Abstract
AIM
In order to improve the biocompatibility of intraocular lenses (IOL), the polymethylmethacrylate (PMMA) IOL was modified with F-heparin.
METHODS
The PMMA IOL was modified with F ions and heparin by the technique of ion beam combined with low temperature and low pressure plasma. The monkeys (20 eyes) with cataract partly were randomly classified into 2 groups and implanted with PMMA IOL and modified IOL respectively for 180 days. All of the eyes were examined by slit-lamp microscope at postoperative 15, 30, 60, 90, 180 days. The extracted IOL was analyzed with computer image analysis, light microscope (LM) and scanning electron microscope (SEM) at postoperative 180 days.
RESULTS
The early inflammatory reactions postoperatively include anterior chamber exudation and aqueous cell count. The modified IOL group showed less than the non-modified IOL group. The late foreign body cell reaction that adhered to the surface of non-modified IOL was more predominant. The morphologic and pathological changes of posterior capsule opacification (PCO) in monkeys' eyes included fibrosis-type, pearl-type and soemmerring's ring. There was a significant difference between the two groups.
CONCLUSION
F-heparin modified IOL has good uveal and capsular biocompatibility.
Keywords: intraocular lenses, F-heparin, surface modification, biocompatibility, Rhesus monkeys
INTRODUCTION
Intraocular lenses (IOL) implantation has become a common procedure for patients with cataract. Polymethylmethacrylate (PMMA) IOL is still the most commonly used hard IOL at present due to their cheapness, long reliability and biocompatible. However the early or late foreign-body reaction and lens epithelial cell complications often occur after cataract surgery. The extent of this cellular reaction and complication depends on several factors such as surgical technique, perioperative treatment, host reaction to the lens, IOL material, design and biocompatibility[1],[2]. There is no doubt that the biocompatibility of the IOL used may be the important factor, particularly the IOL surface, due to its direct interaction with intraocular tissue. Then PMMA IOL was modified to a new-kind surface modified IOL using the matter described below. The purpose of this study was to objectively evaluate the biocompatibility of F-heparin modified IOL, which was implanted into nonhuman primate animal Rhesus monkeys' eyes for 180 days.
MATERIALS AND METHODS
Materials
PMMA IOL (PC 301 UV IOL) and F-heparin modified IOL were adopted in the study. PMMA IOLs were modified with F ions and heparin by the technique of ion beam combined with low temperature and low pressure plasma. The color of modified IOL was light yellow. Ten monkeys were randomly divided into 2 groups: modified group and non-modified IOL group. In modified group 10 eyes (4 eyes with cataract) were implanted with F-heparin modified IOL, while in non-modified group 10 eyes (4 eyes with cataract) were implanted with PMMA IOL.
Methods
Ten monkeys received general anesthesia and were performed with extracapsular cataract extraction (ECCE) and the implantation of PMMA IOL and F-heparin modified IOL respectively by the same surgeon under ophthalmological surgical microscope. All of the eyes were examined by slit-lamp microscope at postoperative 15, 30, 60, 90, 180 days. Anterior chamber exudation, cellular adherence to the IOL surface and posterior capsule opacification (PCO) were observed. The density of the anterior chamber exudation and PCO was graded at four levels (-, +, ++, +++)[3],[4]. The aqueous humor was aspirated from the anterior chamber to calculate cells at different periods postoperatively. The surface of extracted IOL was analyzed with computer image analysis and light microscope (LM). The morphological and pathological changes of posterior capsule opacification (PCO) in monkeys' eyes were observed with light microscope (LM). The cellular adherence to the surface of extracted IOL were analyzed with scanning electron microscope (SEM).
Statistical Analysis
The SPSS 10.0 software (nonparametric and t test) was used for statistical analysis. P<0.05 was considered to be statistically significant.
RESULTS
Early Inflammatory Reaction
At postoperative 15 days the anterior chamber exudation (Table 1) of surgery eyes became prominent, decreased gradually and absorbed. After 30 days the severity of anterior chamber exudation in modified group was less than that in non-modified group (P<0.05). The cellular number (Table 2) of aqueous humor postoperatively was highest at 15 days and recovered at 90 days. At 15, 30, 60 days after the surgery aqueous cell count in non-modified group was much more than that in modified group and there was significant difference statistically.
Table 1. Postoperative Anterior chamber exudation and PCO.
| t(postoperative)/d | Non-modified |
Modified |
P | ||||||
| — | + | ++ | +++ | — | + | ++ | +++ | ||
| Exudate | |||||||||
| 15 | 0 | 6 | 4 | 0 | 0 | 8 | 2 | 0 | 0.342 |
| 30 | 0 | 7 | 3 | 0 | 3 | 7 | 0 | 0 | 0.017 |
| 60 | 4 | 6 | 0 | 0 | 6 | 4 | 0 | 0 | 0.383 |
| 90 | 5 | 5 | 0 | 0 | 8 | 2 | 0 | 0 | 0.170 |
| 180 | 8 | 2 | 0 | 0 | 10 | 0 | 0 | 0 | 0.146 |
| PCO | |||||||||
| 15 | 9 | 0 | 1 | 0 | 10 | 0 | 0 | 0 | 0.317 |
| 30 | 4 | 3 | 2 | 1 | 4 | 6 | 0 | 0 | 0.459 |
| 60 | 2 | 3 | 2 | 3 | 2 | 8 | 0 | 0 | 0.096 |
| 90 | 1 | 3 | 3 | 3 | 1 | 9 | 0 | 0 | 0.021 |
| 180 | 1 | 1 | 5 | 3 | 1 | 9 | 0 | 0 | 0.003 |
(n=10)
Table 2. Postoperative aqueous cell count.
