Abstract
AIM
To detect the effect of connective tissue growth factor (CTGF) on the apoptosis in the diabetic retina with small interfering RNAs (siRNA) targeting CTGF.
METHODS
A total of 60 rats were divided into 6 groups including control group, diabetic 4, 8, 12, 16 weeks groups, and interference group. Diabetic rats were induced by intraperitoneal streptozotocin (STZ). Retinas were obtained from control, diabetic rats and diabetic rats of interference group treated by intravitreal injection of CTGFsiRNA to suppress the expression of CTGF mRNA. Retinal cells apoptosis was detected by Tunnel staining and mRNA expression of CTGF was analyzed by RT-PCR.
RESULTS
The levels of CTGF and the apoptosis in the retinas of diabetic rats were significantly higher than those in the controls. Apoptosis occurred at 4 weeks after a diabetic model being set up, became serious with the diabetes developing, while CTGF elevated at 8 weeks. The apoptosis cell counts increased to 25.8cells/mm2 at 24weeks of diabetes. SiRNA-mediated inhibition of CTGF mRNA resulted in a significant decrease in apoptosis. Significant correlations were found between CTGF and apoptosis in the retina.
CONCLUSION
It was suggested that CTGF might be involved in retinal cells apoptosis which is a characteristic of early diabetic retina. SiRNA targeting CTGF seems to have the advantage of ameliorating retinal cells apoptosis.
Keywords: apoptosis, CTGF, retina, diabetes
INTRODUCTION
Diabetic retinopathy is one of the most common complications of diabetes and a leading cause of vision loss, ultimately resulting in an advanced stage of proliferative retinopathy with neovascularization, fibrovascular proliferation and retinal detachment. At the cellular level, diabetes alters the function and structure of all retinal cell types[1]. The pathogenesis of diabetic retinopathy includes glucose-mediated microvascular damage[2]-[4] and alterations of the neural retina, impaired glial reactivity and apoptotic cell death of retinal cells have been observed in cases of short-term experimental diabetes and in humans with diabetes[5]. Animal studies show accelerated apoptosis of retinal neurons[6], glial activation[7], microvascular cells[8], photoreceptors[9] and microglial cells[10]. It is important to characterize the early pathological processes in the diabetic neural retina before the onset of vascular pathology. More recently, a novel, cysteine rich secreted protein CTGF has been associated with diabetic retinopathies, which is postulated to have prosclerotic, angiogenic and apoptosis induction properties[11],[12] in other tissues. However, there have been few previous reports that have linked the elevated expression of CTGF in diabetic retinas to pathological changes. To investigate the role of diabetes-enhanced CTGF expression in retinal cell apoptosis, diabetic rats were treated with intravitreal injection of CTGFsiRNA.
MATERIALS AND METHODS
Materials
Wistar rats were purchased from Animal Laboratories of China Medical University. Throughout the study animals were given access to food and water in metabolic cages. All animal procedures were in accordance with guidelines set by the Animal Experiment Committee of the China Institutes for Biological Sciences. Wistar rats, male, weighing 180-200g, were randomly divided into 6 groups: control group, diabetic groups at 4 weeks (DM4W), 8 weeks (DM8W), 16 weeks (DM16W), 24 weeks (DM24W) and DM16W interfered with CTGFsiRNA group (n =10 for each group).
Methods
Streptozotocin-induced diabetic rat model
Experimental diabetes was induced by intraperitoneal injection of the β-cell toxin streptozotocin (60mg/kg). Immediately prior to the use, streptozotocin was dissolved in cold 0.1mol/L citrate buffer, pH4.5. Control rats received an injection of 0.1mol/L citrate buffer alone. Blood glucose (BG) levels were measured before and 72 hours after the STZ injection, urinary glucose (UG) measured consequently at the first three days. Only the animals with UG above +++, blood glucose levels>16.7mmol/L were considered diabetes. Body weight, UG, BG and glycated hemoglobin were measured every week. Fifty rats received intraperitoneal injection of the β-cell toxin streptozotocin (60mg/kg). All the animals with UG above +++, blood glucose levels>16.7mmol/L were induced to diabetes. The mean weight and blood glucose were significantly different between non-diabetic and diabetic animals. Diabetic rats had hyperglycemia and increased urinary glucose compared with the normal rats of the control group. At 4 weeks there was no significant effect on body weight and from 8 weeks on, the difference was significant.
