Abstract
AIM
To observe the differences of damage patterns of retinal nerve fiber layer (RNFL) between acute and chronic intraocular pressure (IOP) elevation in primary angle closure glaucoma (PACG) using optical coherence tomography (OCT).
METHODS
Twenty-four patients (48 eyes) with unilateral acute PACG (APACG) attack in the 6 months after admission and 36 patients (64 eyes) with chronic PACG (CPACG) were included in this prospective study. For all cases, IOP has been controlled under 21mmHg after treatment. Using stratus OCT, the RNFL thickness was assessed in eyes with PACG within 3 days, 2 weeks, 1, 3 and 6 months after IOP was controlled. Repeated measures ANOVA was used to examine the changes of RNFL thickness at different time after IOP being controlled in both acute attack eyes and unaffected fellow eyes of APACG and eyes with CPACG.
RESULTS
The mean RNFL thickness for the APACG-attacked eyes increased significantly within 3 days (121.49±23.84)µm after acute onset and then became thinner along with time [(107.22±24.72)µm at 2 weeks,(93.58±18.37)µm at 1 month, (84.10±19.89)µm at 3 months and (78.98±19.17)µm at 6 months]. In APACG-attacked eyes, there were significant differences of average RNFL thickness at 5 different times after IOP was controlled (P<0.001). In the APACG unaffected fellow eyes and CPACG eyes, there were no significant differences in mean RNFL thickness at 5 different times(F=0.450, P=0.104 in APACG unaffected fellow eyes and F=1.558, P=0.200 in CPACG eyes). There was significant difference for interaction between time periods and groups (F=1.912, P=0.003).
CONCLUSION
RNFL damage patterns are different under different IOP elevated courses. In APACG, RNFL was found to be swollen and thickening right after acute attack and then becomes thinning and atrophy along with the time, while RNFL was found to be diffused thinness in CPACG.
Keywords: optical coherence tomography, glaucoma, closed angle, retina, nerve fiber
INTRODUCTION
Glaucoma is the second ocular disease leading to blindness in the world, and it is a progressive optic neuropathy. Glaucomatous fundus morphological changes include glaucomatous optic nerve damage and retinal nerve fiber layer (RNFL) defect which are associated with functional deficit, such as visual field loss. As far as we know, intraocular pressure (IOP) is the major risk reason for the glaucomatous optic nerve damage[1]. It has been shown that RNFL defect precedes visual field loss in primary open angle glaucoma (POAG). Thus RNFL assessment has already become an important parameter for preperimetric diagnosis of POAG and aid opthalmologists to detect the progress of optic nerve damage in glaucoma.
Previous studies about the measurement of RNFL thickness by optical coherence tomography (OCT) mostly focus on diagnosis of POAG[2] but there are few reports concerning about the changes of RNFL in primary angle closure glaucoma(PACG). Furthermore, no data are available for the pathophysiological changes of RNFL under acute and chronic increased IOP courses. In this article, we conducted a comparative study to evaluate the different RNFL damage patterns in acute primary angle closure glaucoma (APACG) and chronic primary angle closure glaucoma (CPACG), in order to find out the difference of RNFL damage patterns between acute and chronic IOP increased in PACG.
MATERIALS AND METHODS
Subjects
Twenty four patients (48 eyes) with AACG and 36 patients (64 eyes) with CCAG were included in this study. All these subjects were successively recruited from Glaucomatous Department of Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China between April 2008 and July 2009. After the procedures and possible outcomes were explained, informed consent was obtained from all patients. The procedures used were conformed to the tenets of the Declaration of Helsinki.
The subjects were classified according to the classification proposed by Kim and Jung[3]. The inclusion criteria for APACG in this study were as follows: an unilateral acute attack accompanied by ocular pain, decreased visual acuity, nausea and vomiting, an elevated IOP over 40mmHg, conjunctival injection, sector atrophy of the iris or corneal epithelial edema, moderate dilated pupil, a closed angle on gonioscopy and the cases with or without glaucomatous visual field damage were included. Criteria for the diagnosis of CPACG were as follows: no history of acute attack, an elevated IOP over 21mmHg, angle closure with peripheral anterior synechiae, and cases with or without glaucomatous visual field damage.
The exclusion criteria were as follows: 1) bilateral attack or acute attack of the fellow eye in the past of APACG subjects; 2) secondary angle closure glaucoma; 3) refraction≥±5.0 D (sphere) and/or 3.0D (cylinder); 4) previous intraocular surgery and other ocular disease and 5) poor-quality of OCT images in which the scanning algorithm failed.
