Abstract
AIM
To investigate the in vitro effects and mechanism of action of cinobufacini on apoptosis of lens epithelial cells (LEC).
METHODS
Rabbit LEC were cultured for 72 hours with cinobufacini at different concentrations(0.0 [control], 0.1, 0.2, 0.3mg/L). The inhibition ratio of cinobufacini acting on LEC was analyzed by ethyl thiazolyl tetrazolium(MTT); the changes in DNA structure, by electrophoresis, and the apoptosis rate, by flow cytometry. The mRNA expression of apoptosis-related genes bcl-2 and bax was examined using the reverse transcription-polymerase chain reaction (RT-PCR).
RESULTS
At concentrations of 0.1mg/L-0.3mg/L, cinobufacini inhibited LEC proliferation. The inhibition ratio increased as the concentration of the drug increased. The typical DNA-ladders on electrophoretic gels were observed for extracts of LEC in the treated groups. The higher the drug concentration (0.1, 0.2, and 0.3mg/L) was, the higher the apoptosis rate (20.47±0.65%, 27.14±0.95%, and 33.49±0.77%, respectively) would be. The apoptosis rates in these groups were significantly different from those of the control group (P<0.01). With the drug concentration increasing, the mRNA expression levels of the pro-apoptotic bax increased, whereas those of the anti-apoptotic bcl-2 decreased.
CONCLUSION
Cinobufacini can notably induce apoptosis of LEC by decreasing the ratio of bcl-2 to bax in vitro. With its low toxicity, this medication may be effective in the prevention and treatment of posterior capsule opacification.
Keywords: cinobutacini, lens epithelial cells, apoptosis, posterior capsule opacification
INTRODUCTION
Posterior capsule opacification (PCO) is the most common complication affecting vision after extracapsular cataract extraction. The prevalence 5 years after surgery has been reported as 30%-40% in adults and 100% in children[1]. Once a PCO forms, the usual treatment is YAG laser posterior capsulotomy, which not only requires expensive laser equipment but can cause complications such as cystoid macular edema, retinal detachment, and glaucoma. In addition, the laser may damage artificial lenses. Therefore, preventing PCO is crucial, because it can seriously affect vision after cataract surgery.Histopatho-logical data have indicated that the primary causes of PCO are the proliferation and transformation of the posterior capsule due to metaplasia of the residual lens epithelial cells (LEC) of the anterior capsule or equator. Anti-cancer drugs such as colchicine have been investigated for the treatment and prevention of PCO. Cytokines and drugs that act on their receptors, cell adhesion molecules, and their ligands are also being studied for treatment and prevention[1],[2]. However, due to the toxicity and expense of these medications, none have been adopted for regular clinical use. Therefore, it may be useful to identify natural products with medicinal effects that can be used to effectively treat or prevent PCO with minimal toxicity. Cinobufacini is a natural anti-cancer drug that inhibits proliferation, induces differentiation, and increases apoptosis in cancer cells. Because of its safety and low toxicity, cinobufacini has been widely used to treat cancer in clinics[3]. In this study, we examined its effects on the growth of LEC and attempted to elucidate its molecular mechanism.
MATERIALS AND METHODS
Materials
Cinobufacini was made by Anhui Jinchan Biochemical Co. Ltd, China (Batch Number 060228-1). The determination of the concentration was based on a standard test of its main component, indole alkaloid. DMEM/F12 culture solution, fetal bovine serum, trypsin EDTA, and ethyl thiazolyl tetrazolium (MTT) assay powder were all purchased from Gibco Inc (USA). The DNA ladders apoptosis-detection kit was purchased from the Biyuntian Biotechnology Company. The RNA extraction kit and Trizol were bought from the Fermentas Co; Taq PCR Master Mixes were bought from the Beijing Tianwei Time Technique Limited Co. The cell apoptosis kit was purchased from Suyi Co. Ltd. We used an enzyme-linked immunosorbent assay (ELISA) reader (BioTek, ELX808; USA), a spectrophotometer (UV-4802type, China), a flow cytometry (Beckman-Coulter ePICS-XL-MCL, USA) and Kodak2000 Gel Imaging System (Kodak, USA). We also used a slit lamp microscope (SLN-3, China), microscope (Olympus BH-2, Japan), and microtome (Bausch & Lomb Inc, USA). Healthy New Zealand white rabbits were sacrificed by ear vein air embolism. The eyeballs were quickly scooped out and the lens anterior membrane was separated. The tissue was cultured at 37°C, in 50mL/L CO2, 950mL/L air, and 100% humidity. When the cultured cells grew to 90% confluence, they were treated with 2.5g/L trypsinase. Cells were subcultured at a ratio of 1:2. The third-generation cells were used in the pharmacologic intervention.