At 4, 8, 16, 24 weeks of diabetes, ten rats were randomly selected from the normal control and diabetic groups and killed with a lethal dose of pentobarbital sodium. Eyes from each rat were rapidly enucleated, one being snap-frozen in liquid nitrogen and stored in -80°C for the consequent RT-PCR, while the contralateral eye was fixed at 4% paraformaldehyde for apoptosis and immunohistochemistry.
Preparation of CTGF siRNA
A double-stranded rat CTGF siRNA was synthesized by personnel at GenePharma (Shanghai, China), as described previously[13],[14]. The sequence of siRNAs targeting rat CTGF gene is shown in Table 1. The resultant siRNA was purified, quantified and suspended in water at a concentration of 50ng/µL, and 0.5µL (10 picomoles) siRNA for CTGF was combined with 0.5µL siRNA transfection reagent (GenePharma Co. Ltd. Shanghai, China) for 20 minutes before injection according to the manufacturer's instructions. Control injection was 1.0µL PBS. The doses of CTGF siRNA used in the present study were chosen according to studies.
Table 1. The sequences of the siRNAs targeting rat CTGF gene.
| Target gene | Sequence |
| CTGF | sense: 5′-CAACUAUGAUGCGAGCCAATT-3′ |
| antisense: 5′-UUGGCUCGCAUCAUAGUUGGG-3′ | |
| Scrambled siRNA | Sense: 5′-UUCUCCGAACGUGUCACGUTT-3′ |
| Antisense: 5′-ACGUGACACGUUCGGAGAATT-3′ |
GenBank accession number NM_022266
Intravitreous injections
Ten diabetic rats at 16 weeks after setting up of the diabetic model were performed intravitreal injection as previously described[15]. Rats were anesthetized with an intraperitoneal injection of 65mg/kg pentobarbital sodium (Sigma, USA). A topical anesthetic (5g/L tetracaine hydrochloride, Santen, Japan) was administered and the pupils were dilated with 10g/L tropicamide before inserting a 30-gauge needle just 2.0mm posterior to the limbus to avoid lens damage. 1µL CTGFsiRNA was injected in right eyes using a 1mL Hamilton syringe. All fellow eyes were injected with Control injections. Then 5g/L topical Tobra-Dex ointment (Alcon, USA) applied to the injected eye for preventing the infection. Rats were killed 1day later, and the eyes were removed. CTGF mRNA levels and apoptosis were examined in retinas.
RNA extraction and RT-PCR
Retina tissues were collected from different groups. RNA was extracted from the retina using Trizol (Invitrogen). RT-PCR was performed according to the manufacturers' instructions. PCR protocol: 2 minutes at 94°C, followed by 32 cycles of 30 seconds at 94°C, 30 seconds at 56°C and 1 minute at 72°C, 2 minutes at 72°C. Relative concentrations of DNA in each specimen was semiquantitated on an Automated Imaging System (Alphainnotech ChemiImager 5500, USA) by the integral absorbance of the product, which was then normalized to the absorbance of β-actin. The data were expressed as the mean transcript/β-actin ratio±SD. The primer of CTGF: 5′-TGT GAAGACATACAGGGCTAA-3′5′-GTTCTCACTTTGGTG GGATAG-3′.