Methods
For APACG subjects, the acute attacks were completely managed by medical therapy followed by peripheral iridotomy or trabeculectomy, and the study was conducted during the follow-up period after the acute episode. For CPACG subjects, peripheral iridotomy or trabeculectomy were performed according to the extent of peripheral anterior synechia (PAS). For all the included subjects, if the IOP rose above 21mmHg at the study visits, tropical anti-glaucoma medications were allowed to use to control the IOP within the level of 21mmHg. There were 5 scheduled study visits: the first was within 3 days after IOP was controlled, and again at 2 weeks, 1, 3 and 6 months afterward. At each visit, all subjects underwent standard visual acuity assessment, IOP measurement with Goldmann applanation tonometry, examination of slit-lamp and ophthalmoscopy. Subjects then underwent OCT examination for RNFL thickness measurement.
RNFL thickness imaging of all study subjects was obtained by the same experienced full-time operators using the Stratus OCT 3000 (Version 4.0.2; Carl Zeiss Meditec, Inc, Dublin, CA). Peripapillary RNFL thickness was automatically calculated by fast RNFL thickness (3.4) of Stratus OCT 3000. Only high-quality images with signal strength of 8 or greater (maximum 10) were included. The following mean RNFL thickness was analyzed: average, superior, nasal, inferior and temporal quadrants.
Statistical Analysis
The statistical analysis was performed using the SPSS version 13.0 for Windows commercial statistical software package (SPSS, Inc, Chicago, IL). Repeated measures ANOVA was used to examine the changes of RNFL thickness at various time points after IOP was controlled in both acute attack eyes and unaffected fellow eyes of APACG and eyes with CPACG. All analyses were conducted at the 0.05 significance level.
RESULTS
The characteristics of the study population were listed in Table 1. This study enrolled 48 eyes of 24 patients with unilateral APACG and 64 eyes of 36 patients with CPACG. There were 6 males and 18 females in APACG group, and 12 males and 24 females in CPACG group; The average age were (58.48±12.43) years in APACG group and (58.86±10.53) years in CPACG group. The statistical differences in the male to female ratio and age were not significant between two groups. The IOP belonged to the APACG and CPACG groups of the 5 scheduled visits were shown in Table 1. No significant statistical differences were found in IOP at any visits between two groups.
Table 1. Comparisons of the characteristics between APACG and CPACG groups.
| Characteristic | APACG Group |
CPACG Group | P | |
| Attacked Eyes | Fellow Eyes | |||
| Male / Female | 6/18 | 12/24 | 0.99 | |
| Age (years) | 58.48±12.43 | 58.86±10.53 | 0.80 | |
| IOP(mmHg) | ||||
| Within 3 days | 16.60±3.08 | 12.13±3.51 | 16.74±4.41 | 0.37 |
| 2 weeks | 12.14±5.22 | 12.74±3.20 | 12.20±2.76 | 0.47 |
| 1 month | 13.38±5.41 | 14.19±3.07 | 11.17±2.26 | 0.52 |
| 3 months | 10.51±6.19 | 13.22±3.85 | 13.08±3.61 | 0.20 |
| 6 months | 13.86±5.24 | 14.80±4.23 | 12.50±3.38 | 0.75 |
APACG: acute primary angle closure glaucoma; CPACG: chronic angle closure glaucoma; IOP: intraocular pressure
Except the RNFL thickness in the temporal quadrant at 1 month visit, the RNFL thickness of temporal, superior, nasal, inferior and average showed a statistically significant difference among attack eyes, fellow eyes of APACG group and eyes of CPACG, respectively (Table 2) at the five different visits.
Table 2. Comparisons of the RNFL thickness between APACG and CPACG groups.