Methods
Rabbit LEC in the logarithmic growth phase were divided into a control group and 3 drug-intervention groups. Cinobufacini was added into the drug-intervention groups until the final concentration was 0.1mg/L, 0.2mg/L, and 0.3mg/L. The cells were then cultured for an additional 72 hours, and the media were changed after 36 hours, maintaining the concentration of the medication.
MTT colorimetry
The rabbit LEC were plated at 5×104 cells/well in 96-well plates and treated with medication, as described above. The experiment was effectively performed six times, simultaneously, as there were six plates for each group. The cell growth was then analyzed by a standard MTT assay. The absorbance at 570nm was measured by an ELISA reader.
DNA structure assay
Cells were inoculated onto 6 plates at 5×105 cells/well. After the group separation, pharmacologic intervention, and treatment with 2.5g/L trypsinase, the cells were centrifuged with their supernatant for 8 minutes at 1300g to produce a cell pellet. The pellet was resuspended in 200µL phosphate buffer saline (PBS). This process was performed according to the manufacturer's instructions for the DNA purification kit. After DNA purification, one-fourth of each sample volume was separated by electrophoresis on a 20g/L agarose gel, which was then stained with Finland dye. These gels were examined and photographed under an ultraviolet lamp after 4 hours of electrophoresis.
Apoptosis rate assay
The rabbit LEC were seeded into 25cm2 culture bottles at 1×106 cells/bottle and treated with the various concentrations of medication. Cells were then collected and evaluated for apoptosis rate by double staining with an annexin V-FITC/PI double staining kit, followed by analysis with a fluorescence-activated cell sorter (FACS). This analysis was performed by using the FL1 and FL3 channels on a Beckman-Coulter ePICS-XL-MCL flow cytometer. To avoid nonspecific fluorescence from dead cells, live cells were gated tightly using forward and side scatter. The analysis was performed once on each of the six repetitions of the cell cultures.
Bcl-2, bax detection
Cells treated with various concentrations of drugs were collected. Total RNA was extracted and purified using Trizol. Complementary DNA (cDNA) was then prepared by reverse-transcription of the total RNA. The RT-PCR was performed using the resultant cDNA as a template and the primers shown in Table 1. The gene amplification conditions were as follows:1)for β-actin: 30 cycles of 94°C for 30s, 56°C for 30s, and 72°C for 30s; 2)for bax: 35 cycles of 94°C for 30s, 62°C for 1 minute, and 72°C for 1 minute; 3)for bcl-2: 33 cycles of 94°C for 30s, 58.8°C for 1 minute, and 72°C for 1 minute. The resultant product was analyzed using 20g/L agarose gel electrophoresis. The concentration of the target DNA band was determined using the Kodak2000 Gel Imaging System. The target gene and actin were quantified using Quantity One 4.4.1 auto analysis software. β -actin (497 bp) was used as an internal control, and the expression abundance of each target gene was calculated as the ratio of absorbance of that gene/absorbance of β -actin.
Table 1. The sequence and amplified fragments of bcl-2, bax, and β-actin.
| Gene-Primer Position | Sequence(5′' to 3′) | Fragment size |
| bax | 391 bp | |
| Upstream | TCTGACGGCAACTTCAACTG | |
| Downstream | CACTGTGACCTGCTCCAGAA | |
| bcl-2 | 361 bp | |
| Upstream | GGAGGATTGTGGCCTTCTTT | |
| Downstream | TCACTTGTGGCTCAGATAGGC | |
| β-actin | 497 bp | |
| Upstream | GCATTGTAACCAACTGGGACG | |
| Downstream | GCGTAACCCTCATAGATGGGC |
Statistical Analysis
The data on the LEC' absorbance, apoptosis rate, and the optical density of the bc1-2 and bax mRNA bands were expressed as mean±standard deviation. SPSS 13.0 software (Chicago, IL, USA) was used for the statistical analysis. The analysis of variance (ANOVA) was used to compare growth inhibition, apoptosis rate, and transcription intensity of bax and bcl-2 under different concentrations of cinobufacini. The relationship between the drug concentration and apoptosis rate was analyzed by the Pearson method. Differences were significant when P <0.05.
RESULTS
LEC Proliferation
The interclass difference of absorbance (0.73±0.06, 0.55±0.04, 0.51±0.05, 0.46±0.05) was statistically significant(F=215.58, P<0.01) among the groups with concentrations from 0.1mg/L to 0.3mg/L. The growth of LEC was inhibited and the inhibition rate increased as the concentration of the drug increased.