In situ cell apoptosis
Apoptotic cells were detected by TUNEL assay with an in situ Cell Apoptosis Detection Kit (Boster, Wuhan, China) according to the kit manufacturer's instructions. After deparaffinization, the sections were treated with proteinase K, incubated with TUNEL reaction mixture and peroxidase-conjugated antibody, stained with the diaminobenzidine solution. To quantify the number of TUNEL-labeled nuclei, we obtained counts and averaged them from five different randomly selected areas of a given coverslip, using an eyepiece graticule grid that represented an area of 400µm×400µm. Thus, to convert values to cells/mm2, each averaged value multiplied by 6.25 (ie. 2.5×2.5). Ten coverslips were analyzed for each treatment and values statistically compared for differences.
Statistical Analysis
All data were expressed as the mean±standard deviation (SD). Statistical analysis was performed by students' t-test using SPSS for windows software (Ver.10.0; SPSS, Inc.). P<0.05 was considered significant.
RESULTS
CTGF mRNA Expression
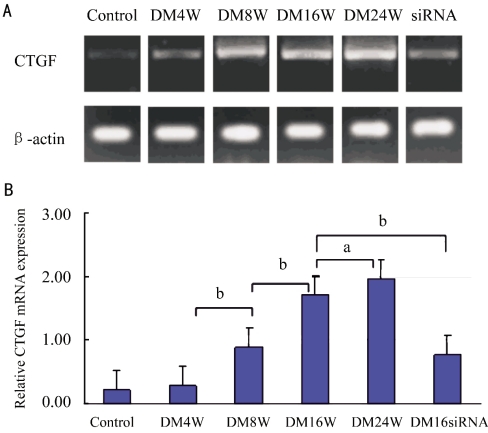
We compared CTGF mRNA expression levels of different groups. There was little CTGF expression in the normal retina and DM4W group, whereas it became stronger in diabetic rat in DM8W (P<0.05). CTGF expression levels were increased twofold in DM16W and DM24W (P<0.01) groups steadily. Figure 1A shows CTGF gene expression in the retina. The statistical analysis demonstrates that CTGF mRNA expression in the diabetic retina is upregulated. The difference is significant shown in the Figure 1B (aP <0.05, bP<0.01). CTGFsiRNA was injected intravitreously in diabetic rats to make the CTGF gene silence. So we chose 16-week groups to inhibit the CTGF expression by intravitreal CTGFsiRNA injection. The results of these experiments showed that the inhibitory efficiency of CTGFsiRNA was 55%. Such inhibition of gene expression was significant (P<0.01), as determined by students' t-test.
Figure 1. Expression of CTGF mRNA in the retina of normal rat, diabetic rats and diabetic retina (n =10).
the difference is significant (aP <0.05, bP <0.01)
Retinal Cell Apoptosis
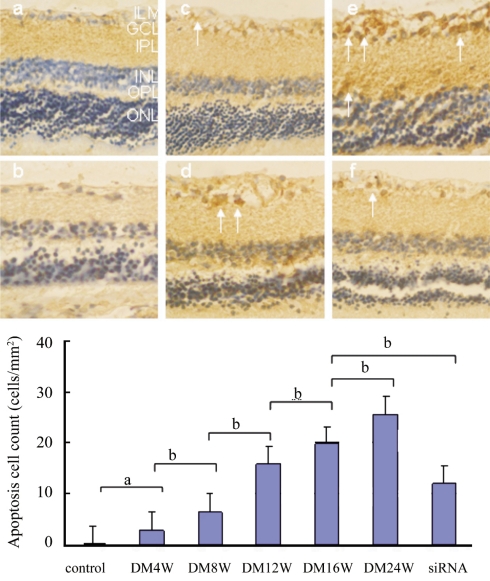
The TUNEL-positive nuclei were identified by a brown reaction product and were found in all regions of the retina. After only 4 weeks, diabetic retinas had few TUNEL-positive nuclei in ganglion cell layer compared to the control (P<0.05). At 8 weeks, the diabetic retinas had more TUNEL-positive nuclei than the control (Figure 2). The count of cell apoptosis is 12.6 cells/mm2 and the positive stained cells included ganglion cells and glial cells. At 24 weeks of diabetes TUNEL-positive nuclei localized in all regions of the retina, including vascular endothelial cells and the cells in inner nuclear layer, and the cell apoptosis counts increased to 25.8cells/mm2. There was a downregulation of apoptosis in interfered retina compared with un-interfered retina. The CTGFsiRNA protects the retina from apoptosis, compared with the un-interfered retina, and the difference was significant (P <0.01).There was a significant correlation between the apoptosis and the expressions of CTGF in diabetic retina (r =0.871, P =0.011).