| Parameters and Time Points | APACG Group |
CPACG Group | P | |
| Attacked Eyes | Fellow Eyes | |||
| Within 3 days | ||||
| Nasal | 96.18±33.76 | 75.63±14.54 | 65.48±17.59 | <0.001 |
| Superior | 160.41±40.41 | 128.19±15.29 | 92.48±32.87 | <0.001 |
| Temporal | 73.65±15.19 | 72.94±11.55 | 63.70±17.80 | <0.001 |
| Inferior | 155.35±33.25 | 135.56±11.96 | 95.14±38.64 | <0.001 |
| Average | 121.49±23.84 | 103.10±10.08 | 80.61±23.96 | <0.001 |
| 2 weeks | ||||
| Nasal | 82.35±35.01 | 72.56±19.74 | 64.13±17.04 | <0.001 |
| Superior | 134.76±37.55 | 123.25±18.42 | 93.92±35.93 | <0.001 |
| Temporal | 70.94±15.52 | 69.75±11.29 | 61.33±17.11 | <0.001 |
| Inferior | 140.94±34.38 | 135.00±15.56 | 93.63±40.48 | <0.001 |
| Average | 107.22±24.72 | 100.11±12.16 | 78.34±25.17 | <0.001 |
| 1 month | ||||
| Nasal | 72.88±17.20 | 77.75±20.40 | 65.81±18.69 | 0.031 |
| Superior | 113.76±28.17 | 124.69±21.17 | 97.00±34.93 | <0.001 |
| Temporal | 63.06±14.01 | 68.63±7.17 | 62.32±17.00 | 0.114 |
| Inferior | 124.53±28.23 | 137.94±18.59 | 95.20±38.59 | <0.001 |
| Average | 93.58±18.37 | 107.86±26.33 | 80.11±24.79 | <0.001 |
| 3 months | ||||
| Nasal | 66.94±16.65 | 79.50±17.55 | 63.20±18.14 | <0.001 |
| Superior | 101.94±31.71 | 128.13±16.07 | 89.59±32.21 | <0.001 |
| Temporal | 59.53±13.02 | 71.00±9.28 | 62.62±17.06 | 0.023 |
| Inferior | 107.88±30.70 | 136.44±17.13 | 90.49±38.11 | <0.001 |
| Average | 84.10±19.89 | 103.85±11.10 | 76.31±24.00 | <0.001 |
| 6 months | ||||
| Nasal | 60.35±16.38 | 77.56±16.16 | 64.04±16.44 | <0.001 |
| Superior | 97.59±25.94 | 124.94±15.72 | 89.14±31.65 | <0.001 |
| Temporal | 58.00±13.43 | 72.63±9.06 | 60.90±16.86 | <0.001 |
| Inferior | 99.71±30.25 | 133.44±18.14 | 90.14±38.17 | <0.001 |
| Average | 78.98±19.17 | 102.22±11.13 | 76.98±24.65 | <0.001 |
APACG: acute primary angle closure glaucoma; CPACG: chronic angle closure glaucoma; RNFL: retinal nerve fiber layer
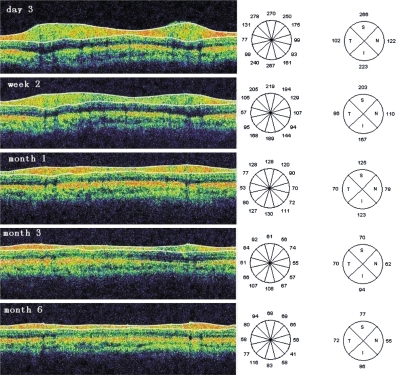
In eyes with APACG attacks, there were significant differences of average RNFL thickness among 5 different times(within 3 days, at 2 weeks, 1, 3 and 6 months) after IOP was controlled (F=11.14, P<0.001=. Moreover, the same significant differences were also found in nasal, superior, temporal and inferior RNFL thickness in APACG-affected eyes (F=5.526, P=0.008 in nasal, F=7.662, P=0.002 in superior, F=16.025, P =0.008 in temporal and F=7.97, P=0.002 in inferior quadrant). By repeated measures ANOVA with least significant difference, there were significant differences at five different times after the episodes (Figure 1).
Figure 1. RNFL thickness in OCT images.
These images belonged to the attacked eye of a 55-year-old female patient with acute primary angle closure glaucoma. These images were taken at 3 days, 2 weeks, 1, 3 and 6 months after acute attack was controlled. The average RNFL (µm) thickness were 178.28 at day 3, 141.40 at week 2, 99.01 at month 1, 74.04 at month 3, and 67.20 at month 6
In the APACG unaffected fellow eyes and CPACG eyes, there were no significant differences in average RNFL thickness at five different time points (F=0.450, P=0.104 in APACG unaffected fellow eyes and F=1.558, P=0.200 in CPACG eyes). And there were no significant differences in each quadrant's RNFL thickness in APACG-unaffected fellow eyes (P>0.05) and CPACG eyes during five visits with least significant difference (P>0.05).