| Group |
t(Postoperation)/d |
||||
| 15 | 30 | 60 | 90 | 80 | |
| Non-modified | 75.2±17.3 | 29.3±9.8 | 12.0±3.7 | 4.0±2.3 | 1.40±1.0 |
| Modified | 51.9±14.2b | 20.4±4.3a | 8.4±3.0a | 3.2±1.5 | 1.0±1.0 |
aP<0.05, bP<0.01 vs Non-modified
(mean±SD, n=10)
Morphological Observation of Adherent Cells
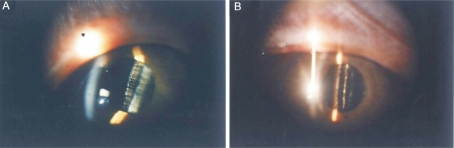
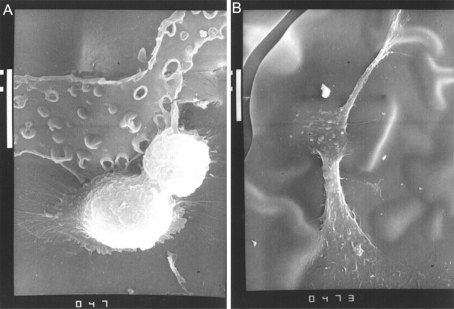
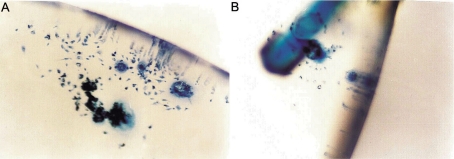
Under slit-lamp microscope we found that the cellular adherence to surface of modified IOL was less than that of non-modified IOL (Figure 1). The analyses of the surface of IOL by LM and SEM also provided the above results at 180 days postoperatively. And the surface-modified IOL had deposition of fine granular proteins on the membrane, but the surface of non-modified IOL deposited fibrous reticular proteins on the membrane by SEM (Figure 2). Cellular adhesion was less on the IOL optic and more on the IOL border by LM (Figure 3). By computer analysis the largest deposited cells on the IOL were giant cells with the average area of 620.06µm2. The most deposited cells on IOL were macrophages with the average area of 99.99µm2.
Figure 1. Surface deposited of IOL.
A: non-modified; B: modified
Figure 2. Surface adherence of IOL.
A: non-modified (SEM×3 000); B: modified (SEM×2 000)
Figure 3. Surface adherence of IOL.
A: non-modified (LM×30); B: modified (LM×30)
Posterior Capsule Opacification
Under slit-lamp microscoppe posterior capsule opacification (PCO) appeared at 15 days and was obvious at 90 days postoperatively in the eyes of monkeys. In the modified group the degree of PCO was lower than that in the non-modified group at 90 days and 180 days (Table 1). At 180 days postoperatively histopathologic observsation found the morphological changes of posterior capsule opacification (PCO) in monkeys' eyes had fibrosis-type, pearl-type and soemmerring's ring.
DISCUSSION
Biocompatibility means a compatibility to life and is defined as “the capability of a prosthesis implanted in the body to exist in harmony with tissue without causing deleterious changes”[5],[6]. As implantation material, the interation of an IOL with the intraocular environment is influenced by its material surface properties[7]. The hydrophilicity or hydrophobicity of IOL surface can be used as a reliable parameter for predicting biocompatibility. The purpose of our study was to improve the biocompatibility of PMMA IOL by surface modification. With the surface modification, the surface of F-heparin modified PMMA IOL had the hydrophilicity of heparin-surface-modified PMMA IOL and the hydrophobicity of F-surface-modified PMMA IOL, which formed a balance structure of hydrophilicity and hydrophobicity[8]. The color of the modified IOL appeared light yellow, which was nearly similar to nature color of old lenses. The evaluation of biocompatibility of a new-type surface modification IOL included uveal biocompatibility and capsular biocompatibility. In particularly we chose nonhuman primate animal Rhesus monkeys as experimental animals implanted with PMMA IOL and F-heparin modified PMMA IOL, following up 180 days.