Figure 2. Tunnel positive cells (arrows) appeared in diabetic retina.
a: normal retina; b: diabetic 4 weeks; c: diabetic 8 weeks; d: diabetic 16 weeks; e: diabetic 24 weeks; f: diabetic 16 weeks interfered by CTGFsiRNA. The internal limiting membrane (ILM), the ganglion cell layer (GCL), the inner plexiform layer (IPL), the outer plexiform layer (OPL), and the outer nuclear layer (ONL).The difference is significant (aP <0.05, bP <0.01)
DISCUSSION
The present study indicates there is an increased expression of the CTGF gene level and the apoptosis of early diabetes and the degree of increasing became stronger with the diabetic development. The diabetic retinas showed apoptosis in ganglion cells layer chiefly. We can come to a conclusion that this change is a very early marker of diabetes-induced retinal changes and it occurred before the onset of visible vascular lesions. Then we used siRNA targeting with CTGF to silence the CTGF gene, which is a valuable tool for investigating the function of gene products in tissues[16]-[18]. CTGFsiRNA could effectively down-regulate the expression of CTGF in diabetic rat retinas, and a significant inhibition of apoptosis in the retina occurred by the interfered with CTGFsiRNA. There was a strong correlation between apoptosis and CTGF in diabetic rat retinas. So we demonstrated CTGF might be affected on the apoptosis in diabetic retina of rat. Increased apoptosis is implicated in several other diabetic complications such as neuronial apoptosis in neuropathy, cardiomyocyte apoptosis in card-iomyopathy, and mesangial cell apoptosis in nephropathy. Diabetes can affect capillaries, neurons, and glia[19] within the retina and alters the function and structure of all retinal cell types. Much evidence had shown the apoptosis in the ganglion cells layer, the inner nuclear layer[20], and photoreceptors and microvascular cells in diabetic retina. Direct diabetes damage to glial cell or neuronal metabolism would directly impact neurotransmission[21] and may lead to apoptosis of retinal neurons and visual field defects. Indeed, retinal axons are lost before the onset of visible vascular lesions[22]. Recent reports also demonstrate that impaired local responses on the multifocal electroretinograms predict subsequent development of vascular lesions[23]. Vision depends on neuronal function, so most forms of vision impairment with clear ocular media must include neuronal dysfunction definitely. Further work is needed to determine how alterations in ganglion, glial, microglial, and neuronal cell interactions reduce the quality of vision.
