on eye with human being. Moreover, guinea pig is easy to raise and operate, so currently it has extensively used to make myopia model. Generally, experimental myopia was induced by form deprivation[4],[8],[9] and defocus[10]. Studies show that animal's eyeball has subjective emmetropia process after birth. The most convincing example is that eye makes compensated adaptation to lens refraction[11]-[15], which indicates that emmetropia process is vision dependant. Therefore, the study on mechanism of guinea pig LIM will benefit the study on human myopia mechanism. In this report, we constructed guinea pig LIM to study mechanism of myopia and seeking effective treatment to myopia.
The results in this study illustrate that at 4-week old age, guinea pig's eye is under hypermetropia status and hypermetropia degree gradually decreases with the time. Lens could increase eye axial length on guinea pig at this age and thus induces high degree myopia. Thus, the time period from birth to mature is critical for vision development. Regular vision development and emmetropia process rely on vision. Lens could cause myopia by increasing eye axial length.
Changes of 5-HT level in LIM retina, choroids and sclera
5-HT was found in the late forties in twentieth century. Ten years later, 5-HT was found in animal central nervous system and possessing neurotransmitter function. In the late fifties, peripheral nervous system was also found to contain 5-HT different receptor subtypes. Eye also contains 5-HT neurons or receptors. 5-HT plays import role in eye's function, it could change retinal, increase intraocular pressure, contract ocular blood vessels and improve mitosis. During experimental myopia formation, retina, choroids and sclera will make corresponding change. 5-HT immunoreactivity was localized to a subset of amacrine cell bodies in the inner nuclear layer of the retina, and to two synaptic strata in the inner plexiform layer. George et al[16] shows that 5-HT functions as stimulant in LIM model, although high dose of 5-HT plays inhibitory role. In the model of LIM, the number of amacrine cell containing 5-HT in retina increased 12.5%. In this report, we tested 5-HT level in retina, choroids and sclera from LIM group, LIM control group and normal control group guinea pig with HPLC. We found 5-HT existed in guinea pig retina, choroids and sclera. The order of 5-HT level in these structures from high to low is choroids, sclera and retina. Furthermore, 5-HT level in retina, choroids and sclera all increased congruously in LIM. We hypothesize that 5-HT participates in the formation of LIM in guinea pig and triggers myopia formation.
5-HT2A receptor mRNA expression in retina, choroids and sclera of LIM guinea pig
5-HT's function varies upon binding on its different receptors. In 1979, two 5-HT receptors were identified in rabbit brain, 5- HT1 and 5-HT2. Subsequently, 5-HT1A, 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT4, 5-HT5A, 5-HT6 and 5-HT7 were also found. 5-HT receptors locate on the membrane of animal neuron and other cells. 5-HT receptors are activated only after binding with ligand and then with 5-HT. Except that 5-HT3 receptor's ligand is ion channel, all other receptors are trans-membrane receptor conjugated with G-protein and work by activating intracellular secondary messenger[17].
Yan et al[3] detected the mRNA expression levels of 5-HT1A, 5-HT2A, 5-HT2C, 5-HT3 and 5-HT7 receptor subtypes on retina of rats by RT-PCR. The results indicate 5-HT7 and 5-HT2A receptor have the highest expression level, 5-HT2C, 5-HT3 have intermediate expression, 5-HT1A shows the lowest expression, and no other receptors subtype are found to have positive expression. 5-HT2 receptors are conjugated with Gq/G11 and regulate cell function by lowering intracellular IP3 and DAG level. 5-HT2 has three subtypes, 5-HT2A, 5-HT2B, 5-HT2C. 5-HT2A has roles in regulating muscle contraction, vessel contraction/relaxation, hallucination generation and aggression. George et al[16] injected 5-HT antagonist, selective 5-HT2 receptor antagonist and 5-HT into vitreous body ventricle of form deprivation and LIM chicken models to observe their impact on eye development. They found 5-HT antagonist reduced LIM eyeball elongation by half, but had not impact on forming deprivation model eye. Selective 5-HT2 receptor antagonist only had slight impact on LIM eyeball length increase.
The results in the report showed that 5-HT2A mRNA expressed in retina, choroids and sclera in guinea pig eyes. It was up-regulated in retina, choroids and sclera of guinea pig LIM eyes and this up-regulation was consistent with the increase of 5-HT level in retina, choroids and sclera, which indicates 5-HT participates in the formation of LIM by binding with 5-HT2A receptor. We presume that part neural signals release by LIM retina work on choroids and sclera, manipulate sclera cell proliferation and differentiation, which leads to sclera elongation and further causes myopia. However, the specific pathway for myopia formation still needs more research.
REFERENCES
- 1.Zhao J, Mao J, Luo R, Li F, Munoz SR, Ellwein LB. The progression of refractive error in shool-age children: shunyi district, China. Am J Ophthalmol. 2002;134(5):735–743. doi: 10.1016/s0002-9394(02)01689-6. [DOI] [PubMed] [Google Scholar]
- 2.Hong H, Tian YJ, Liu P, Zhang W. The study of etiology in form deprivation in chicken. J Lab Anim Sci Manag. 2004;21(2):18–19. [Google Scholar]
- 3.Liu Y, Wu SX, Wang WN, Yan H, Yang XG. 5-HT receptor subtypes mRNA expression in rat retina: RT-PCR study. Jiefangjun Yixue Zazhi. 2005;30(5):413–415. [Google Scholar]
- 4.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatalli dfusion in monkeys. Nature 1977. 1977;66(5597):66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 5.Hodos W, Fitzke FW, Hayes BP, Holden AL. Experimental myopia in chicks: ocular refraction by electroretinography. Invest Ophthalmol Vis Sci. 1985;6(10):1423–1430. [PubMed] [Google Scholar]
- 6.Siegwart JT, Norton TT. Refractive and ocular changes in treeshrews raised with plus or minus lenses. Invest Ophthalmol Vis Sci. 1993;4(suppl):1208–1208. [Google Scholar]
- 7.Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerves eetion in chicks. Vision Res. 1996;36(7):1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- 8.Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201(4362):1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- 9.Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaiaglis) Brain Res. 1977;124(1):154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- 10.Schaeffel F, Bartmann M, Hagel G, Zrenner E. Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res. 1995;35(9):1247–1264. doi: 10.1016/0042-6989(94)00221-7. [DOI] [PubMed] [Google Scholar]
- 11.Irving EL, Callender MG, Sivak JG . Inducing myopia, hyperopia and astigmatism in chicks. Optom Vis Science. 1991;68(5):364–368. doi: 10.1097/00006324-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Judge SJ, Graham B. Differential ocular growth of infant marmoset induced by optical anisometropia combined with alternating occlusion. J Physiol (Lond) 1995;485(1):27–28. [Google Scholar]
- 13.McFadden S, Wallman J. Guinea pig eye growth compensation for spectacle lenses. Invest Ophthalmol Vis Sci. 1995;36:3504. [Google Scholar]
- 14.Hung LF, Crawford MLJ, Smith EL. Spectacle lense salter eye growth and the refractive status of young monkeys. Nature Medicine. 1995;1(8):761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- 15.Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991;31(4):717–734. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- 16.George A, Schmid KL, Pow DV. Retinal serotonin, eye growth and myopia development in chick. Exp Eye Res. 2005;81(5):616. doi: 10.1016/j.exer.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Glennon R, Dukat M, Westkaemper RB. Serotonin receptor lubtypes and ligands. Neuropsychopharmacology Westkaemper: The Fifth Generation of Progress 2000 [Google Scholar]