The early tissue response to IOL implantation after cataract surgery comprised an inflammatory response, including anterior chamber exudation and aqueous humor cell response. This response was mainly related to the breakdown of the blood-aqueous barrier by surgical irritation. The experimental results showed that the modified IOL group showed less than the non- modified IOL group and had a significant difference statistically. Uveal biocompatibility is defined as the reaction of the uvea to the IOL[1],[9]-[12]. The foreign-body cellular reaction can be seen as the most important parameter for uveal biocompatibility. Under slit-lamp microscope, LM, SEM, the surface cellular adherence to modified IOL was mild. Postoperatively 180 days, the IOL extracted were analyzed with computer image analysis, LM and SEM, showed that the adherent cells were mainly macrophages, epithelioid, small round cells and giant cells. And there was fibrous proteinic membrane covering on the surface of IOL. This indicated a prolonged inflammatory reaction of the uvea to the IOL. The follow-up 180 days animal experiment showed the modified IOL better uveal biocompatibility. Capsular biocompatibility is defined as the reaction of LECs and the capsular to IOL material and design[1],[9]-[12]. The main determinants of capsular biocompatibility were lens epithelium cell outgrowth, anterior and posterior capsular opacification and capsular contraction. Under slit-lamp microscope we observed the extent of PCO during the follow-up period postoperatively. There was significant difference between two groups, more lens epithelial cell outgrowth with PMMA IOL. The modified group revealed better capsular biocompatibility. Histopathologic findings showed that the morphological changes of PCO in monkeys' eyes were same as those in human beings, including fibrosis-type, pearl-type and soemmerring's ring.
Therefore the study on material modification is important for development of materials science. Our experiment by surface modification to change surface property got a new-type surface modified IOL, which was implanted into Rhesus Monkeys' eyes for 180 days in vivo and provided optimal uveal and capsular biocompatibility.
REFERENCES
- 1.Abela-Formanek C, Amon M, Schild G, Schauersberger J, Heinze G, Kruger A. Uveal and capsular biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses. J Cataract Refract Surg. 2002;28:50–61. doi: 10.1016/s0886-3350(01)01122-1. [DOI] [PubMed] [Google Scholar]
- 2.Tehrani M, Dick HB, Wolters B, Pakula T, Wolf E. Material properties of various intraocular lenses in an experimental study. Ophthalmologica. 2004;218:57–63. doi: 10.1159/000074568. [DOI] [PubMed] [Google Scholar]
- 3.Zetterstrom C, Lundvall A, Olivestedt G. Exfoliation syndrome and heparin surface modified intraocular lenses. Acta Ophthalmologica. 1992;70:91–95. doi: 10.1111/j.1755-3768.1992.tb02097.x. [DOI] [PubMed] [Google Scholar]
- 4.Spängberg M, Kihlström I, Björklund H, Bjurström S, Lydahl E, Larsson R. Improved biocompatibility of intraocular lenses by heparin surface modification: a 12-month implantation study in monkeys. J Cataract Refract Surg. 1990;16:170–177. doi: 10.1016/s0886-3350(13)80726-2. [DOI] [PubMed] [Google Scholar]
- 5.Amon M. Biocompatibility of Intraocular Lenses. J Cataract Refract Surg. 2001;27:178–179. doi: 10.1016/s0886-3350(01)00742-8. [DOI] [PubMed] [Google Scholar]
- 6.Mamalis N. Intraocular lens biocompatibility. J Cataract Refract Surg. 2002;28:1–2. doi: 10.1016/s0886-3350(01)01283-4. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder AC, Lingenfelder C, Seitz B, Grabowy U, Spraul WC, Gatzioufas Z, Herrmann M. Impact of fibronectin on surface properties of intraocular lenses. Graefes Arch Clin Exp Ophthalmol. 2009;247:1277–1283. doi: 10.1007/s00417-009-1130-6. [DOI] [PubMed] [Google Scholar]
- 8.Gu HQ, Xu GF. The translation publishing company of science and technology in Tianjin; 1993. Biomedical materials science; pp. 179–184. [Google Scholar]
- 9.Schild G, Amon M, Abela-Formanek C, Schauersberger J, Bartl G, Kruger A. Uveal and capsular biocompatibility of a single-piece, sharp-edged hydrophilic acrylic intraocular lens with collagen (Collamer): 1-year results. J Cataract Refract Surg. 2004;30:1254–1258. doi: 10.1016/j.jcrs.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 10.Roesel M, Heinz C, Heimes B, Koch JM, Heiligenhaus A. Uveal and capsular biocompatibility of two foldable acrylic intraocular lenses in patients with endogenous uveitis---a prospective randomized study. Graefes Arch Clin Exp Ophthalmol. 2008;246:1609–1615. doi: 10.1007/s00417-008-0886-4. [DOI] [PubMed] [Google Scholar]
- 11.Werner L. Biocompatibility of intraocular lens materials. Curr Opin Ophthalmology. 2008;19:41–49. doi: 10.1097/ICU.0b013e3282f20132. [DOI] [PubMed] [Google Scholar]
- 12.Richter-Mueksch S, Kahraman G, Amon M, Schild-Burggasser G, Schauersberger J, Abela-Formanek C. Uveal and capsular biocompatibility after implantation of sharp-edged hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in eyes with pseudoexfoliation syndrome. J Cataract Refract Surg. 2007;33:1414–1418. doi: 10.1016/j.jcrs.2007.05.009. [DOI] [PubMed] [Google Scholar]