CTGF is a cysteine-rich matricellular protein belonging to the CCN family of proteins, which have many diverse functions such as angiogenesis, fibrosis and apoptosis and so on. Recently, various studies have shown that CTGF expression at the mRNA or protein level in retina has previously been demonstrated in vivo in diabetic rat[24] and human[25], as well as in cultured retinal microvascular cells[26]. CTGF has been shown to be upregulated in the retina together with endothelial cell death. These are believed to be the result of metabolic changes caused by hyperglycemia and advanced glycation end products (AGEs)[27]. Overexpression of CTGF in cultured human aortic smooth muscle cells, a cell type closely related to pericytes and mesangial cells induces apoptosis by activating caspase 3[28]. Moreover, the involvement of Cyr61 and CTGF in pericyte detachment and anoikis was implicated in the pathogenesis of DR[29]. Cyr61- and CTGF-induced apoptosis was mediated through the intrinsic pathway and involved the expression of genes that have been functionally grouped as p53 target genes. Expression of the matrix metalloproteinase-2 gene, a known target of p53, was increased in pericytes overexpressing either Cyr61 or CTGF. Inhibition of matrix metallopro-teinase-2 had, at least in part, a protective effect against Cyr61- and CTGF-induced apoptosis. Thus, it is possible that up-regulation of CTGF may contribute to inducing apoptosis, especially in the vascular endothelium cells, ganglion cells, perhaps included the glial cells. This up-regulation leads to the loss of retinal cells as a critical early event. And then the vascular cells affected suggest that cell loss may be a direct result of a consequence of widespread vascular disease in the etiology of diabetic retinopathy[30]. But it is indicated that most of the apoptotic cells in the retina are not endothelial cells or pericytes. Judging from such different findings, there might be an unidentified mechanism modulating the apoptosis in the diabetic retina.
Together, these studies leave little doubt that apoptosis is the earliest detectable changes in diabetes. Regardless of whether the initial events begin in blood vessels or neural cells, the clinical stage of diabetic retinopathy manifest cellular, histological, and functional features of a retinal neuropathy[31]. To the best of our knowledge, there is no evidence that a primary, selective defect in vascular cells is sufficient to cause diabetic retinopathy. Clearly, it is essential to treat both the vascular and neural elements of the retina to preserve vision. This concept permits a new paradigm for understanding the mechanism of vision impairment in diabetes and provides therapeutic targets that are directly linked to vision[32],[33]. In summary, this study suggests that CTGF may be involved in apoptosis which is a characteristic of early diabetic retina. siRNA targeting CTGF seems to have the advantage of ameliorating retina apoptosis directly or indirectly. This study provides evidence treatment strategies that intravitreous injection of siRNA containing potentially therapeutic transgenes treatment[34],[35]. However, it remains unclear how up-regulated expression of CTGF in the diabetic retina and the exact mechanism leading to apoptosis in STZ rats should be further investigated. Meanwhile, we must be cautious in interpreting these findings, because animal models of diabetic retinopathy do not exhibit advanced retinal lesions such as those seen in the man.
Footnotes
Foundation item: Supported by the Educational Office of Liaoning Province, China(No.20060994)
REFERENCES
- 1.Abu El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci. 2004;45(8):2760–2766. doi: 10.1167/iovs.03-1392. [DOI] [PubMed] [Google Scholar]
- 2.Hudson BI, Schmidt AM. RAGE: a novel target for drug intervention in diabetic vascular disease. Pharm Res. 2004;21(7):1079–1086. doi: 10.1023/b:pham.0000032992.75423.9b. [DOI] [PubMed] [Google Scholar]