DNA Electrophoresis
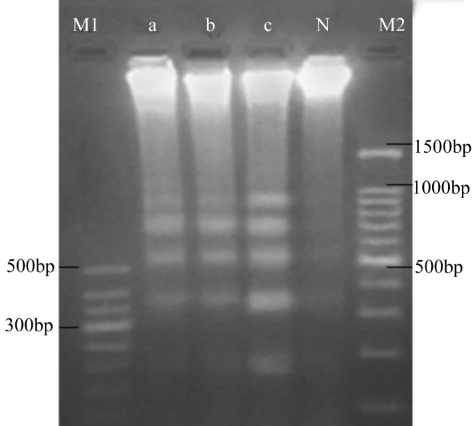
Electrophoresis of DNA extracts from LEC that were not exposed to cinobufacini showed a DNA zone only near the starting point of the lane. In contrast, typical DNA ladders were apparent for extracts from LEC in the drug-treated groups. In addition, the intensities of the DNA bands-most notably, those close to the 180-200bp level-increased as the concentration of cinobufacini increased (Figure 1).
Figure 1. DNA electrophoresis of extracts from LEC treated with cinobufacini.
M1: Marker 1; M2: Marker 2;N, a,b, c: 0, 0.1mg/L, 0.2mg/L, and 0.3mg/L cinobufacini respectively
Apoptosis Rate
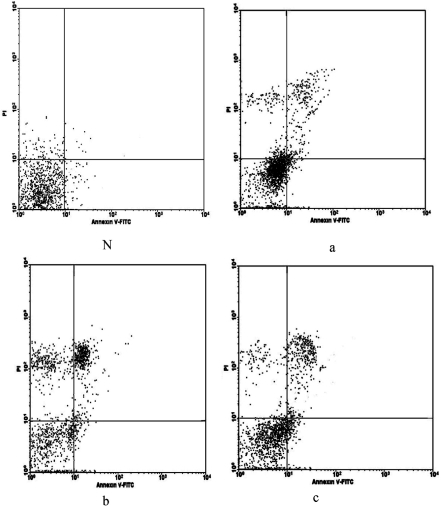
Apoptosis rates in the drug-intervention groups were much higher than those in the untreated groups (F=244.76, P<0.01, Figure 2). The apoptosis rate was positively correlated with the drug concentration (r=0.961, P<0.01, Pearson method).
Figure 2. Dot plots from FACS analysis showing apoptosis of LEC cultured with different concentrations of cinobufacini.
Lower left quadrant: apoptotic cells. N, a, b, c: 0, 0.1mg/L, 0.2mg/L, 0.3mg/L
bcl-2, bax Expresion
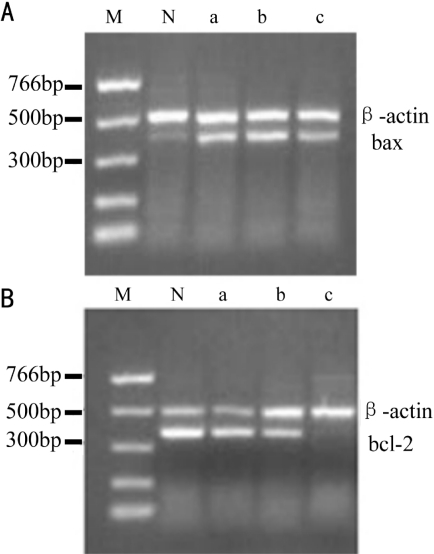
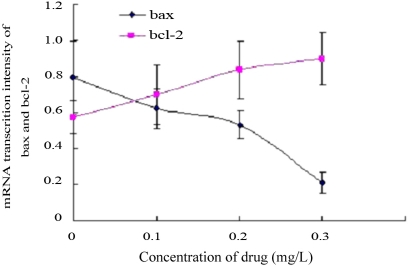
Amplified segments of mRNA concordant with 391bp (bax) and 361bp (bcl-2) were evident in every group (Figure 3); both of these segments were followed by another concordant with 497bp (β -actin). Interclass differences in the mRNA transcription intensity of bax and bcl-2 in different groups were statistically significant (bax, F=185.65, P<0.01; bcl-2, F=126.85, P<0.01). With the drug concentration increasing, the mRNA expression levels of the pro-apoptotic bax increased, whereas those of the anti-apoptotic bcl-2 decreased(Figure 4).
Figure 3. mRNA transcription intensity of A: bax, β-actin and B: bcl-2, β-actin of LEC.
M: Marker; N: untreated; a: 0.1mg/L cinobufacini; b: 0.2mg/L cinobutacini; c: 0.3mg/L cinobutacini
Figure 4. Comparison of mRNA transcription intensity of bax and bcl-2.