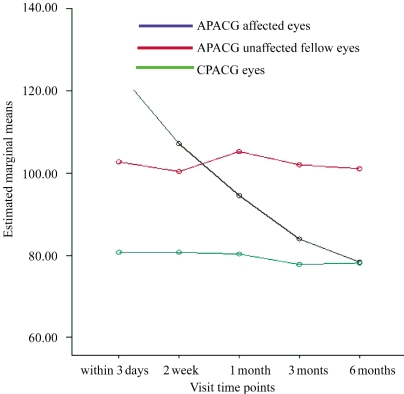
Figure 2 showed the change curves of average RNFL thickness with time of APACG affected eyes, fellow eyes and CPACG eyes. There was significant difference for interaction between time periods and groups (F=1.912, P = 0.003). This indicated that the changes of RNFL thickness of the APACG affected, fellow eyes and CPACG eyes were different.
Figure 2. The change curves of average RNFL thickness with time of acute primary angle closure glaucoma affected eyes, fellow eyes and chronic primary angle closure glaucoma eyes.
DISCUSSION
OCT-derived RNFL thickness, though different from actual histological measurement, has been shown to possess good correlation with histological measurement[4]. Various imaging technologies have been introduced for an objective assessment of RNFL, and using these current test methods such as fundus photography, retina analyzer and laser polarimetry (SLP, GDx-VCC), loss of optic nerve fibers are found to precede detectable visual field[5]-[7]. Quigley et al[8] showed that up to 40% to 50% of optic nerve fibers could be lost in the absence of a visual field defect. In POAG, the IOP raised pattern displays a mild progression, which is the same situation in the RNFL damage pattern. It has already been reported that RNFL thickness gradually decreases while visual field defect increases with the development of POAG, and RNFL thickness has a good correlation with visual field defect[2],[9].
The pattern and severity of RNFL damage is not merely a function of a “snapshot” IOP alone, it is progressive and related to the duration of the insult[10]. As a result, developing glaucomatous RNFL damage at different IOP raised courses may vary from different types of glaucoma. As we know, although APACG and CPACG have the similar anatomical structure, they present different IOP elevated courses. If the pupillary block is marked, pronounced iris bombe leads to complete appositional obstruction of the trabecular meshwork and thus IOP is increased rapidly resulting in the development of APACG, which is accompanied by ocular pain, headache, and blurred vision. On the other hand, CPACG is the glaucoma in which the peripheral iris is in apposition to the trabecular meshwork with a gradual progression of the adhesion and IOP is raised gradually, with subjective symptoms rarely present which IOP elevation is similar to the pattern of POAG[11]. So PACG would be a nice choice to investigate the different pattern of RNFL damage under acute or chronic IOP elevated patterns. As patients with PACG are not allowed to perform OCT (OCT1 and OCT2) scans, there are very few studies focus on the damage pattern of RNFL in PACG. Now Stratus OCT (OCT3) is a convenient invention for patients with PACG, since it can be performed under natural pupil. It has been reported that if the pupillary diameter is not less than 3mm, Stratus OCT-derived RNFL thickness is not affected by the pupillary diameter[12]. Therefore, ordering to get the RNFL thickness of the patients with PACG, we measured RNFL thickness of PACG eyes by Stratus OCT under natural pupil condition in this article.
Most of the previous studies in evaluating the optic nerve damage pattern of PACG mainly focus on the visual field examination. Because the visual acuity is often low after APACG attack, it makes visual field examination difficult and unreliable in these patients. Furthermore, as visual field examination is a kind of psychophysical examination, its result is affected by subjective factors.
Although visual field damage under automated perimetry may not be found in the relatively early period after remission of an APACG attack, such acute stress can lead to the RNFL changes[13],[14]. Our study showed that the change of RNFL after an APACG attack was a dynamic process. The RNFL of APACG-attacked eyes were observed to be edema and the RNEL thickness increased significantly than that of the unaffected fellow eyes right after an episode. Two weeks later, the edema of RNFL subsidised and the RNEL thickness decreased to a level nearly to that of the fellow eyes. One month after acute attack, the RNFL thickness decreased progressively till to 6 month, which suggested that the damage of RNFL happened immediately after APACG attack. Similar to POAG, the IOP elevation in CPACG presents a more relative moderate course than in APACG. The increased IOP can cause the apoptosis of ganglion cells and thinness of RNFL thickness. Although both of the RNFL damage in CPACG and APACG are caused by pathological IOP elevation, the former shows a much rather moderate course. In this study, we found that the RNFL thickness in CPACG was decreased before IOP controlled, which was similar to that of POAG. After IOP controlled, the RNFL thickness was stable.