- 3.Barile GR, Pachydaki SI, Tari SR, Lee SE, Donmoyer CM, Ma W, Rong LL, Buciarelli LG, Wendt T, Hörig H, Hudson BI, Qu W, Weinberg AD, Yan SF, Schmidt AM. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46(8):2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6(4):511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 5.Kita T, Hata Y, Kano K, Miura M, Narkao S, Noda Y, Shimokawa H, Ishibashi T. Transforming growth factor-β2 and connective tissue growth factor in proliferative vitreoretinal diseases: possible involvement of hyalocytes and therapeutic potential of Rho kinase inhibitor. Diabetes. 2007;56(1):231–238. doi: 10.2337/db06-0581. [DOI] [PubMed] [Google Scholar]
- 6.Park SH, Park JW, Park SJ, Kim KY, Chung JW, Chun MH, Oh SJ. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia. 2003;46:1260–1268. doi: 10.1007/s00125-003-1177-6. [DOI] [PubMed] [Google Scholar]
- 7.Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes: the Penn State Retina Research Group. Invest Ophthalmol Vis Sci. 2000;41:3561–3568. [PubMed] [Google Scholar]
- 8.Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-α production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008;172(5):1411–1418. doi: 10.2353/ajpath.2008.071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SH, Park JW, Park SJ, Kim KY, Chung JW, Chun MH, Oh SJ. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia. 2003;46(9):1260–1268. doi: 10.1007/s00125-003-1177-6. [DOI] [PubMed] [Google Scholar]
- 10.Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54(5):1559–1565. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Yang R, Tinner B, Choudhry A, Schutze N, Chaqour B. Cysteine-rich protein 61 and connective tissue growth factor induce deadhesion and anoikis of retinal pericytes. Endocrinology. 2008;149(4):1666–1677. doi: 10.1210/en.2007-1415. [DOI] [PubMed] [Google Scholar]
- 12.Van Setten G, Berglin L, Blalock TD, Schultz G. Detection of connective tissue growth factor in subretinal fluid following retinal detachment: possible contribution to subretinal scar formation, preliminary results. Ophthalmic Res. 2005;37(6):289–292. doi: 10.1159/000087698. [DOI] [PubMed] [Google Scholar]
- 13.Zhou XD, Xiong MM, Tan FK, Guo XJ, Arnett FC. SPARC, an upstream regulator of connective tissue growth factor in response to transforming growth factor β stimulation. Arthritis Rheum. 2006;54(12):3885–3889. doi: 10.1002/art.22249. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Tan FK, Guo X, Wallis D, Milewicz DM, Xue S, Arnett FC. Small interfering RNA inhibition of SPARC attenuates the profibrotic effect of transforming growth factor in cultured normal human fibroblasts. Arthritis Rheum. 2005;52(1):257–261. doi: 10.1002/art.20785. [DOI] [PubMed] [Google Scholar]
- 15.Aranda J, Rivera JC, Jeziorski MC, Riesgo-Escovar J, Nava G, López-Barrera F, Quiróz-Mercado H, Berger P, Martìnez de la Escalera G, Clapp CR. Prolactins are natural inhibitors of angiogenesis in the retina. Invest Ophthalmol Vis Sci. 2005;46(8):2947–2953. doi: 10.1167/iovs.05-0173. [DOI] [PubMed] [Google Scholar]
- 16.Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, Bennett J, Tolentino MJ. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9(5):210–216. [PubMed] [Google Scholar]
- 17.Kim B, Tang Q, Biswas PS, Xu J, Schiffelers RM, Xie FY, Ansari AM, Scaria PV, Woodle MC, Lu P, Rouse BT. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol. 2004;165(6):2177–2185. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Twigg SM, Cooper ME. The time has come to target connective tissue growth factor in diabetic complications. Diabetologia. 2004;47(6):965–968. doi: 10.1007/s00125-004-1423-6. [DOI] [PubMed] [Google Scholar]