DISCUSSION
Although many methods for preventing PCO have been investigated, no effective measure has emerged. Antitumor medications may be effective in preventing and treating PCO because an important aspect of treatment depends on inhibiting the multiplication and transference of LEC[2]. One such antitumor medicine is cinobufotalin, which has been effective against primary liver, lung, gastric, and esophageal cancers[3]-[6]. This medicine may have wider applications, because it can inhibit the synthesis of RNA and DNA, induce tumor cell apoptosis, suppress angiogenesis, and enhance the immune response. Most importantly, it is rather safe because it has a low toxicity. As a result, cinobufotalin may be an ideal for preventing and treating PCO. In this study, we found that cinobufacini, a derivative of cinobufatalin, can inhibit the proliferation of rabbit LEC at concentrations between 0.1mg/L and 0.3mg/L. In addition, these concentrations can induce apoptosis of the LEC in a dose-dependent manner. Furthermore, the inhibition ratios and apoptosis rates were concordant with each other at each concentration, indicating that the drug inhibits LEC proliferation by inducing apoptosis.
Many oncogenes and tumor-suppressor genes are involved in apoptosis. Among them, the Bcl-2 gene family is regarded as the most important contributor[7]. Members of the Bcl-2 family include the genes for anti-apoptotic factors (Bcl-2 [the representative member of this group], Bcl-x1, Mcl-1, etc.), pro-apoptotic factors (Bax [representative member of this group], Bak, etc.), and BH3 domain- containing factors (Bim, Bid, etc.). Bax can induce calcium release and cell apoptosis, while Bcl-2 can inhibit apoptosis. Since over-expressed Bax can counteract the anti-apoptotic effect of Bcl-2 molecules by forming a heterodimer between them, the ratio of Bcl-2 to Bax determines whether survival or apoptosis dominates after the stimulation of apoptotic activity. Our study indicates that cinobufacini can increase bax expression but inhibit bcl-2 expression in a dose-dependent manner in rabbit LEC.
In summary, our study indicated that cinobufacini can induce LEC apoptosis by decreasing the ratio of bcl-2 to bax. Therefore, cinobufacini may be effective in treating and preventing PCO.
Footnotes
Foundation items: Supported by major research project of traditional Chinese medicine of Fujian Province of China(No.wzzb0606); professor development fund project of Fujian Medical University of China(No. 2006-js6033).
REFERENCES
- 1.Fernandez V, Fragoso MA, Billotte C, Lamar P, Orozco MA, Dubovy S, Willcox M, Parel JM. Efficacy of various drugs in the prevention of posterior capsule opacification: experimental study of rabbit eyes. 2004;30(12):2598–2605. doi: 10.1016/j.jcrs.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Miyamoto T, Saika S, Okada Y, Ishida-Nishikawa I, Sumioka T, Fujita N, Ohnishi Y. Topical exposure of mitomycin C reduces opacification of the residual anterior lens capsule and lenticular regeneration after vitrectomy and lensectomy in rabbits. Graefes Arch Clin Exp Ophthalmol. 2004;242(4):327–331. doi: 10.1007/s00417-003-0839-x. [DOI] [PubMed] [Google Scholar]
- 3.Meng ZQ, Shen YH, Yang PY, Newman R, Bei WY, Zhang Y, Ge YQ, Cohen L, Kurzrock R, Liu LM. Phase I study of cinobufacini in hepatocellular carcinoma, non-small cell lung cancer, and pancreatic cancer: a preliminary report. China Oncology. 2007;17(5):376–379. [Google Scholar]
- 4.Kurosawa M, Tani Y, Nishimura S, Numazawa S, Yoshida T. Distinct PKC isozymes regulate bufalin-induced differentiation and apoptosis in human monocytic cells. Am J Physiol Cell Physiol. 2001;280(3):459–464. doi: 10.1152/ajpcell.2001.280.3.C459. [DOI] [PubMed] [Google Scholar]
- 5.Kurosawa M, Numazawa S, Tani Y, Yoshida T. ERK signaling mediates the induction of inflammatory cytokines by bufalin in human monocytic cells. Am J Physiol Cell Physiol. 2000;278(3):500–508. doi: 10.1152/ajpcell.2000.278.3.C500. [DOI] [PubMed] [Google Scholar]
- 6.Enomoto A, Rho MC, Komiyama K, Hayashi M. Inhibitory effects of bufadienolides on interleukin-6 in MH-60 cells. J Nat Prod. 2004;67(12):2070–2072. doi: 10.1021/np049950e. [DOI] [PubMed] [Google Scholar]
- 7.Tsukahara S, Yamamoto S, Tin-Tin-Win-Shwe. Ahmed S, Kunugita N, Arashidani K, Fujimaki H. Inhalation of low-level formaldehyde increase the bcl-2/bax expression ratio in the hippocampus of immunologically sensitized mice. Neuroimmunomodulation. 2006;13(2):63–68. doi: 10.1159/000094829. [DOI] [PubMed] [Google Scholar]