Since it is formidable to observe the histological and structural changes of RNFL in human eyes with PACG in vivo, most theories about how the RNFL damage in PACG almost derive from animal experiments or autopsy. Degeneration of axons, near and within the lamina cribosa, is thought by many investigators to be the basic damage in glaucoma. In APACG, the abrupt IOP elevation compress the vessels in the prelaminar region[15]. With the reduction in optic nerve head blood flow, there are irreversible ischemic and hypoxia damage and reperfusion injury to the optic disk and axons. Low perfusion pressure (blood pressure minus IOP), without causing infarction, interferes with nutrition and the normal physiology of axons in the area of lamina cribosa[16]-[20]. Reduced blood supply also interferes with glial astrocytic transmission from capillaries of nutritions, such as adenosine triphosphate, essential to the physiology of axons[21],[22]. As mechanical compression and vascular insufficiency prolongs, rapid orthograde and retrograde axonal transport is decreased within the optic nerve head at the level of the scleral lamina cribrosa[23],[24]. This decrease is not due to a simple slowdown of transport, but results from a total block of rapid transport in some axons, and without impairment in other axons, which cause axons swelling and papilla edema. Knox et al[25] explained such optic nerve edema phenomenon after sudden IOP elevation was the result of blockage at the lamina cribosa where axons cannot distend because of dense connective tissue and that the continued inflow of axoplasmic substance causes swellings. The ganglion cells are unable to tolerate the severe blockade of transport, and then axon death occurs. Our results also indicated that the RNEL was swollen immediately right after attack and then atrophy in APACG. The RNFL damage patterns under acute IOP elevation in PACG observed by OCT were similar to the results derived from animal experiments and autopsy. Different from APACG, the IOP elevation course is gradually and moderately in CPACG. The dysfunction of axonal transport is decreased rather than ceased. This may be the reason for why moderate IOP elevation did not cause retinal edema in CPACG. The image of OCT showed RNFL thickness significant thinning, especially in superior and inferior quadrants, which is likely to POAG[2]. And after IOP was controlled, the RNFL thickness does not decrease progressively.
In this study, we have used OCT to observe the RNFL damage patterns of PACG subject with or without acute attack, and we have found that the RNFL damage patterns are different in acute and chronic IOP elevated courses. In APACG, the RNFL appears to be swollen and thickening right after acute attack and then becomes thinning and atrophy along with time since the IOP elevates suddenly and abruptly. While in CPACG, the RNFL shows to be diffused thinness as the IOP elevates moderately and gradually. This detection of progressive changes in the RNFL thickness after APACG may offer some insight into the pathogenesis of damage caused by acute raised IOP in this condition. RNFL observation also is a better way to evaluate the prognosis of PACG.
REFERENCES
- 1.Araie M, Sekine M, Suzuki Y. Factors contributing to the progression of visual damage in eyes with normal tention glaucoma. Ophthalmology. 1994;101:1440–1444. doi: 10.1016/s0161-6420(94)31153-5. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Ling Y, Luo R, Ge J, Zheng X. Optical coherence tomography in measuring retinal nerve fiber layer thickness in normal subjects and patients with open-angle glaucoma. Chin Med J. 2001;114:524–529. [PubMed] [Google Scholar]
- 3.Kim YY, Jung HR. Clarifying the nomenclature for primary angle-closure glaucoma. Surv Ophthalmol. 1997;6:125–136. doi: 10.1016/s0039-6257(97)00023-4. [DOI] [PubMed] [Google Scholar]
- 4.Skaf M, Bernardes AB, Cardillo JA, Costa RA, Melo LA, Jr, Castro JC, Varma R. Retinal nerve fibre layer thickness profile in normal eyes using third-generation OCT. Eye. 2006;20:431–439. doi: 10.1038/sj.eye.6701896. [DOI] [PubMed] [Google Scholar]
- 5.Zangwill L, Berry CA, Garden VS, Weinreb RN. Reproducibility of retardation measurements with the nerve fibre analyzer II. J Glaucoma. 1997;6:384–389. [PubMed] [Google Scholar]