- 19.Gaucher D, Chiappore JA, Pâques M, Simonutti M, Boitard C, Sahel JA, Massin P, Picaud S. Microglial changes occur without neural cell death in diabetic retinopathy. Vision Res. 2007;47(5):612–623. doi: 10.1016/j.visres.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci. 2006;47(7):3143–3150. doi: 10.1167/iovs.05-1376. [DOI] [PubMed] [Google Scholar]
- 21.Varkonyi TT, Peto T, Degi R, Lengyel C, Janàky M, Kempler P, Lonovics J. Impairment of visual evoked potentials: an early central manifestation of diabetic neuropathy? Diabetes Care. 2002;25(9):1661–1662. doi: 10.2337/diacare.25.9.1661. [DOI] [PubMed] [Google Scholar]
- 22.Ozdek S, Lonneville YH, Onol M, Yetkin I, Hasanreiso_lu BB. Assessment of nerve fiber layer in diabetic patients with scanning laser polarimetry. Eye. 2002;16(6):761–765. doi: 10.1038/sj.eye.6700207. [DOI] [PubMed] [Google Scholar]
- 23.Han Y, Bearse MA, Jr, Schneck ME, Barez S, Jacobsen CH, Adams AJ. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45(3):948–954. doi: 10.1167/iovs.03-1101. [DOI] [PubMed] [Google Scholar]
- 24.Tikellis C, Cooper ME, Twigg SM. Connective tissue growth factor is up-regulated in the diabetic retina: amelioration by angiotensin-converting enzyme inhibition. Endocrinology. 2004;145(2):860–866. doi: 10.1210/en.2003-0967. [DOI] [PubMed] [Google Scholar]
- 25.Kuiper EJ, Witmer AN, Klaassen I, Oliver N, Goldschmeding R, Schlingemann RO. Differential expression of connective tissue growth factor in microglia and pericytes in the human diabetic retina. Br J Ophthalmol. 2004;88(8):1082–1087. doi: 10.1136/bjo.2003.032045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuma K, Naruse K, Suzuma I, Takahara N, Ueki K, Aiello LP, King GL. Vascular endothelial growth factor induces expression of connective tissue growth factor via KDR, Flt1, and phosphatidylinositol 3-kinase-akt-dependent pathways in retinal vascular cells. J Biol Chem. 2000;275(52):40725–40731. doi: 10.1074/jbc.M006509200. [DOI] [PubMed] [Google Scholar]
- 27.Lorenzi M, Gerhardinger C. Early cellular and molecular changes induced by diabetes in the retina. Diabetologia. 2001;44(7):791–804. doi: 10.1007/s001250100544. [DOI] [PubMed] [Google Scholar]
- 28.Hishikawa K, Nakaki T, Fujii T. Connective tissue growth factor induces apoptosis via caspase 3 in cultured human aortic smooth muscle cells. Eur J Pharmacol. 2000;392:19–22. doi: 10.1016/s0014-2999(00)00115-1. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Yang R, Tinner B, Choudhry A, Schutze N, Chaqour B. Cysteine-rich protein 61 and connective tissue growth factor induce deadhesion and anoikis of retinal pericytes. Endocrinology. 2008;149(4):1666–1677. doi: 10.1210/en.2007-1415. [DOI] [PubMed] [Google Scholar]
- 30.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47:253–262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- 31.Layton CJ, Chidlow G, Casson RJ, Wood JP, Graham M, Osborne NN. Monocarboxylate transporter expression remains unchanged during the development of diabetic retinal neuropathy in the rat. Invest Ophthalmol Vis Sci. 2005;46(8):2878–2885. doi: 10.1167/iovs.04-1458. [DOI] [PubMed] [Google Scholar]
- 32.Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- 33.Joussen AM, Poulaki V, Mitsiades N, Cai WY, Suzuma I, Pak J, Ju ST, Rook SL, Esser P, Mitsiades CS, Kirchhof B, Adamis AP, Aiello LP. Suppression of Fas-FasL-induced endothelial cell apoptosis prevents diabetic blood-retinal barrier breakdown in a model of streptozotocin-induced diabetes. FASEB J. 2003;17(1):76–78. doi: 10.1096/fj.02-0157fje. [DOI] [PubMed] [Google Scholar]
- 34.Shimo T, Nakanishi T, Nishida T, Asano M, Sasaki A, Kanyama M, Kuboki T, Matsumura T, Takigawa M. Involvement of CTGF, a hypertrophic chondrocyte-specific gene product, in tumor angiogenesis. Oncology. 2001;61(4):315–322. doi: 10.1159/000055339. [DOI] [PubMed] [Google Scholar]
- 35.Cooper ME, Bonnet F, Oldfield M, Jandeleit-Dahm K. Mechanisms of diabetic vasculopathy: an overview. Am J Hypertens. 2001;14:475–486. doi: 10.1016/s0895-7061(00)01323-6. [DOI] [PubMed] [Google Scholar]