- 6.Niessen AG, Van Den Berg TJ, Langerhorst CT, Greve EL. Retinal nerve fiber layer assessment by scanning laser polarimetry and standardized photography. Am J Ophthalmol. 1996;121:484–493. doi: 10.1016/s0002-9394(14)75422-4. [DOI] [PubMed] [Google Scholar]
- 7.Weinreb RN, Shakiba S, Zangwill L. Scanning laser polarimetry to measure the nerve fiber layer of normal and glaucomatous eyes. Am J Ophthalmol. 1995;119:627–636. doi: 10.1016/s0002-9394(14)70221-1. [DOI] [PubMed] [Google Scholar]
- 8.Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema and toxic neuropathy. Arch Ophthalmol. 1982;100:135–146. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- 9.Sihota R, Sony P, Gupta V, Dada T, Singh R. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci. 2006;47:2006–2010. doi: 10.1167/iovs.05-1102. [DOI] [PubMed] [Google Scholar]
- 10.Jay JL, Murdoch JR. The rate of visual field loss in untreated primary open angle glaucoma. Br J Ophthalmol. 1993;77:176–178. doi: 10.1136/bjo.77.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon KC, Won LD, Cho HJ, Yang KJ. Ultrasound biomicroscopic changes after laser iridotomy or trabeculectomy in angle-closure glaucoma. Korean J Ophthalmol. 2004;18:9–14. doi: 10.3341/kjo.2004.18.1.9. [DOI] [PubMed] [Google Scholar]
- 12.Huang JJ, Liu X, Zeng YF, Zheng XP. Effect of pupil size on measurement of retinal nerve fiber layer thickness using optical coherence tomography. Journal of Sun Yat-sen University (med) 2006;27:212–215. [Google Scholar]
- 13.Msagoff Z, Aung T, Ang LP, Chew PT. Long-term clinical course of primary angle-closure glaucoma in an Asian population. Ophthalmology. 2000;107:2300–2304. doi: 10.1016/s0161-6420(00)00385-7. [DOI] [PubMed] [Google Scholar]
- 14.Aung T, Ang LP, Chan SP, Chew PT. Acute primary angle-closure: long-term intraocular pressure outcome in Asian eyes. Am J Ophthalmol. 2001;131:7–12. doi: 10.1016/s0002-9394(00)00621-8. [DOI] [PubMed] [Google Scholar]
- 15.Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and edema of the optic disc. Br J Ophthalmol. 1969;53:721–748. doi: 10.1136/bjo.53.11.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–783. [PubMed] [Google Scholar]
- 17.Quigley H, Anderson DR. The dynamics and location of axonal transport blockade by acute intraocular pressure elevation in primate optic nerve. Invest Ophthalmol. 1976;15:606–616. [PubMed] [Google Scholar]
- 18.Minckler DS, Bunt AH, Johnson GW. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Invest Ophthalmol Vis Sci. 1977;16:426–441. [PubMed] [Google Scholar]
- 19.Minckler DS, Bunt AH, Klock IB. Radiographic and cytochemical ultrastructural studies of axoplasmic transport in the monkey optic nerve head. Invest Ophthalmol Vis Sci. 1978;17:33–50. [PubMed] [Google Scholar]
- 20.Minckler DS. Correlation between anatomic features and axonal transport in primate optic nerve head. Trans Am Ophthalmol Soc. 1986;84:429–451. [PMC free article] [PubMed] [Google Scholar]
- 21.Lampert PW, Vogel MH, Zimmerman LE. Pathology of the optic nerve in experimental acute glaucoma. Electron microscopic studies. Invest Ophthalmol. 1968;7:199–213. [PubMed] [Google Scholar]
- 22.Lampert PW. A comparative electron microscopic study of reactive, degenerating, regenerating and dystrophic axons. J Neuropathol Exp Neurol. 1967;26:345–368. doi: 10.1097/00005072-196707000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Quigley HA, Anderson DR. Distribution of axonal transport blockade by acute intraocular pressure elevation in the primate optic nerve head. Invest Ophthalmol Vis Sci. 1977;16:640–644. [PubMed] [Google Scholar]
- 24.Quigley HA, Guy J, Anderson DR. Blockade of rapid axonal transport: effect of intraocular pressure elevation in primate optic nerve. Arch Ophthalmol. 1979;97:525–531. doi: 10.1001/archopht.1979.01020010269018. [DOI] [PubMed] [Google Scholar]
- 25.Knox DL, Eagle RC, Jr, Green WR. Optic nerve hydropic axonal degeneration and blocked retrograde axoplasmic transport: histopathologic features in human high-pressure secondary glaucoma. Arch Ophthalmol. 2007;125:347–353. doi: 10.1001/archopht.125.3.347. [DOI] [PubMed] [Google Scholar]